Introduction
Globally, lung cancer is the major cause of
malignancy-related mortality, and its incidence is on the rise in
many countries (1). Histologically,
lung cancer is classified as small-cell lung cancer (SCLC) and
non-small cell lung cancer (NSCLC). Due to the high ability of
rapid growth and early distant metastasis, the prognosis of SCLC is
considered be the poorest of all lung cancer types. Its 5-year
survival rate is less than 2% (2,3).
Approximately two-thirds of SCLC patients present obvious
metastatic disease (4). In
particular, bone is one of the most common sites of metastasis.
Osteolytic metastases are incurable and are often associated with
skeletal-related events including pain, hypercalcemia, fracture and
nerve compression syndromes, all of which decrease the quality of
life of patients (5). However, the
mechanism underlying the bone metastatic capacity of SCLC remains
obscure. Bone metastasis occurs as a sequence of complex processes
involving multiple interactions of cancer cells with host cellular
and extracellular microenvironments. Various molecules, such as
adhesion molecules, cytokines (6),
chemokines (7,8), hormones (9) and their receptors have been reported
to play important roles in these processes.
SBC-3 and SBC-5 cell lines demonstrate a similar
genetic background to human SCLC but a different potential for bone
metastasis. SBC-5 has a higher capability of bone metastasis than
SBC-3 (10–12). Consequently, this pair of cell lines
is widely used as cell models in the research of the molecular
pathogenesis of bone metastasis in human SCLC.
In our laboratory, we previously explored the
preliminary mechanism of bone metastasis using SCLC cell lines
(SBC-3 and SBC-5). We found that calcineurin Aα and zinc finger
E-box binding homeobox 1 (ZEB1) were closely related to the
osteotropic metastasis of SCLC. In addition, the
epithelial-mesenchymal transition (EMT) pathway was observed to
participate in the process of bone metastasis in SCLC (13–15).
However, the exact mechanism is still unknown.
MGr1-Ag, also termed as the 37-kDa laminin receptor
precursor (37LRP), is the precursor of the metastasis-associated
67-kDa laminin receptor (67LR). It exhibits high laminin-binding
activity and appears to be a ribosomal protein which is essential
for protein synthesis (16,17). Increased research interest has been
stimulated by the observation that MGr1-Ag is a multifunctional
protein that is required for cell differentiation, migration and
growth. We and other researchers have consistently observed it in
invasive and metastatic cancers, and it is associated with poor
prognosis (18–20). Therefore, we proposed that MGr1-Ag
may play a role in the process of bone metastasis in SCLC.
EMT has been proposed as a key step during carcinoma
progression and metastatic development. Its hallmark is the loss of
polarized organization and downregulation of epithelial molecular
markers (e.g. E-cadherin, α-catenin, and β-catenin), and at the
same time, upregulation of mesenchymal proteins (e.g. fibronectin,
sm-actin and vimentin) (21,22).
According to previous research, the worst prognosis of SCLC is
associated with the presence of cells with a mesenchymal character
(23). This indicates that EMT
plays an important role in SCLC cell metastasis.
In the present study, we first observe that MGr1-Ag
was highly expressed in bone-metastatic SCLC cell line SBC-5.
Subsequently we investigated the effects of the upregulation or
knockdown of MGr1-Ag expression on cell invasion and migration
in vitro as well as the bone metastatic ability of SCLC cell
lines in vivo. Additionally, changes in cell morphology and
the expression of EMT markers (E-cadherin and fibronectin) were
observed. The results demonstrated that MGr1-Ag contributes to an
EMT-like transformation, invasion and metastasis in bone-metastatic
SCLC. Therefore, we present initial evidence that MGr1-Ag promotes
the occurrence of bone metastasis in SCLC via the EMT pathway.
Materials and methods
Cell culture
The human SCLC cell lines SBC-3 and SBC-5 were gifts
from Professor Saburo Sone and Seiji Yano (University of Tokushima
School of Medicine, Japan) (11).
