Introduction
Breast cancer is the leading cancer among women in
the industrialized world and 15–20% of breast cancer tumors feature
an overexpression and/or amplification of human epidermal growth
factor receptor-2 (HER2). HER2, also termed HER2/neu, ErbB2 or
p185HER2, is one of four tyrosine kinase receptors of the HER
family which includes HER1 (EGFR), HER2, HER3 and HER4. The HER2
gene is located on chromosome 17 and encodes HER2, which is a
185-kDa glycoprotein composed of an intracellular tyrosine kinase
domain, a transmembrane domain, and an extracellular domain with a
yet unknown ligand (1). Activation
of the HER2 pathway is presumably driven by the binding of
heregulins to HER3 and HER4 or EGF to HER1 and the subsequent
hetero-dimerization with HER2, which leads to the activation of the
downstream pathway (2).
Overexpression of the HER2 protein and/or
amplification of the HER2 gene leads to tumor cell proliferation
and is associated with an aggressive tumor and a poor prognosis
(3,4). Furthermore, HER2
overexpression/amplification predicts the effect of HER2-targeted
therapy (e.g., trastuzumab and lapatinib) in combination with
chemotherapy in both the metastatic and adjuvant setting, and
several studies have demonstrated that the addition of trastuzumab
reduces the risk of recurrence in HER2-positive breast cancer
patients by approximately 50% (5–7).
Several studies have reported the discordance of
HER2 status between primary and recurrent disease years after the
primary treatment in 3–14% of the cases (8–10).
This has led to the hypothesis that an additional group of patients
may benefit from HER2-targeted therapy in the adjuvant setting.
However, it remains unclear whether the observed discordance of
HER2 status is due to heterogeneity of the primary tumor, acquired
HER2 expression during the course of the disease, or limited
sensitivity of the assay leading to misclassification of a modest,
but clinically relevant HER2 overexpression. Currently, patients
are selected for adjuvant HER2-targeted therapy based on
immunohistochemistry (IHC) and fluorescence- or chromogenic in
situ hybridization (FISH, CISH) (11). In addition to these
semi-quantitative tests, quantitative real-time PCR and
microarray-based RNA expression analysis of HER2 have emerged over
the past decade, delivering quantitative estimates of HER2 DNA and
RNA expression (12–14).
In this study, tissue HER2 status was determined by
a quantitative immunoassay using ADVIA Centaur. This assay is able
to analyze a larger tumor amount, whereby the influence of tumor
heterogeneity is reduced compared to IHC/FISH. By using this
method, an additional 9% of patients were classified as
HER2-positive compared to the conventional IHC/FISH methods as
reported in a previous study by Olsen et al(15). The clinical relevance of this
information however, is unknown and therefore, the aim of the
present study was to perform a clinical evaluation of the
quantitative Centaur assay. We wished to examine the hypothesis
that the clinical outcome is poorer in a group of patients defined
as tissue HER2-positive by Centaur only, but not treated with
adjuvant HER2-targeted therapy, compared to patients defined as
HER2-positive by IHC/FISH and therefore treated with adjuvant
HER2-targeted therapy.
Patients and methods
Study population and patient samples
This prospective cohort study was performed in a
single center cancer hospital. Women eligible for primary surgery
for breast cancer stages I-IIIA were included after written
informed consent. The study was approved by the Regional Scientific
Ethics Committee for Southern Denmark (project identification
number S-VF-20040101). Tumor tissue samples and autologous
reference tissue samples were obtained from 415 breast cancer
patients between 2004 and 2010. Surgery was performed in accordance
with the guidelines from the Danish Breast Cancer Cooperative Group
(DBCG).
The tissue samples were obtained within 1 h after
surgery. One part of the sample was fixed in formalin and
paraffin-embedded for IHC/FISH analysis. The other part of the
sample was snap-frozen in liquid nitrogen and stored in a local
biobank at −80°C for later Centaur analysis. A dedicated
pathologist verified the presence of tumor tissue in the tumor
sample and the lack of tumor tissue in the autologous reference
tissue. Reference tissue was taken at least 1 cm away from tumor
tissue whenever possible.
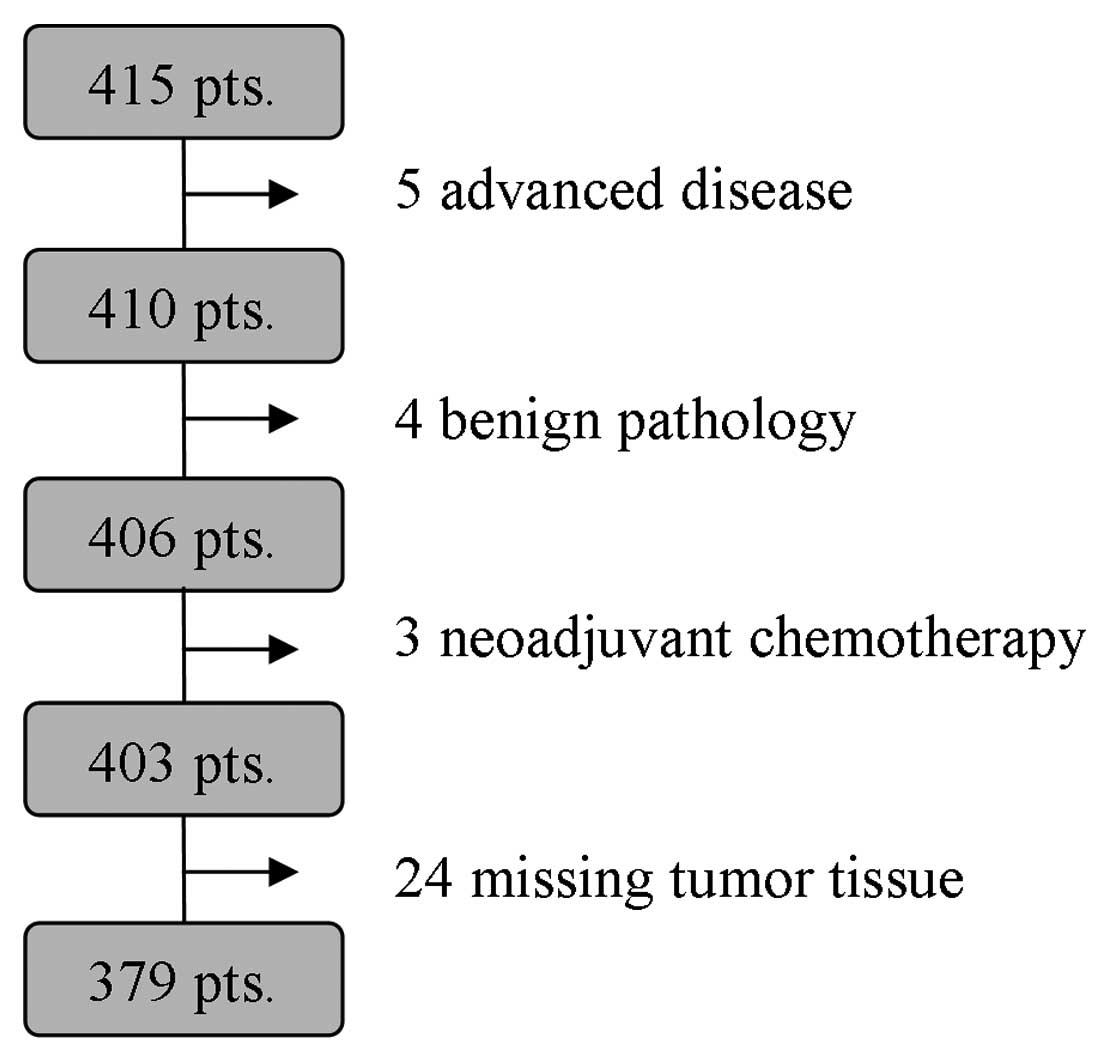
