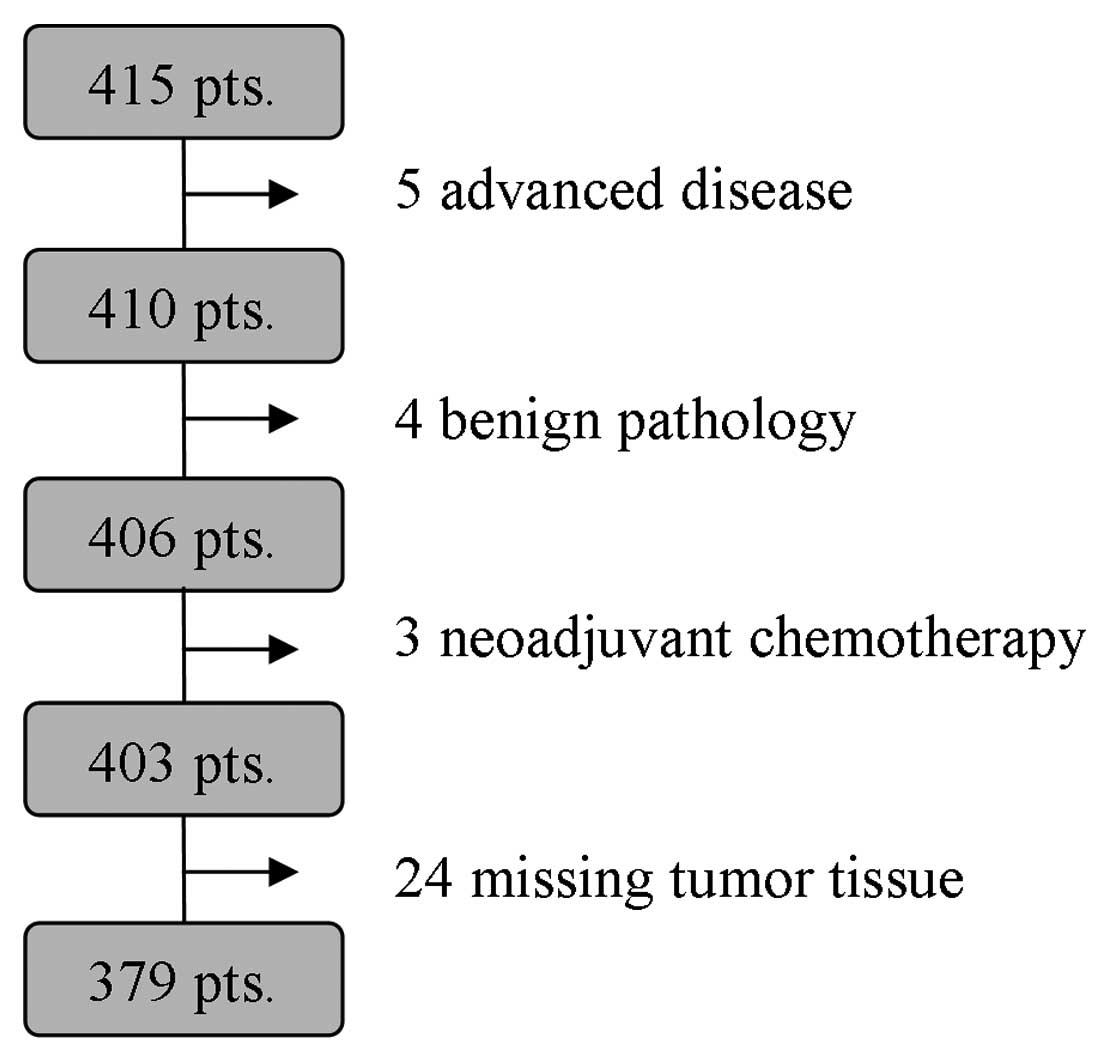
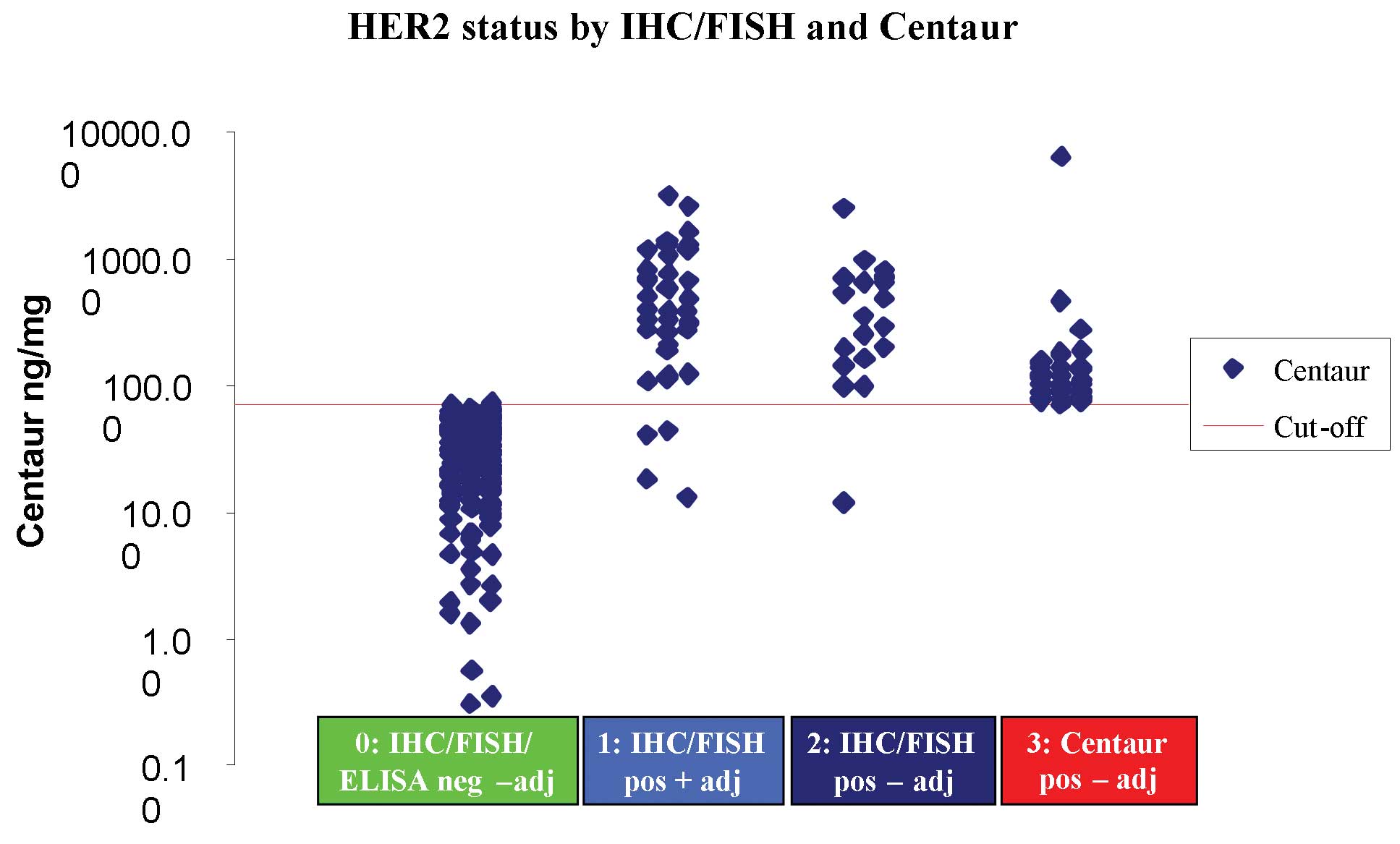
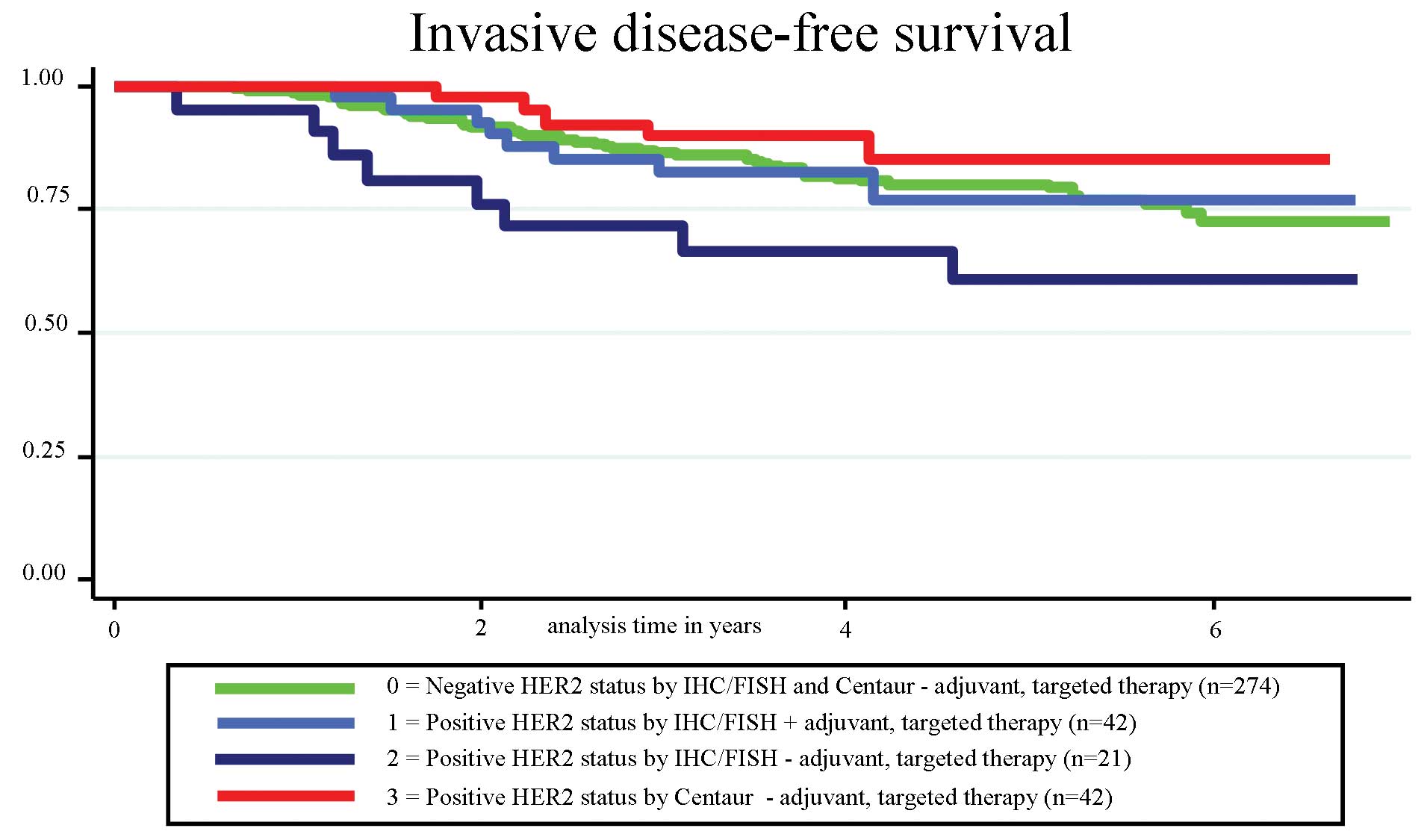
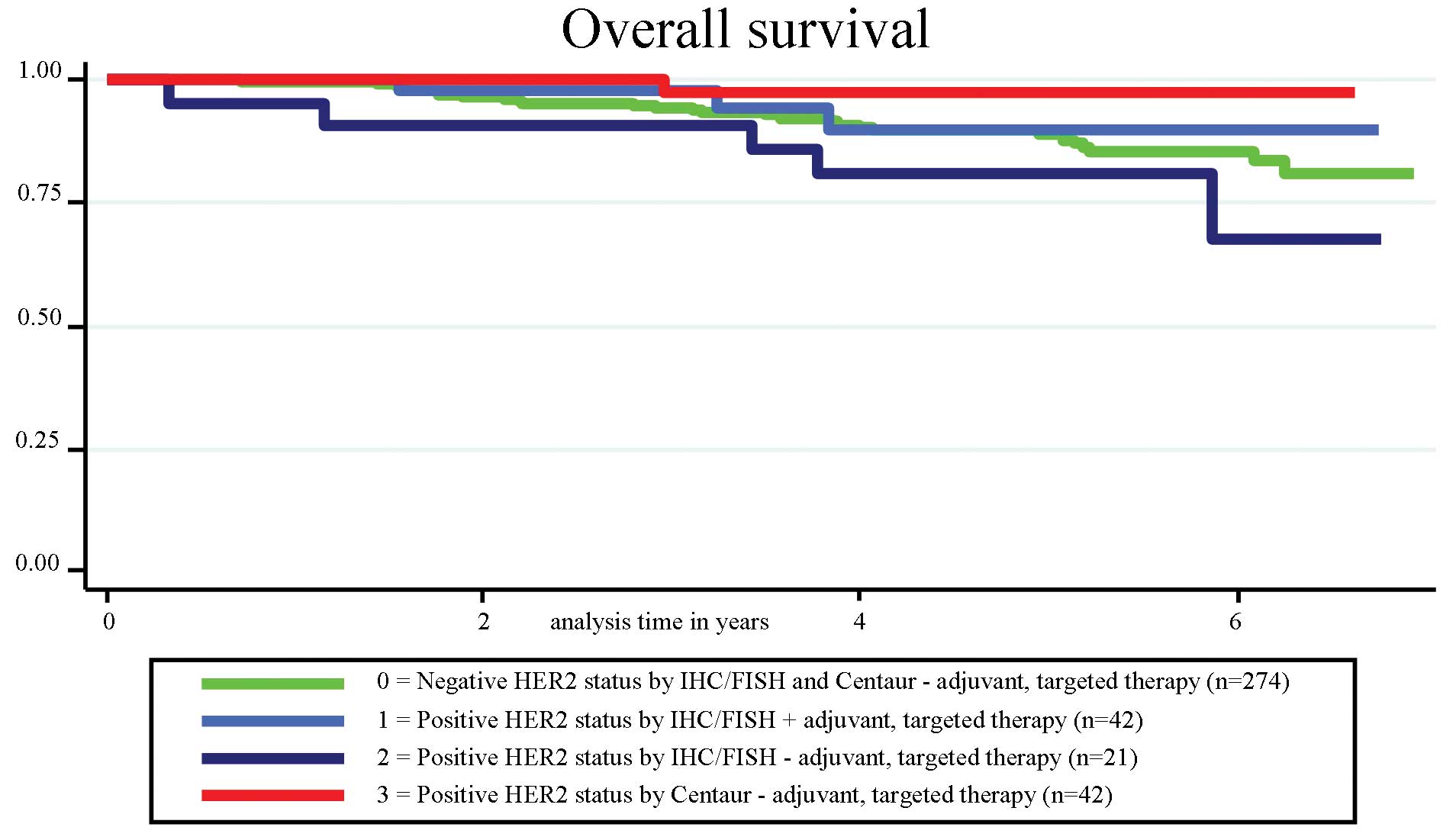
|
1
|
Kurebayashi J: Biological and clinical
significance of HER2 overexpression in breast cancer. Breast
Cancer. 8:45–51. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carney WP, Neumann R, Lipton A, et al:
Potential clinical utility of serum HER-2/neu oncoprotein
concentrations in patients with breast cancer. Clin Chem.
49:1579–1598. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pauletti G, Dandekar S, Rong H, et al:
Assessment of methods for tissue-based detection of the HER-2/neu
alteration in human breast cancer: a direct comparison of
fluorescence in situ hybridization and immunohistochemistry. J Clin
Oncol. 18:3651–3664. 2000.PubMed/NCBI
|
|
4
|
Slamon DJ: Human breast cancer:
correlation of relapse and survival with amplification of the
HER-2/neu oncogene. Science. 235:177–182. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Slamon DJ, Leyland-Jones B, Shak S, et al:
Use of chemotherapy plus a monoclonal antibody against HER2 for
metastatic breast cancer that overexpresses HER2. N Engl J Med.
