Introduction
DNA double-strand breaks (DSBs) are generally
assumed to play a major role in radiation-induced cell death
(1). Phosphorylation of the histone
protein H2AX (γ-H2AX) is one of the earliest markers of DNA damage
after ionizing radiation. These γ-H2AX ionizing radiation-induced
foci (IRIF), which appear already at 3 min after irradiation and
increase in time reaching a maximum at 20–30 min after irradiation,
have been reported to mark the locations of DNA DSBs (2–7). After
the breaks are rejoined, γ-H2AX is dephosphorylated again. The
disappearance of the foci is related to repair of the DNA (8).
An important factor in responses of cells to
irradiation is potentially lethal damage repair (PLDR). Repair of
PLD is usually complete between 6 and 12 h after irradiation
(9). PLDR can be studied with
delayed plating experiments of plateau-phase cultures. Survival of
cells plated after a delay of 24 h following irradiation is
compared with survival of cells plated directly after irradiation
(10–13). In cells demonstrating PLDR, cell
survival is enhanced if the cells are allowed to remain undisturbed
for some time after irradiation before they are assayed for colony
formation (10,11). Several studies have demonstrated
that PLDR can be influenced by the status of the tumor protein 53
(TP53) (14–18). However, some studies have suggested
that PLDR does not depend on functional TP53 (19–21).
Survival curves are commonly described and analyzed
using the linear-quadratic (LQ) model: S(D)/S(0) = exp
-(αD+βD2) (22–24). Studies investigating the repair of
potentially lethal damage are critical as factors influencing PLDR
may alter tumor radiocurability. The advantage of using the LQ
model is that changes in PLDR can be determined quantitatively by
analyses of the linear parameter α, describing the low dose range
of the survival curve, separately from the parameter β dominating
the high dose range (10,25–27).
Analysis of survival curves from numerous studies has shown that
PLDR is most clearly demonstrated by changes of the linear
parameter α (24,28–30).
The relationship between radiation sensitivity and
the induction of γ-H2AX IRIF is not always clear. Foci of γ-H2AX
are also induced by factors other than ionizing radiation, such as
during the process of replication. Not all cell lines have similar
numbers of γ-H2AX foci after equal radiation doses. Several studies
showed that the induction of γ-H2AX foci is not directly correlated
with the cellular survival after radiation (4,31).
However, it has been demonstrated that there is a correlation
between the number of residual DNA DSBs at 10 h after irradiation
and cell survival (32,33). Previous studies suggested that the
induction of γ-H2AX after single and fractionated irradiation
appears to be a useful marker of cellular radiosensitivity
(8,34).
The aim of the present study was to establish
whether PLDR is correlated with repair of DNA DSB. As the status of
TP53 is important for PLDR (16),
the level of PLDR was determined in three prostate cell lines with
different TP53 status. Then, the induction of γ-H2AX foci after a 2
Gy dose was determined directly and 24 h after radiation. The data
indicate correlations between TP53 status and PLDR, and decay of
γ-H2AX foci and the level of PLDR.
Materials and methods
Cell cultures
Human prostate cancer cell lines with different
status of TP53 were used: LNCaP, wt TP53; PC3, TP53 null; and
DU145, mut TP53, as previously described (35–37).
All three cell lines were cultured in RPMI-1640 medium (Gibco,
Invitrogen) supplemented with 10% fetal calf serum, 100 U/ml
penicillin/streptomycin and 1 mM glutamine in a humidified
atmosphere of 5% CO2/95% air. The doubling time of all
three cell lines was 24 h.
