Introduction
The current clinical treatments for adults with
acute lymphoblastic leukemia (ALL) yield positive initial
responses, but treatment-related mortality (TRM) is common and the
risk of a relapse is relatively high. The cure rate of adult ALL
scarcely exceeds 40%, and TRM is between 5 and 10% (1). Long periods of hospitalization and
serious morbidity for ALL are common. Leukemic relapse in adult ALL
remains a major therapeutic problem, and it has no suitable salvage
therapy with low side-effects, not even hematopoietic stem cell
transplantation, which produces both short- and long-term toxicity
(2,3). It is, therefore, critical to develop
new therapeutics for adult ALL treatment.
Indirubin is the active ingredient of Dang Gui Long
Hui Wan, from a mixture of herbal medicines customarily used in
traditional Chinese medicine to treat chronic myelocytic leukemia
(CML) (4). Clinical trials in China
(5,6) showed that indirubin causes almost no
toxicity in the liver, kidneys, or bone marrow. It has also been
reported that the indirubin derivative acts a potent inhibitor of
cyclin-dependent kinases (CDKs) such as CDK1 and CDK2 (4,7,8). The
indirubin-induced arrest of the G1 or G2/M phase of the cell cycle
in a variety of cultured cell types, including chronic leukemia
cells (K562), has also been studied (4,9,10).
Additionally, indirubin and its derivatives induce apoptosis in a
variety of human tumor cells: breast cancer (7,11),
melanoma (12,13), oral-area carcinoma (9,10,14),
lung cancer (10) and promyelocytic
leukemia (HL-60) (15) cells.
Nevertheless, the functional effect of indirubin on ALL remains
unknown.
In the present study, we specifically focused on the
cytotoxicity of indirubin-3′-monoxime (I3M) and its mechanism of
cell death in B cell lymphoblast leukemia cells and chronic myeloid
leukemia cells. We also investigated the hematotoxicity of I3M by
analyzing the survival of normal human granulocytes and
lymphocytes.
Materials and methods
Isolation of lymphocytes and
granulocytes
Buffy coats from healthy donors were obtained from
the Tainan Blood Center, Taiwan Blood Services Foundation. Approval
was obtained from the Chiayi Christian Hospital Institutional
Review Board for these studies. Informed consent was obtained
according to the Declaration of Helsinki. Peripheral blood
mononuclear cells (PBMCs) were isolated using density gradient
centrifugation (Ficoll-Paque; GE Healthcare Bio-Sciences AB,
Uppsala, Sweden). After washing, the cells were incubated in Petri
dishes at 37°C in an atmosphere of 5% CO2 for 90 min.
Non-adherent cells were separated from adherent monocytes by
moderate aspiration and used as lymphocytes. Flow cytometry
(FACSan; BD Biosciences, Palo Alto, CA, USA) showed that >85% of
this lymphocyte population stained positive for CD3-antibody (data
not shown). Granulocytes were isolated using the MACS®
(Miltenyi Biotec, Auburn, CA, USA) online general protocol.
Briefly, after the PBMCs had been isolated, the thin, white
cell-layer over the erythrocytes was collected and washed in
Dulbecco’s phosphate-buffered saline (DPBS). To lyse the remaining
red blood cells, they were incubated with three times the volume of
lysis buffer [155 mM NH4Cl, 10 mM KHCO3 and
0.1 mM EDTA (pH: 7.3)] for 5 min at room temperature. After they
had been centrifuged and washed, the granulocytes were finally
obtained. The granulocyte population used in the experiments was
CD45-antibody-positive. Flow cytometry showed that the percentage
of granulocytes was >88%.
Cell culture
Human CML K562 and human ALL JM1 cells were obtained
from the Bioresource Collection and Research Center, Taiwan. The
K562 cells were cultured in Iscove’s modified Dulbecco’s medium
(IMDM) supplemented with 10% fetal bovine serum (FBS) and 4 mM
L-glutamine (Gibco/Invitrogen, Grand Island, NY, USA); the JM1
cells were kept in IMDM supplemented with 10% FBS, 4 mM L-glutamine
and 0.05 mM 2-mercaptoethanol (Sigma-Aldrich, St. Louis, MO, USA)
at 37°C in a humidified atmosphere containing 5% CO2 in
air.
The primary lymphocytes and the granulocytes were
cultured in RPMI-1640 medium (Gibco/Invitrogen) supplemented with
10% FBS, 2 mM L-glutamine and penicillin (100 U/ml) streptomycin
(100 μg/ml) (Gibco/Invitrogen) at 37°C in an atmosphere of 5%
CO2 in air.
