Introduction
Hepatocellular carcinoma (HCC) is one of the most
common solid tumors worldwide, particularly in East Asia and in
Sub-Saharan Africa (1,2). It is the second most common cause of
cancer-related mortality in men, and the sixth in women (3). Currently, the only curative
therapeutic options for early-stage HCC are surgical interventions,
including percutaneous ablation, hepatic resection, and liver
transplantation. Fewer than 12% of diagnosed HCC patients are
eligible for curative therapies in developing countries (4). Survival of HCC varies widely, and
similar clinicopathological characteristics are likely attributable
to heterogeneous biological behavior of tumor cells (5). Although recent studies have found some
abnormal gene expressions in HCC that could serve as prognostic
markers, knowledge of molecules that could help forecast early
recurrence of HCC is limited.
Recently, Bill et al(6) found that cytohesin-2 was significantly
overexpressed in human lung cancer, and inhibition of cytohesin
could lead to reduction of lung cancer xenografts via activation of
cytoplasmic ErbB receptors. Cytohesin-2 is part of the cytohesin
family, which are guanine nucleotide exchange factors (GEFs) for
ADP ribosylation factors (ARFs) that belong to the family of small
Ras-like GTPases. As in the case of other small GTPases, ARF
function critically depends on activation by GEFs (7). Thus, since ARFs are involved in
controlling cytoskeletal dynamics, cell migration, vesicular
traffic, and signaling (8,9), cytohesins are key regulators of these
processes. Cytohesin was initially reported in 1996 by Kolanus
et al(10). However, recent
studies have focused on physiological action, and the function of
cytohesin in pathological states, particularly in cancer, is
limited (11). Cytohesin critically
affects cytoplasmic conformational activators of ErbB receptors,
which are phosphoinositide 3-kinase (PI3K)/Akt/mTOR pathway in
HEK-293 cells. Recognized abnormalities in HCC include aberrant
signaling through the PI3K/AKT and mTOR pathways (12). Cytohesin-2 overexpression in human
lung cancer is reportedly correlated with auto-phosphorylation of
EGFR (6); EGFR auto-phosphorylation
was also identified in HCC (13).
Lim et al(11) reported
cytohesin-2 as critical to the insulin and insulin-like growth
factor (IGF) pathway in HepG2 cells. Another study showed the role
of cytohesin-2 in vascular endothelial growth factor
(VEGF)-dependent initiation of angiogenesis by regulating VEGF
Receptor (VEGFR)-2 internalization in endothelial cells (14).
In the present study, we examined the expression of
cytohesin-2 gene and protein in 80 samples of paired tumor and the
surrounding non-tumorous liver tissue, and these samples were
collected from 40 patients with HCC. The correlations between
cytohesin-2 expression and the clinicopathological characteristics
were evaluated, and the prognostic significance of cytohesin-2 in
HCC was also elucidated.
Materials and methods
Patients and tissue specimens
The study group consisted of 40 HCC patients who
underwent surgery (hepatic resection) at the First Hospital of
Xi'an Jiaotong University, China (from 2008 to 2009). All tumors
and surrounding non-tumorous liver tissues were collected at
surgical resection and stored immediately at −80°C until analysis.
All specimens were confirmed histologically. Written informed
consent, as required by the Institutional Review Board, was
obtained from all patients. The clinicopathological profiles of the
patients enrolled in the study were collected. The study was
approved by the Local Medical Ethics Committee.
Antibodies
Polyclonal rabbit anti-cytohesin-2 (10405-1-AP) and
polyclonal rabbit anti-β-actin (10497-1-AP) were obtained from
Proteintech Group (Chicago, IL, USA). Goat anti-mouse and goat
anti-rabbit IgG (H+L), peroxidase conjugated were obtained from
Pierce Biotechnology (USA).
RNA preparation and reverse
transcription
Total RNA was extracted from the HCC and surrounding
non-tumorous liver tissue samples with RNAfast200 (Fastagen
Biotechnology, Shanghai, China). The amount of RNA was measured
spectrophotometrically by absorbance at 260 nm. First-strand cDNA
was generated from RNA with the RevertAid First Strand cDNA
Synthesis kit from Fermentas (Shanghai, China).
Quantitative real-time polymerase chain
reaction (qRT-PCR)
qRT-PCR was performed in a Bio-Rad Real-time iQ5
System (Bio-Rad, Philadelphia, PA, USA) using SYBR Premix Ex Taq II
(Takara, Dalian, China). Thermocycling was carried out in a final
volume of 20 μl containing 1.0 μl of the cDNA sample, 100 nM each
of the cytohesin-2 or β-actin primers (forward and
reverse), and 10 μl of SYBR Premix Ex Taq II (including TaqDNA
polymerase, reaction buffer, and deoxynucleotide triphosphate
mixture). The primers for qRT-PCR were: cytohesin-2,
forward, 5′-acggtgccatgactgaggtg-3 and reverse,
5′-tgctgaaggtcagtgtgacgtg-3′; β-actin, forward,
5′-tgtccaccttccagcagatgtg-3′ and reverse,
5′-agtcctcggccacattgtgaac-3′ (all produced by Takara). The PCR
amplification consisted of 40 cycles (95°C for 5 sec, 55°C for 30
sec) after an initial denaturation step (95°C for 10 sec). To
correct for differences in both quality and quantity between
samples, β-actin was used as an internal control. The
targets were obtained from the same mRNA preparations. Melting
curves were performed to ensure only one product was amplified.
Cytohesin-2 mRNA expression score
The relative amount of cytohesin-2 in mRNA
from HCC (T) and the surrounding non-tumorous liver tissues (N)
that were normalized to β-actin mRNA was calculated. The
cytohesin-2 mRNA expression score in each tissue was defined as
2−ΔΔCt; ΔΔCt =
(CtCytohesin-2-Ctβ-actin)T-(CtCytohesin-2-Ctβ-actin)N.
