Introduction
Parathyroid hormone-related protein (PTHrP) was
originally identified as a major factor responsible for humoral
hypercalcemia in malignant tumors such as lung and breast
carcinomas (1,2). PTHrP produced by tumors and other
cells binds to the common PTH/PTHrP receptor in osteoblasts and
activates expression of receptor activator of the NF-κB ligand
(RANKL), which promotes the maturation of pre-osteoclasts into
osteoclasts which consequently induces bone resorption and
hypercalcemia (3,4). PTHrP is produced by certain malignant
tumors, and is involved in malignant conversion of breast, colon
and prostate cancers by increasing cell proliferation, survival,
adhesion, migration and invasion (5–7). We
previously reported that PTHrP is highly expressed in oral
carcinoma cell lines and that it promotes malignancy of oral
cancers (8).
Mucoepidermoid carcinoma is the most common
malignant tumor of the major and minor salivary glands, accounting
for ~30% of salivary gland malignancies (9,10). Its
clinical behavior is highly variable and ranges from slow-growing
and indolent to locally aggressive and highly metastatic (11,12).
Histologically, mucoepidermoid carcinoma is comprised of 3
different cell types: mucinous cells, intermediate cells and
epidermoid cells. Their growth patterns range from cystic to solid
to infiltrative. These parameters have been incorporated into
several different grading systems that have been correlated with
prognosis and, therefore, play an important role in treatment
decisions (9). However, various
cases of mucoepidermoid carcinoma with poor prognoses are estimated
to have low-grade malignancy upon histological examination.
Therefore, we evaluated the PTHrP expression in mucoepidermoid
carcinoma and herein discuss its role in malignancy.
Materials and methods
Patients and tissue samples
Twenty-one patients who consulted the Department of
Oral Surgery, Hokkaido University Hospital, and who were diagnosed
as having mucoepidermoid carcinoma were examined. Informed consent
was obtained from the patients prior to the samples being used. The
experiment was conducted under the ethical guidelines of Hokkaido
University Hospital. TNM classification was carried out according
to the UICC criteria, and tumors were graded according to the World
Health Organization guidelines of 2005.
Western blotting
Human oral squamous cell carcinoma cell lines HSC2,
HSC3 and HSC4 [Japanese Collection of Research Bioresources (JCRB),
Osaka, Japan) were used in the present study. PC-3, a prostate
carcinoma cell line, was used as a positive control. The cells were
maintained in Dulbecco’s modified Eagle’s medium (DMEM)
supplemented with 10% fetal bovine serum (FBS). The cells were
lysed in lysis buffer [10 mM Tris-HCl (pH 7.4), 5 mM EDTA, 150 mM
NaCl, 10% glycerol, 1% Triton X-100, 0.1% SDS and protease
inhibitor cocktail] (Roche Diagnostics, Indianapolis, IN, USA) for
20 min on ice and clarified by microcentrifugation. The supernatant
was subjected to SDS-PAGE and transferred to polyvinylidene
difluoride membranes (Bio-Rad Laboratories, Hercules, CA, USA). A
PTHrP rabbit polyclonal antibody (Y201; Yanaihara Institute Inc.,
Shizuoka, Japan) was used for detection of PTHrP in the cultured
oral squamous cell carcinoma cell lines.
Immunohistochemical analysis
Immunohistochemical detection of PTHrP, α smooth
muscle actin (αSMA) and CD34 was conducted using paraffin-embedded
tissue sections. Sections (5-μm) were deparaffinized and
rehydrated. They were immersed in 3% hydrogen peroxide in distilled
water for 5 min to block endogenous peroxidase activity followed by
1% BSA in phosphate-buffered saline (PBS) for 10 min. They were
then exposed to the primary rabbit polyclonal antibody for PTHrP,
αSMA and CD34 for 1 h at room temperature. After washing with PBS
three times, Simple Stain MAX PO (Nichirei Biosciences, Tokyo,
Japan) was used for 30 min, and sections were visualized with
EnVision Plus kits/HRP (Dako, Tokyo, Japan) at room temperature.
The peroxidase reaction products were developed with
3,3′-diaminobenzidine, and the sections were counterstained with
hematoxylin.
The PTHrP expression ratio of mucoepidermoid
carcinoma was calculated by counting the number of positive tumor
cells over the total number of tumor cells at a magnification of
×400 in three different areas. The PTHrP expression ratio of
mucoepidermoid carcinoma was measured using Nanozoomer with NDP
view software (Hamamatsu Photonics, Hamamatsu, Japan) and expressed
as: PTHrP expression of mucoepidermoid carcinoma (%) × area of each
type of cell.
Statistical analysis
Data concerning the PTHrP expression ratio of
mucoepidermoid carcinoma were analyzed and compared with the
two-sample t-test for differences in means. The criterion for
statistical significance was P<0.05.