Cell viability assay
The inhibitory effect of I3M on cell viability was
determined using a WST-1 colorimetric assay (Roche Diagnostics,
Mannheim, Germany). The assay was set up in 96-well culture plates
with triplicate wells for each experimental condition. JM1 cells
(1×105/well) and K562 cells (3×104/well) were
treated with a series of concentrations of I3M (7.5–35 μM) for 24
and 48 h. WST-1 was then added to each well and the cells were
incubated for 2 h at 37°C in the dark. This assay is based on the
conversion of the tetrazolium salt
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2-tetrazolium
by mitochondrial dehydrogenase to a formazan product. The
absorbance at 450 nm was measured using a microplate reader. Cell
viability is expressed as a percentage of population growth with a
standard deviation (SD) relative to that of untreated control
cells. For apoptotic analyses, the cells were incubated with
benzyloxycarbonyl-Val-Ala-Asp-(OMe)-fluoromethylketone (Z-VAD-FMK)
(Sigma-Aldrich) for 1 h prior to I3M treatments.
Cell cycle analysis
K562 and JM1 cells were treated with 10 or 20 μM of
I3M for 24 or 48 h. After washing with PBS, the cells were fixed in
cold 70% ethanol at 4°C for 6 h, centrifuged at 300 g for 10 min,
and then stained in PBS containing 40 μg/ml of propidium iodide
(PI) (Sigma-Aldrich) and 100 μg/ml of RNase A (Invitrogen) for 30
min at room temperature. The flow cytometer was used for cell cycle
analysis. The histogram of the cell cycle distribution was then
analyzed (ModFit LT; Verity Software House, Topsham, ME, USA). The
percentage of cells in the subdiploid region (sub-G1) represented
cells undergoing apoptotic DNA fragmentation.
Activity of caspase-3 assay
Analysis of the enzymatic activity of caspase-3 in
K562 and JM1 cells was carried out using a caspase-3/CPP32
colorimetric assay kit (BioVision, Mountain View, CA, USA). K562
(3×105/ml) and JM1 (1×106/ml) cells were
treated with or without various concentrations of I3M for 48 h, and
were then centrifuged. Chilled cell lysis buffer (50 μl) was added
to the cell pellet, which was then incubated on ice for 10 min.
After the protein concentration had been analyzed, an additional
100 μg of protein was mixed with 2X reaction buffer and
DEVD-p-nitroanilide (pNA) substrate. The incubation was carried out
at 37°C for 2 h. This assay was based on detecting the chromophore
pNA after it had been cleaved from the labeled substrate DEVD-pNA.
pNA light emission was quantified using a microtiter plate at 405
nm. The activity of caspase-3 in I3M-treated cells was determined
comparing their absorbance values with those of untreated control
cells.
Assessing cell necrosis
After K562 cells (3×104) and JM1 cells
(1×105) had been incubated with or without various
concentrations of I3M for 24 or 48 h, their necrotic cell death was
quantitatively measured by releasing lactate dehydrogenase (LDH)
into the culture supernatants (CytoTox 96®
Non-Radioactive Cytotoxicity Assay kit; Promega, Madison, WI, USA).
During the loss of membrane integrity and cell necrosis, LDH
expression increased. The culture supernatants (50 μl/well) were
transferred to an enzymatic assay plate, mixed with reconstituted
substrate, and then incubated for 30 min at room temperature in the
dark. After stop solution had been added, the absorbance was
recorded at 492 nm. Maximal LDH release occurred after the control
cells had been lysed for 45 min at room temperature.
Western blotting for LC3-II formation,
PARP-1 cleavage, and beclin-1 antibody expression
JM1 and K562 cells were treated with or without I3M
for 24 and 48 h. To assess the protein expression, the whole cell
extract was harvested in lysis buffer (1% NP-40, 0.5% sodium
deoxycholate, 0.1% SDS and a protease inhibitor mixture) on ice for
20 min. Following centrifugation, the protein concentration was
analyzed using a BCA assay (Thermo Fisher Scientific, Fremont, CA,
USA), and subjected to sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE). After the whole cell extract had
undergone western blotting, the SDS-PAGE PVDF membrane was blocked
with antibodies against LC3 (Novus Biologicals, Littleton, CO,
USA), PARP-1/2 (H-250), or beclin-1 (Santa Cruz Biotechnology,
Santa Cruz, CA, USA) and then incubated with secondary antibodies:
anti-goat IgG antibody or anti-rabbit IgG antibody (Jackson
ImmunoResearch, West Grove, PA, USA). Actin or α-tubulin
(Sigma-Aldrich) was used as an internal control.
Statistical analysis
Each experiment was repeated at least 6 times. Data
are the means ± SD. Single values represent the mean optical
density (OD) value of triplicate cultures and are given as a
percentage of the control (= 100% proliferation). A two-tailed
Student’s t-test was used for statistical analysis.