The expression score <1 was identified as low cytohesin-2
mRNA expression, and >1 was identified as high
cytohesin-2 mRNA expression (there was no score =1)
(15).
Immunohistochemical assay (IHC)
For IHC, fresh tissues were fixed in 10% neutral
buffered formalin for 16 h at 4°C and then placed in a Thermo
Shandon tissue processor, and embedded in paraffin. Sections were
heated in a 60°C oven, and wax removed by three changes of xylene,
followed by a graded ethanol series (100, 95, 90 and 80%) before
being subjected to a final wash in double-distilled H2O.
After quenching endogenous peroxidase activity with 3%
H2O2 for 10 min and blocking with BSA for 30
min, sections were incubated at 4°C overnight with primary antibody
against cytohesin-2 at a dilution of 1:100. Detection of
cytohesin-2 was achieved with the Envision-horseradish peroxidase
system (DakoCytomation, Glostrup, Denmark). All slides were
counterstained with Gill's Hematoxylin for 1 min, dehydrated, and
mounted for light microscopic evaluation independently by two
experienced pathologists. A cutoff point of 25% was used in our
statistical analysis; sections were classified as negative, weak
(0–25%) or strong (≥ 25%) (16).
The staining intensity and average percentage of positive liver
cells were assayed for 10 independent high magnification (x400)
fields. The total score was calculated by multiplying the staining
intensity and the percentage of positive liver cells. Sections with
a total score of >1 were defined as exhibiting positive staining
for cytohesin-2 (17).
Protein preparation
Samples were homogenized in ice-cold RIPA buffer [1X
phosphate-buffered saline (PBS), 1% Nonidet P-40, 0.5% sodium
deoxycholate, 1% sodium dodecyl sulfate (SDS)] with protease
inhibitors (Xianfeng Biotech, Xi'an China), then incubated on ice
for 20 min and centrifuged at 12,000 rpm for 15 min at 4°C to
specification. The supernatant was quantified by Bradford BCA
protein assay and stored at −80°C until further use.
Western blot analysis
The protein samples (20 μg) were separated by 10%
SDS-PAGE; proteins were then transferred to polyvinylidene fluoride
(PVDF) membranes which were blocked for 1 h with 1% BSA in
Tris-buffered saline with Tween-20 (TBST) buffer (20 mM/l Tris, pH
7.6, 100 mM/l NaCl, 0.1% Tween-20). This was followed by incubation
with primary antibodies, including polyclonal antibody against
cytohesin-2 (1:1,000 dilution) and β-actin (1:1,000 dilution) in
TBST buffer containing 1% BSA, at 4°C overnight. After washing
three times with TBST buffer, membranes were incubated with a
horseradish peroxidase-conjugated goat anti-rabbit IgG and goat
anti-rabbit IgG as secondary antibodies (1:10,000 dilution) for 1 h
at room temperature. After the membranes were washed four times
again in TBST buffer, reactions were visualized with the ECL
detection system (Millipore, Billerica, MA, USA). Values for
cytohesin-2 were normalized to β-actin expression; cytohesin-2
protein values were compared between HCC and adjacent non-tumorous
liver tissue. Ratios >1 were identified as higher cytohesin-2
protein expression; ratios ≤1 were identified as lower cytohesin-2
expression (18). All western blot
analyses were repeated at least three times.
Follow-up
The follow-up duration was defined as the interval
between the date of surgery and the date of death or last
follow-up. The study was censored on 31 January 2012. The median
follow-up time was 25.32 months (range, 15–36). All patients were
followed up every 1–3 months in the first year and every 3–6 months
thereafter. The follow-up protocol included physical examination,
serum α-fetoprotein (AFP) level, chest X-ray, abdominal
ultrasonography and computed tomography. Computed tomography and/or
magnetic resonance imaging and/or positron emission tomography were
performed when intrahepatic relapse or distant metastasis was
suspected.
Statistical analysis
All statistical analyses were carried out using the
SPSS 16.0 statistical software package (SPSS Inc., Chicago, IL,
USA). The associations between cytohesin-2 expression and
clinicopathological parameters were analyzed using Student's
t-test. Overall and disease-free survival curves were generated
using the Kaplan-Meier method; differences between curves were
assessed by the log-rank test. Independent prognostic factors were
estimated by the Cox proportional hazards stepwise regression
model. All P-values were 2-sided. P<0.05 was considered to
indicate statistically significant differences.
