Introduction
Resorcinol is a non-isoprenoid lipid found in a
range of plant and bacterial species; they exert non-specific
antioxidant and anti-mutagenic effects and regulate proliferation
(1). Chemical analogs of these
lipids have demonstrated anticancer effects in animal models of
colon (2), lung (3) and pancreas tumor (4). 4-hexylresorcinol (4-HR) is also a
chemical analog of these lipids (1).
In microorganisms, chemical analogs of 4-HR occur in
dormant cysts (5,6) and resting cyst-like cells (7,8).
Exogenous administration of these auto-regulatory factors and their
analogs-alkyl resorcinol induces dormancy in bacteria (9); these compounds are similarly active in
eukaryotic cells such as ras-transformed fibroblasts (10). Therefore, 4-HR may also inhibit the
growth of tumor cells, altering their physiological state and
activity. In a previous report, 4-HR showed a preventive effect in
mononuclear cell leukemia, hepatocellular neoplasm, and circulatory
system tumors (11,12). Recently, combination therapy of 4-HR
and cisplatin was shown to have synergistic effects in
nasopharyngeal carcinoma (13).
4-HR inhibits the NF-κB pathway via the suppression of
transglutaminase-2 (13,14). 4-HR also accelerates tumor
differentiation by the suppression of E2F2 and E2F3 (15).
The aim of the present study was to determine the
potential antitumor effects of 4-HR as a single agent on oral
squamous cell carcinoma (OSCC). Additionally, the change of
intracellular calcium following 4-HR application and drug
interaction with calcium channel blockers were demonstrated. We
used in vitro SCC-9 cell culture and in vivo
xenograft model systems and assessed cell viability, intracellular
calcium tracking and apoptosis.
Materials and methods
Cell cultures and MTT assay
SCC-9 was grown to confluence in Ham’s
F12/Dulbecco’s modified Eagle’s medium (Gibco BRL, Gaithersburg,
MD, USA) containing 1% penicillin/streptomycin, fibroblast growth
factor-2 (100 μg/ml) and 10% fetal calf serum (FCS). Primary
cultured human gingival fibroblasts (PHGF) were used as controls.
4-HR (Sigma, St. Louis, MO, USA) was added to confluent cells to
final concentrations of 1, 5 or 10 μg/ml.
Cell viability quantification at 48 h after 4-HR
application was assessed by tetrazolium salt
3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay as previously described (16). Briefly, cells were incubated with
MTT solution (Cell Proliferation kit I; Roche Molecular
Biochemicals, Mannheim, Germany) in 6-well plates for 4 h at room
temperature. Formazan crystals were solubilized overnight, and the
product was estimated by measuring absorbance at 590 nm with a
Victor Multilabel counter (Perkin-Elmer Wallac GmbH, Freiburg,
Germany).
Apoptosis and caspase-3/7 assays
Apoptosis was determined with the Annexin V-FITC
Apoptosis Detection kit I (BD Biosciences, San Jose, CA, USA)
following a 2-h incubation with 4-HR. The caspase assay was
performed with a commercial kit (Caspase-Glo® 3/7 assay,
Promega, Madison, WI, USA) at 30 min after 4-HR was added. Cell
culture in medium lacking 4-HR was used as negative control.
Calcium tracking with confocal microscopy
and calcium channel study
To monitor calcium, SCC-9 cells were treated with
Fluo-4 NW calcium assay kit (Molecular Probes, Eugene, OR, USA),
and then visualized using a laser scanning confocal microscope
(Leica Microsystems Heidelberg GmbH, Mannheim, Germany) as
previously described (17).
Calcium channel antagonists (blockers) were used to
confirm the role of calcium in 4-HR-mediated proliferation and
apoptosis. SCC-9 cells were exposed to Norvasc (0.1, 0.5 or 1
μg/ml; Pfizer Korea, Seoul, Korea), and 4-HR (10 μg/ml) was added 2
h later; control cells did not receive Norvasc. The MTT cell
proliferation assay was performed 24 h after 4-HR treatment.
Subsequently, SCC-9 cells were treated with calcium channel
antagonists (Norvasc or diltiazem; 1 μg/ml) for 2 h before exposure
to 4-HR. Diltiazem was obtained from Sigma. The caspase-3/7 assay
was performed 30 min after 4-HR application.