The human lung fibroblast (HLF) cell line, used as a control, was
from the Shanghai Cell Bank (Shanghai, China). The cells were
cultured in RPMI-1640 medium (Gibco, Carlsbad, CA, USA) containing
100 U/ml penicillin, 100 μg/ml streptomycin and 10% fetal calf
serum (FCS). Cultures were incubated in a humidified atmosphere
with 5% CO2 at 37°C.
Plasmid constructs and transfection
To upregulate and downregulate the expression of
MGr1-Ag in the SCLC SBC-3 and SBC-5 cell lines. MGr1-Ag-targeting
oligonucleotides for generating cDNA were designed from full-length
MGr1-Ag by Shanghai GeneChem Co., Ltd. Their sequences were
forward, 5′-GAGGATCCCCGGGTACCGGTCGCCACCATGCCGGA GCCCTTGAT-3′ and
reverse, 5′-TCACCATGGTGGCGACCG GAGACCAGTCAGTGGTTGCTC-3′. Three
pairs of MGr1-Ag-specific siRNAs were also designed (Shanghai
GeneChem Co., Ltd.): MGr1-Ag siRNA1 forward, 5′-cgggaGATCCTG
AAGAGATTGAAATTCAAGAGATTTCAATCTCTTCAGG ATCtcTTTTTg-3′ and reverse,
5′-aattcaaaaagaGATCCTGAA GAGATTGAAATCTCTTGAATTTCAATCTCTTCAGGAT
Ctc-3′; siRNA2 forward, 5′-CcggcaCTCCTGGAACCTTCACT
AATTCAAGAGATTAGTGAAGGTTCCAGGAGtgTTTTg-3′ and reverse,
5′-aattcaaaaacaCCTCTTGAATTAGTGAAGGTT CCAGGAGtg-3′; siRNA3 forward,
5′-CcggtaCCTACCATTGC GCTGTGTATTCAAGAGATACACAGCGCAATGGAGGtaT
TTTTg-3′ and reverse, 5′-aattcaaaaataCCTACCATTGCGCTG
TGTATCTCTTGATACACAGCGCAATGGTAGGta-3′. After testing overexpression
and knockdown efficiencies, stem-loop oligonucleotides were
synthesized and cloned into the lentivirus-based vector pSicoR
(Addgene). A non-targeting (scrambled) stem-loop DNA pSicoR vector
was generated as a negative control. Lentiviral particles were
prepared as described previously (24). The overexpression and knockdown
efficiencies were then confirmed by western blot analysis and
real-time PCR. SBC-3 cells transfected with MGr1-Ag cDNA or empty
vector were designated SBC-3/MGr and SBC-3/pc. SBC-5 cells
transfected with siRNA-lentivirus-MGr1-Ag or scrambled RNA duplex
were designated SBC-5/MGr-si and SBC-5/scr.
Real-time reverse transcription
(RT)-polymerase chain reaction (PCR)
Total cellular RNA was extracted using TRIzol
reagent from the cells following the different treatments, and was
then reverse transcribed using the SuperScript® III
RT-PCR system (both from Invitrogen, USA), according to the
manufacturer’s instructions. Synthesized cDNA was used as a
template for the TaqMan real-time PCR technique to quantify mRNA
expression by using a qPCR core kit (Eurogentec) and an ABI Prism
7700 sequence detection system (PE Applied Biosystems). TaqMan PCR
primers for each gene were designed as in a previous study
(20). The primer sequences
included: β-actin forward, 5′-GGCGGCACCACCATGTACCCT-3′ and reverse,
5′-AGGGGCCGGACTCGTCATACT-3′; MGr1-Ag forward,
5′-GCAGCAGGAACCCACTTAGG-3′ and reverse,
5′-GGCAGCAGCAAACTTCAGC-3′.
Western blot analysis
After pretreatment according to experimental demand,
the cells were washed three times with ice cold phosphate-buffered
saline (PBS). Then, the cytosolic extracts were extracted with
lysis buffer (62.5 mM Tris-HCl, pH 6.8, 2% SDS, 5%
2-mercaptoethanol, 10% glycerol and protease inhibitor cocktail).