A total of 36 patients were excluded from the final
analysis due to advanced disease, benign pathology, neoadjuvant
chemotherapy, or missing tumor tissue (flow diagram, Fig. 1). The mean age of the remaining 379
patients was 60 years (34–91 years). A total of 272 patients
(71.8%) were treated with breast-conserving surgery, while 107
(28.2%) had a mastectomy. Only 17 patients (4.5%) had bilateral
synchronous breast cancer. The majority of patients (330 patients,
87.1%) had invasive ductal carcinoma, 25 patients (6.6%) had
invasive lobular carcinoma, and 24 patients (6.3%) had other types
of breast cancer.
End points
End points were defined according to the standard
definitions by Hudis et al(16) Invasive disease-free survival (IDFS)
was defined as the time from primary surgery to one of the
following events: ipsilateral invasive breast tumor recurrence,
regional invasive breast cancer recurrence, distant recurrence,
death by any cause, contralateral invasive breast cancer, or second
primary non-breast invasive cancer. This definition excludes all
types of carcinoma in situ [ductal carcinoma in situ
(DCIS), lobular carcinoma in situ (LCIS) and all in
situ cancers of non-breast sites] and squamous or basal cell
skin cancers. Overall survival (OS) was defined as the time from
primary surgery to death by any cause (includes death from breast
cancer, non-breast cancer or unknown causes).
Clinical and histological data
Pathological data were obtained from the DBCG
database and verified in the local database at the Department of
Pathology, Vejle Hospital, Vejle, Denmark. Clinical IDFS data were
obtained from the local electronic health records and the
nationwide online electronic health records holding data from all
Danish hospitals. OS data were obtained from the nationwide Danish
Civil Registration System, which contains basic personal data on
all residents in Denmark.
Tissue HER2 determination
Homogenization of the tissue samples and
determination of the tissue HER2 status and estrogen receptor (ER)
status have been described in detail in a previous study of ours
[Olsen et al(17)]. The
quantitative detection of HER2 tissue concentration was determined
using fresh-frozen tumor tissue and autologous reference tissue by
means of commercially available HER2/neu assay using the ADVIA
Centaur system (Siemens Healthcare Diagnostics, Deerfield, IL,
USA). This automated immunoassay uses two monoclonal antibodies
(TA-1 and NB-3) for the detection of HER2 protein. The
chemiluminescence signal is directly proportional to the quantity
of HER2 protein in the sample. Each assay was controlled by two
commercial controls (Siemens Healthcare Diagnostics) and one
in-house serum pool. The assay shows an acceptable inter-assay
coefficient of variation (CV) between 4.4 and 5.6%.
Tissue HER2 status was determined on
paraffin-embedded tumor tissue using the IHC and FISH methods. The
tumors were considered HER2-positive if defined IHC3+ or
IHC2+ combined with FISH ≥2. IHC analysis was assessed
by HercepTest™ (DakoCytomation, Glostrup, Denmark). IHC0 and
IHC1+ were considered HER2-negative, whereas
IHC3+ was defined as HER2-positive. IHC2+ was
considered borderline and in these cases HER2 expression was
further determined using the HER2 FISH pharmDx™ kit
(DakoCytomation). The threshold for overexpression was a ratio
equal to or exceeding 2.0 between the HER2 gene copy number and the
chromosome 17 centromere.
ER staining was carried out using an anti-human ER
monoclonal antibody (clone 1D5; DakoCytomation) and visualized by
the SuperSensitive polymer-HRP IHC kit (Biogenex Laboratories Inc.,
San Ramon, CA, USA). Tumors with a nuclei staining ≥10% were
considered ER-positive according to the contemporary DBCG
guidelines.
Statistical methods
We enrolled 400 patients to detect a 25% absolute
reduction in the risk of IDFS events from 45 to 20% with 80% power
and a two-sided significance level of 5%. The statistical analyses
were carried out using Stata version 11 software (StataCorp LP, TX,
USA). Kaplan-Meier curves and the log-rank test were used to
compare all time-to-event end points. Multivariate IDFS and OS
analyses were performed using the Cox proportional hazards
regression model. Fisher’s exact test and McNemar’s test were used
to compare categorical data. ROC curves were used to investigate
the cut-off for the quantitative Centaur assay. For all tests,
two-sided P-values <0.05 were considered statistically
significant.