Results
Cytohesin-2 expression in HCC and
surrounding non-tumorous liver tissue specimens
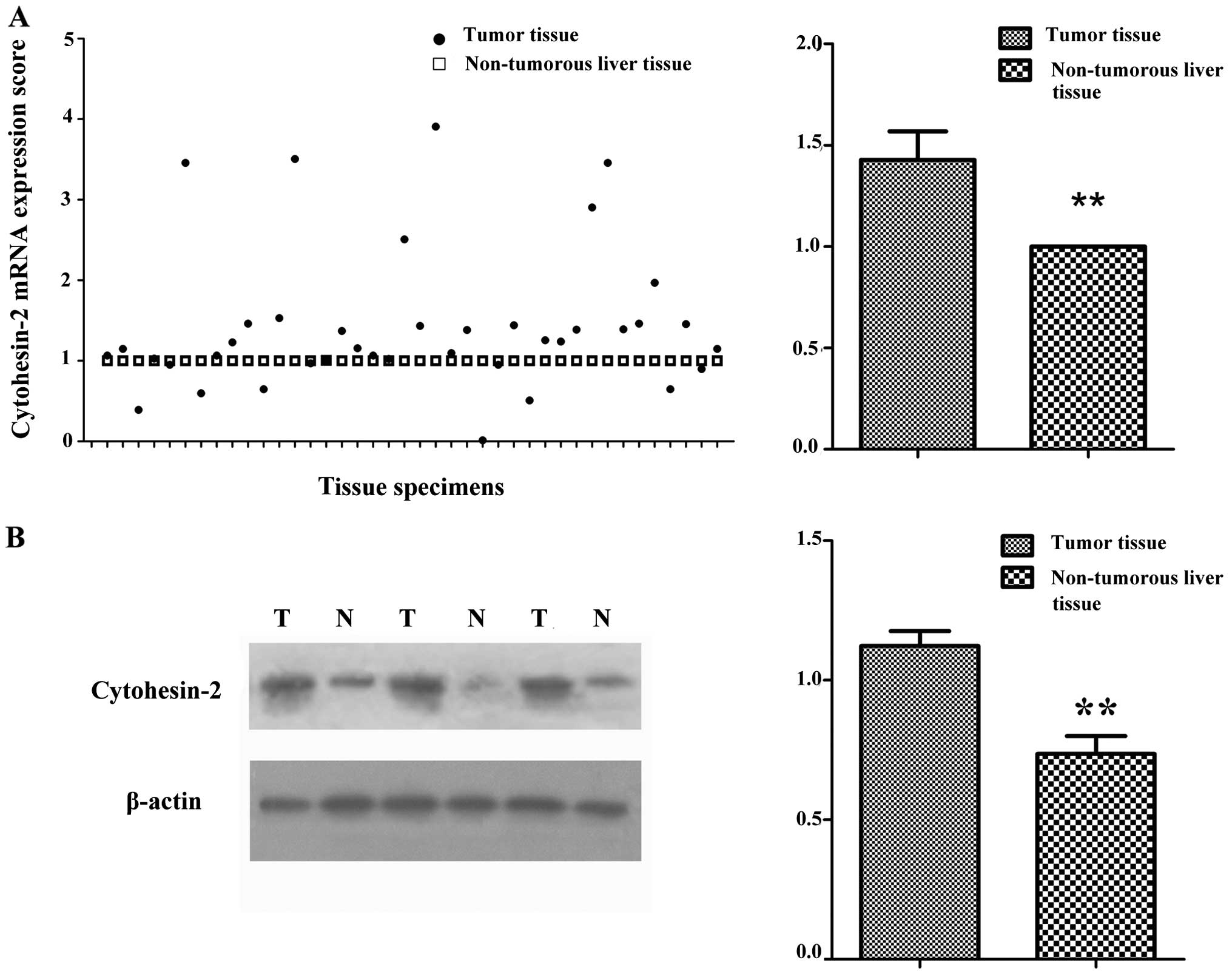
We first examined cytohesin-2 expression in 40 HCC
patient specimens and surrounding non-tumorous liver tissues, using
qRT-PCR and western blot analysis (Fig.
1). Cytohesin-2 mRNA expression was between 0.13 and 3.97
(average ± SD, 1.43±0.89) in HCC tissues, which was higher than in
surrounding non-tumorous liver tissues (P=0.005) (Table I). Cytohesin-2 protein expression
was between 0.21 and 1.56 (average ± SD, 1.12±0.33) in HCC tissues,
which was higher than in surrounding non-tumorous liver tissues
(average ± SD, 0.70±0.39; P=0.009) (Table I and Fig. 1). Protein expression was consistent
with that of mRNA in the same monitored samples (data not shown).
Similar results were obtained from the same batch of HCC tissues
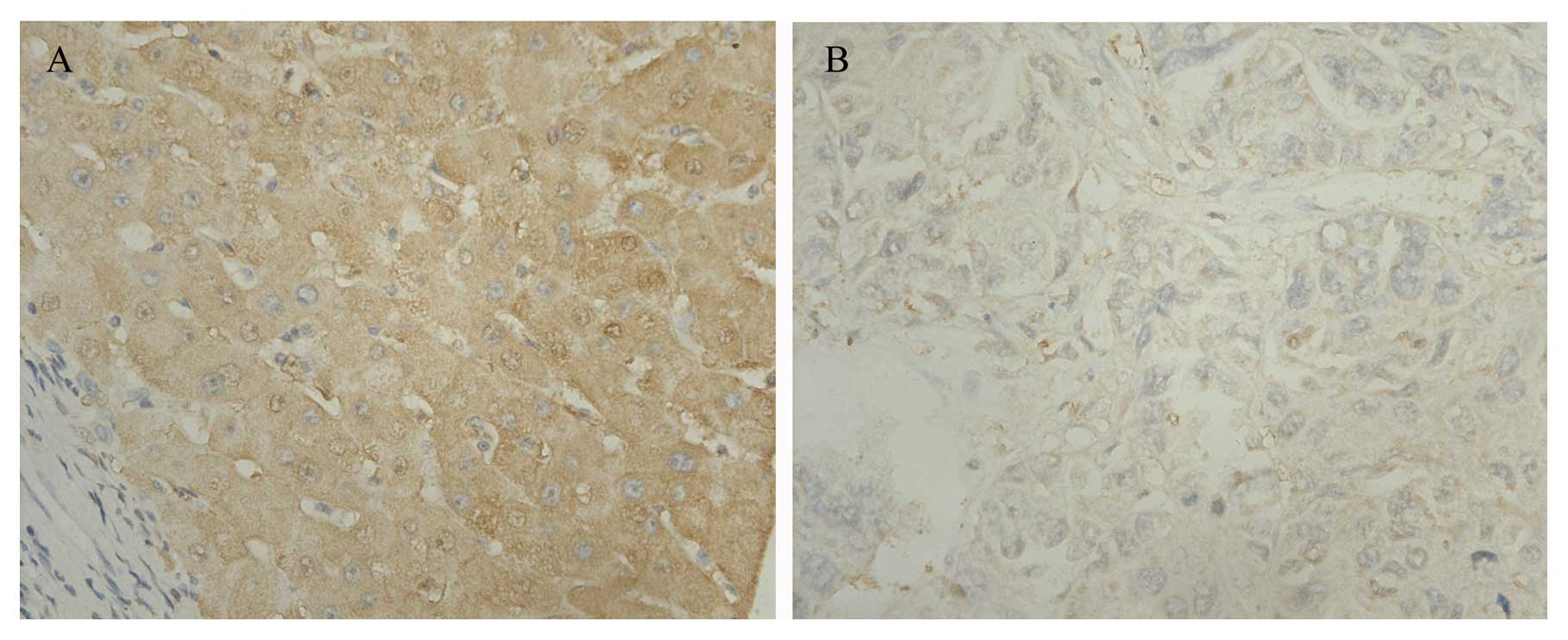
using IHC, which indicated cytohesin-2 to be more highly expressed
in tumors than in surrounding non-tumorous liver tissues.