Western blotting
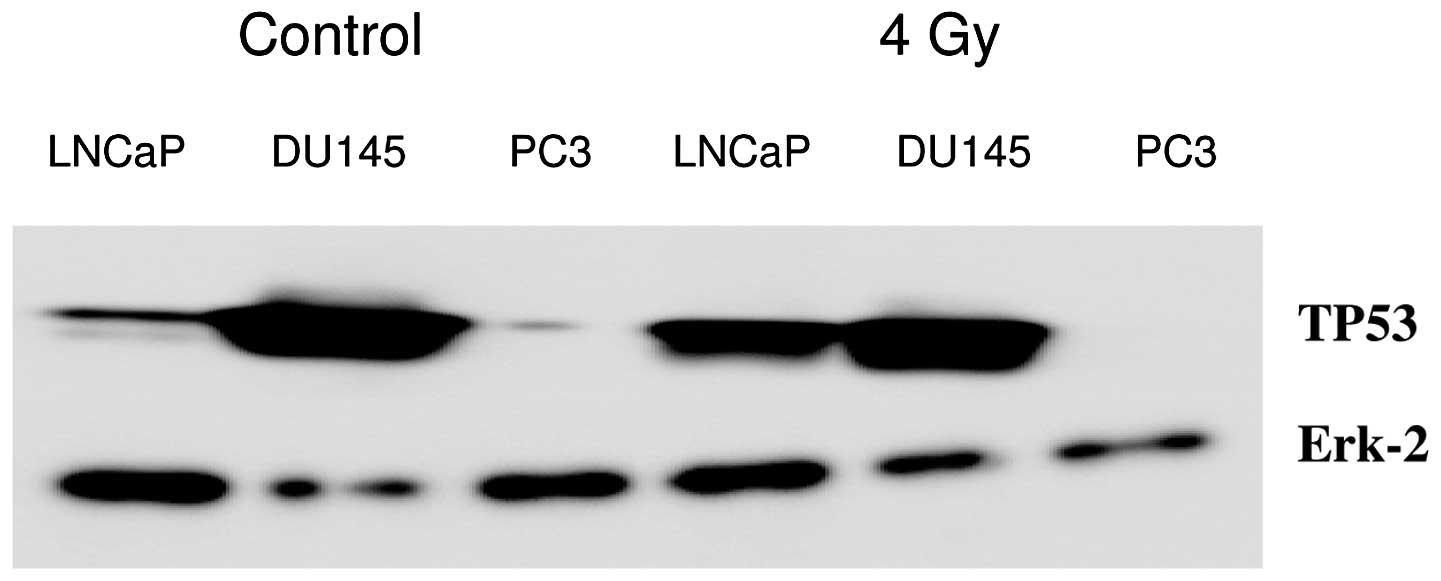
Western blotting of TP53 induction at 4 h after 4 Gy
is shown in Fig. 1. In LNCaP cells,
TP53 was induced at 4 h after 4 Gy; in DU145 cells, the mutated
TP53 protein was present before and after irradiation; and in PC3
cells, no TP53 was detected. Erk-2 protein was used for loading
control.
Radiation treatment
Confluent cultures of cells growing in monolayers
were irradiated at 37°C in a waterbath and 5% CO2/95%
air was supplied during irradiation. Irradiation was performed with
a Stabilipan 250 KeV X-ray machine (Siemens, Germany). For
determination of γ-H2AX foci, a radiation dose of 2 Gy was applied
and for the survival experiments cells were exposed to single doses
of 0, 2, 4, 6 and 8 Gy. The distance between the focus and the
culture dish was 9 cm. A 0.5-mm Cu filter was used and the dose
rate was ~3 Gy/m.
Clonogenic survival
Directly and 24 h after irradiation, cells were
trypsinized and replated for clonogenic survival assay in
appropriate cell numbers in 6-well macroplates (38,39).
Subsequently, cells were incubated for 10 days. Surviving colonies
were fixated and stained with glutaraldehyde-crystal violet
solution and counted. Survival curves were analyzed using SPSS
statistical software (Chicago, IL, USA) by means of fit of data by
weighted linear regression, according to the linear-quadratic
formula: S(D)/S(0) = exp-(αD+βD2) (10,27,40,41).
In the formula, the S(D) is the survival at dose D and S(0) is the
survival at dose 0. As a measure of PLDR, the ratio PLD-α is
calculated as the ratio of the value of linear parameter α of cells
immediately plated (ip) after irradiation and cells delayed plated
(dp) 24 h after irradiation.
Immunohistochemistry for γ-H2AX
We counted the number of γ-H2AX foci in cells that
were grown on cover slides (42,43).
The cover slides (21×26 mm) were sterilized with alcohol and were
placed in 60-mm cell culture dishes. The cells were reseeded at a
density of 2.5×105 cells on cell culture dishes
containing sterile cover slides and were grown until a confluent
layer was obtained. The cells were then irradiated. The number of
γ-H2AX foci was determined 30 min and 24 h after irradiation.
Following irradiation, cells were washed with
phosphate-buffered saline (PBS) and fixed in PBS containing 2%
para-formaldehyde for 15 min. After three further washes with PBS,
cells were treated with PBS containing 0.1% Triton X-100 and 1% FCS
(TNBS) for 30 min to permeabilize the cells.
A primary mouse monoclonal anti-γ-H2AX antibody
(Millipore) was diluted 1:100 in TNBS. Fixed, permeabilized cells
on the cover slides were incubated with 50 μl primary antibody
under a parafilm strip for 90 min at room temperature. Cells were
then washed with PBS for ~5 min and the parafilm strip was removed.