344:783–792. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Piccart-Gebhart MJ, Procter M,
Leyland-Jones B, et al: Trastuzumab after adjuvant chemotherapy in
HER2-positive breast cancer. N Engl J Med. 353:1659–1672. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Romond EH, Perez EA, Bryant J, et al:
Trastuzumab plus adjuvant chemotherapy for operable HER2-positive
breast cancer. N Engl J Med. 353:1673–1684. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gong Y, Booser DJ and Sneige N: Comparison
of HER-2 status determined by fluorescence in situ hybridization in
primary and metastatic breast carcinoma. Cancer. 103:1763–1769.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zidan J, Dashkovsky I, Stayerman C, et al:
Comparison of HER-2 overexpression in primary breast cancer and
metastatic sites and its effect on biological targeting therapy of
metastatic disease. Br J Cancer. 93:552–556. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gancberg D, Di LA, Cardoso F, et al:
Comparison of HER-2 status between primary breast cancer and
corresponding distant metastatic sites. Ann Oncol. 13:1036–1043.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wolff AC, Hammond ME, Schwartz JN, et al:
American Society of Clinical Oncology/College of American
Pathologists guideline recommendations for human epidermal growth
factor receptor 2 testing in breast cancer. J Clin Oncol.
25:118–145. 2007. View Article : Google Scholar
|
|
12
|
Tse C, Brault D, Gligorov J, et al:
Evaluation of the quantitative analytical methods real-time PCR for
HER-2 gene quantification and ELISA of serum HER-2 protein and
comparison with fluorescence in situ hybridization and
immunohistochemistry for determining HER-2 status in breast cancer
patients. Clin Chem. 51:1093–1101. 2005.PubMed/NCBI
|
|
13
|
Bergqvist J, Ohd JF, Smeds J, et al:
Quantitative real-time PCR analysis and microarray-based RNA
expression of HER2 in relation to outcome. Ann Oncol. 18:845–850.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Egervari K, Toth J, Nemes Z and Szollosi
Z: An alternative and reliable real-time quantitative PCR method to
determine HER2/neu amplification in breast cancer. Appl
Immunohistochem Mol Morphol. 17:247–254. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Olsen DA, Ostergaard B, Bokmand S, et al:
HER-2 protein concentrations in breast cancer cells increase before
immunohistochemical and fluorescence in situ hybridization analysis
turn positive. Clin Chem Lab Med. 45:177–182. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hudis CA, Barlow WE, Costantino JP, et al:
Proposal for standardized definitions for efficacy end points in
adjuvant breast cancer trials: the STEEP system. J Clin Oncol.
25:2127–2132. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Olsen DA, Bechmann T, Ostergaard B, et al:
Increased concentrations of growth factors and activation of the
EGFR system in breast cancer. Clin Chem Lab Med. 50:1809–1818.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Konecny G, Untch M, Arboleda J, et al:
Her-2/neu and urokinase-type plasminogen activator and its
inhibitor in breast cancer. Clin Cancer Res. 7:2448–2457.
2001.PubMed/NCBI
|
|
19
|
Dowsett M, Procter M, Caskill-Stevens W,
et al: Disease-free survival according to degree of HER2
amplification for patients treated with adjuvant chemotherapy with
or without 1 year of trastuzumab: the HERA Trial. J Clin Oncol.
27:2962–2969. 2009.PubMed/NCBI
|
|
20
|
Gilcrease MZ, Woodward WA, Nicolas MM, et
al: Even low-level HER2 expression may be associated with worse
outcome in node-positive breast cancer. Am J Surg Pathol.
33:759–767. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Viale G: Be precise! The need to consider
the mechanisms for CEP17 copy number changes in breast cancer. J
Pathol. 219:1–2. 2009.
|
|
22
|
Müller V, Thomssen C, Karakas C, et al:
Quantitative assessment of HER-2/neu protein concentration in
breast cancer by enzyme-linked immunosorbent assay. Int J Biol
Markers. 18:13–20. 2003.PubMed/NCBI
|
|
23
|
Palmieri C, Shah D, Krell J, et al:
Management and outcome of HER2-positive early breast cancer treated
with or without trastuzumab in the adjuvant trastuzumab era. Clin
Breast Cancer. 11:93–102. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tovey SM, Brown S, Doughty JC, et al: Poor
survival outcomes in HER2-positive breast cancer patients with
low-grade, node-negative tumours. Br J Cancer. 100:680–683. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sawaki M, Mukai H, Tokudome N, et al:
Safety of adjuvant trastuzumab for HER-2-overexpressing elderly
breast cancer patients: a multicenter cohort study. Breast Cancer.
19:253–258. 2012. View Article : Google Scholar : PubMed/NCBI
|