According to the standard protocol for western blot analysis, equal
amounts of protein (50 μg) were separated by 12% SDS-polyacrylamide
gel electrophoresis (SDS-PAGE) and electrotransferred onto
polyvinylidene difluoride (PVDF) membranes. The proteins were
hybridized with primary antibodies against MGr1-Ag (1:200;
developed by Bioss Biotech, Beijing, China), E-cadherin (1:500),
fibronectin (1:500; both from BD Biosciences, Franklin Lakes, NJ,
USA) or β-actin (1:4,000; Sigma, USA). After staining with an
HRP-linked secondary antibody (GE Healthcare Bio-Science, USA), the
protein bands were detected by the ECL chemiluminescence system
(Amersham Phamacia Biotech, USA).
In vitro invasion and migration
assays
In vitro cell migration assays were performed
as described previously (13) using
Transwell chambers (8-μm pore size; Costar). Cells were allowed to
grow to ~75–80% confluence and were serum-starved for 24 h. After
detachment with trypsin, cells were washed with PBS, resuspended in
serum-free medium, and 250 μl of the cell suspension
(2×105 cells/ml) was added to the upper chamber.
Complete medium was added to the bottom wells of the chamber. The
cells that had not migrated were removed from the upper face of the
filters using cotton swabs, and the cells that had migrated to the
lower face of the filters were fixed with 5% glutaraldehyde
solution and stained with 0.5% solution of toluidine blue in 2%
sodium carbonate. Images of three random ×10 fields were captured
from each membrane, and the number of migratory cells was counted.
The mean of triplicate assays for each experimental condition was
used. Similar inserts coated with Matrigel (BD Biosciences) were
used to determine the invasive potential in the invasion assay.
Wound healing assay
The wound healing assay was performed as described
previously (25). In brief, cells
were seeded in 6-well plates and allowed to grow to ~90% confluence
before wounding with a 200 μl plastic tip across the monolayer
cells. Debris was removed by washing three times with PBS, and then
cells were cultured with fresh medium containing 5% fetal bovine
serum. After 48 h, images were captured by phase-contrast
microscope. Each experiment was performed in triplicate and
repeated three times.
Immunofluorescence
Cells transfected with the indicated expression
vectors were plated on sterile microscope coverslips. After 24 h,
cells were washed and fixed in acetone/methanol (1:1) solution for
3 min on ice. Then the coverslips were rinsed and incubated with
primary antibodies specific for E-cadherin or fibronectin (1:500;
BD Biosciences) for 1 h at room temperature. After washing, the
coverslips were incubated with Alexa 488-conjugated rabbit
anti-mouse IgG (Pierce Biotechnology, Inc., Rockford, IL, USA) for
1 h. Finally, cells were examined and photographed using a confocal
inverted microscope (Axiovert 200M; Zeiss, Oberkochen, Germany). To
monitor BrdU incorporation, cells were pulse labeled with BrdU for
40 min and stained with DAPI according to the protocols supplied
with the Detection Kit I (Roche Applied Science, Basel,
Switzerland).
Tail-vein bone metastatic assay
Female NOD/scid mice, 3–4 weeks old, were purchased
from HFK Bioscience Co., Ltd. (Beijing, China) and maintained in
our animal facilities under specific pathogen-free conditions. The
mice were divided into groups and injected through the tail vein
with 1×106 cells in 0.1 ml PBS and monitored for overall
health and total body weight. Five weeks after injection, the mice
were sacrificed. The number of sites of bone metastases on the body
surface was counted. Sites of bone metastases were serially
sectioned for X-ray analysis and observed under a light microscope.
Each experimental group contained 5 mice. All animals were handled
using best humane practices and cared for in accordance with
National Institutes of Health Animal Care and Use Committee
guidelines.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 11.0; Chicago, IL, USA). Assays for
characterizing cell phenotypes were analyzed using Student’s
t-test. The Wilcoxon rank sum test was used to determine the
significance of differences in bone metastasis numbers between
groups. P<0.05 was considered to indicate a statistically
significant result.