Subsequently, cells were washed 2 times with TNBS.
Cells on cover slides were incubated with 50 μl
secondary antibody anti-Mouse Cy3 (Jackson) (1:100 in TNBS) under a
parafilm strip for 30 min at room temperature. Cells were then
washed 2–3 times with TNBS for ~5 min and the parafilm strip was
removed. Nuclei were stained with DAPI (2.5 μg/ml) for 5 min and
embedded in Vectashield. Then, cover slides were sealed to
microscope slides. Rubber cement was used to seal the whole
construct.
γ-H2AX foci scoring
Digital image analysis was performed to determine
the number of γ-H2AX IRIF. Fluorescent photomicrographs of γ-H2AX
foci were obtained using Image Pro Plus software. Stack images of
cells were obtained using a Leica DM RA HC Upright Microscope
equipped with a CCD camera. Stack images of 50 cells/sample were
captured using Image Pro Plus software. One stack image consisted
of 23 slices with a 300-nm interval between the slices along the
Z-axis. Images were then processed and the number of foci in cells
was scored using custom-made software (42–45).
All experiments were carried out in triplicates,
independently from each other. Numbers of foci in unirradiated
control cells were subtracted from numbers in irradiated samples.
S-phase cells were excluded using an EdU
(5-ethynyl-2′-deoxyuridine) staining (Invitrogen, Eugene, OR, USA)
to mark these cells. The ratio of the number of γ-H2AX foci at 30
min and 24 h after irradiation was calculated as a measure of foci
decay resulting from repair of DNA DSBs.
Results
To assess TP53 status of the used cell lines,
western blot analysis was performed of TP53 induction after 4 Gy
radiation dose (Fig. 1). In LNCaP
cells, wt TP53 induction was visible; in the DU145 cells, mutant
TP53 was present; and in the PC3 cells, no TP53 was observed.
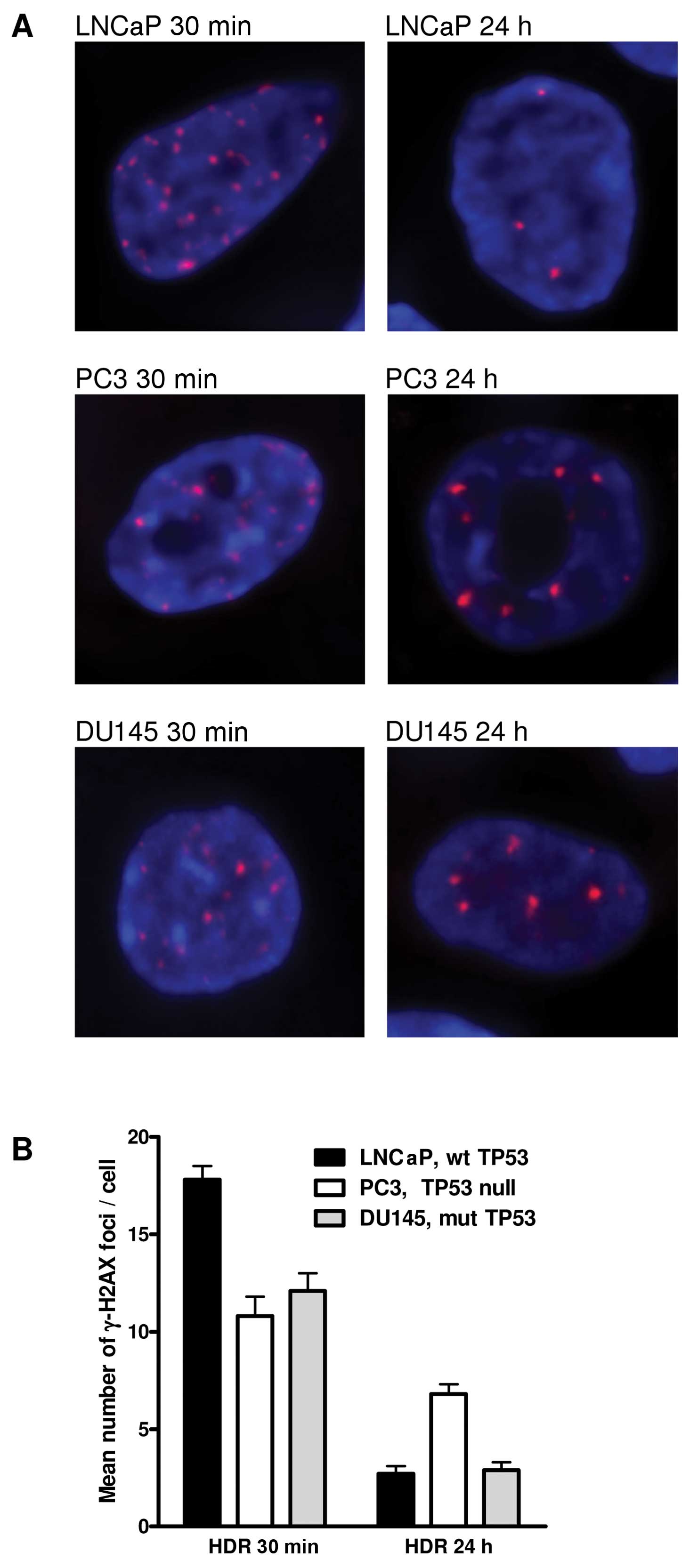
Fig. 2 shows the
radiation-induced number of γ-H2AX foci in the different prostate
cancer cells. At 30 min after irradiation, LNCaP cells had the
highest number and PC3 cells had the lowest number of γ-H2AX foci.