Results
Patient characteristics
Final analysis included 379 patients. At the
clinical cut-off date (September 19, 2011) the median follow-up was
3.9 years for IDFS and 4.2 years for OS. We compared the clinical
outcome in four groups of patients defined by HER2 status
(determined by IHC/FISH and Centaur) and adjuvant HER2-targeted
therapy. The four groups were defined as follows: Group 0, patients
defined as tissue HER2-negative by IHC/FISH and ADVIA Centaur and
therefore not offered HER2-targeted therapy. Group 1, patients
defined as tissue HER-positive by IHC/FISH and therefore offered
HER2-targeted therapy. Group 2, patients defined as tissue
HER2-positive by IHC/FISH, but not offered HER2-targeted therapy,
as they were older than 60 years and therefore only received
endocrine treatment when adjuvant treatment was required according
to the recommendations by the contemporary DBCG guidelines. Group
3, patients defined as tissue HER2-positive by ADVIA Centaur, but
not by IHC/FISH and therefore not offered HER2-targeted
therapy.
Table I shows
patient demographics and clinical characteristics in the four
groups. Clinical prognostic factors were significantly better in
group 3 compared to group 1 as regards tumor grade, axillary nodal
status and ER status (P=0.001, 0.026 and <0.001, respectively;
Fisher’s exact test). Furthermore, Table I shows that the majority of patients
in groups 1 and 2 were also defined as tissue HER2-positive by
ADVIA Centaur. Likewise, differences in adjuvant treatment were
observed as indicated in Table II.
Significantly fewer patients in group 3 compared to group 1
received adjuvant chemotherapy (P<0.001; Fisher’s exact
test).
 | Table IDemographics and clinical
characteristics in the four groups of patients defined by HER2
status (determined by IHC/FISH and Centaur), +/− adjuvant
HER2-targeted therapy. |
Table I
Demographics and clinical
characteristics in the four groups of patients defined by HER2
status (determined by IHC/FISH and Centaur), +/− adjuvant
HER2-targeted therapy.
| Group 0 HER2-neg −
adj (n=274) | Group 1 HER2-pos
IHC/FISH + adj (n=42) | Group 2 HER2-pos
IHC/FISH − adj (n=21) | Group 3 HER2-pos
Centaur − adj (n=42) | |
|---|
|
|
|
|
| |
|---|
| Characteristic | No. | % | No. | % | No. | % | No. | % | P-valuea |
|---|
| Age |
| <40 years | 6 | 2.2 | 4 | 9.5 | | | | | |
| 40–59 years | 111 | 40.5 | 25 | 59.5 | 4 | 19.0 | 23 | 54.8 | |
| ≥60 years | 157 | 57.3 | 13 | 31.0 | 17 | 81.0 | 19 | 45.2 | 0.091 |
| Type of surgery |
|
Breast-conserving | 192 | 70.1 | 30 | 71.4 | 16 | 76.2 | 34 | 81.0 | |
| Mastectomy | 82 | 29.9 | 12 | 28.6 | 5 | 23.8 | 8 | 19.0 | 0.443 |
| Tumor type |
| Ductal | 236 | 86.1 | 38 | 90.5 | 19 | 90.5 | 37 | 88.1 | |
| Lobular | 21 | 7.7 | 2 | 4.8 | | | 2 | 4.8 | |
| Others | 17 | 6.2 | 2 | 4.8 | 2 | 9.5 | 3 | 7.1 | 1.000 |
| Tumor grade |
| Grade 1 | 68 | 24.8 | 1 | 2.4 | 1 | 4.8 | 12 | 28.6 | |
| Grade 2 | 132 | 48.2 | 20 | 47.6 | 7 | 33.3 | 21 | 50.0 | |
| Grade 3 | 56 | 20.4 | 17 | 40.5 | 11 | 52.4 | 6 | 14.3 | |
| Unknown | 18 | 6.6 | 4 | 9.5 | 2 | 9.5 | 3 | 7.1 | 0.001 |
| Tumor size |
| T1 ≤20 mm | 130 | 47.4 | 17 | 40.5 | 9 | 42.9 | 24 | 57.1 | |
| T2 >0 ≤50
mm | 138 | 50.4 | 24 | 57.1 | 12 | 57.1 | 18 | 42.9 | |
| T3 >50 mm | 6 | 2.2 | 1 | 2.4 | | | | | 0.190 |
| Nodal status |
| N0, 0 nodes | 127 | 46.4 | 18 | 42.9 | 11 | 52.4 | 26 | 61.9 | |
| N1, 1–3 nodes | 106 | 38.7 | 12 | 28.6 | 8 | 38.1 | 14 | 33.3 | |
| N2, 4–9 nodes | 28 | 10.2 | 8 | 19.0 | 2 | 9.5 | 1 | 2.4 | |
| N3, ≥10 nodes | 13 | 4.7 | 4 | 9.5 | | | 1 | 2.4 | 0.026 |
| ER status |
| Negative | 38 | 13.9 | 18 | 42.9 | 6 | 28.6 | 3 | 7.1 | |
| Positive | 236 | 86.1 | 24 | 57.1 | 15 | 71.4 | 39 | 92.9 |
<0.001 |
| HER2 IHC/FISH |
| Negative | 274 | | | | | | 42 | | |
| Positive | | | 42 | | 21 | | | | NA |
| HER2 Centaur |
| Negative <72
ng/mg | 274 | | 4 | | 1 | | | | |
| Positive ≥72
ng/mg | | | 38 | | 20 | | 42 | | NA |
 | Table IIAdjuvant therapy in the four groups
of patients defined by HER2 status (determined by IHC/FISH and
Centaur), +/− adjuvant HER2-targeted therapy. |
Table II
Adjuvant therapy in the four groups
of patients defined by HER2 status (determined by IHC/FISH and
Centaur), +/− adjuvant HER2-targeted therapy.