Scanning and transmission electron
microscopy
Following a 24-h incubation with 10 μg/ml 4-HR,
SCC-9 cell suspension was applied to copper grids and dried in a
vacuum to prepare for scanning electron microscopy (SEM). SCC-9
cells treated with 10 μg/ml cisplatin for 24 h were included as a
positive control. Prior to transmission electron microscopy (TEM)
analysis, cells were harvested by centrifugation at 300 × g for 10
min, and then dehydrated and fixed (18). Specimens were polymerized in Spurr
(Epon 812) resin, cut on an ultratome (Leica, Uppsala, Sweden),
stained with lead citrate, and mounted on copper grids. SEM and TEM
were performed using a JEOL microscope (Tokyo, Japan) operating at
accelerating voltage 15.0 kv and magnification ×7000.
Tumor xenograft model
Male nude mice (BALB/cAnNCrj-nu/nu) were purchased
from Charles River Japan Inc. (Shin-Yokohama, Japan). Seventeen
mice were subcutaneously injected with SCC-9 cells
(2.5×106); all subsequently developed tumors. Commencing
on the following day, two experimental groups (each group, n=6)
received daily intraperitoneal injections of 4-HR (10 mg/kg of body
weight) for 16 days, while the control group (n=5) received daily
injections of the vehicle (normal saline). Another group of mice
received daily intraperitoneal injections of 4-HR (10 mg/kg of body
weight) plus diltiazem (20 mg/kg of body weight) for 16 days
(19,20). Tumors were measured in two
dimensions with calipers every 2 or 3 days, and tumor volumes were
calculated with the following formula: volume = a ×
b2/2, where a is the tumor measurement at its widest
point and b is the measurement perpendicular to a. Tumor weight was
determined at the time of sacrifice.
Statistical analysis
The difference between the untreated control and the
drug-treated group in each experiment was compared by independent
sample t-test. Inhibitory concentration 50 (IC50) was
calculated at 48 h after 4-HR administration by linear regression
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
4-HR inhibits SCC-9 cell
proliferation
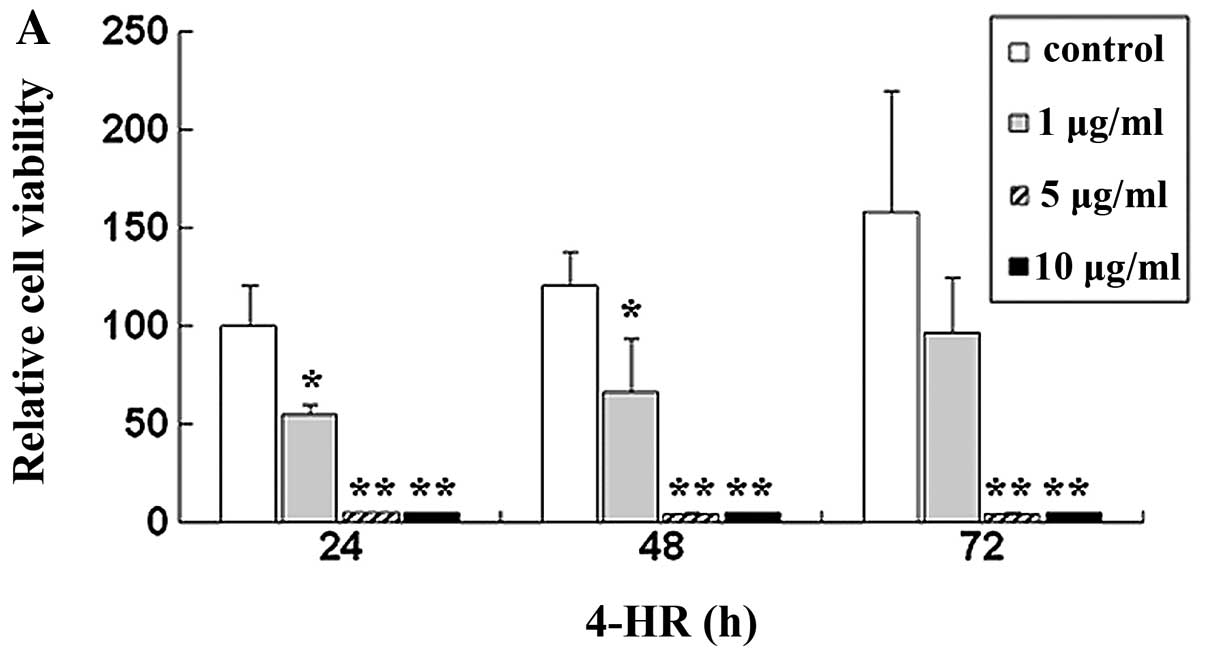
Initially, we determined the effect of 4-HR on SCC-9
cell viability. Relative cell viability after treatment with 1, 5
and 10 μg/ml 4-HR was 55.0, 5.2 and 4.7%, respectively, compared
with control (P=0.043, 0.007 and 0.007) (Fig. 1A). By contrast, 4-HR exerted only a
slight effect on PHGF (Fig. 1B).