Results
I3M is cytotoxic and decreases cell
viability in ALL and CML cells
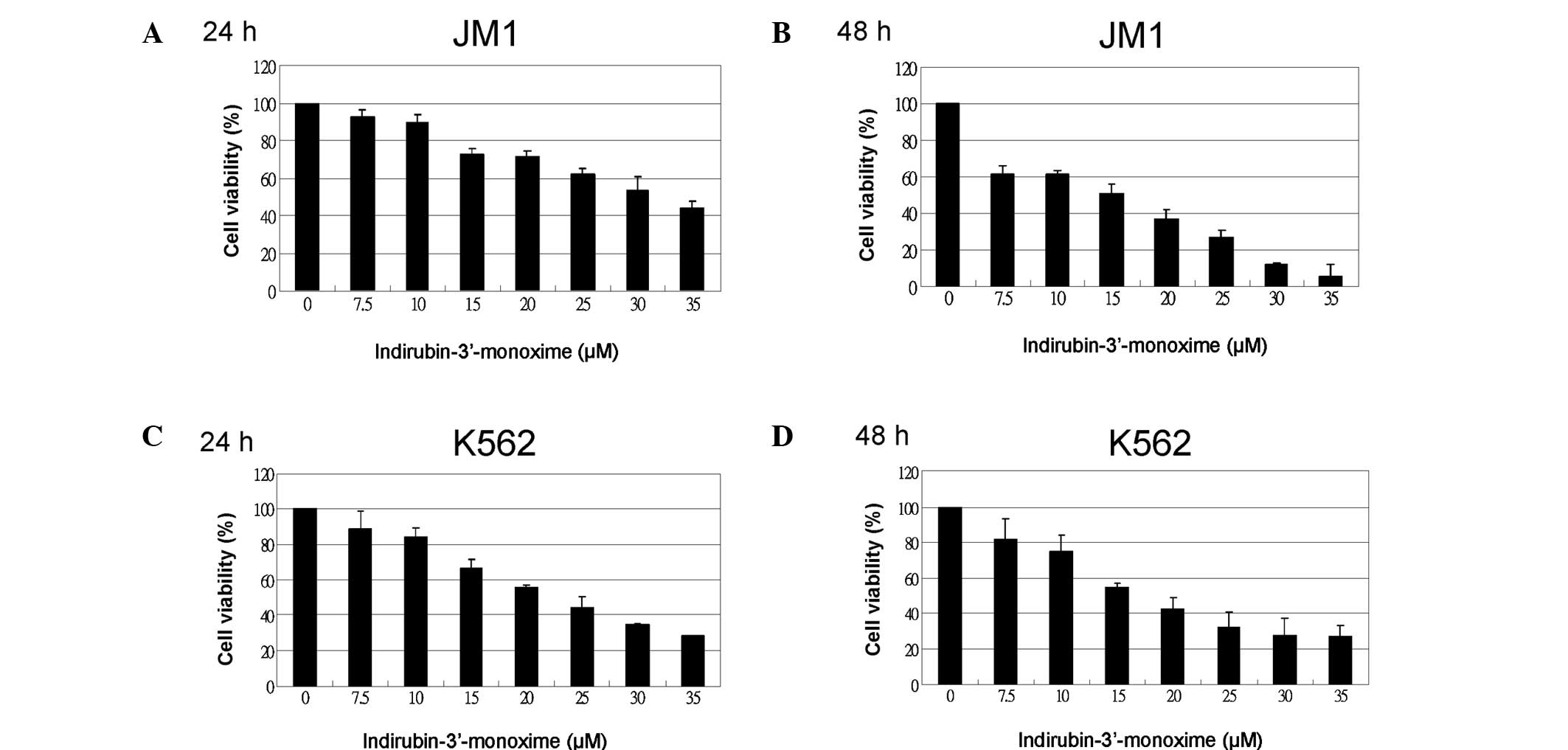
A WST-1 assay of cell viability was used to
determine whether I3M is cytotoxic to JM1 (ALL) cells. Differences
in the antitumor effects of I3M were compared in JM1 cells and K562
(CML) cells. I3M significantly dose-dependently reduced cell
viability in JM1 and K562 cells (Fig.
1). At 48 h, the 50% inhibitory concentration (IC50)
value of I3M against JM1 and K562 cells was below 20 μM. The 48-h
treatment was more cytotoxic than the 24-h treatment, particularly
in JM1 cells.
I3M arrests human JM1 and K562 cells in
the G2/M phase of the cell cycle
To assess the effect of I3M on the cell cycle, JM1
and K562 cells that had been treated with 10 or 20 μM I3M for 24
and 48 h were analyzed. Cell cycle analysis showed that I3M
preferentially arrested JM1 and K562 cells in the G2/M phase
(Fig. 2).
I3M induces apoptosis in JM1 and K562
cells
The increasing proportions of JM1 and K562 cells in
the sub-G1 phase particularly indicated that I3M induced apoptotic
cell death, which increased with time and concentration in both
types of leukemia cells (Fig. 2).