| Group 0 HER2-neg −
adj (n=274) | Group 1 HER2-pos
IHC/FISH + adj (n=42) | Group 2 HER2-pos
IHC/FISH − adj (n=21) | Group 3 HER2-pos
Centaur − adj (n=42) | |
|---|
|
|
|
|
| |
|---|
| Adjuvant
therapy | No. | % | No. | % | No. | % | No. | % | P-valuea |
|---|
| Chemotherapy |
| No | 171 | 62.4 | 1 | 2.4 | 19 | 90.5 | 23 | 54.8 | |
| CEF | 42 | 15.3 | 13 | 31.0 | | | 4 | 9.5 | |
| CMF | 1 | 0.4 | 1 | 2.4 | 2 | 9.5 | | | |
| EC + D | 47 | 17.2 | 27 | 64.3 | | | 14 | 33.3 | |
| DC | 13 | 4.7 | | | | | 1 | 2.4 |
<0.001 |
| HER2-targeted |
| No | 274 | 100.0 | | | 21 | 100.0 | 42 | 100.0 | |
| Herceptin | | | 28 | 66.7 | | | | | |
| Neratinibb | | | 4 | 9.5 | | | | | |
| Lapatinibb | | | 10 | 23.8 | | | | |
<0.001 |
| Endocrine |
| No | 70 | 25.5 | 17 | 40.5 | 7 | 33.3 | 11 | 26.2 | |
| Tam | 45 | 16.4 | 7 | 16.7 | 1 | 4.8 | 6 | 14.3 | |
| Tam + AIc | 107 | 39.1 | 7 | 16.7 | 7 | 33.3 | 16 | 38.1 | |
| AI | 52 | 19.0 | 11 | 26.2 | 6 | 28.6 | 9 | 21.4 | 0.165 |
| Radiotherapy |
| No | 49 | 17.9 | 4 | 9.5 | 6 | 28.6 | 6 | 14.3 | |
| Yes | 225 | 82.1 | 38 | 90.5 | 15 | 71.4 | 36 | 85.7 | 0.738 |
HER2 status determined by IHC/FISH and
ADVIA Centaur
Paraffin-embedded and fresh-frozen tumor tissue
samples were available from all 379 patients for IHC/FISH and ADVIA
Centaur analyses. The cut-off value of 72 ng/mg protein for ADVIA
Centaur HER2 positivity was determined on available autologous
reference tissue from 371 out of 403 patients applying a 97.5%
confidence interval (CI). As shown in our previous study [Olsen
et al(17)], we found the
median value of HER2 to be significantly higher in the tumor tissue
(median, 42.3 ng/mg; range, 0–6158.2 ng/mg) than in autologous
reference tissue (2.6 ng/mg; range, 0–862.8 ng/mg; P<0.0001;
Wilcoxon signed rank test on 367 patients with both tumor and
autologous reference tissue).
Using the cut-off value of 72 ng/mg, the
quantitative ADVIA Centaur defined 100 out of the 379 patients
(26.4%) as tissue HER2-positive, whereas only 63 patients (16.6%)
were defined HER2-positive by the use of IHC/FISH (P<0.0001;
McNemar’s test). The ADVIA centaur misclassified only 5 out of the
63 patients defined as tissue HER2-positive by IHC/FISH as shown in
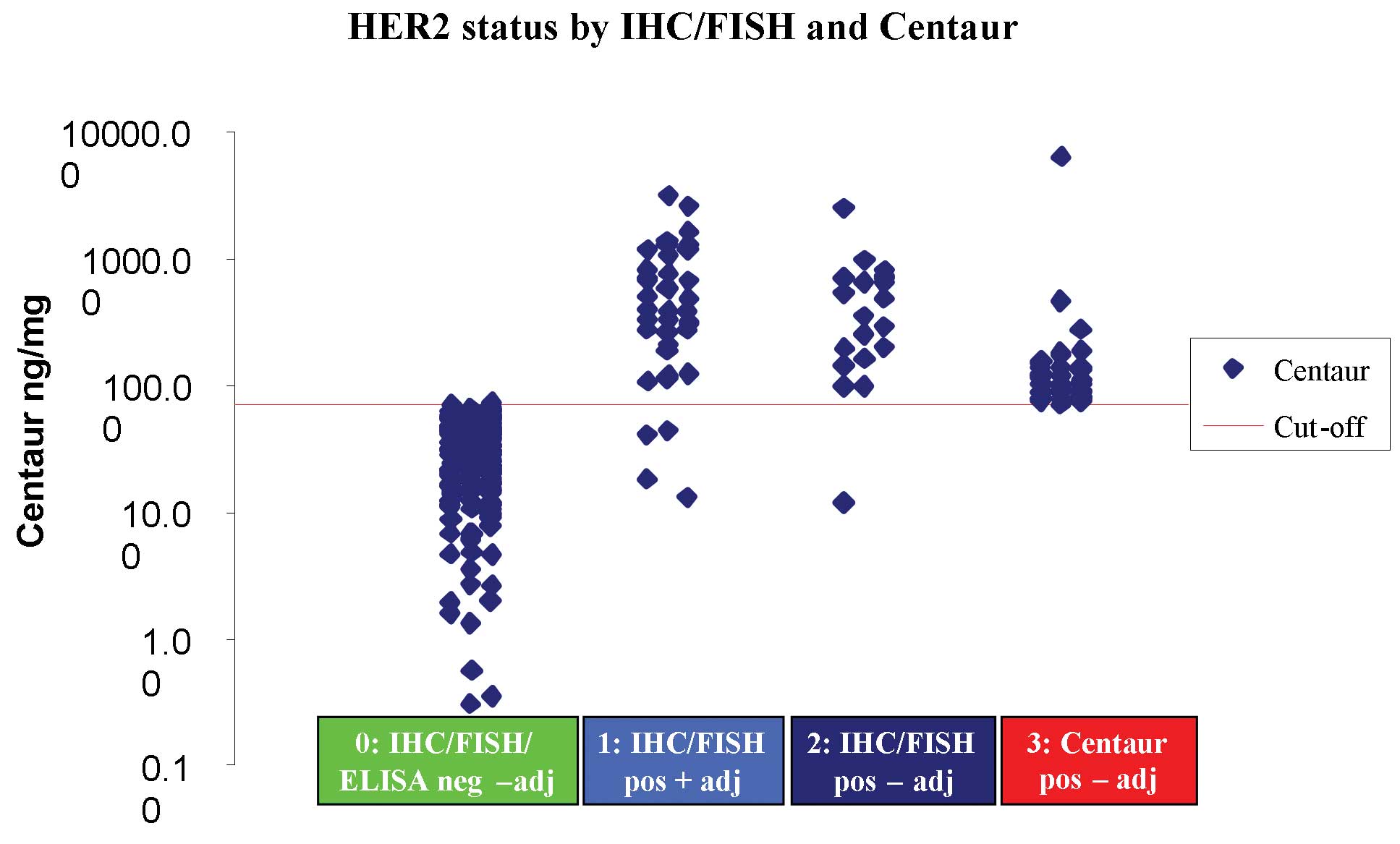
Table I. Fig. 2 shows the distribution of ADVIA
Centaur values according to the four different groups; it should be
noted that only five values in groups 1 and 2 are below the cut-off
value of 72 ng/mg.