Results
MGr1-Ag is overexpressed in the
bone-metastatic SCLC cell line
Previous studies have shown that the SCLC cell
lines, SBC-3 and SBC-5, have similar genetic background but
different potential for bone metastasis. SBC-5 has significantly
higher capability of bone metastasis than SBC-3 (10,11).
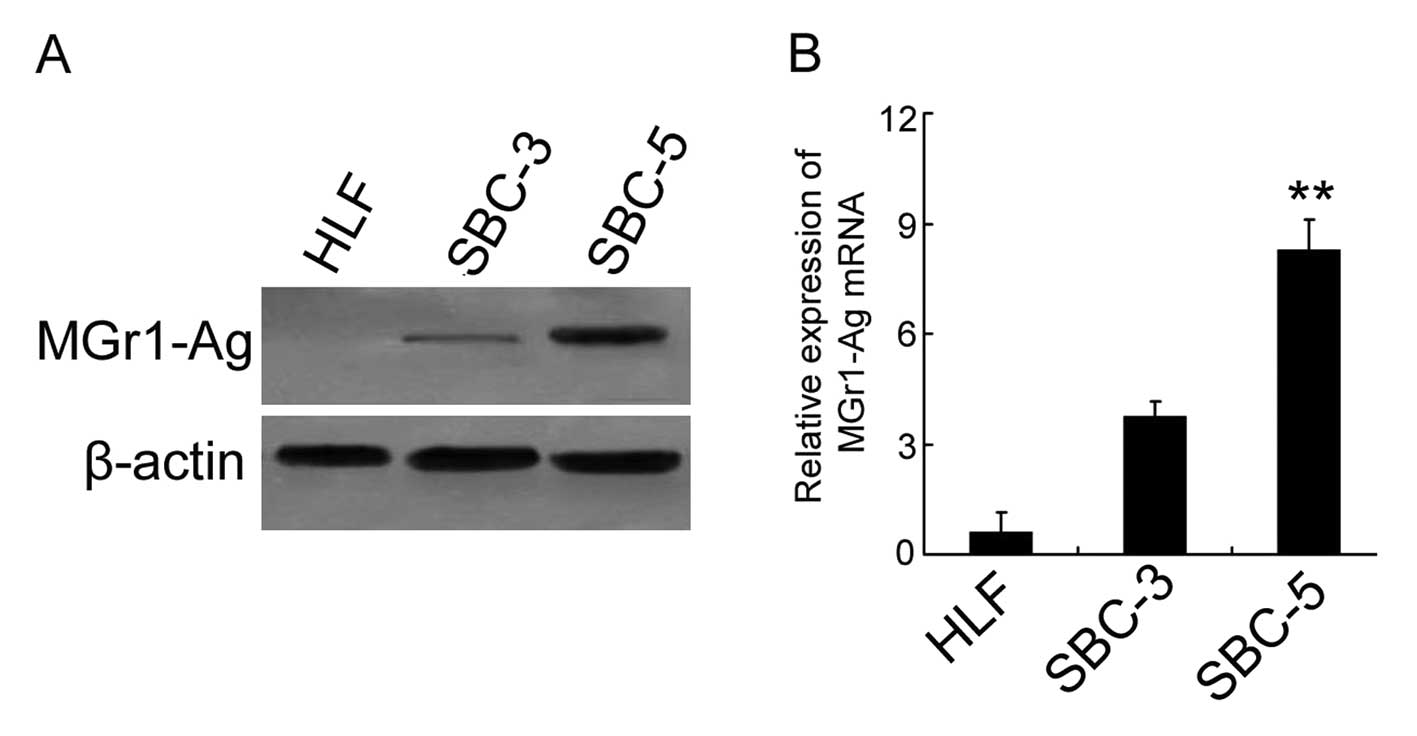
To detect the differential expression of MGr1-Ag in these cell
lines, western blot analysis and real-time PCR were performed.