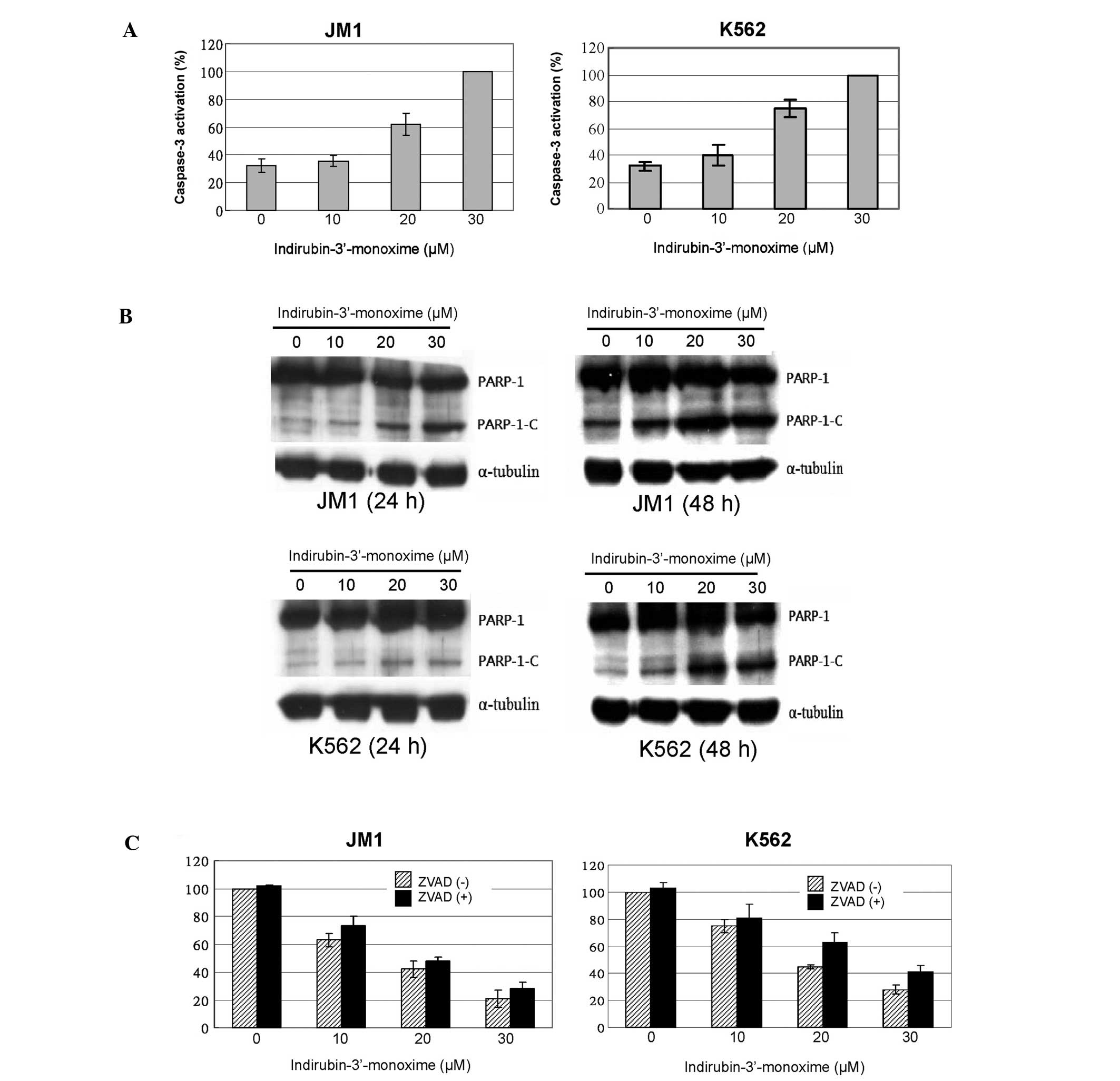
Since caspase-3 activation is involved in apoptosis, we next
assessed the activation of caspase-3 in I3M-treated JM1 and K562
cells. Dose-dependent activation of caspase-3 was detected in both
JM1 and K562 cell types (Fig. 3A).
In addition, I3M triggered the proteolytic cleavage of PARP-1, a
signature event during apoptosis, particularly after 48 h in cells
treated with 20 and 30 μM of I3M (Fig.
3B). This suggests that I3M caused apoptosis. However, when we
added the pan-caspase inhibitor Z-VAD-FMK to I3M-treated cell
cultures, I3M-induced cell death was only partially inhibited
(Fig. 3C).