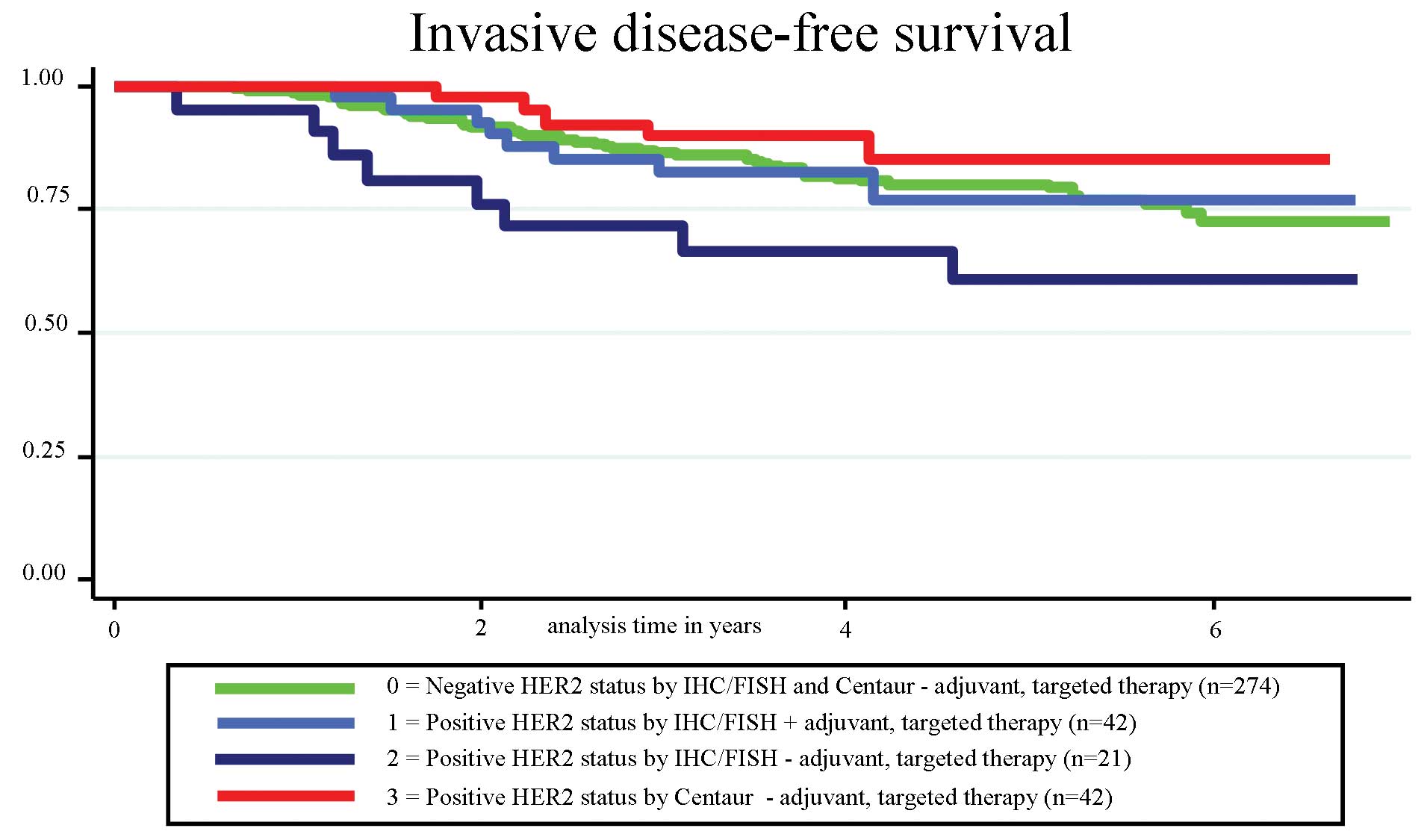
Invasive disease-free survival
Follow-up was available for all 379 patients in the
final analysis. At a median follow-up of 3.9 years (0.3–6.9 years),
there were 74 events in the four groups, 45 distant recurrence, 11
regional invasive breast cancer recurrence or
ipsilateral/contralateral invasive breast cancer, 10 died from
non-breast cancer/unknown cause, and eight had second primary
non-breast invasive cancer. No significant difference in IDFS was
found among the four groups (P=0.159; log-rank). Surprisingly, we
found a significantly greater number of events in group 2 compared
to group 3 (P=0.025; log-rank), with eight events in 21 patients in
the IHC/FISH-positive group not receiving adjuvant HER2-targeted
treatment (group 2) and only five events in 42 patients in the
Centaur-positive group not receiving adjuvant HER2-targeted
treatment (group 3) (Fig. 3).