Cytohesin-2 was located in the cytoplasm (Fig. 2).
 | Table IClinicopathological characteristics
and cytohesin-2 expression in HCC. |
Table I
Clinicopathological characteristics
and cytohesin-2 expression in HCC.
| Clinicopathological
characteristic | Variable | No. of cases | Cytohesin-2 mRNA
expression scores (means ± SD) | P-value | Cytohesin-2 protein
expression scores (means ± SD)b | P-value |
|---|
| Gender | Male | 31 | 1.50±0.96 | 0.345 | 1.42±0.88 | 0.408 |
| Female | 9 | 1.18±0.49 | | 1.16±0.46 | |
| Age (years) | >60 | 10 | 1.67±0.88 | 0.32 | 1.30±0.84 | 0.412 |
| ≤60 | 30 | 1.35±0.89 | | 1.55±0.74 | |
| Background liver
status | With cirrhosis | 37 | 1.44±0.90 | 0.712 | 1.38±0.83 | 0.702 |
| Without
cirrhosis | 3 | 1.24±0.73 | | 1.19±0.59 | |
| Maximal tumor size
(mm) | <50 | 28 | 1.46±0.94 | 0.757 | 1.39±0.87 | 0.753 |
| >50 | 12 | 1.36±0.78 | | 1.30±0.68 | |
| α-Fetoprotein
(ng/ml) | ≤400 | 20 | 1.06±0.38 | 0.007a | 1.04±0.35 | 0.01a |
| >400 | 20 | 1.79±1.09 | | 1.69±1.00 | |
| Tumor number | Single | 33 | 1.39±0.88 | 0.593 | 1.33±0.82 | 0.511 |
| Multiple | 7 | 1.59±0.93 | | 1.55±0.78 | |
| Histology | Well and Mod | 24 | 1.49±0.96 | 0.621 | 1.40±0.82 | 0.771 |
| Poor | 16 | 1.34±0.78 | | 1.32±0.82 | |
| Capsule
formation | + | 27 | 1.55±1.02 | 0.203 | 1.49±0.94 | 0.174 |
| − | 13 | 1.17±0.40 | | 1.11±0.36 | |
| Vascular
invasion | + | 9 | 2.26±1.26 | 0.001a | 2.09±1.19 | 0.01a |
| − | 31 | 1.18±0.56 | | 1.15±0.52 | |
| TNM stage | I | 30 | 1.52±0.98 | 0.241 | 1.45±0.89 | 0.239 |
| II, III, IV | 10 | 1.14±0.45 | | 1.10±0.41 | |
Correlation between cytohesin-2
expression and clinicopathological parameters
Subsequently, clinicopathological data were
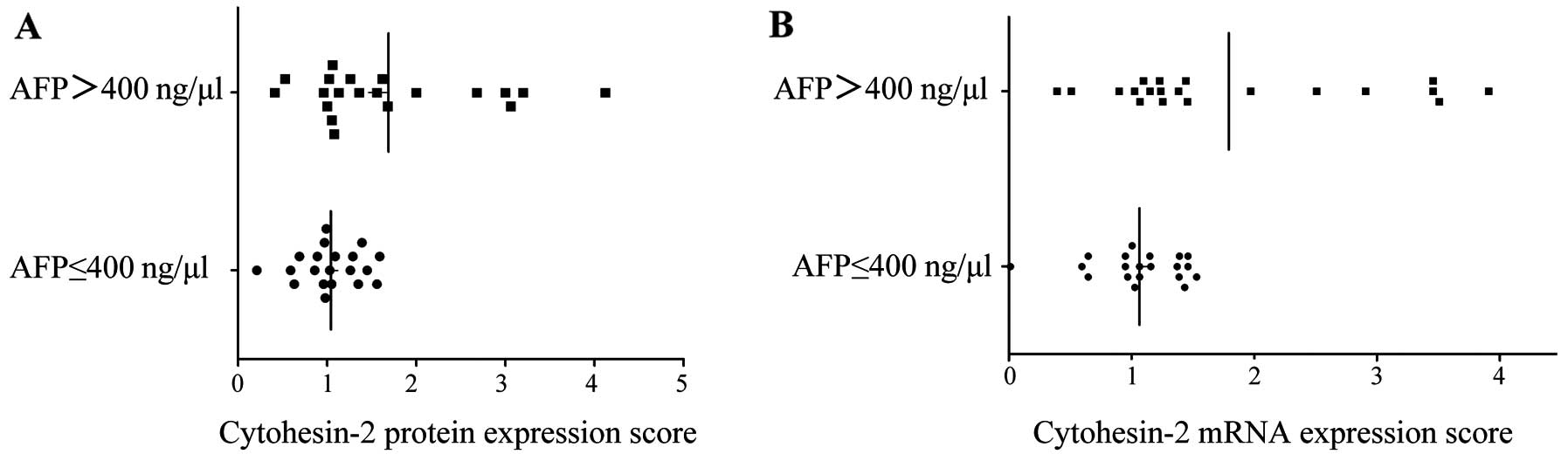
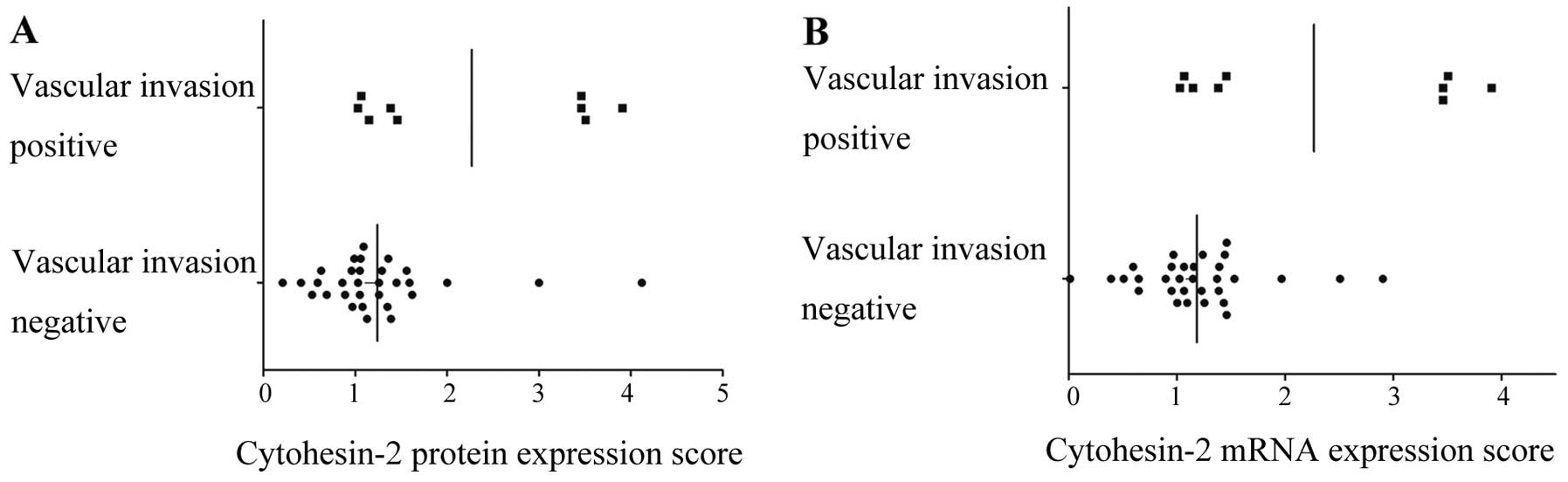
correlated with the cytohesin-2 expression. Cytohesin-2 protein
expression was significantly associated with high AFP (P=0.01)
(Table I and Fig. 3A) and vascular invasion (P=0.01)
(Table I and Fig. 4A). The bivariate correlation showed
AFP and cytohesin-2 protein expression were positively correlated
(correlation coefficient=0.417, P=0.01). Similar results were
observed when we checked cytohesin-2 protein expression and
vascular invasion (correlation coefficient=0.361, P=0.022). There
was no relationship between cytohesin-2 expression and other