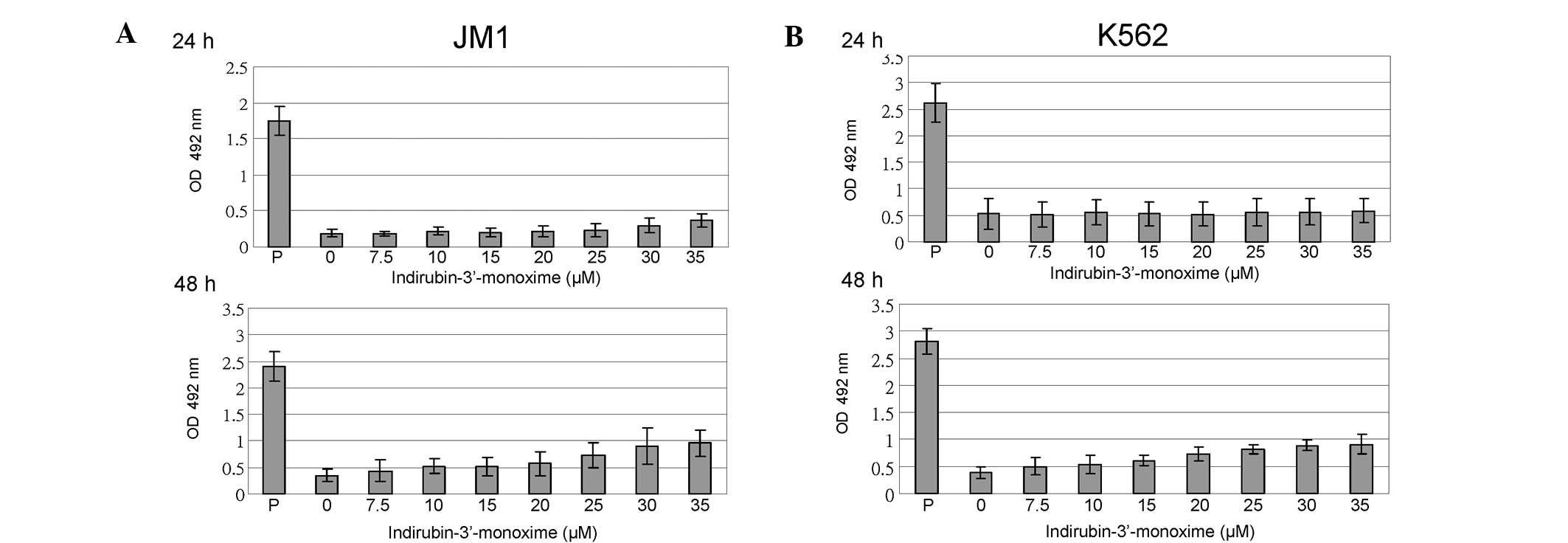
I3M does not induce cell necrosis in JM1
or K562 cells
To clarify the data presented in Fig. 3C, we examined whether I3M induced a
type of cell death other than apoptosis. Therefore, we analyzed the
effect of I3M on cell necrosis. The results showed that even after
a high dose of I3M (35 μM), the amount of LDH released into the
culture medium by JM1 and K562 cells was significantly lower than
that released by the positive controls (Fig. 4).
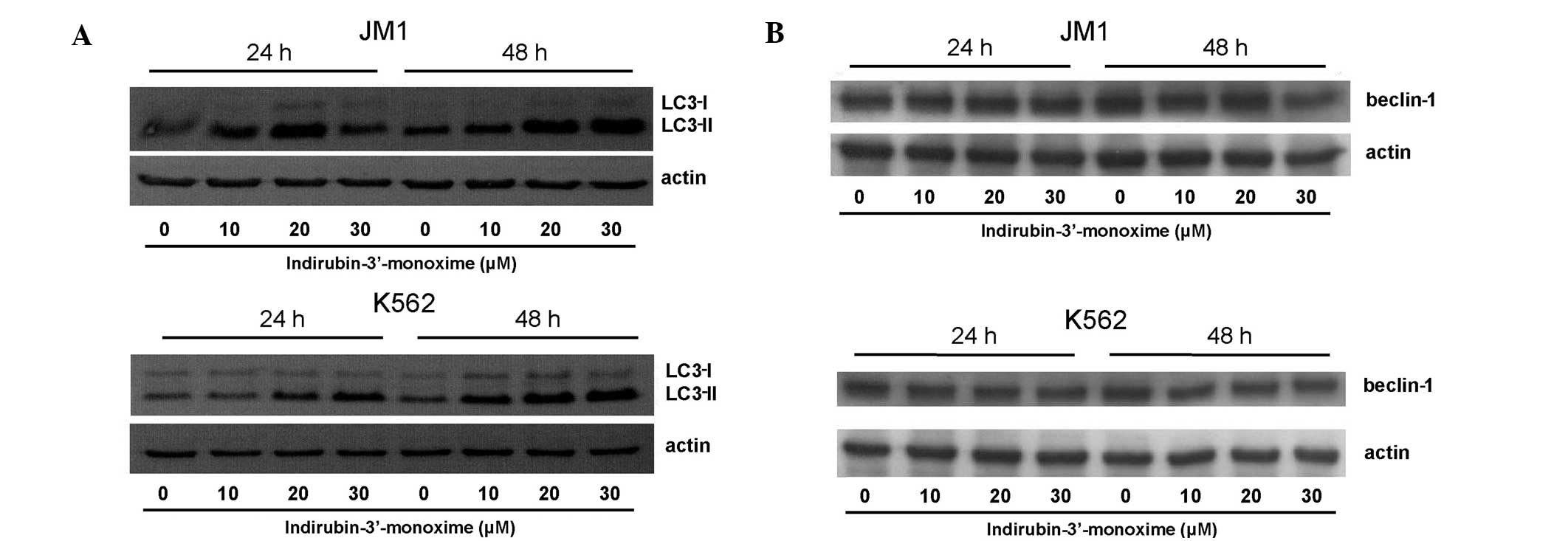
I3M induces autophagic cell death in JM1
and K562 cells
We next investigated whether I3M induced autophagy.