Results
Clinical features of the cases
examined
The clinical features of the cases are shown in
Table I. There were 12 male and 9
female patients. The mean age of the patients was 56 years (range,
23–75 years). There were a wide variety of primary sites. The
primary sites of the tumors were the parotid gland in 4 patients,
submandibular gland in 1, sublingual gland in 1 and minor salivary
glands in the other 15 cases. TNM classification was performed
according to the guidelines of the International Union Against
Cancer TNM classification system. Patients were followed up for 5
years with regard to the prognosis, lymph node metastasis and/or
tumor recurrence. The TNM classification of tumor size was T1 in 5
(24%) patients, T2 in 10 (47%), T3 in 4 (19%) and T4 in 2 (10%)
patients. Nodal status was N0 in 17 patients, N1 in 1 and N2 in 3
patients. Five patients presented with subsequent metastases
including regional lymph nodes or distant organs and tumor
recurrence during the 5-year follow-up period.
 | Table IClinical features of the
mucoepidermoid carcinoma cases examined. |
Table I
Clinical features of the
mucoepidermoid carcinoma cases examined.
| Case no. | Gender | Age (years) | Primary site | cTNM | Subsequent
metastasis/recurrence |
|---|
| 1 | Female | 66 | Parotid gland | T2N0M0 | |
| 2 | Male | 41 | Palate | T2N0M0 | |
| 3 | Female | 35 | Buccal mucosa | T2N0M0 | Metastasis |
| 4 | Female | 47 | Buccal mucosa | T3N0M0 | |
| 5 | Female | 71 | Buccal mucosa | T1N0M0 | |
| 6 | Male | 71 | Parotid gland | T3N0M0 | |
| 7 | Male | 64 | Sublingual gland | T2N0M0 | Metastasis |
| 8 | Male | 63 | Submandibular
gland | T3N2bM0 | |
| 9 | Male | 74 | Tongue | T1N0M0 | |
| 10 | Male | 60 | Alveolar part of
mandible | T2N0M0 | |
| 11 | Male | 61 | Floor of the
mouth | T2N0M0 | Metastasis |
| 12 | Male | 54 | Floor of the
mouth | T4N2cM0 | |
| 13 | Male | 75 | Palate | T2N0M0 | Metastasis |
| 14 | Female | 23 | Parotid gland | T2N0M0 | |
| 15 | Male | 54 | Floor of the
mouth | T4N1M0 | Recurrence |
| 16 | Male | 41 | Tongue | T1N0M0 | |
| 17 | Female | 47 | Parotid gland | T2N0M0 | |
| 18 | Female | 53 | Alveolar part of
mandible | T2N0M0 | |
| 19 | Female | 48 | Lip | TT1N0M0 | |
| 20 | Male | 69 | Palate | T1N0M0 | |
| 21 | Female | 64 | Alveolar part of
mandible | T3N2cM0 | |
PTHrP protein is expressed in oral
squamous cell carcinoma cell lines
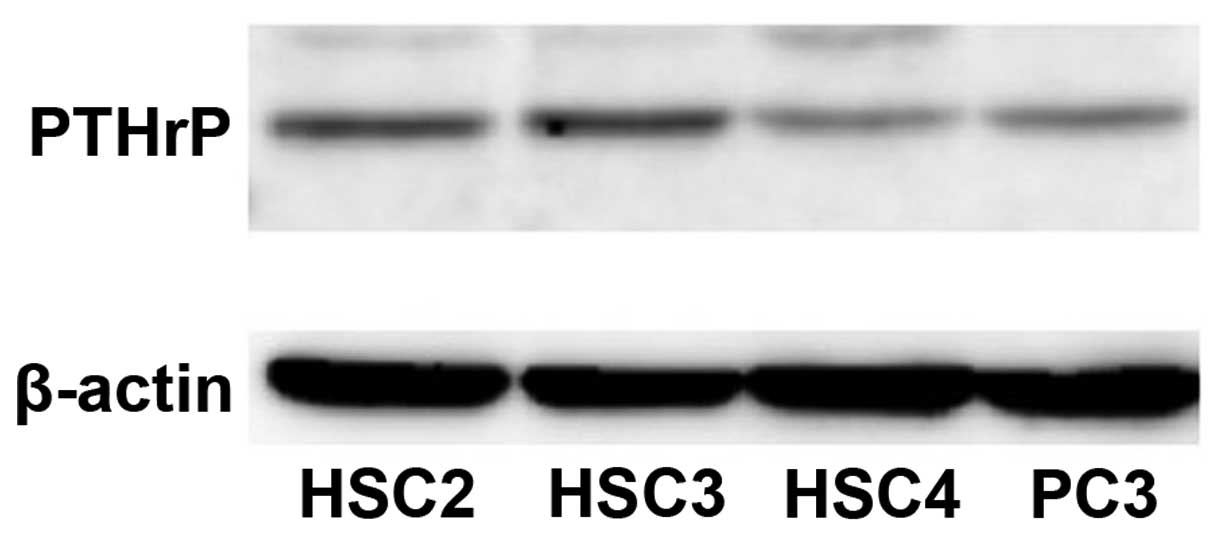
To address the role of PTHrP in oral epithelial
tumors, we first examined, using western blotting, whether PTHrP
was expressed in oral carcinoma cell lines, HSC2, HSC3, HSC4. All
of the cell lines expressed PTHrP protein at levels that were
almost equal to or higher than the level in PC-3, the positive
control prostate carcinoma cell line (Fig. 1).