On the contrary, 24 h post-treatment PC3 cells had the highest
number and LNCaP cells had the lowest number of foci. The DU145
cells had intermediate numbers of foci for both post-irradiation
conditions. Initial number of foci at 30 min after irradiation
ranged between 10 and 18 foci/cell. At 24 h after treatment, the
numbers ranged between 2.7 and 7 foci/cell. The decline in foci
number was the highest for LNCaP cells and the lowest for PC3
cells.
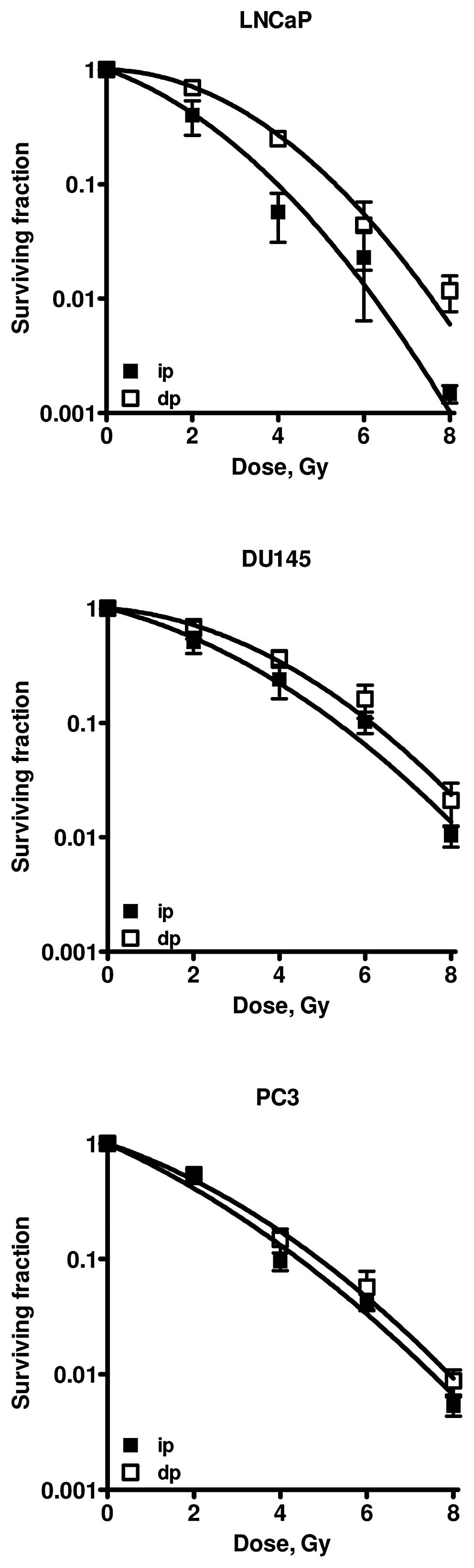
Survival curves of the different cell lines are
presented in Fig. 3. LNCaP and
DU145 cells clearly showed increased survival after dp as compared
to ip cells after irradiation. Survival curves of PC3 cells plated
immediately and 24 h after irradiation do not show any difference.