LC3-II formation dose-dependently increased in JM1 and K562 cells
24 and 48 h after treatment (Fig.
5); however, the expression of beclin-1, the other
autophagy-related protein, did not.
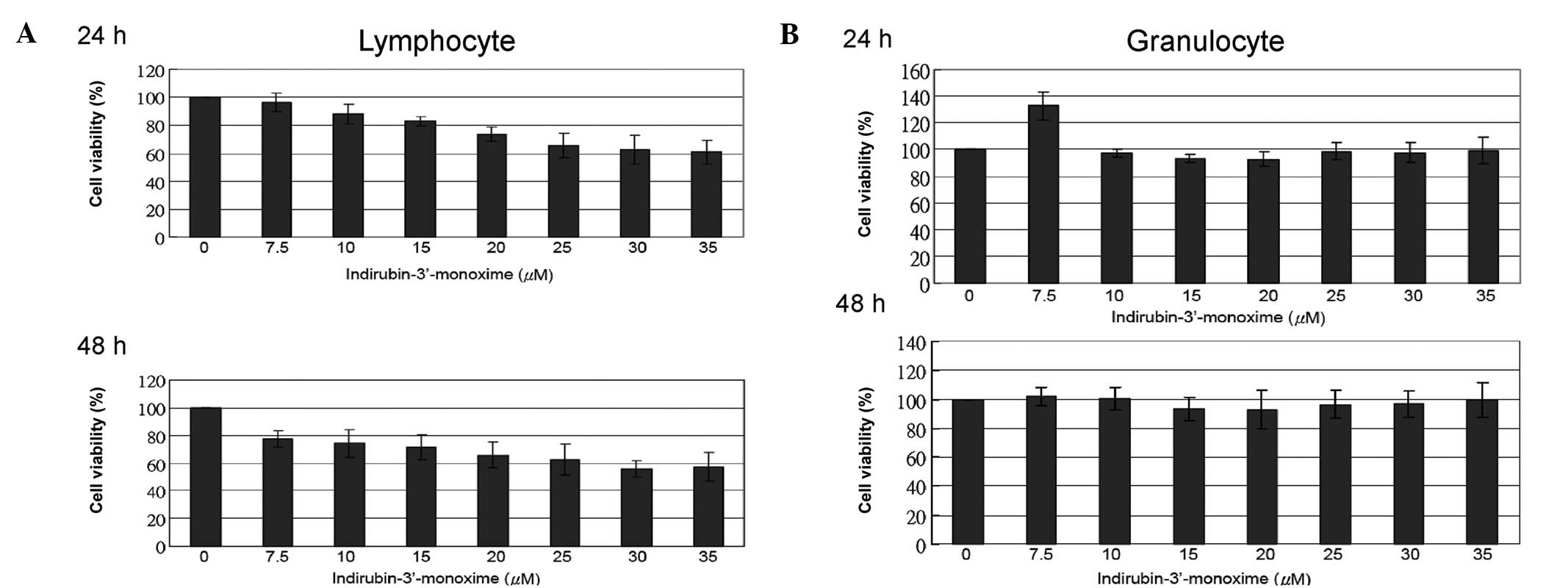
I3M marginally induces cytotoxicity in
primary lymphocytes, but not in primary granulocytes
Primary lymphocytes and granulocytes isolated from
human peripheral blood were treated with the indicated
concentrations of I3M. Cytotoxicity was evaluated using a WST-1
assay. The cell viability of primary lymphocytes treated with the
IC50 value (20 μM) of I3M were only marginally affected;
24-h treatment was 73.9±5.1% and 48-h treatment was 65.9±9.3%
(Fig. 6A), and that of granulocytes
was not significantly affected. Increasing the concentration of I3M
to 35 μM and prolonging the treatment time to 48 h did not induce
cell death (Fig. 6B).
Discussion
In the present study, we found that I3M
dose-dependently increased cell death in human ALL JM1 cells and
human CML K562 cells. These effects on cell viability included
cell-cycle arrest and the induction of apoptosis and autophagy.
Previous studies have shown that several indirubin
derivatives lead to significant G2/M cell-cycle arrest. One study
(4) reported that I3M suppressed
K562 cell proliferation by inducing the arrest of cell development
in the G2/M phase, which supports our observation that I3M
significantly induced a G2/M phase arrest in JM1 and K562 cells.
Moreover, the cell-cycle results showed an increasing sub-G1
population, and, with the caspase-3 activation, demonstrated a
dose-dependent induction of apoptosis. In general, during
apoptosis, caspase-3 activation leads to PARP fragmentation and
cleavage. We also found PARP-1 cleavage in the present study. After
using the pan-caspase inhibitor Z-VAD-FMK to block caspase
activation, I3M-induced cell death was only slightly reduced. This
suggested that, in addition to apoptosis, I3M induces cell death
using other mechanisms as well.