IC50 was 2.94 μg/ml for SCC-9. However, IC50
of PHGF was 49.30 μg/ml.
4-HR induces apoptosis of SCC-9
cells
Subsequently, we determined whether 4-HR could
induce apoptosis. Following treatment with 4-HR (10 μg/ml), SCC-9
cells were considerably smaller and rounder compared with control
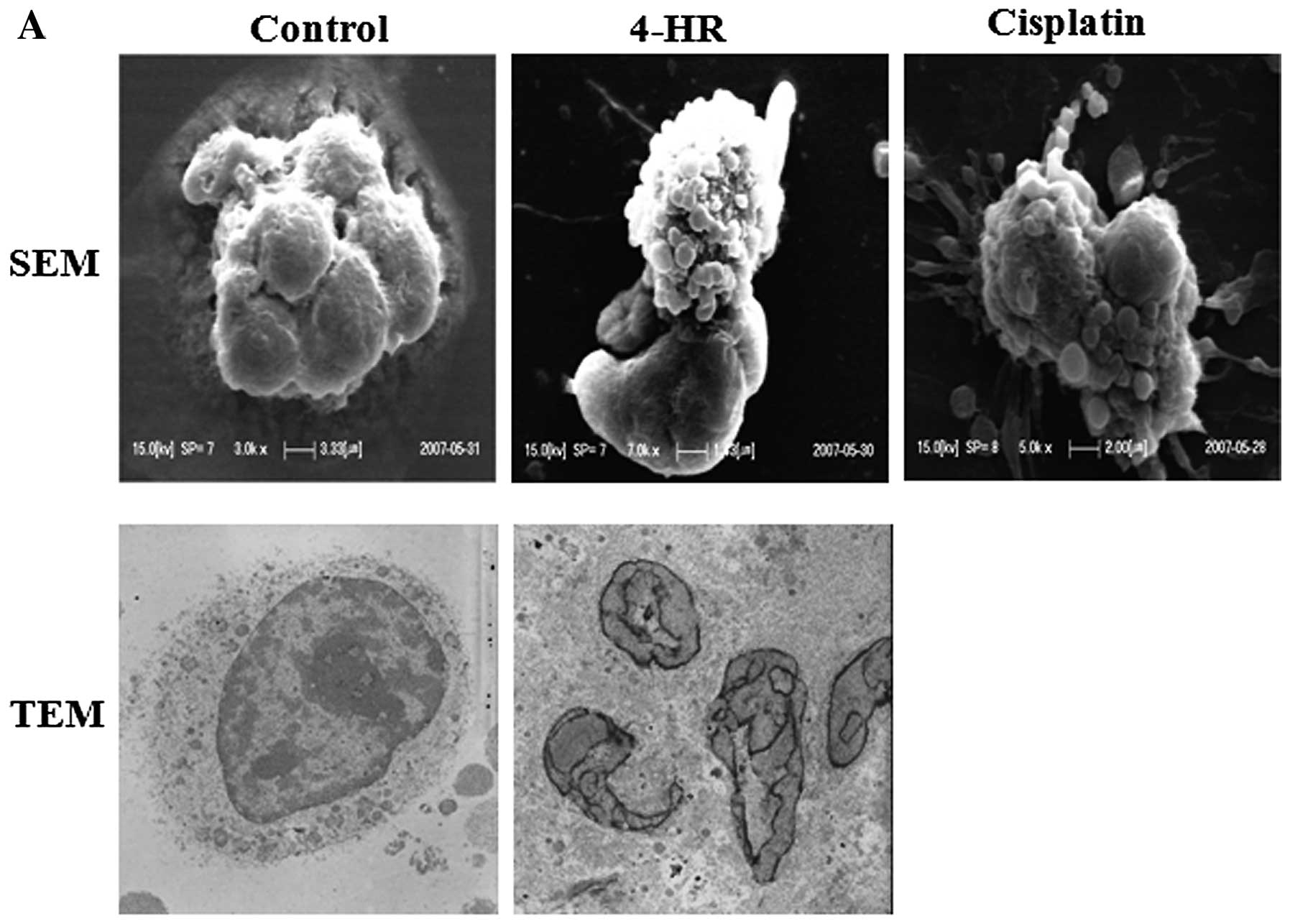
cells (data not shown), suggesting apoptosis. SEM and TEM
examinations revealed: i) the appearance of apoptotic bodies around
cells following 4-HR treatment (10 μg/ml), which was similar to
cells treated with cisplatin, and ii) cleaved or fragmented nuclei
with finger-like projections, which is a typical feature of
apoptosis (Fig. 2A). By contrast,
control cells exhibited smooth surfaces and intact
intra-cytoplasmic structures and nuclei. Following treatment with
4-HR (5 μg/ml), SCC-9 cells became stainable with Annexin V
(Fig. 2B), most likely due to
membrane changes, a primary event in apoptosis. The proportion of
apoptotic cells was significantly increased in 5 and 10 μg/ml 4-HR
compared to the untreated control (Fig.