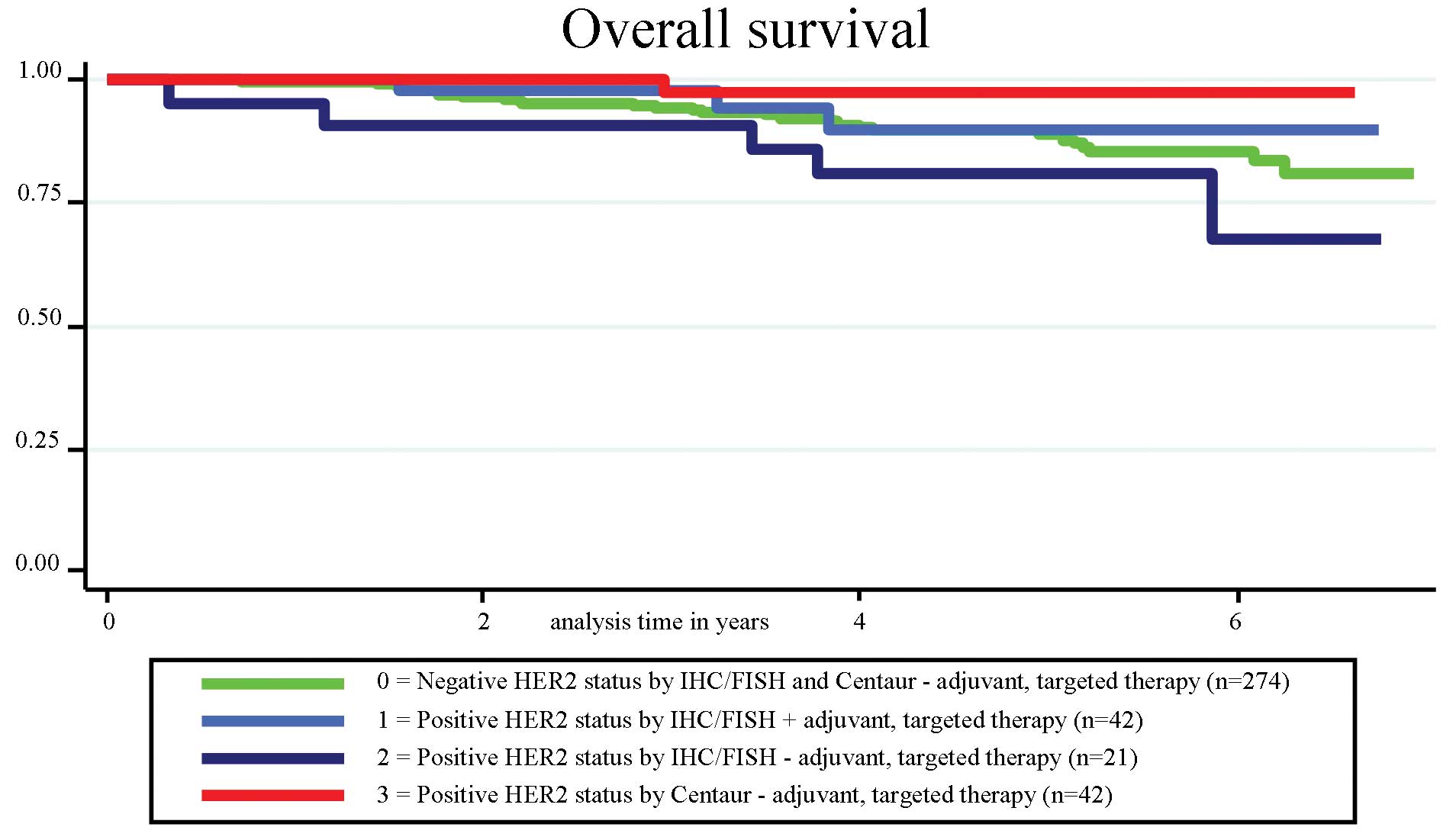
Overall survival
At a median follow-up of 4.2 years (0.3–6.9 years),
the four groups had a total of 39 events (29 died from breast
cancer and 10 died from non-breast cancer/unknown cause). As for
IDFS, no significant difference in survival was observed among the
four groups (P=0.150; log-rank); however, a significantly higher
number of deaths was observed in group 2 compared to group 3
(P=0.020; log-rank) (Fig. 4).
Univariate and multivariate analysis
Table III shows
the results of the univariate analysis with dichotomized variables,
identifying tumor grade (grade of <3 vs. grade 3), tumor size
(≤20 vs. >20 mm), axillary node status (negative vs. positive)
and estrogen receptor status (negative vs. positive) as
statistically significant for OS, whereas only tumor grade was
statistically significant for IDFS. HER2 status (negative vs.
positive) was not significant in the univariate analysis, neither
when determined by IHC/FISH nor by Centaur (<72 vs. ≥72
ng/mg).
 | Table IIIUnivariate analysis of prognostic
factors for IDFS and OS in the 379 patients. |
Table III
Univariate analysis of prognostic
factors for IDFS and OS in the 379 patients.
| Factor | IDFS P-value | OS P-value |
|---|
| Group | 0.159 | 0.150 |
| Age, <60 vs. ≥60
years | 0.207 | 0.055 |
| Tumor grade, grade
<3 vs. grade 3 | <0.001 | <0.001 |
| Tumor size, ≤ 20
vs. >20 mm | 0.058 | 0.009 |
| Lymph nodes,
neg/pos | 0.206 | 0.012 |
| ER status,
neg/pos | 0.111 | 0.049 |
| HER2 IHC/FISH,
neg/pos | 0.228 | 0.522 |
| HER2 Centaur,
<72 vs. ≥72 ng/mg | 0.651 | 0.670 |
The variables in the multivariate analysis included
group (also representing HER2 status), age, tumor grade, tumor
size, axillary node status and ER-status as outlined in Table IV. In the multivariate analysis for
IDFS, the only independent prognostic marker was tumor grade
(P=0.011). In the multivariate analysis for OS age (P=0.048), tumor
grade (P=0.010) and axillary node status (P=0.025) were independent
prognostic markers. Groups determined by HER2 status were not an
independent prognostic factor in the above analysis.