Human lung fibroblasts (HLFs) (normal cells without bone-metastatic
ability) were used as the negative control in each experiment. Data
from the western blot analysis showed that the protein expression
level of MGr1-Ag in SBC-5 cells was distinguishably higher than
that in the SBC-3 cells (Fig. 1A).
Similarly, the differential expression of MGr1-Ag mRNA was
confirmed by data from real-time PCR (P<0.01 vs. SBC-3)(Fig. 1B). These results indicated that
MGr1-Ag overexpression was closely associated with the
bone-metastatic ability of SCLC cells.
Upregulated expression of MGr1-Ag in
SBC-3 cells enhances cell invasion and migration in vitro, and
increases the number of bone metastases in vivo
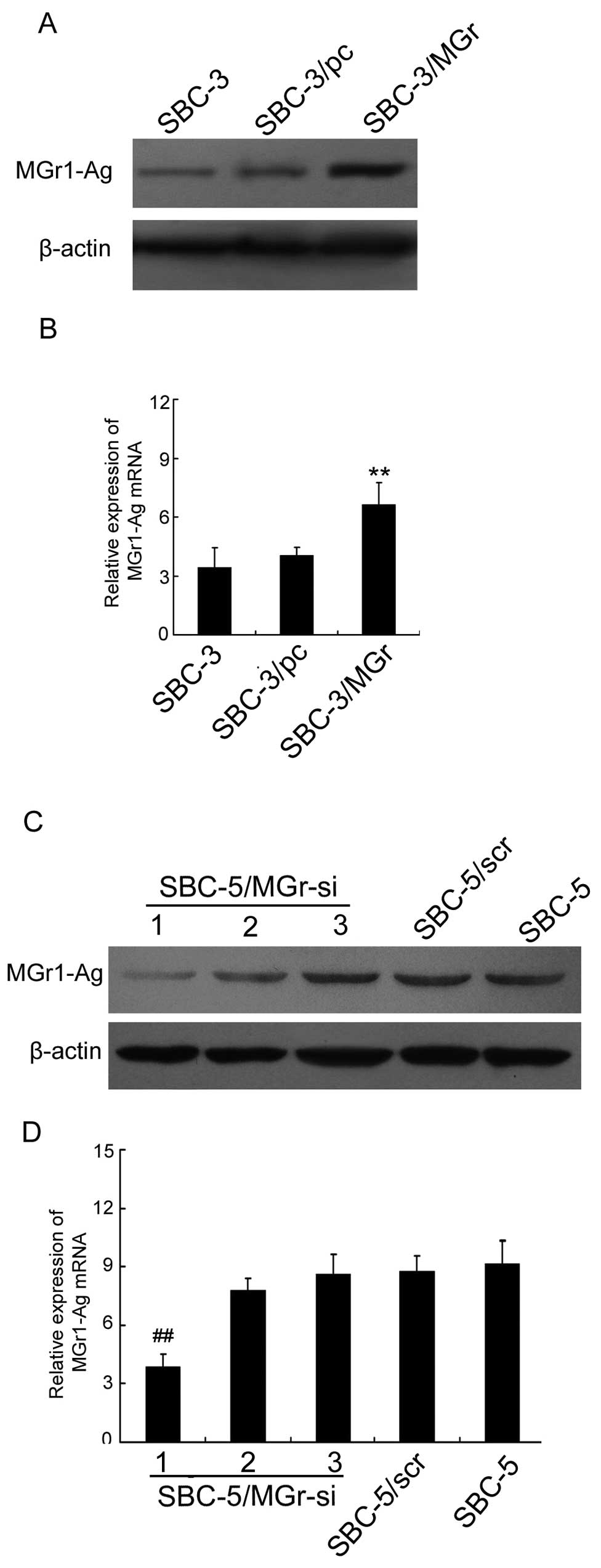
To further explore the effect of MGr1-Ag on bone
metastasis in SCLC, MGr1-Ag stably transfected cell line SBC-3/MGr
was constructed according to Materials and methods. The SBC-3/pc
cell line was employed as the control-transfected cells. Western
blot analysis and real-time PCR were performed to test the
efficiencies of MGr1-Ag upregulation. The data revealed that
MGr1-Ag expression was markedly increased in the SBC-3/MGr cells
when compared with that in SBC-3/pc cells at the protein and mRNA
levels, respectively (P<0.01 vs. SBC-3/pc) (Fig. 2A and B). Subsequently, we detected
the invasive and migratory ability of each cell group in
vitro. The results from the Transwell assays showed that
SBC-3/MGr cells demonstrated increased migratory and invasive
abilities compared with the control cells (P<0.01 vs. SBC-3/pc)
(Fig. 3A and C). Meanwhile, data
from the wound healing assay revealed that, compared with SBC-3/pc
cells, SBC-3/MGr cells exhibited a much greater migratory capacity
to repair the wound (Fig. 3E-a and
-b).