2C). To confirm 4-HR-mediated apoptosis, caspase-3/7 activity
was determined. Caspase-3/7 activity was dose-dependently increased
by 4-HR in SCC-9 cells (Fig. 2D,
right panel), while its activity was marginally increased in PHGF
from 20 μg/ml of 4-HR (Fig. 2D,
left panel). These results indicate that 4-HR selectively induces
apoptosis of SCC-9 cells at lower concentrations than PHGF.
Differential effects of 4-HR on
intracellular calcium signaling in SCC-9 cells
Calcium is known to play role in cell proliferation,
differentiation and apoptosis; therefore, we assessed calcium
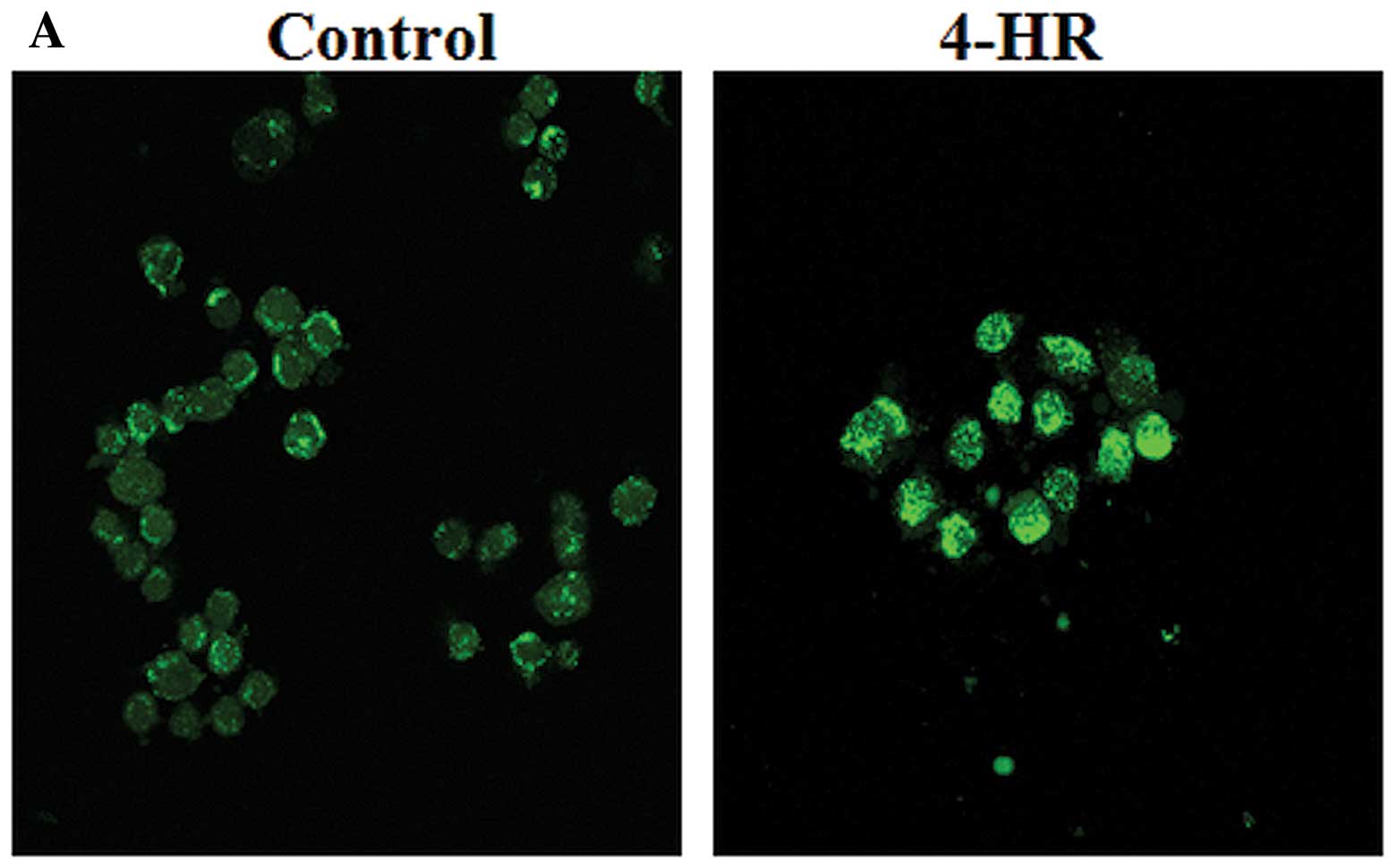
uptake in SCC-9 cells. As shown in Fig.