 | Table IVMultivariate analysis of prognostic
factors for IDFS and OS in 379 patients. |
Table IV
Multivariate analysis of prognostic
factors for IDFS and OS in 379 patients.
| IDFS | OS |
|---|
|
|
|
|---|
| Factor | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Group |
| 0, HER2-neg −
adj | 1.00 | | 1.00 | |
| 1, IHC/FISH pos +
adj. | 0.94
(0.43–2.05) | 0.879 | 0.72
(0.21–2.49) | 0.602 |
| 2, IHC/FISH pos −
adj. | 1.49
(0.69–3.21) | 0.306 | 1.29
(0.49–3.39) | 0.602 |
| 3, Centaur pos −
adj. | 0.65
(0.26–1.62) | 0.352 | 0.27
(0.04–2.01) | 0.202 |
| Age |
| <60 years | 1.00 | | 1.00 | |
| ≥60 years | 1.35
(0.83–2.22) | 0.231 | 2.04
(1.00–4.15) | 0.048 |
| Tumor grade |
| Grade 1, 2,
unknown | 1.00 | | 1.00 | |
| Grade 3 | 1.94
(1.16–3.24) | 0.011 | 2.48
(1.24–4.97) | 0.010 |
| Tumor size |
| ≤20 mm | 1.00 | | 1.00 | |
| >20 mm | 1.32
(0.80–2.17) | 0.624 | 1.80
(0.85–3.83) | 0.124 |
| Axillary node
status |
| Negative | 1.00 | | 1.00 | |
| Positive | 1.33
(0.82–2.17) | 0.245 | 2.32
(1.11–4.83) | 0.025 |
| ER status |
| Negative | 1.00 | | 1.00 | |
| Positive | 0.78
(0.43–1.41) | 0.411 | 0.60
(0.28–1.29) | 0.195 |
ROC curve analysis
We also investigated the ability of the quantitative
Centaur assay to discriminate between patients with or without
recurrence. The ROC curve analyses resulted in an almost straight
line with an area under the ROC curve of 0.49, which implies that
the Centaur assay was not able to discriminate between patients at
all. In accordance with this finding, there were no statistically
significant differences observed between the mean Centaur value in
patients with or without recurrence in any of the four groups of
patients in this study (data not shown).
Discussion
In the current study, we reject the hypothesis that
the clinical outcome is worse in a group of patients defined as
tissue HER2-positive by Centaur only and not treated with adjuvant
HER2-targeted therapy compared to patients defined as HER2-positive
by IHC/FISH and treated with adjuvant HER2-targeted therapy. In
fact, the best outcome was observed in the group of patients
defined as HER2-positive by Centaur only. In contrast to this
finding, Konecny et al(18)
demonstrated an association between HER2 overexpression by ELISA
and shorter disease-free survival (DFS) in a cohort of 587 patients
with primary breast cancer prior to the era of HER2-targeted
therapy. Currently, adjuvant HER2-targeted therapy is the standard
of care for HER2-positive breast cancer patients. Scrutiny of the
clinically relevant question as to whether we could increase the
number of patients eligible for adjuvant HER2-targeted therapy
could only be done in a design such as the present one followed by
a prospective intervention study.
A number of studies have indicated the existence of
an additional group that may benefit from adjuvant HER2-targeted
therapy. First, as shown in a previous study [HERceptin Adjuvant
(HERA) trial], among patients defined as HER2-positive by IHC and
FISH (IHC2+ and FISH+), a significant
improvement in clinical outcome was observed in patients treated
with adjuvant chemotherapy plus trastuzumab for one year (19). Second, Gilcrease et
al(20) demonstrated that even
a low-level HER2 expression (IHC1+) can be associated
with a worse outcome in node-positive patients. Finally, the study
by Viale raised an ongoing discussion regarding the
misclassification of some patients by FISH using HER2/CEP17 ratio
instead of relating the HER2 copy number to the cell count
(21).
The cut-off value of 72 ng/mg used in this study is
consistent with the cut-off value of 400 fmol/mg (~74 ng/mg) found
by Konecny et al(18), who
optimized the cut-off value to provide the maximum separation of
patients according to DFS. On the other hand, Müller et
al(22) applied ROC statistics
to optimize their cut-off value (42 ng/mg) according to the FISH
results. If we had used this lower cut-off value, we would have
defined 51% of the patients as HER2-positive (193 out of 379
patients). In contrast to these two studies, we aimed to find a
novel and more sensitive assay and therefore chose to investigate
Centaur HER2 as a biological variable and defined the cut-off value
in our study according to the autologous reference tissue by
applying a 97.5% CI.
Another unexpected, although interesting finding of
this study was that the HER2-positive patients in group 2 (age ≥
60) had a worse outcome than expected, possibly due to the lack of
adjuvant HER2-targeted therapy. This emphasizes the need for a
change in clinical practice where many elderly patients are not
selected for adjuvant chemotherapy and HER2-targeted therapy based
on age only. Furthermore, Palmieri et al(23) reported a worse outcome in patients
who were not offered HER2-targeted therapy due to a clinical
judgment that the breast cancer was low-risk. Likewise, Tovey et
al(24) emphasized the need for
HER2-targeted therapy even in low-grade, node-negative tumors.
Sawaki et al(25) showed
that elderly patients tolerated trastuzumab well, which highlights
the need for a new view of elderly patients and adjuvant
HER2-targeted therapy.
In conclusion, the main finding of the present study
is that quantitative the detection of HER2 concentration using
Centaur does not define a new group of patients eligible for
HER2-targeted therapy. Therefore, tissue HER2 status defined by
IHC/FISH analysis remains the gold standard. HER2 amplification is
presumably the decisive factor, which, in addition to an
overexpression of the HER2 protein, leads to the aggressive nature
of HER2-positive tumors. Further studies are therefore warranted in
order to identify novel methods of detecting this
amplification.