clinicopathological variables, including gender, age, tumor size,
liver cirrhosis, TNM stage, tumor number, tumor capsule, and
Edmondson-Steiner grade. All patients were infected with hepatitis
B virus, as hepatitis B surface antigen (HBsAg) status was not
mentioned. Cytohesin-2 mRNA expression was also associated with
high AFP level (P=0.007) (Table I
and Fig. 3B) and vascular invasion
(P=0.001) (Table I and Fig. 4B).
Prognostic of HCC subtypes defined by
cytohesin-2 level
During the course of follow-up, 30/40 patients
(75.0%) were found with intrahepatic recurrence, 4 patients (10%)
developed distant metastases. Twenty-four patients (60%) succumbed
to cancer-related causes, 8 patients (20%) succumbed to liver
cirrhosis-related diseases (such as hepatic failure and upper
gastrointestinal hemorrhage), 3 patients (7.5%) succumbed to
diseases unrelated to cancer, and 5 patients (12.5%) were still
alive. We selected gender, age, tumor size, liver cirrhosis, TNM
stage, tumor number, tumor capsule, and Edmondson-Steiner grade,
cytohesin-2 as prognostic factors for the analysis of overall
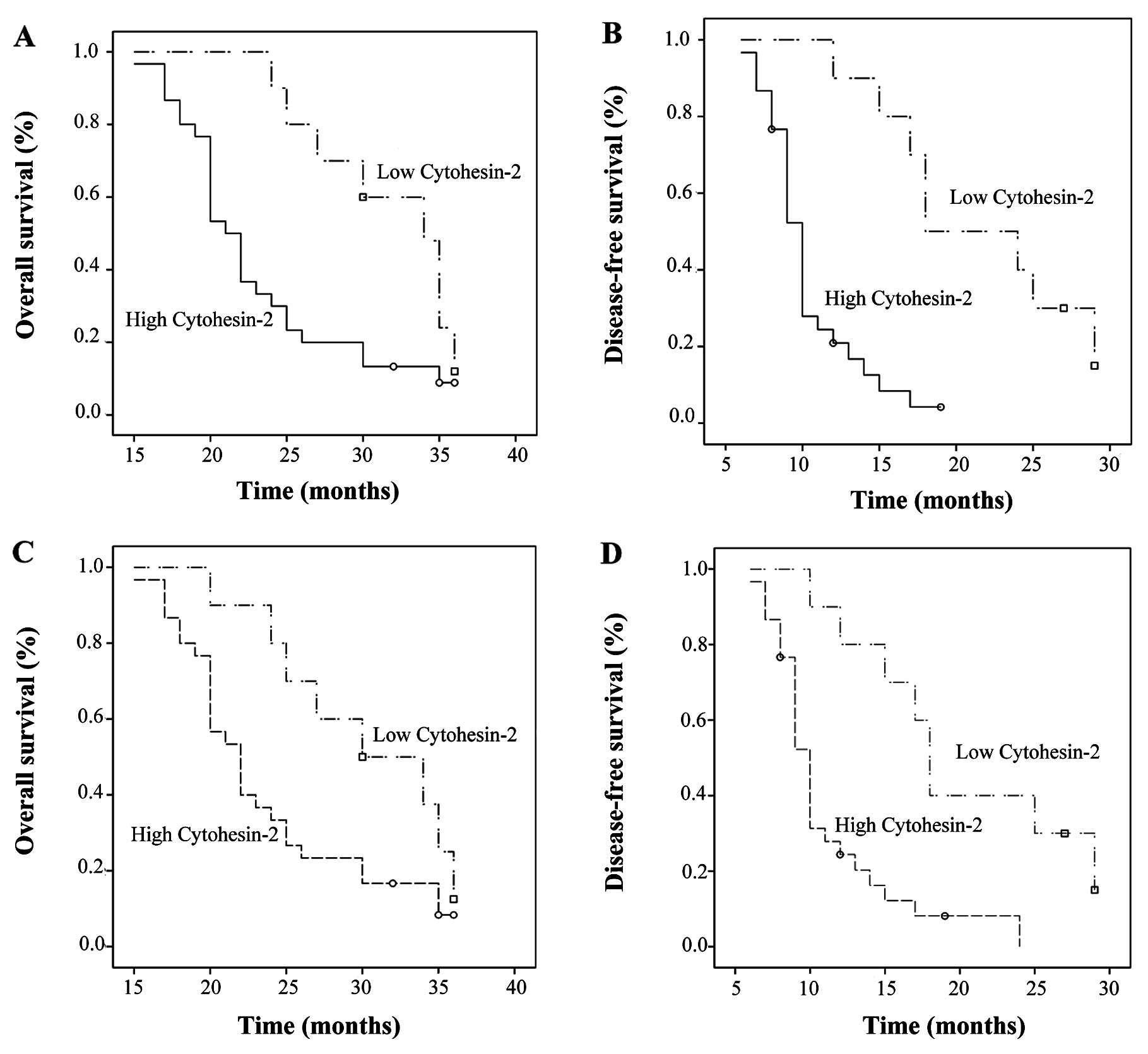
survival (OS) and disease-free survival (DFS). Significant OS and
DFS advantages were observed in patients with low cytohesin-2
protein expression. Median survival was 31.72 months (24–36) of the
low-level group, which was significantly longer than that of the
high-level group (23.19 months) (15–36; P=0.005). DFS was 21.40
months (9–29) in the low-level group, which was significantly
longer than that of the high-level group (10.17 months) (6–19;
P<0.01).
Stratified univariate and multivariate
analysis
In a univariate analysis model, cytohesin-2 mRNA and
protein expression were significantly associated with OS (P=0.022;
P=0.048, respectively) and DFS (P<0.01; P=0.001, respectively)
(Fig. 5). The similar result was
obtained when we performed multivariate analysis, and it showed
that cytohesin-2 protein expression level was associated with OS
(P=0.009) and DFS (P=0.002) (Table
II). Cytohesin-2 mRNA expression level as a factor for
multivariate analysis was then examined, and we found the similar
result as cytohesin-2 protein expression level as a factor we
showed in Table II. Since survival
may be associated with TNM stage, we stratified the data according