Values of the linear and quadratic parameters, α and β, the PLD-α
ratio (= αip/αdp) as a measure of PLDR, the
ratio of the number of γ-H2AX foci at 30 min and 24 h after
irradiation, and the TP53 status of the different cell lines are
presented in Table I. The decay of
foci correlates well with PLDR. In Table II, the surviving fractions and the
number of foci after 2 Gy are given for immediately and delayed
plated cells. It can be observed that in almost all cases, high
survival levels correlated with low residual foci numbers and, vice
versa, low survival levels correlated with high residual numbers of
foci.
 | Table ILQ parameters α and β, PLD-α, ratio of
foci decay and the TP 53 status of the different prostate cancer
cell lines. |
Table I
LQ parameters α and β, PLD-α, ratio of
foci decay and the TP 53 status of the different prostate cancer
cell lines.
| LQ parameter cell
line | α,
Gy−1 | β,
Gy−2 | PLDR-α | Ratio foci
decay | TP53 status |
|---|
| LNCaP |
| ip | 0.31±0.09 | 0.08±0.03 | 10.3±3.9 | 6.9±0.3 |
TP53+ |
| dp | 0.03±0.01 | 0.08±0.02 | | | |
| DU145 |
| ip | 0.22±0.06 | 0.04±0.01 | 3.1±1.6 | 4.2±0.7 | TP53 mutated |
| dp | 0.07±0.03 | 0.05±0.01 | | | |
| PC3 |
| ip | 0.39±0.04 | 0.03±0.01 | 1.3±0.22 | 1.6±0.2 | TP53 null |
| dp | 0.30±0.04 | 0.04±0.01 | | | |
 | Table IISurviving fraction and the number of
radiation-induced γ-H2AX foci after 2 Gy. |
Table II
Surviving fraction and the number of
radiation-induced γ-H2AX foci after 2 Gy.
| LNCaP | DU145 | PC3 |
|---|
|
|
|
|
|---|
| 2 Gy | Surviving
fraction | No. of foci | Surviving
fraction | No. of foci | Surviving
fraction | No. of foci |
|---|
| ip | 0.39±0.10 | 17.8±0.7 | 0.55±0.08 | 12.1±0.9 | 0.41±0.05 | 10.8±1.0 |
| dp | 0.70±0.15 | 2.7±0.4 | 0.71±0.10 | 2.9±0.4 | 0.46±0.05 | 6.8±0.5 |
Discussion
The three prostate tumor cell lines examined in this
study differ in their TP53 status. The TP53 status was confirmed
with western blotting. In LNCaP cells, TP53 was induced 4 h after 4
Gy irradiation; in DU145 cells, the mutated TP53 protein was
present before and after irradiation; in PC3 cells, TP53 was not
detected at all. Earlier studies reported that an intact TP53
status is required for repair of potentially lethal damage
(14–17). Therefore, the level of PLDR was
investigated in the three cells lines. The LNCaP cells with wt TP53
protein clearly demonstrated PLDR. As expected, in the DU145 cell
line harbouring mutated TP53 PLDR was reduced and in the PC3 cells
(TP53 null) PLDR was not seen at all.
Furthermore, the present data demonstrates that the
decay of γ-H2AX foci after 2 Gy radiation dose correlates with
PLDR. Phosphorylation of H2AX occurs rapidly after induction of DNA
DSB. The γ-H2AX foci have been suggested to be a valid measure for
radiosensitivity and the disappearance of the foci might be related
to the repair of DNA damage following radiation treatment (8). It has already been shown by MacPhail
et al(46) that the decay of
γ-H2AX foci is associated with cell survival and repair of DSB.
Yoshikawa et al(31)
suggested that there was no close correlation between residual foci
and radiosensitivity in some tumor cell lines. However, Yoshikawa
et al(31) only studied
survival of cells plated immediately after irradiation. In our
study, we irradiated cells and plated them both directly and 24 h
after irradiation in order to study PLDR. The cell line with the
highest PLDR was also found to have the largest decay in number of
foci, resembling a more proficient repair of DNA DSB. This is also
corroborated by our observation that higher surviving fractions
after 2 Gy correlated with a lower number of residual γ-H2AX foci
in the prostate cell lines (Table
II).
The linear-quadratic model is based on well-accepted
biophysical concepts, involving the assumption that lethal damage
can be induced by single-particle tracks and by interaction of
damage from multiple particles.
In a review of published data on the dependence of
different types of lethal damage on the linear energy transfer of
ionizing particles, Barendsen (23,25,26)
derived evidence that sublethal lesions and potentially lethal
lesions show similar RBE-LET (relative biological effect-linear
energy transfer) relationships as DNA DSBs, with only a relatively
low RBE at the optimal LET (27).
This is evidently different from the high RBE values commonly
derived for unrepairable lethal lesions and chromosome aberrations.