3A, intracellular calcium was significantly increased in SCC-9
cells after 4-HR treatment (10 μg/ml) compared with control cells.
Furthermore, increased intracellular calcium was observed
specifically in SCC-9 cells, but not in normal dermal fibroblasts
(Fig. 3B). In SCC-9 cells, 4-HR
treatment delayed the decrease in intracellular calcium after peak
concentration; however, in normal dermal fibroblasts, peak calcium
levels were much lower, and 4-HR did not affect the kinetics of the
calcium response. Notably, 4-HR suppressed calcium oscillation in
both SCC-9 cells and normal dermal fibroblasts (Fig. 3).
Calcium blockers were used to confirm the essential
role of calcium uptake. As shown in Fig. 3C, the 4-HR anti-proliferative effect
was inhibited by Norvasc (0.1–1 μg/ml; P<0.001); cellular
proliferation was increased ~3-fold compared with the 4-HR control.
Calcium channel blockers Norvasc and diltiazem also blocked
4-HR-mediated caspase-3/7 activity in SCC-9 cells (P<0.001 in 5
and 10 μg/ml of 4-HR; Fig. 3D).
These results confirm that 4-HR-mediated effects are due, in part,
to increased intracellular calcium.
4-HR reduces tumor formation in a
xenograft in vivo model
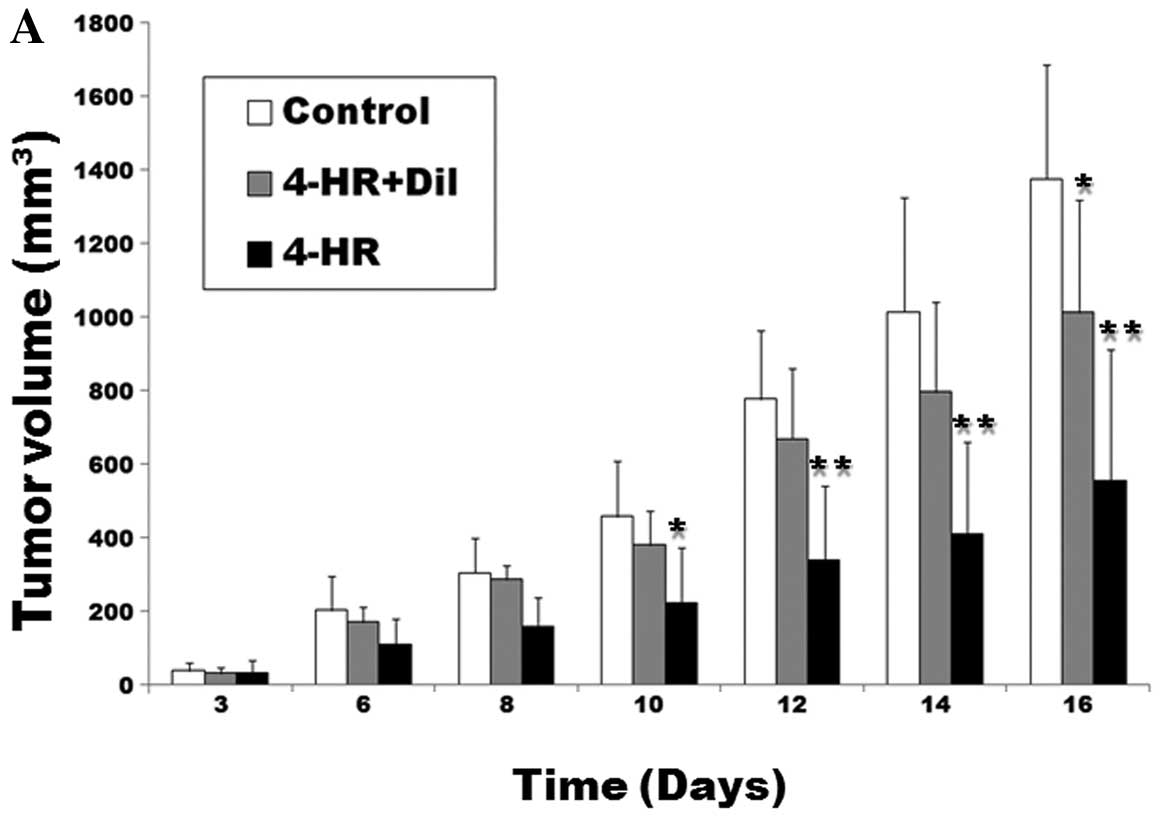
Next, we tested 4-HR antitumor effects in the
xenograft model. SCC-9 cells were injected subcutaneously into nude
mice. In mice receiving daily 4-HR treatment (10 mg/kg) for 16
days, tumor mass was markedly smaller compared with the control
group (P=0.003; Fig. 4A). In mice
receiving concomitant application of 4-HR and diltiazem, tumor mass
was significantly larger compared with the 4-HR group (P=0.038).
There was no statistically significant difference between the
control and the 4-HR + diltiazem group (P>0.05). At the time of
necropsy, the average mass weight was 0.94±0.27 g/mouse in the
control group (Fig. 4B), 0.72±0.21
g/mouse in the 4-HR + diltiazem group and 0.47±0.23 g/mouse in the
4-HR group. When compared to the control, the 4-HR group was
statistically significantly different (P=0.007). Taken together,
these results indicate that 4-HR inhibits tumor cell proliferation
in mouse tumor xenografts and concomitant application of calcium
channel blocker partly reverses the antitumor effect of 4-HR.
Discussion
In the present study, we demonstrated that 4-HR