to TNM stage and investigated the prognostic value of cytohesin-2
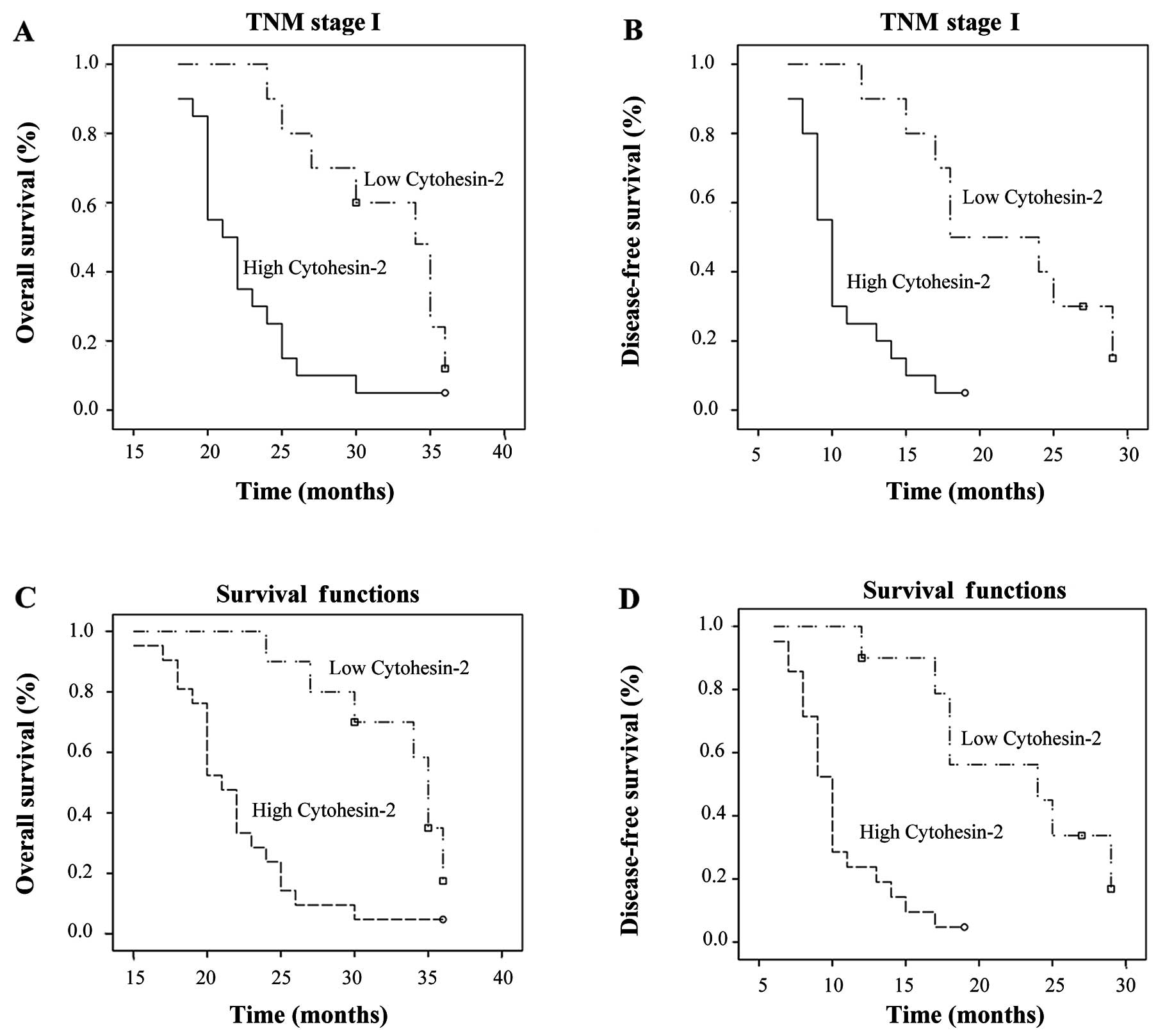
for TNM stage I patients; there were no significant differences in
the patient background profiles. For the 30 TNM stage I patients,
significant correlations were found between cytohesin-2 expression
and OS and DFS. Cytohesin-2 was an independent prognostic factor
for survival in TNM stage I patients. Patients with high
cytohesin-2 mRNA and protein expression had poorer OS (P=0.015;
P<0.01) and DFS (P<0.01; P<0.01) compared to those with
low cytohesin-2 expression in TNM stage I (Fig. 6).
 | Table IIMultivariate analysis of factors
contributing to overall survival and disease-free survival in HCC
patients. |
Table II
Multivariate analysis of factors
contributing to overall survival and disease-free survival in HCC
patients.
| Overall
survival | Disease-free
survival |
|---|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Tumor size | 1.375
(1.158–1.892) | 0.026 | 2.911
(1.203–7.043) | 0.018 |
| Tumor number | 2.921
(0.958–8.910) | 0.060 | 3.613
(1.112–11.733) | 0.033 |
| Histology | 5.499
(1.897–15.938) | 0.002 | 2.498
(0.996–6.267) | 0.051 |
| Vascular
invasion | 3.560
(1.449–8.571) | 0.006 | 4.044
(1.270–12.882) | 0.018 |
| Cytohesin-2 protein
expression level | 3.251
(1.338–7.902) | 0.009 | 5.558
(1.897–16.284) | 0.002 |
Discussion
HCC is one of the most common solid tumors in
mainland China, with an annual incidence of 24 per 100,000 people.
The mortality rate of HCC is as high as its morbidity rate
(19), due to its high recurrence
rate which is as high as 54% at 5 years, even for early-stage HCC
treated with radical resection (20). Molecular markers for HCC recurrence
are limited in early-stage disease, although some molecules have
been identified as prognostic markers. For this reason, identifying
genetic alterations that allow estimation of early HCC recurrence
are important.
Cytohesin-2 is reported to be overexpressed in human
lung cancer (6), and to participate
in the IGF pathway in HepG2 cells (11). However, to our knowledge,
cytohesin-2 expression in HCC has yet to be reported. In this
study, we analyzed cytohesin-2 mRNA and protein expressions in 40
HCC patients and correlated them with clinicopathological
characteristics and prognoses to determine whether this biomarker