The hypothesis was proposed that sublethal lesions and potentially
lethal lesions are both DNA-DSBs. The present results on the decay
of γ-H2AX foci, which mark DNA DSBs, and the correlation with PLD
repair are consistent with this hypothesis. Potentially lethal
lesions contribute only a part to the linear parameter α in the LQ
model and are similarly repairable as sublethal damage. In earlier
studies, we demonstrated a correlation between survival,
chromosomal aberrations and PLDR, which was shown by a decrease in
the value of α with higher survival and lower number of chromosomal
aberrations (12,13). Herein, we further demonstrated that
there is an association between PLDR α and a decrease of DNA DSB,
which strengthens the biological basis of the LQ model. However,
our study remains to be confirmed in an isogenic system with cells
only different in TP53 status.
Acknowledgements
The authors thank the Maurits and Anna de Kock and
the Nijbakker Morra foundations for sponsoring the fluorescence
microscopes with software to study the γ-H2AX foci. The Dutch
Cancer Foundation (nos. UVA 2008-4019 and UVA 2012-5540) and the
Stichting Vanderes are acknowledged for financing personnel
support.
References
|
1
|
Frankenberg-Schwager M: Induction, repair
and biological relevance of radiation-induced DNA lesions in
eukaryotic cells. Radiat Environ Biophys. 29:273–292. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aten JA, Stap J, Krawczyk PM, van Oven CH,
Hoebe RA, Essers J and Kanaar R: Dynamics of DNA double-strand
breaks revealed by clustering of damaged chromosome domains.
Science. 303:92–95. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lau P, Baumstark-Khan C, Hellweg CE and
Reitz G: X-irradiation-induced cell cycle delay and DNA
double-strand breaks in the murine osteoblastic cell line OCT-1.
Radiat Environ Biophys. 49:271–280. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beels L, Werbrouck J and Thierens H: Dose
response and repair kinetics of gamma-H2AX foci induced by in vitro
irradiation of whole blood and T-lymphocytes with X- and
gamma-radiation. Int J Radiat Biol. 86:760–768. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vandersickel V, Depuydt J, Van Bockstaele
B, Perletti G, Philippe J, Thierens H and Vral A: Early increase of
radiation-induced γH2AX foci in a human Ku70/80 knockdown cell line
characterized by an enhanced radiosensitivity. J Radiat Res.
51:633–641. 2010.
|
|
6
|
Mosconi M, Giesen U, Langner F, Mielke C,
Dalla Rosa I and Dirks WG: 53BP1 and MDC1 foci formation in HT-1080
cells for low- and high-LET microbeam irradiations. Radiat Environ
Biophys. 50:345–352. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zlobinskaya O, Dollinger G, Michalski D,
Hable V, Greubel C, Du G, Multhoff G, Röper B, Molls M and Schmid
TE: Induction and repair of DNA double-strand breaks assessed by
gamma-H2AX foci after irradiation with pulsed or continuous proton
beams. Radiat Environ Biophys. 51:23–32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Olive PL and Banáth JP: Phosphorylation of
histone H2AX as a measure of radiosensitivity. Int J Radiat Oncol
Biol Phys. 58:331–335. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hall EJ and Garcia AJ: Radiobiology for
the Radiologist. 6th edition. Lippincott Williams & Wilkins;
Philadelphia, PA: 2006
|
|
10
|
Franken NA, van Bree C, Kipp JB and
Barendsen GW: Modification of potentially lethal damage in
irradiated Chinese hamster V79 cells after incorporation of
halogenated pyrimidines. Int J Radiat Biol. 72:101–109. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Franken N, van Bree C, Streefkerk J, Kuper
I, Rodermond H, Kipp J and Barendsen G: Radiosensitization by
iodo-deoxyuridine in cultured SW-1573 human lung tumor cells. Oncol
Rep. 4:1073–1076. 1997.PubMed/NCBI
|
|
12
|
Franken NA, Ruurs P, Ludwików G, van Bree
C, Kipp JB, Darroudi F and Barendsen GW: Correlation between cell
reproductive death and chromosome aberrations assessed by FISH for
low and high doses of radiation and sensitization by
iodo-deoxyuridine in human SW-1573 cells. Int J Radiat Biol.