strongly inhibited SCC-9 cell proliferation compared with normal
fibroblasts, and induced apoptosis. Furthermore, these in
vitro effects were reproducible in xenografts after SCC-9 cell
implantation in nude mice. The antitumor effect of 4-HR was partly
reversed by the application of calcium channel blockers both in
vitro and in vivo.
4-HR also induced the apoptosis of PHGF, although at
a significantly higher concentration (>20 μg/ml; Fig. 2D), which is in accordance with
previous results (21). 4-HR
targets transformed cells via an unknown mechanism. Although this
study focused on SCC-9 cells, we also observed the inhibitory
action of 4-HR on gastric adenocarcinoma, breast cancer, lung
cancer, and hepatoma cells (data not shown), suggesting that 4-HR
may be applicable to other types of cancer. Selective tumor cell
apoptosis is one objective in developing anticancer drugs. In the
present study, we demonstrated that 4-HR stimulated apoptosis of
SCC-9 cells, but not of PHGF.
4-HR increases epithelial cell differentiation in
SCC-9 cells (15). The calcium is
important in the epithelial cell differentiation (22). 4-HR inhibits TG-2 activity (14) and the activity of TG-2 is also
calcium dependent (23). Therefore,
the molecular mechanisms of the observed 4-HR-mediated effects may
be partly dependent on calcium. Calcium uptake is due, in part, to
upregulation of voltage-dependent calcium channels; calcium channel
blockers attenuated 4-HR-mediated cellular effects include protein
kinase C-α, which plays an important role in calcium-induced
keratinocyte differentiation (22).
The effects of 4-HR on cancer cell proliferation and
differentiation may also be due to non-specific interactions with
other proteins (24), changes in
membrane permeability (25), and/or
antioxidant and, thus, anti-mutagenic activities (26). A possible mechanism concerning the
different uptake of calcium ion by 4-HR in OSCC cells and
fibroblasts might be related to the glutamate receptor. The gene
expression of ionotropic glutamate receptors was decreased by 4-HR
application (data not shown). Glutamate receptor is related to
calcium oscillation (27,28) and OSCC highly expresses glutamate
receptor (29). However, the
glutamate receptor-related hypothesis remains to be confirmed in
further experimental studies.