PTHrP expression in mucoepidermoid
carcinomas is related to cancer metastasis and recurrence
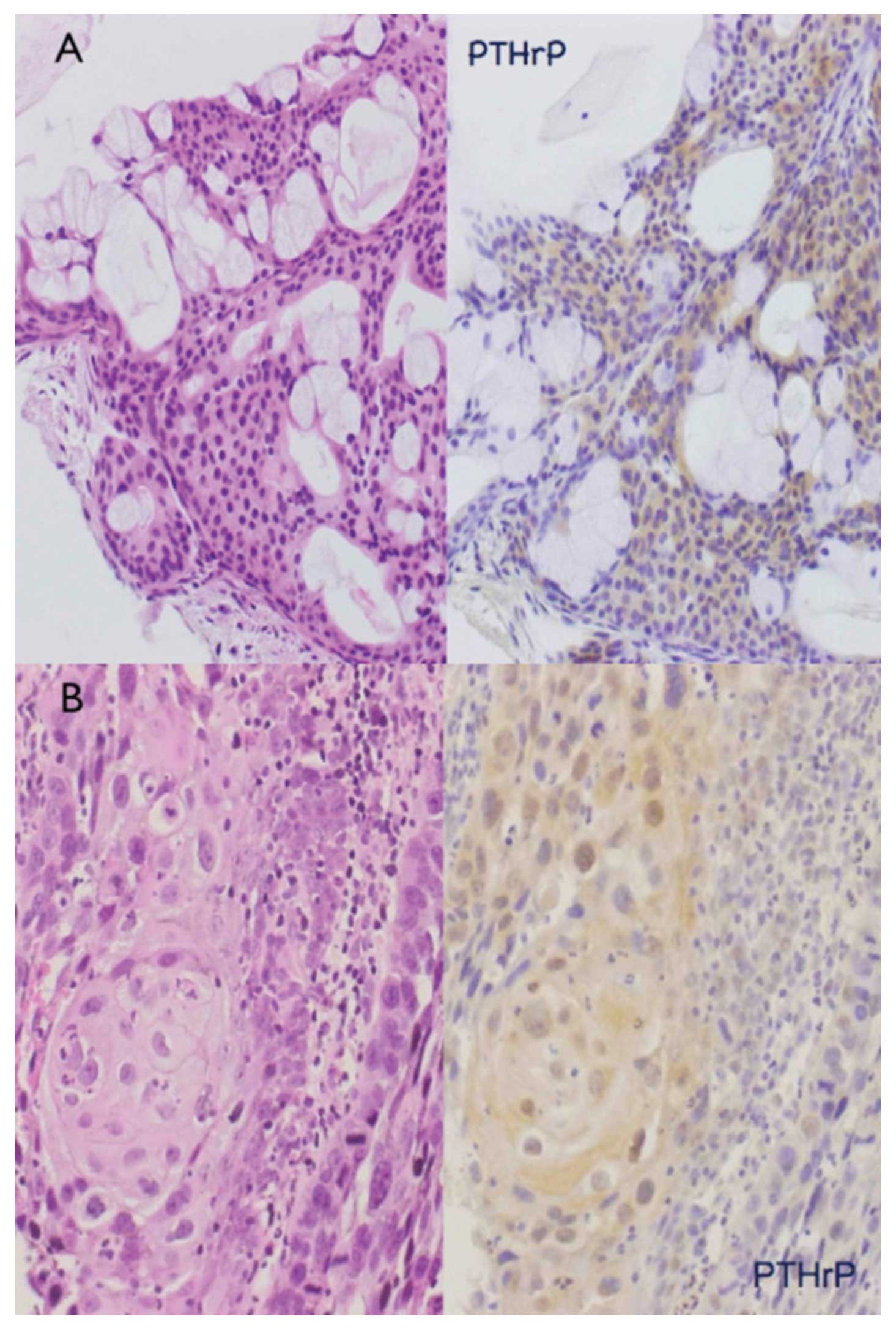
Immunohistochemical detection of PTHrP was performed
for 21 cases of mucoepidermoid carcinoma. Cytoplasmic
PTHrP-positive signals were observed in 17 of the 21 cases, and the
remaining 4 were PTHrP-negative. PTHrP-positive signals were noted
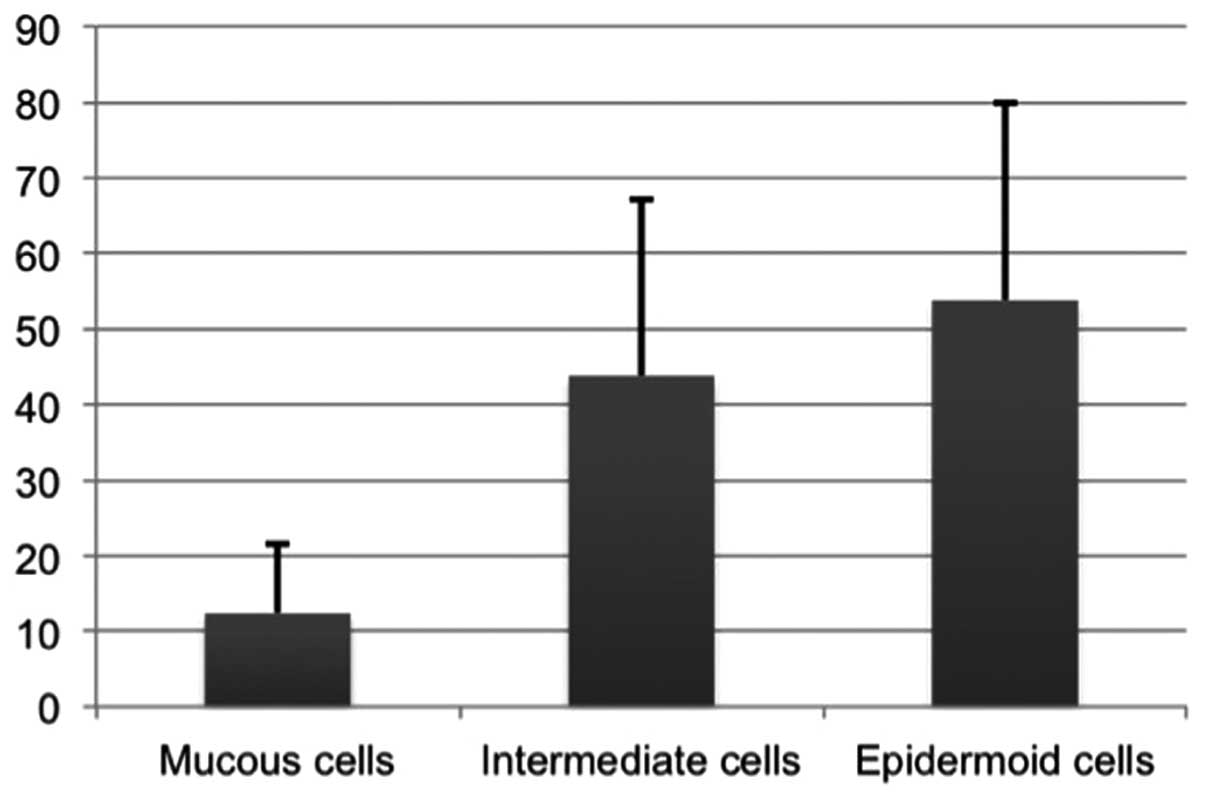
predominantly in intermediate cells (Fig. 2A) and epidermoid cells (Fig. 2B); PTHrP-positive signals were