could predict disease outcome. Cytohesin-2 expression in HCC tissue
was significantly higher than in surrounding non-tumorous tissue,
as Bill et al(6) found in
human lung cancer. Markedly, high cytohesin-2 expression was
significantly correlated with more aggressive cancer, in terms of
shorter OS and DFS, AFP and vascular invasion, which are putative
clinicopathological markers for HCC development, invasiveness and
unfavorable prognosis (21). These
data indicate that high cytohesin-2 expression occurs in HCC and is
associated with an aggressive invasion phenotype, and that
cytohesin-2 expression correlated with AFP which is a biomarker for
proliferation (22). This finding
is in agreement with previous studies; for example, Lim et
al(11) reported that
cytohesin-2 is involved in insulin and IGF pathways and promotes
HepG2 proliferation. Cytohesin-2 promotes tumor proliferation and
this function was identified in human lung cancer as SecinH3 which
targets the Sec7 domain of the cytohesins (23) and reduces the growth of
EGFR-dependent lung tumor xenografts (6). Cytohesin-2 expression correlated with
vascular invasion; this was in accordance with the finding of
Mannell et al (14) that
cytohesin-2 affects VEGF-dependent initiation of angiogenesis by
regulating VEGFR-2 internalization in endothelial cells.
Cytohesin-2 scores in HCC with vascular invasion were used to
separate samples into two groups. No statistical difference in
intensity of vascular invasion was found between the high- and
low-score groups. Furthermore, in the low-score group, there was
one case in which a portal vein tumor thrombus (PVTT) protruded
into the first main portal vein branch beyond the resection line
for >1 cm (19), and the others
were located in the hepatic resection area or protruded into the
first main portal vein branch beyond the resection line for <1
cm. Nevertheless, in the high-score group, two cases of PVTT
protruded into the first main portal vein branch beyond the
resection line for >1 cm, and the PVTT extended into the main
portal vein in one case. The lack of statistical difference may be
due to the small number of samples, as patients with both HCC and
PTVV have diminished access to hepatectomy. The effect of
cytohesin-2 in angiogenesis is a future research priority for
us.