There are at least three types of cell death:
necrosis, apoptosis and autophagy (16). Most studies have indicated that
indirubin induces apoptosis in diverse cancer cells. However, Ribas
et al(17,18) showed that 7-bromoindirubin-3′-oxime,
an indirubin analog, triggered caspase-independent cell death,
i.e., a specific type of necrotic cell death. In the present study,
however, we found that I3M did not induce necrotic cell death in
leukemia cells.
Autophagy is caspase-independent programmed cell
death (19). Microtubule-associated
protein 1 light chain 3 (LC3), an autophagosomal ortholog of yeast
Atg8, is a novel marker for autophagy. LC3-I is localized in the
cytoplasm, whereas LC3-II aggregates on the membrane of
autophagosomes (20,21). Indeed, we found in I3M-treated ALL
cells and CML cells the first evidence of the typical phenomenon of
autophagy, i.e., significant upregulation of LC3-II formation.
Autophagy may trigger the upregulation of beclin-1, since beclin-1
is involved in the formation of autophagosomes (22). Compared with untreated cells,
beclin-1 expression was not significantly upregulated in
I3M-treated JM1 and K562 cells in the present study. Nonetheless,
autophagy occurs independently of beclin-1 (23–26),
which suggests that I3M-induced autophagy might also not affect
beclin-1 expression. Future studies are required to confirm this
hypothesis.
Patients with leukemia produce an abnormal number of
leukocytes. When clinical therapy decreases the number of
granulocytes and healthy lymphocytes in the blood, it often leads
to serious infection and mortality. Therefore, clinical cancer
therapy must be as non-cytotoxic as possible to healthy leukocytes,
particularly for leukemia therapy. PHA-stimulated peripheral blood
lymphocytes are dose-dependently sensitive to I3M treatment
(27); however, I3M is cytotoxic to
unstimulated lymphocytes only in high doses. These data do not
conflict with our findings. Consequently, lymphocyte viability was
only marginally affected in the present study. Due to their innate
immunity, granulocytes are central in defending the host from
infection by other organisms. This is the first study of the
effects of I3M on healthy primary granulocytes. The dose of I3M
that we used had no cytotoxic effect on granulocytes.
In conclusion, we showed that I3M was cytotoxic to
K562 and JM1 cells, and that it had no significant effect on the
cell viability of primary lymphocytes and granulocytes. This
finding might provide a first step for clinical trials with
low-level side-effects in ALL treatment.
Acknowledgements
The authors thank the Tainan Blood Center, Taiwan
Blood Services Foundation, for providing buffy coats. This study
was supported by grant R96-11 from the Ditmanson Medical Foundation
Chia-Yi Christian Hospital Research Program.
References
|
1
|
Pui CH and Evans WE: Treatment of acute
lymphoblastic leukemia. N Engl J Med. 354:166–178. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fielding AK, Richards SM, Chopra R, et al:
Outcome of 609 adults after relapse of acute lymphoblastic leukemia
(ALL); an MRC UKALL12/ECOG 2993 study. Blood. 109:944–950. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tavernier E, Boiron JM, Huguet F, et al:
Outcome of treatment after first relapse in adults with acute
lymphoblastic leukemia initially treated by the LALA-94 trial.
Leukemia. 21:1907–1914. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hoessel R, Leclerc S, Endicott JA, et al:
Indirubin, the active constituent of a Chinese antileukemia
medicine, inhibits cyclin-dependent kinases. Nat Cell Biol.
1:60–67. 1999.PubMed/NCBI
|
|
5
|
Institute of Haematology, Chinese Academy
of Medical Sciences. Experimental and clinical studies of indirubin
in the treatment of CML. Chinese J Intern Med. 18:83–88. 1979.
|
|
6
|
Cooperative Group of Clinical Therapy of
Indirubin. Clinical studies of 314 cases of CML treated with
indirubin. Chinese J Intern Med. 1:132–135. 1980.
|
|
7
|
Marko D, Schatzle S, Friedel A, Genzlinger
A, Zankl H, Meijer L and Eisenbrand G: Inhibition of
cyclin-dependent kinase 1 (CDK1) by indirubin derivatives in human
tumour cells. Br J Cancer. 84:283–289. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jautelat R, Brumby T, Schäfer M, Briem H,
Eisenbrand G, Schwahn S, Krüger M, Lücking U, Prien O and
Siemeister G: From the insoluble dye indirubin towards highly
active, soluble CDK2-inhibitors. Chembiochem. 6:531–540. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim SA, Kim SW, Chang S, Yoon JH and Ahn
SG: 5′-nitro-indirubinoxime induces G2/M cell cycle arrest and
apoptosis in human KB oral carcinoma cells. Cancer Lett. 274:72–77.