present in 12% of mucous-producing cells, 43% of intermediate cells
and 53% of epidermoid cells on average (Fig. 3). Thus, the PTHrP-positive cell
percentages were significantly higher in intermediate cells and
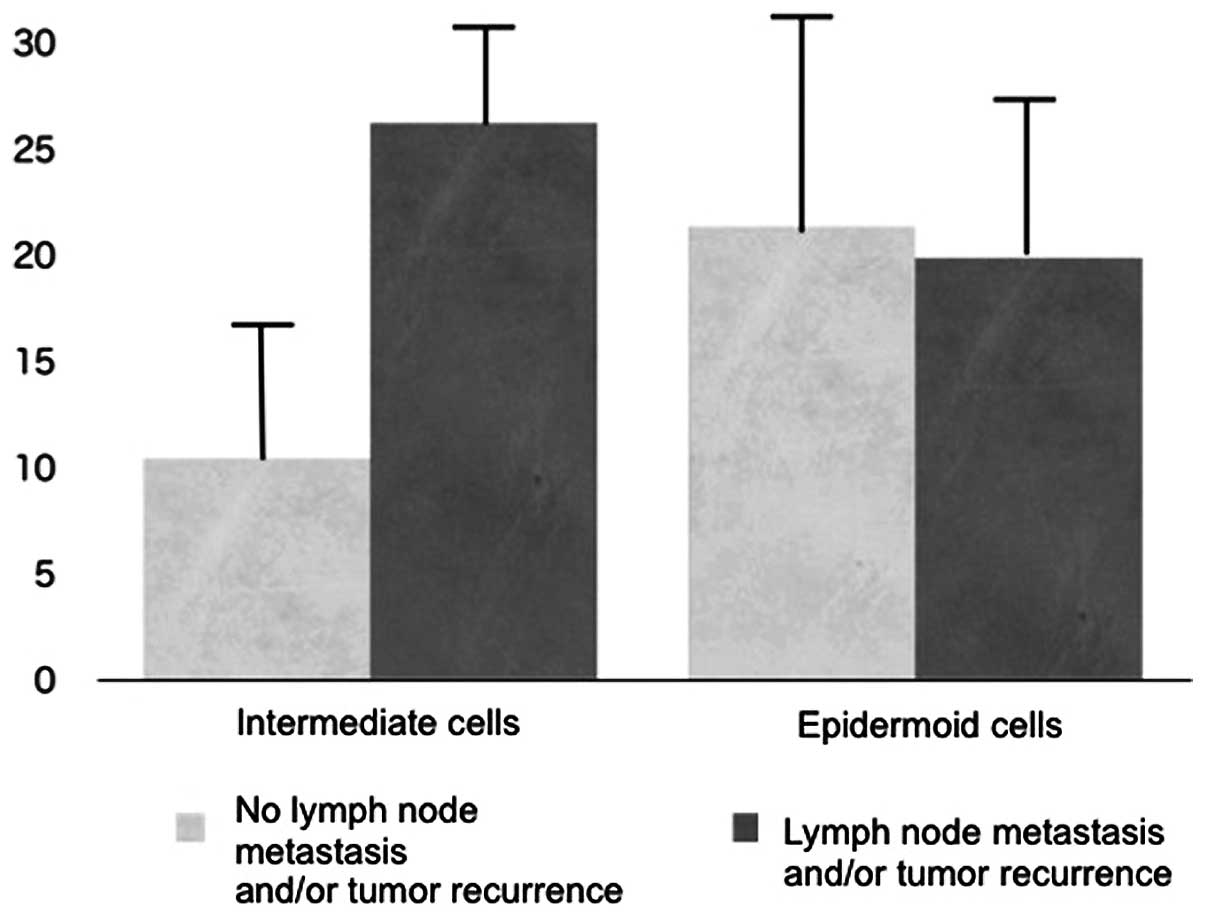
epidermoid cells. Subsequently, the relationship between the PTHrP
expression ratio of intermediate and epidermoid cells in
mucoepidermoid carcinoma and cancer behavior was investigated.