75:293–299. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Franken NA, van Bree C, Veltmaat MA,
Ludwików G, Kipp JB and Barendsen GW: Increased chromosome exchange
frequencies in iodo-doxyuridine-sensitized human SW-1573 cells
after γ-irradiation. Oncol Rep. 6:59–63. 1999.PubMed/NCBI
|
|
14
|
Schwartz JL, Jordan R, Kaufmann WK, Rasey
J, Russell KJ and Weichselbaum RR: Evidence for the expression of
radiation-induced potentially lethal damage being a p53-dependent
process. Int J Radiat Biol. 76:1037–1043. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schwartz JL, Rasey J, Wiens L, Jordan R
and Russell KJ: Functional inactivation of p53 by HPV-E6
transformation is associated with a reduced expression of
radiation-induced potentially lethal damage. Int J Radiat Biol.
75:285–291. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Franken NA, van Bree C, ten Cate R, van
Oven CH and Haveman J: Importance of TP53 and RB in the repair of
potentially lethal damage and induction of color junctions after
exposure to ionizing radiation. Radiat Res. 158:707–714. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Franken NA, van Bree C and Haveman J:
Differential response to radiation of TP53-inactivated cells by
overexpression of dominant-negative mutant TP53 or HPVE6. Radiat
Res. 161:504–510. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Devlin HL, Mack PC, Burich RA, Gumerlock
PH, Kung HJ, Mudryj M and deVere White RW: Impairment of the DNA
repair and growth arrest pathways by p53R2 silencing enhances DNA
damage-induced apoptosis in a p53-dependent manner in prostate
cancer cells. Mol Cancer Res. 6:808–818. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Danielsen T, Smith-Sørensen B, Grønlund
HA, Hvidsten M, Børresen-Dale AL and Rofstad EK: No association
between radiosensitivity and TP53 status, G1 arrest or protein
levels of p53, myc, ras or raf in human melanoma lines. Int J
Radiat Biol. 75:1149–1160. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van Bree C, Savonije JH, Franken NA,
Haveman J and Bakker PJ: The effect of p53-function on the
sensitivity to paclitaxel with or without hyperthermia in human
colorectal carcinoma cells. Int J Oncol. 16:739–744.
2000.PubMed/NCBI
|
|
21
|
van Bree C, Franken NA, Rodermond HM,
Stalpers LJ and Haveman J: Repair of potentially lethal damage does
not depend on functional TP53 in human glioblastoma cells. Radiat
Res. 161:511–516. 2004.
|
|
22
|
Barendsen GW: Dose fractionation, dose
rate and iso-effect relationships for normal tissue responses
(review). Int J Radiat Oncol Biol Phys. 8:1981–1997. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Barendsen GW: RBE-LET relationships for
different types of lethal radiation damage in mammalian cells:
comparison with DNA dsb and an interpretation of differences in
radiosensitivity. Int J Radiat Biol. 66:433–436. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Barendsen GW: Parameters of
linear-quadratic radiation dose-effect relationships: dependence on
LET and mechanisms of reproductive cell death. Int J Radiat Biol.
71:649–655. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barendsen GW: Sublethal damage and DNA
double strand breaks have similar RBE-LET relationships: evidence
and implications. Int J Radiat Biol. 63:325–330. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barendsen GW: The relationships between
RBE and LET for different types of lethal damage in mammalian
cells: biophysical and molecular mechanisms (review). Radiat Res.
139:257–270. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Barendsen GW, van Bree C and Franken NA:
Importance of cell proliferative state and potentially lethal
damage repair on radiation effectiveness: Implications for combined
tumor treatments (Review). Int J Oncol. 19:247–256. 2001.PubMed/NCBI
|
|
28
|
Franken NA, Hovingh S, Rodermond H,
Stalpers L, Barendsen GW and Crezee J: Radiosensitization with
chemotherapeutic agents and hyperthermia: Effects on the
linear-quadratic parameters of radiation cell survival curves. J
Cancer Sci Ther S. 5:002 View Article : Google Scholar : 2011.
|
|
29
|
Franken NA, Hovingh S, Oei A, Cobussen P,
Bergs JW, van Bree C, Rodermond HM, Stalpers LJ, Kok P, Barendsen
GW and Crezee J: Radiosensitization with hyperthermia and
chemotherapeutic agents: Effects on linear-quadratic parameters of
radiation cell survival curves (Review). Ionizing Radiation/Book 1.
Mitsuru N. Intech Publisher; 2012
|
|
30
|
van Bree C, Franken NA, Bakker PJ,
Klomp-Tukker LJ, Barendsen GW and Kipp JB: Hyperthermia and
incorporation of halogenated pyrimidines: radiosensitization in
cultured rodent and human tumor cells. Int J Radiat Oncol Biol
Phys. 39:489–496. 1997.PubMed/NCBI
|
|
31
|
Yoshikawa T, Kashino G, Ono K and Watanabe
M: Phosphorylated H2AX foci in tumor cells have no correlation with
their radiation sensitivities. J Radiat Res. 50:151–160. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dikomey E and Brammer I: Relationship
between cellular radiosensitivity and non-repaired double-strand
breaks studied for different growth states, dose rates and plating
conditions in a normal human fibroblast line. Int J Radiat Biol.