2009.
|
|
10
|
Yoon JH, Kim SA, Kwon SM, Park JH, Park
HS, Kim YC, Yoon JH and Ahn SG: 5′-Nitro-indirubinoxime induces G1
cell cycle arrest and apoptosis in salivary gland adenocarcinoma
cells through the inhibition of Notch-1 signaling. Biochim Biophys
Acta. 1800:352–358. 2010.
|
|
11
|
Shi R, Li W, Zhang X, Zhang Y, Peng H, Xie
Y, Fan D, Liu R, Liu X and Xiong D: A novel indirubin derivative
PHII-7 potentiates adriamycin cytotoxicity via inhibiting
P-glycoprotein expression in human breast cancer MCF-7/ADR cells.
Eur J Pharmacol. 669:38–44. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kunz M, Driller KM, Hein M, Hohensee I,
Ramer R, Hinz B, Berger A, Eberle J and Langer P: Synthesis of
thia-analogous indirubin N-Glycosides and their influence on
melanoma cell growth and apoptosis. ChemMedChem. 5:534–539.
2010.PubMed/NCBI
|
|
13
|
Berger A, Quast SA, Plötz M, Hein M, Kunz
M, Langer P and Eberle J: Sensitization of melanoma cells for death
ligand-induced apoptosis by an indirubin derivative. Enhancement of
both extrinsic and intrinsic apoptosis pathways. Biochem Pharmacol.
81:71–81. 2011. View Article : Google Scholar
|
|
14
|
Kameswaran TR and Ramanibai R:
Indirubin-3-monooxime induced cell cycle arrest and apoptosis in
Hep-2 human laryngeal carcinoma cells. Biomed Pharmacother.
63:146–154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Suzuki K, Adachi R, Hirayama A, Watanabe
H, Otani S, Watanabe Y and Kasahara T: Indirubin, a Chinese
anti-leukaemia drug, promotes neutrophilic differentiation of human
myelocytic leukemia HL-60 cells. Br J Haematol. 130:681–690. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Okada H and Mak TW: Pathways of apoptotic
and non-apoptotic death in tumour cells. Nat Rev Cancer. 4:592–603.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ribas J, Bettayeb K, Ferandin Y, et al:
7-Bromoindirubin-3′-oxime induces caspase-independent cell death.
Oncogene. 25:6304–6318. 2006.
|
|
18
|
Ribas J, Yuste VJ, Garrofé-Ochoa X, Meijer
L, Esquerda JE and Boix J: 7-Bromoindirubin-3′-oxime uncovers a
serine protease-mediated paradigm of necrotic cell death. Biochem
Pharmacol. 76:39–52. 2008.
|
|
19
|
Kondo Y, Kanzawa T, Sawaya R and Kondo S:
The role of autophagy in cancer development and response to
therapy. Nat Rev Cancer. 5:726–734. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoshioka A, Miyata H, Doki Y, Yamasaki M,
Sohma I, Gotoh K, Takiguchi S, Fujiwara Y, Uchiyama Y and Monden M:
LC3, an autophagosome marker, is highly expressed in
gastrointestinal cancers. Int J Oncol. 33:461–468. 2008.PubMed/NCBI
|
|
21
|
Kabeya Y, Mizushima N, Ueno T, Yamamoto A,
Kirisako T, Noda T, Kominami E, Ohsumi Y and Yoshimori T: LC3, a
mammalian homologue of yeast Apg8p, is localized in autophagosome
membranes after processing. EMBO J. 19:5720–5728. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang J: Beclin 1 bridges autophagy,
apoptosis and differentiation. Autophagy. 4:947–948. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu JH, Horbinski C, Guo F, Watkins S,
Uchiyama Y and Chu CT: Regulation of autophagy by extracellular
signal-regulated protein kinases during
1-methyl-4-phenylpyridinium-induced cell death. Am J Pathol.
170:75–86. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim I, Rodriguez-Enriquez S and Lemasters
JJ: Selective degradation of mitochondria by mitophagy. Arch
Biochem Biophys. 462:245–253. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mordier S, Deval C, Béchet D, Tassa A and
Ferrara M: Leucine limitation induces autophagy and activation of
lysosome-dependent proteolysis in C2C12 myotubes through a
mammalian target of rapamycin-dependent signaling pathway. J Biol
Chem. 275:29900–29906. 2000. View Article : Google Scholar
|
|
26
|
Kanazawa T, Taneike I, Akaishi R,
Yoshizawa F, Furuya N, Fujimura S and Kadowaki M: Amino acids and
insulin control autophagic proteolysis through different signaling
pathways in relation to mTOR in isolated rat hepatocytes. J Biol
Chem. 279:8452–8459. 2004. View Article : Google Scholar
|
|
27
|
Kagialis-Girard S, Mialou V, Chebel A,
Chien WW, Tigaud I, Mokdad F, Badiou C and Ffrench M: Inhibition of
normal lymphocyte proliferation by indirubin-3′-monoxime: a
multifactorial process. Leuk Lymphoma. 48:605–615. 2007.PubMed/NCBI
|