There was no significant relationship between the PTHrP expression
ratio in epidermoid cells and tumor malignancy; however, a
significant correlation was observed between the PTHrP expression
ratio in intermediate cells and 7 cases of primary and subsequent
tumor metastasis and/or recurrence (Fig. 4).
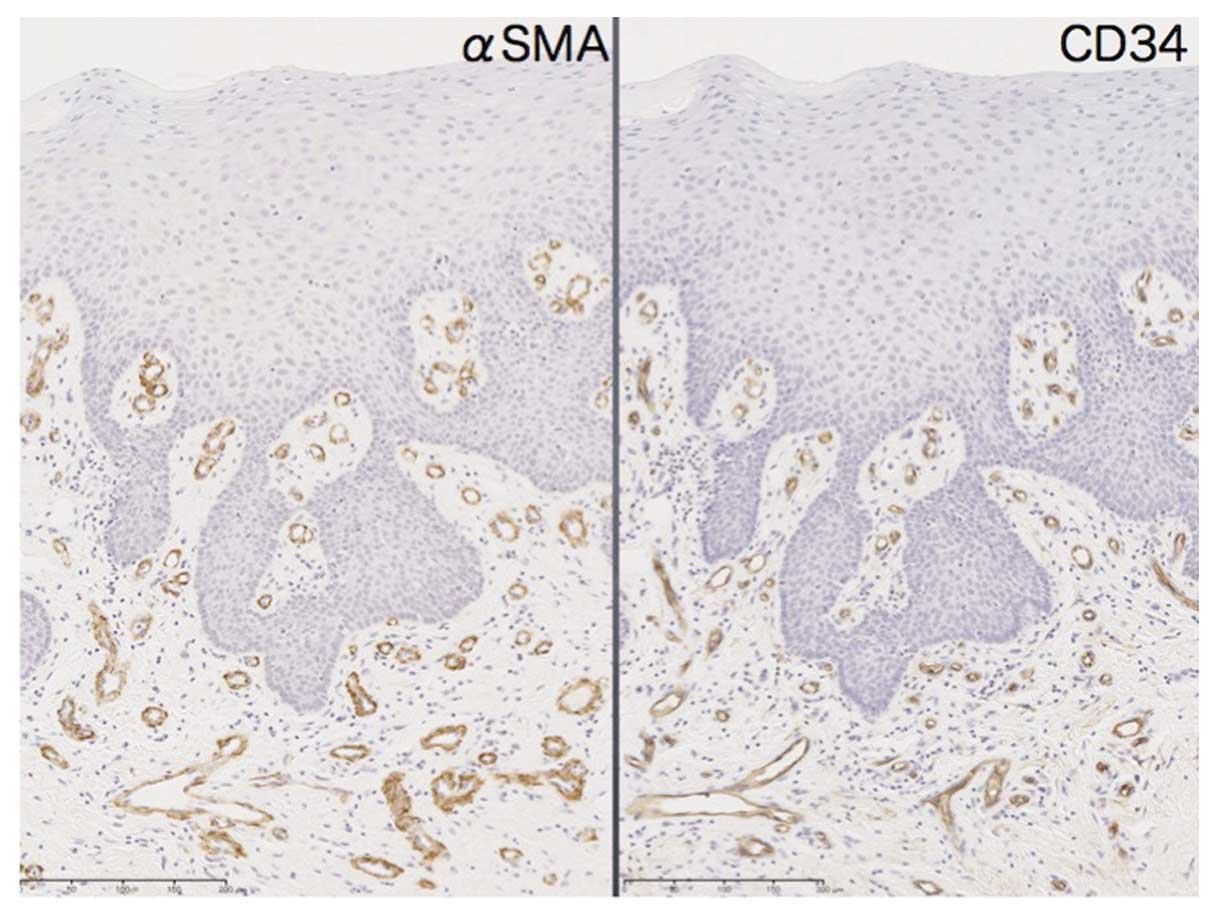
Cancer-associated fibroblast (CAF) induction was
estimated by αSMA expression in cancer stromal tissue. In normal
mucosa, αSMA expression was almost equal to the distribution of
CD34-positive vascular tissue and no obvious induction of CAFs was