The effects of 4-HR on apoptosis are dose-dependent;
relatively high doses (5–10 μg/ml) of 4-HR caused the rupture of
cellular membranes. This is similar to the effect of conventional
anticancer drugs, which are typically toxic and induce apoptosis in
cancer cells (30). However, the
anti-proliferative effect of 4-HR at a low concentration (1 μg/ml)
was not accompanied by cytotoxicity or apoptosis. In the previous
16 days, toxicology and carcinogenesis studies demonstrated that
oral doses of 4-HR up to 500 mg/kg did not affect the survival of
experimental animals (12). In the
present study, the effective dose of 4-HR was significantly lower
(10 mg/kg body weight) than a previous animal study (12). However, prolonged use of 4-HR causes
nephropathy and osteosclerosis in humans (31) as well as in animals (11). This may be due to increased
intracellular calcium concentrations and the inhibition of the
NF-κB pathway (13).
Collectively, our results suggest that 4-HR has
strong antitumor effects by inhibiting calcium channel oscillation
and inducing apoptosis. The antitumor effects of 4-HR were partly
reversed by the application of calcium channel blockers.
Acknowledgements
The authors thank Dr Janet S. Stein for the critical
reading of the manuscript. This study was supported by a grant from
the Next-Generation BioGreen21 Program (Center for Nutraceutical
and Pharmaceutical Materials no. PJ009013), Rural Development
Administration, Republic of Korea.
References
|
1
|
Kozubek A and Tyman JHP: Resorcinolic
lipids, the natural non-isoprenoid phenolic amphiphiles and their
biological activity. Chem Rev. 99:1–25. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mutoh M, Takahashi M, Fukuda K, et al:
Suppression of cyclooxygenase-2 promoter-dependent transcription
activity in colon cancer cells by chemopreventive agents with a
resorcin-type structure. Carcinogenesis. 21:959–963. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hasegawa R, Furukawa F, Toyoda K, et al:
Inhibitory effect of antioxidants on
N-bis(2-hydroxypropyl)nitrosamine-induced lung
carcinogenesis in rats. Jpn J Cancer Res. 81:871–877. 1990.
|
|
4
|
Maruyama H, Amamura T, Nakae D, et al:
Effects of catechol and its analogs on pancreatic carcinogenesis
initiated by N-nitrosobis(2-oxopropyl)amine in Syrian
hamsters. Carcinogenesis. 12:1331–1334. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chin D, Boyle GM, Porceddu S, Theile DR,
Parsons PG and Coman WB: Head and neck cancer: past, present and
future. Expert Rev Anticancer Ther. 6:1111–1118. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Braakhuis BJ, Tabor MP, Kummer JA, Leemans
CR and Brakenhoff RH: A genetic explanation of Slaughter’s concept
of field cancerization: evidence and clinical implications. Cancer
Res. 63:1727–1730. 2003.
|
|
7
|
Reusch RN and Sadoff HL:
5-n-Alkylresorcinols from encysting Azotobacter vinelandii:
isolation and characterization. J Bacteriol. 139:448–453.
1979.PubMed/NCBI
|
|
8
|
Reusch RN and Sadoff HL: Novel lipid
components of the Azotobacter vinelandii cyst membrane.
Nature. 302:268–270. 1983. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Osipov GA, El’-Registan GI, Svetlichnyi
VA, Kozlova AN and Duda VV: Chemical nature of the autoregulating
factor d1 in Pseudomonas carboxydoflava. Mikrobiologiia.
54:186–190. 1985.(In Russian).
|
|
10
|
El’-Registan GI, Tsyshnatii GV, Duzha MV,
Pronin SV and Mitiushina LL: Regulation of Pseudomonas
carboxydoflavagrowth and development by specific endogenous
factors. Mikrobiologiia. 49:561–565. 1980.(In Russian).
|
|
11
|
Chhabra RS, Huff JE, Haseman J, Hall A,
Baskin G and Cowan M: Inhibition of some spontaneous tumors by
4-hexylresorcinol in F344/N rats and B6C3F1 mice. Fundam Appl
Toxicol. 11:685–690. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
National Toxicology Program. NTP
toxicology and carcinogenesis studies of 4-hexylresorcinol (CAS No.
136-77-6) in F344/N rats and B6C3F1 mice (Gavage
Studies). Natl Toxicol Program Tech Rep Ser. 330:1–166.
1988.PubMed/NCBI
|
|
13
|
Kim SG, Lee SW, Park YW, Jeong JH and Choi
JY: 4-hexylresorcinol inhibits NF-κB phosphorylation and has a
synergistic effect with cisplatin in KB cells. Oncol Rep.