Acknowledgements
The study was financed by the Vejle Hospital
Research Foundation. HER2 kits were kindly granted by Siemens
Healthcare Diagnostics (Deerfield, IL, USA). The authors thank Sara
Egsgaard and Camilla Davidsen for their laboratory work, and Karin
Larsen for proofreading.
Notes
[1] Conflicts of
interest. I.B. and T.B. have received remuneration for two
lectures on serum HER2 from Siemens Healthcare Diagnostics.
References
|
1
|
Kurebayashi J: Biological and clinical
significance of HER2 overexpression in breast cancer. Breast
Cancer. 8:45–51. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carney WP, Neumann R, Lipton A, et al:
Potential clinical utility of serum HER-2/neu oncoprotein
concentrations in patients with breast cancer. Clin Chem.
49:1579–1598. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pauletti G, Dandekar S, Rong H, et al:
Assessment of methods for tissue-based detection of the HER-2/neu
alteration in human breast cancer: a direct comparison of
fluorescence in situ hybridization and immunohistochemistry. J Clin
Oncol. 18:3651–3664. 2000.PubMed/NCBI
|
|
4
|
Slamon DJ: Human breast cancer:
correlation of relapse and survival with amplification of the
HER-2/neu oncogene. Science. 235:177–182. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Slamon DJ, Leyland-Jones B, Shak S, et al:
Use of chemotherapy plus a monoclonal antibody against HER2 for
metastatic breast cancer that overexpresses HER2. N Engl J Med.
344:783–792. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Piccart-Gebhart MJ, Procter M,
Leyland-Jones B, et al: Trastuzumab after adjuvant chemotherapy in
HER2-positive breast cancer. N Engl J Med. 353:1659–1672. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Romond EH, Perez EA, Bryant J, et al:
Trastuzumab plus adjuvant chemotherapy for operable HER2-positive
breast cancer. N Engl J Med. 353:1673–1684. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gong Y, Booser DJ and Sneige N: Comparison
of HER-2 status determined by fluorescence in situ hybridization in
primary and metastatic breast carcinoma. Cancer. 103:1763–1769.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zidan J, Dashkovsky I, Stayerman C, et al:
Comparison of HER-2 overexpression in primary breast cancer and
metastatic sites and its effect on biological targeting therapy of
metastatic disease. Br J Cancer. 93:552–556. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gancberg D, Di LA, Cardoso F, et al:
Comparison of HER-2 status between primary breast cancer and
corresponding distant metastatic sites. Ann Oncol. 13:1036–1043.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wolff AC, Hammond ME, Schwartz JN, et al:
American Society of Clinical Oncology/College of American
Pathologists guideline recommendations for human epidermal growth
factor receptor 2 testing in breast cancer. J Clin Oncol.
25:118–145. 2007. View Article : Google Scholar
|
|
12
|
Tse C, Brault D, Gligorov J, et al:
Evaluation of the quantitative analytical methods real-time PCR for
HER-2 gene quantification and ELISA of serum HER-2 protein and
comparison with fluorescence in situ hybridization and
immunohistochemistry for determining HER-2 status in breast cancer
patients. Clin Chem. 51:1093–1101. 2005.PubMed/NCBI
|
|
13
|
Bergqvist J, Ohd JF, Smeds J, et al:
Quantitative real-time PCR analysis and microarray-based RNA
expression of HER2 in relation to outcome. Ann Oncol. 18:845–850.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Egervari K, Toth J, Nemes Z and Szollosi
Z: An alternative and reliable real-time quantitative PCR method to
determine HER2/neu amplification in breast cancer. Appl
Immunohistochem Mol Morphol. 17:247–254. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Olsen DA, Ostergaard B, Bokmand S, et al:
HER-2 protein concentrations in breast cancer cells increase before
immunohistochemical and fluorescence in situ hybridization analysis
turn positive. Clin Chem Lab Med. 45:177–182. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hudis CA, Barlow WE, Costantino JP, et al:
Proposal for standardized definitions for efficacy end points in
adjuvant breast cancer trials: the STEEP system. J Clin Oncol.
25:2127–2132. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Olsen DA, Bechmann T, Ostergaard B, et al:
Increased concentrations of growth factors and activation of the
EGFR system in breast cancer. Clin Chem Lab Med. 50:1809–1818.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Konecny G, Untch M, Arboleda J, et al:
Her-2/neu and urokinase-type plasminogen activator and its
inhibitor in breast cancer. Clin Cancer Res. 7:2448–2457.
2001.PubMed/NCBI
|
|
19
|
Dowsett M, Procter M, Caskill-Stevens W,
et al: Disease-free survival according to degree of HER2
amplification for patients treated with adjuvant chemotherapy with
or without 1 year of trastuzumab: the HERA Trial. J Clin Oncol.
27:2962–2969. 2009.PubMed/NCBI
|
|
20
|
Gilcrease MZ, Woodward WA, Nicolas MM, et
al: Even low-level HER2 expression may be associated with worse
outcome in node-positive breast cancer. Am J Surg Pathol.
33:759–767. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Viale G: Be precise! The need to consider
the mechanisms for CEP17 copy number changes in breast cancer. J
Pathol. 219:1–2. 2009.
|
|
22
|
Müller V, Thomssen C, Karakas C, et al:
Quantitative assessment of HER-2/neu protein concentration in
breast cancer by enzyme-linked immunosorbent assay. Int J Biol
Markers. 18:13–20. 2003.PubMed/NCBI
|
|
23
|
Palmieri C, Shah D, Krell J, et al:
Management and outcome of HER2-positive early breast cancer treated
with or without trastuzumab in the adjuvant trastuzumab era. Clin
Breast Cancer. 11:93–102. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tovey SM, Brown S, Doughty JC, et al: Poor
survival outcomes in HER2-positive breast cancer patients with
low-grade, node-negative tumours. Br J Cancer. 100:680–683. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sawaki M, Mukai H, Tokudome N, et al:
Safety of adjuvant trastuzumab for HER-2-overexpressing elderly
breast cancer patients: a multicenter cohort study. Breast Cancer.
19:253–258. 2012. View Article : Google Scholar : PubMed/NCBI
|