observed (Fig. 5). In contrast,
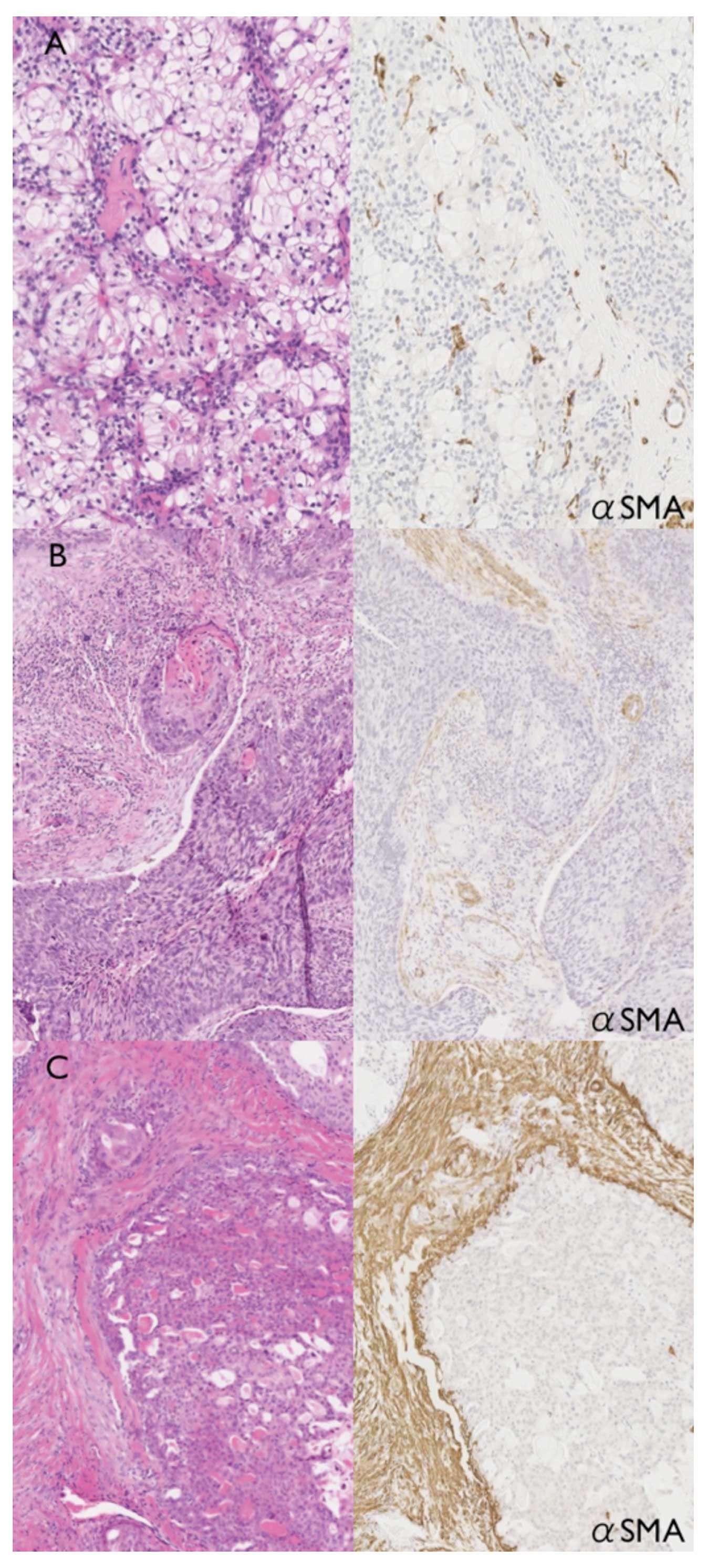
αSMA-positive CAFs were induced in stromal tissue of mucoepidermoid
carcinoma. Slight induction of CAFs was observed around
mucous-producing cancer cells (Fig.
6A). CAFs were more abundant in stromal tissue around
epidermoid cancer cells (Fig. 6B)
and intense αSMA-positive CAFs were widely observed in stromal
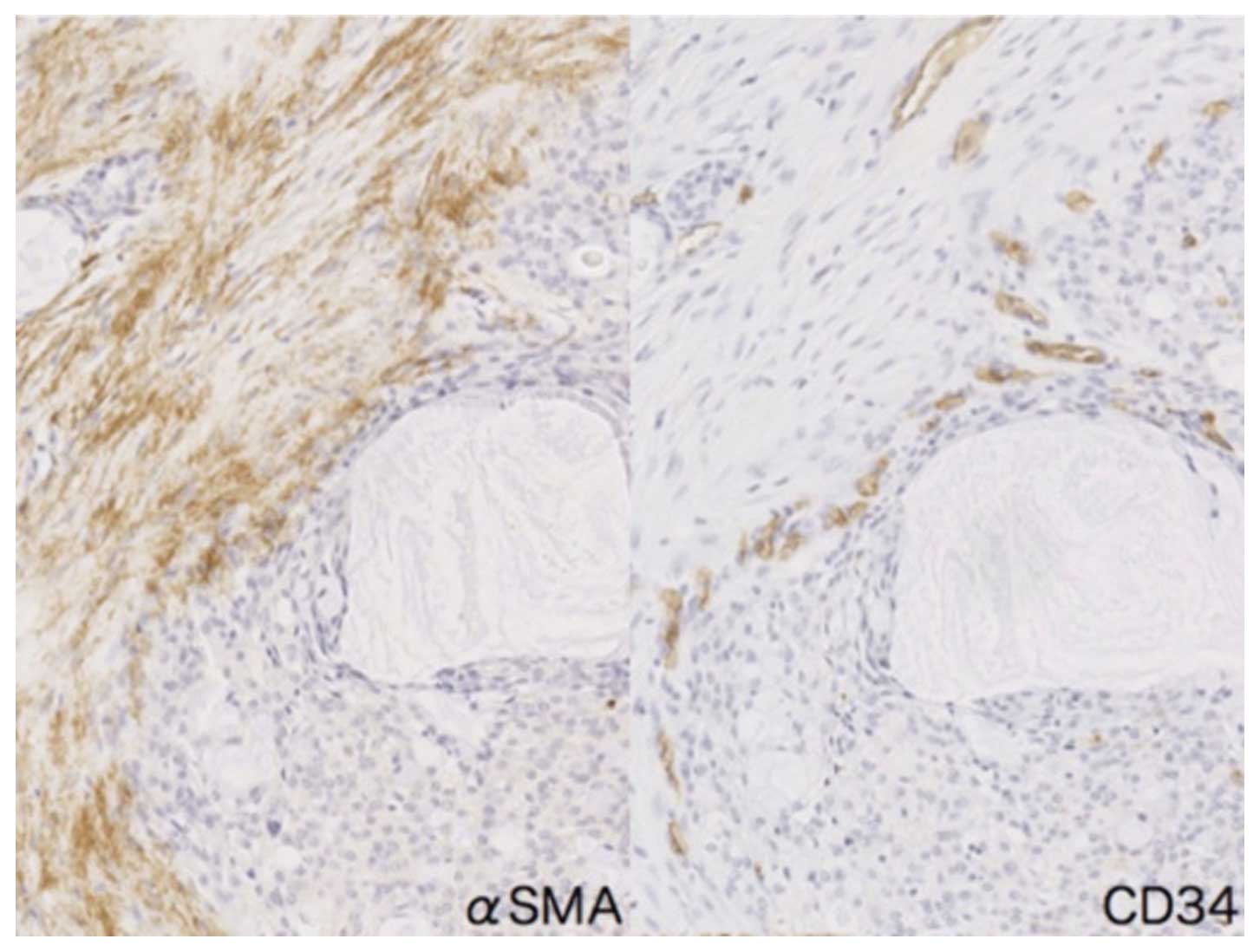
tissue around intermediate cancer cells (Fig. 6C). In addition, numerous
CD34-positive small vessels were present adjacent to αSMA-positive
CAFs in mucoepidermoid carcinoma (Fig.