Clinical stage is the most important factor in the
prognosis of HCC patients. The International Union Against Cancer's
TNM staging system is the most widely used. However, it is
difficult for a surgeon to predict which individual TNM stage I
patients will suffer early recurrence following curative treatment.
Although several molecular markers have been shown to possess
potential predictive significance, biomarkers that could identify
patients with TNM stage I HCC who are likely to respond
optimistically to curative excision remain substantially limited.
Our stratified analysis showed that cytohesin-2 expression had
clear prognostic value for OS and DFS in these patients, indicating
that cytohesin-2 could be used as a predictive tool to identify
patients with TNM stage I HCC at high risk of recurrence.
High cytohesin-2 expression in HCC tissues predicts
poor prognosis, which suggests that cytohesin-2 affects the HCC
malignancy process. This information could identify high-risk HCC
patients who may benefit from more intensive treatment and
follow-up care following resection of primary tumors. Further
studies are required to gain insight into the underlying biology of
cytohesin-2 in HCC, and to develop new therapies targeted at
cytohesin-2.
Acknowledgements
The authors thank Dr Ting Lei and Professor Gui-Hua
Zhuang for the histologic confirmation of specimens and statistical
analyses. This study was completed in the Key Laboratory of
Forensic Medicine of the Ministry of Health of China, Xi'an
Jiaotong University. The authors thank all the patients who
participated in this study.
References
|
1
|
El-Serag HB and Rudolph L: Hepatocellular
carcinoma: epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. Ca Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
3
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. Ca Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
4
|
Kitisin K, Pishvaian MJ, Johnson LB and
Mishra L: Liver stem cells and molecular signaling pathways in
hepatocellular carcinoma. Gastrointest Cancer Res. 1(Suppl 2):
S13–S21. 2007.PubMed/NCBI
|
|
5
|
Dragani TA: Risk of HCC: Genetic
heterogeneity and complex genetics. J Hepatol. 52:252–257. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bill A, Schmitz A, Albertoni B, et al:
Cytohesins are cytoplasmic ErbB receptor activators. Cell.
143:201–211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bos JL, Rehmann H and Wittinghofer A: GEFs
and GAPs: Critical elements in the control of small G proteins.
Cell. 129:865–877. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Casanova JE: Regulation of arf activation:
the sec7 family of guanine nucleotide exchange factors. Traffic.
8:1476–1485. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kolanus W: Guanine nucleotide exchange
factors of the cytohesin family and their roles in signal
transduction. Immunol Rev. 218:102–113. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kolanus W, Nagel W, Schiller B, et al:
Alpha L beta 2 integrin/LFA-1 binding to ICAM-1 induced by
cytohesin-1, a cytoplasmic regulatory molecule. Cell. 86:233–242.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lim J, Zhou M, Veenstra TD and Morrison
DK: The CNK1 scaffold binds cytohesins and promotes insulin pathway
signaling. Genes Dev. 24:1496–1506. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thomas M: Molecular targeted therapy for
hepatocellular carcinoma. J Gastroenterol. 44:136–141. 2009.
View Article : Google Scholar
|
|
13
|
Kannangai R, Sahin F and Torbenson MS:
EGFR is phosphorylated at Ty845 in hepatocellular carcinoma. Mod
Pathol. 19:1456–1461. 2006.PubMed/NCBI
|
|
14
|
Mannell HK, Pircher J, Chaudhry DI, et al:
ARNO regulates VEGF-dependent tissue responses by stabilizing
endothelial VEGFR-2 surface expression. Cardiovasc Res. 93:111–119.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shirahata A, Fan W, Sakuraba K, et al:
MACC 1 as a marker for vascular invasive hepatocellular carcinoma.
Anticancer Res. 31:777–780. 2011.PubMed/NCBI
|
|
16
|
Wang L, Chen S, Zhang M, et al: Legumain:
A biomarker for diagnosis and prognosis of human ovarian cancer. J
Cell Biochem. 113:2679–2686. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tu K, Zheng X, Zan X, Han S, Yao Y and Liu
Q: Evaluation of Fbxw7 expression and its correlation with the
expression of c-Myc, cyclin E and p53 in human hepatocellular
carcinoma. Hepatol Res. 42:904–910. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tan NS, Michalik L, Di-Poi N, et al:
Essential role of Smad3 in the inhibition of inflammation-induced
PPAR beta/delta expression. EMBO J. 23:4211–4221. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen XP, Qiu FZ, Wu ZD, et al: Effects of
location and extension of portal vein tumor thrombus on long-term
outcomes of surgical treatment for hepatocellular carcinoma. Ann
Surg Oncol. 13:940–946. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cherqui D, Laurent A, Mocellin N, et al:
Liver resection for transplantable hepatocellular carcinoma:
long-term survival and role of secondary liver transplantation. Ann
Surg. 250:738–746. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cammà C, Schepis F, Orlando A, et al:
Transarterial chemoembolization for unresectable hepatocellular
carcinoma: meta-analysis of randomized controlled trials.
Radiology. 224:47–54. 2002.PubMed/NCBI
|
|
22
|
Fujioka M, Nakashima Y, Nakashima O and
Kojiro M: Immunohistologic study on the expressions of
α-fetoprotein and protein induced by vitamin K absence or
antagonist II in surgically resected small hepatocellular
carcinoma. Hepatology. 34:1128–1134. 2001.
|
|
23
|
Hafner M, Schmitz A, Grüne I, et al:
Inhibition of cytohesins by SecinH3 leads to hepatic insulin
resistance. Nature. 444:941–944. 2006. View Article : Google Scholar : PubMed/NCBI
|