76:773–781. 2000. View Article : Google Scholar
|
|
33
|
Banáth JP, Klokov D, MacPhail SH, Banuelos
CA and Olive PL: Residual gammaH2AX foci as an indication of lethal
DNA lesions. BMC Cancer. 10:42010.PubMed/NCBI
|
|
34
|
Klokov D, MacPhail SM, Banáth JP, Byrne JP
and Olive PL: Phosphorylated histone H2AX in relation to cell
survival in tumor cells and xenografts exposed to single and
fractionated doses of X-rays. Radiother Oncol. 80:223–229. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
DeWeese TL, Shipman JM, Dillehay LE and
Nelson WG: Sensitivity of human prostatic carcinoma cell lines to
low dose rate radiation exposure. J Urol. 159:591–598. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Geldof AA and Slotman BJ: Radiosensitizing
effect of cisplatin in prostate cancer cell lines. Cancer Lett.
101:233–239. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Geldof AA, Plaizier MA, Duivenvoorden I,
Ringelberg M, Versteegh RT, Newling DW and Teule GJ: Cell cycle
perturbations and radiosensitization effects in a human prostate
cancer cell line. J Cancer Res Clin Oncol. 129:175–182.
2003.PubMed/NCBI
|
|
38
|
Franken NAP, Rodermond HM, Stap J, Haveman
J and van Bree C: Clonogenic assay of cells in vitro. Nat Protoc.
1:2315–2319. 2006. View Article : Google Scholar
|
|
39
|
Bergs JW, Franken NA, ten Cate R, van Bree
C and Haveman J: Effects of cisplatin and gamma-irradiation on cell
survival, the induction of chromosomal aberrations and apoptosis in
SW-1573 cells. Mutat Res. 594:148–154. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Franken NA, van Bree C, Veltmaat MA,
Rodermond HM, Haveman J and Barendsen GW: Radiosensitization by
bromodeoxyuridine and hyperthermia: analysis of linear and
quadratic parameters of radiation survival curves of two human
tumor cell lines. J Radiat Res. 42:179–190. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Franken NA, ten Cate R, van Bree C and
Haveman J: Induction of the early response protein EGR-1 in human
tumour cells after ionizing radiation is correlated with a
reduction of repair of lethal lesions and an increase of repair of
sublethal lesions. Int J Oncol. 24:1027–1031. 2004.
|
|
42
|
Franken NA, ten Cate R, Krawczyk PM, Stap
J, Haveman J, Aten J and Barendsen GW: Comparison of RBE values of
high-LET α-particles for the induction of DNA-DSBs, chromosome
aberrations and cell reproductive death. Radiat Oncol.
6:642011.
|
|
43
|
Franken NA, Hovingh SE, ten Cate R,
Krawczyk PM, Stap J, Hoebe R, Aten J and Barendsen GW: Relative
biological effectiveness of high linear energy transfer α-particles
for the induction of DNA-double-strand breaks, chromosome
aberrations and reproductive cell death in SW-1573 lung tumour
cells. Oncol Rep. 27:769–774. 2012.
|
|
44
|
Ludwików G, Xiao Y, Hoebe RA, Franken NA,
Darroudi F, Stap J, Van Oven CH, Van Noorden CJ and Aten J:
Induction of chromosome aberrations in unirradiated chromatin after
partial irradiation of a cell nucleus. Int J Radiat Biol.
78:239–247. 2002.PubMed/NCBI
|
|
45
|
Bergs JW, Krawczyk PM, Borovski T, ten
Cate R, Rodermond HM, Stap J, Medema JP, Haveman J, Essers J, van
Bree C, Stalpers LJ, Kanaar R, Aten J and Franken NA: Inhibition of
homologous recombination by hyperthermia shunts early double strand
break repair to non-homologous end-joining. DNA Repair (Amst).
12:38–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
MacPhail SH, Banáth JP, Yu TY, Chu EH,
Lambur H and Olive PL: Expression of phosphorylated histone H2AX in
cultured cell lines following exposure to X-rays. Int J Radiat
Biol. 79:351–358. 2003. View Article : Google Scholar : PubMed/NCBI
|