7).
Discussion
Mucoepidermoid carcinoma was formerly classified as
a benign tumor termed the mucoepidermoid tumor. Most patients have
a favorable outcome, yet some patients succumb to the disease.
Therefore, mucoepidermoid carcinoma was reclassified as a malignant
tumor in the 1992 WHO classification because of its capacity for
metastasis regardless of the macroscopic and histologic appearance.
Mucoepidermoid carcinoma is categorized as a low- or high-grade
malignancy with respect to local recurrence and metastatic ability
(13). These criteria were followed
until the 2005 classification (9).
A grading system with low- to high-grade malignancy using five
histopathological features is now utilized. It can be reproducible
in defining the prognosis of mucoepidermoid carcinoma patients;
however, there are some exceptions concerning tumor grading and
prognoses. Thus, it is necessary to establish new methods that can
estimate the exact potential for tumor malignancy.
PTHrP was purified from a human lung cancer cell
line, and was shown to have biological activities similar to
parathyroid hormone (PTH). PTHrP is correlated with the humoral
hypercalcemia of malignancy (2).
Clinical evidence supports another important role for PTHrP in
malignancy as a mediator of the bone destruction associated with
osteolytic metastasis (7,14). PTHrP expression by breast carcinoma
cells may provide a selective growth advantage to bone due to its
ability to stimulate osteoclastic bone resorption (1). Furthermore, growth factors such as
transforming growth factor-β (TGF-β), which are abundant in bone
matrix, are released and activated by osteoclastic bone resorption
and may enhance PTHrP expression and tumor cell growth (14,15).
Moreover, PTHrP overexpression was found to increase mitogenesis
and decrease apoptosis in a human breast cancer cell line. Clones
of MCF-7 cells that overexpress wild-type PTHrP show significantly
higher laminin adhesion and migration. This indicates that PTHrP
may play a role in breast cancer metastasis by upregulating
proinvasive integrin expression, and controlling PTHrP production
in breast cancer may provide a therapeutic benefit (7).
In the present study, PTHrP was detected in oral
squamous cell carcinoma cell lines, and this raised the possibility
that PTHrP may be involved in oral malignancies. Mucoepidermoid
carcinoma is composed of mucous-producing, epidermoid (squamoid)
cells and those of the intermediate type. We hypothesized that
epidermoid (squamoid) cells show a higher level of PTHrP
expression, and we found that PTHrP expression was predominantly
observed both in epidermoid and intermediate cells. Few signals
were observed in mucous-producing cells in the mucoepidermoid
carcinoma cases. PTHrP-expressing cell volumes were measured by
morphometry, and in the cancer cases where intermediate cells
abundantly expressed PTHrP there was a significant association with
malignancy, including lymph node metastasis and/or tumor recurrence
during the 5-year follow-up period. These results indicate that
PTHrP is actually expressed in mucoepidermoid carcinoma, and PTHrP
expression in intermediate cells is closely related to
malignancy.
Recently, the microenvironment surrounding tumor
cells has attracted much attention. Stromal cells have been thought
to be composed of normal cells; however, according to Hida and
colleagues, tumor endothelial cells have abnormalities and
different phenotypes compared to normal endothelial cells (16–18).
Fibroblasts in cancer stromal tissue were also shown to have
different phenotypes and have been termed ‘cancer-associated
fibroblasts (CAFs) (19,20). CAFs have been shown to produce
TGF-β, which induces epithelial-mesenchymal transition (EMT)
(21). We hypothesised that PTHrP
affects the extracellular matrix and plays a role in changing the
extracellular matrix to CAFs. These cells were induced in stromal
tissue in the mucoepidermoid carcinoma cases, and were most often
present around intermediate cells of the mucoepidermoid carcinoma.