26:1527–1532. 2011.PubMed/NCBI
|
|
14
|
Kim SG, Jeong JH, Park YW, et al:
4-hexylresorcinol inhibits transglutaminase-2 activity and has
synergistic effects along with cisplatin in KB cells. Oncol Rep.
25:1597–1602. 2011.PubMed/NCBI
|
|
15
|
Kim SG, Kim AS, Jeong JH, Choi JY and
Kweon H: 4-hexylresorcinol stimulates the differentiation of SCC-9
cells through the suppression of E2F2, E2F3 and Sp3 expression and
the promotion of Sp1 expression. Oncol Rep. 28:677–681.
2012.PubMed/NCBI
|
|
16
|
Grossi F and Aita M: Bevacizumab and
non-small-cell lung cancer: starving the enemy to survive. Expert
Opin Biol Ther. 7:1107–1119. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Trollinger DR, Cascio WE and Lemasters JJ:
Selective loading of Rhod 2 into mitochondria shows mitochondrial
Ca2+ transients during the contractile cycle in adult
rabbit cardiac myocytes. Biochem Biophy Res Comm. 236:738–742.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guejes L, Zurgil N, Deutsch M, Gilburd B
and Shoenfeld Y: The influence of different cultivating conditions
on polymorphonuclear leukocyte apoptotic processes in vitro, I: the
morphological characteristics of PMN spontaneous apoptosis.
Ultrastruct Pathol. 27:23–32. 2003. View Article : Google Scholar
|
|
19
|
El-Azab MF and Moustafa YM: Influence of
calcium channel blockers on anticonvulsant and antinociceptive
activities of valproic acid in pentylenetetrazole-kindled mice.
Pharmacol Rep. 64:305–314. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luszczki JJ, Trojnar MK, Trojnar MP, et
al: Effects of three calcium channel antagonists (amlodipine,
diltiazem, and verapamil) on the protective action of lamotrigine
in the mouse maximal electroshock-induced seizure model. Pharmacol
Rep. 59:672–682. 2007.
|
|
21
|
Il’inskaya ON, Kolpakov AI, Mulyukin AL,
Dreyer F and El’-Registan GI: Effects of membrane-active microbial
autoregulators on the growth of cultured ras-transformed
fibroblasts. Appl Biochem Microbiol. 36:473–477. 2000.PubMed/NCBI
|
|
22
|
Yang LC, Ng DC and Bikle DD: Role of
protein kinase Cα in calcium induced keratinocyte differentiation:
defective regulation in squamous cell carcinoma. J Cell Physiol.
195:249–259. 2003.
|
|
23
|
Jung HJ, Chen Z, Wang M, et al: Calcium
blockers decrease the bortezomib resistance in mantle cell lymphoma
via manipulation of tissue transglutaminase activities. Blood.
119:2568–2578. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rimando AM, Dayan FE and Streibig JC: PSII
inhibitory activity of resorcinolic lipids from Sorghum bicolor. J
Nat Prod. 66:42–45. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kozubek A and Demel RA: Permeability
changes of erythrocytes and liposomes by 5-(n-alk(en)yl)
resorcinols from rye. Biochim Biophys Acta. 603:220–227. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hladyszowski K, Zubik L and Kozubek A:
Quantum mechanical and experimental oxidation studies of
pentadecylresorcinol, olivetol, orcinol and resorcinol. Free
Radical Res. 28:359–368. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bezzi P, Carmignoto G, Pasti L, et al:
Prostaglandins stimulate calcium-dependent glutamate release in
astrocytes. Nature. 391:281–285. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Parri HR, Gould TM and Crunelli V:
Spontaneous astrocytic Ca2+ oscillations in situ drive
NMDAR-mediated neuronal excitation. Nat Neurosci. 4:803–812. 2001.
View Article : Google Scholar
|
|
29
|
Choi SW, Park SY, Hong SP, Pai H, Choi JY
and Kim SG: The expression of NMDA receptor 1 is associated with
clinicopathological parameters and prognosis in the oral squamous
cell carcinoma. J Oral Pathol Med. 33:533–537. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schwerdt G, Freudinger R, Schuster C,
Weber F, Thews O and Gekle M: Cisplatin-induced apoptosis is
enhanced by hypoxia and by inhibition of mitochondria in renal
collecting duct cells. Toxicol Sci. 85:735–742. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Robinson HM Jr: Effective antifungal drugs
and indications for their use. Med Clin North Am. 51:1181–1188.
1967.PubMed/NCBI
|