The precise mechanism of development of mucoepidermoid carcinoma
remains obscure. However, intermediate cells are thought to be less
differentiated than the other cell types, and they are consequently
the source of the other cell types in mucoepidermoid carcinoma
(22). Our results indicate that
PTHrP-expressing mucoepidermoid carcinoma induces CAFs in stromal
tissue, in particular around intermediate cancer cells. This may
account for the malignant potential of intermediate cells, and
suggests that PTHrP expression is a prognostic factor for
mucoepidermoid carcinoma.
Acknowledgements
This study was supported, in part, by grants-in-aid
for Scientific Research from the Ministry of Education, Culture,
Sports, Science and Technology of Japan.
References
|
1
|
Burtis WJ, Wu T, Bunch C, et al:
Identification of a novel 17,000-dalton parathyroid hormone-like
adenylate cyclase-stimulating protein from a tumor associated with
humoral hypercalcemia of malignancy. J Biol Chem. 262:7151–7156.
1987.PubMed/NCBI
|
|
2
|
Moseley JM, Kubota M, Diefenbach-Jagger H,
et al: Parathyroid hormone-related protein purified from a human
lung cancer cell line. Proc Natl Acad Sci USA. 84:5048–5052. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Itoh K, Udagawa N, Matsuzaki K, et al:
Importance of membrane- or matrix-associated forms of M-CSF and
RANKL/ODF in osteoclastogenesis supported by SaOS-4/3 cells
expressing recombinant PTH/PTHrP receptors. J Bone Miner Res.
15:1766–1775. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kayamori K, Sakamoto K, Nakashima T, et
al: Roles of interleukin-6 and parathyroid hormone-related peptide
in osteoclast formation associated with oral cancers: significance
of interleukin-6 synthesized by stromal cells in response to cancer
cells. Am J Pathol. 176:968–980. 2010. View Article : Google Scholar
|
|
5
|
Theman TA and Collins MT: The role of the
calcium-sensing receptor in bone biology and pathophysiology. Curr
Pharm Biotechnol. 10:289–301. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mula RV, Bhatia V and Falzon M: PTHrP
promotes colon cancer cell migration and invasion in an integrin
α6β4-dependent manner through activation of Rac1. Cancer Lett.
298:119–127. 2010.PubMed/NCBI
|
|
7
|
Shen X, Qian L and Falzon M: PTH-related
protein enhances MCF-7 breast cancer cell adhesion, migration, and
invasion via an intracrine pathway. Exp Cell Res. 294:420–433.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamada T, Tsuda M, Ohba Y, et al: PTHrP
promotes malignancy of human oral cancer cell downstream of the
EGFR signaling. Biochem Biophys Res Commun. 368:575–581. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goode RK and El-Naggar AK: Mucoepidermoid
carcinoma. Barnes L, Eveson JW, Reichart P and Sidransky D: IARC
Press; Lyon: pp. 219–220. 2005
|
|
10
|
Spiro RH, Huvos AG, Berk R, et al:
Mucoepidermoid carcinoma of salivary gland origin. A
clinicopathologic study of 367 cases. Am J Surg. 136:461–468. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Seethala RR: An update on grading of
salivary gland carcinomas. Head Neck Pathol. 3:69–77. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rapidis AD, Givalos N, Gakiopoulou H, et
al: Mucoepidermoid carcinoma of the salivary glands. Oral Oncol.
43:130–136. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seifert G and Sobin LH: Histological
typing of salivary gland tumours. Springer-Verlag; Tokyo: 1992
|
|
14
|
Guise TA: Parathyroid hormone-related
protein and bone metastases. Cancer. 80:1572–1580. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Johnson RW, Nguyen MP, Padalecki SS, et
al: TGF-β promotion of Gli2-induced expression of parathyroid
hormone-related protein, an important osteolytic factor in bone
metastasis, is independent of canonical hedgehog signaling. Cancer
Res. 71:822–831. 2010.
|
|
16
|
Hida K, Hida Y, Amin DN, et al:
Tumor-associated endothelial cells with cytogenetic abnormalities.
Cancer Res. 64:8249–8255. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hida K and Klagsbrun M: A new perspective
on tumor endothelial cells: unexpected chromosome and centrosome
abnormalities. Cancer Res. 65:2507–2510. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Amin DN, Hida K, Bielenberg DR, et al:
Tumor endothelial cells express epidermal growth factor receptor
(EGFR) but not ErbB3 and are responsive to EGF and to EGFR kinase
inhibitors. Cancer Res. 66:2173–2180. 2006. View Article : Google Scholar
|
|
19
|
Orimo A, Gupta PB, Sgroi DC, et al:
Stromal fibroblasts present in invasive human breast carcinomas
promote tumor growth and angiogenesis through elevated SDF-1/CXCL12
secretion. Cell. 121:335–348. 2005. View Article : Google Scholar
|
|
20
|
Micke P and Ostman A: Tumour-stroma
interaction: cancer-associated fibroblasts as novel targets in
anti-cancer therapy? Lung Cancer. 45(Suppl 2): S163–S175. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mink SR, Vashistha S, Zhang W, et al:
Cancer-associated fibroblasts derived from EGFR-TKI-resistant
tumors reverse EGFR pathway inhibition by EGFR-TKIs. Mol Cancer
Res. 8:809–820. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Azevedo RS, Almeida OP, Kowalski LP, et
al: Comparative cytokeratin expression in the different cell types
of salivary gland mucoepidermoid carcinoma. Head Neck Pathol.
2:257–264. 2008. View Article : Google Scholar : PubMed/NCBI
|