Furthermore, tail-vein bone metastatic assays were
adopted to examine the bone-metastatic ability of SBC-3, SBC-3/pc
and SBC-3/MGr cells in vivo. Compared with control cells
(SBC-3/pc), the injection of SBC-3/MGr cells led to a significant
increase in bone metastatic lesions (P<0.01 vs. SBC-3/pc)
(Table I and Fig. 3F-a and -b). Taken together, these
results suggest that MGr1-Ag overexpression promotes the invasion,
migration and bone-metastatic abilities of SCLC cells both in
vitro and in vivo.
 | Table IBone metastatic lesions in the
NOD/scid mice model after inoculation of the indicated cells
through the tail vein. |
Table I
Bone metastatic lesions in the
NOD/scid mice model after inoculation of the indicated cells
through the tail vein.
| Group | Incidence | Median (range) |
|---|
| SBC-3 | 0/5 | 0 (0–0) |
| SBC-3/pc | 0/5 | 0 (0–0) |
| SBC-3/MGr | 4/5 | 3 (0–5)a |
| SBC-5 | 5/5 | 9 (8–12) |
| SBC-5/scr | 5/5 | 11 (8–13) |
| SBC-5/MGr-si | 3/5 | 4 (0–7)b |
Suppressed expression of MGr1-Ag in SBC-5
cells inhibits cell invasion and migration in vitro, and decreases
the number of bone metastases in vivo
To further confirm the effect of MGr1-Ag in bone
metastasis of SCLC, RNA interference was employed to knockdown the
expression of MGr1-Ag in the SBC-5 cell line. The knockdown
efficiency was confirmed by western blot analysis and real-time
PCR. MGr1-siRNA1 effectively downregulated MGr1-Ag in SBC-5 cells
at the protein and mRNA level, while the effect of MGr1-siRNA2 and
MGr1-siRNA3 was minima (P<0.01 vs. SBC-5/scr) (Fig. 2C and D). Thus, SBC-5 cells
transfected with siRNA1 (termed SBC-5/MGr-si) were used in the
subsequent investigations. SBC-5/scr cells were used as control.
Next, cell invasion and migration in the in vitro assays
showed that downregulation of MGr1-Ag in SBC-5 cells significantly
inhibited their migratory and invasive abilities compared with
control cells (P<0.01 vs. SBC-5/scr) (Fig. 3B and D). The wound healing assay was
also performed in SBC-5 cells. As shown in Fig. 3E-c and -d, knockdown of MGr1-Ag
notably reduced the migratory capacity of SBC-5 cells to repair the
wound. Meanwhile, to investigate the effect of MGr1-Ag knockdown on
the ability of bone metastasis, SBC-5/MGr-si cells were injected
into NOD/scid mice and assayed for the development of bone
metastatic lesions. Compared with the control group, the injection
of SBC-5/MGr-si cells led to a significant decrease in the number
of bone metastatic lesions (P<0.01 vs. SBC-5/scr) (Table I and Fig. 3F-c and -d). These results suggest
that, both in vitro and in vivo, inhibition with
siRNA targeting MGr1-Ag had the potential to suppress the invasion,
migration and bone-metastatic ability of SCLC cells.
EMT is involved in MGr1-Ag induced bone
metastasis in SCLC
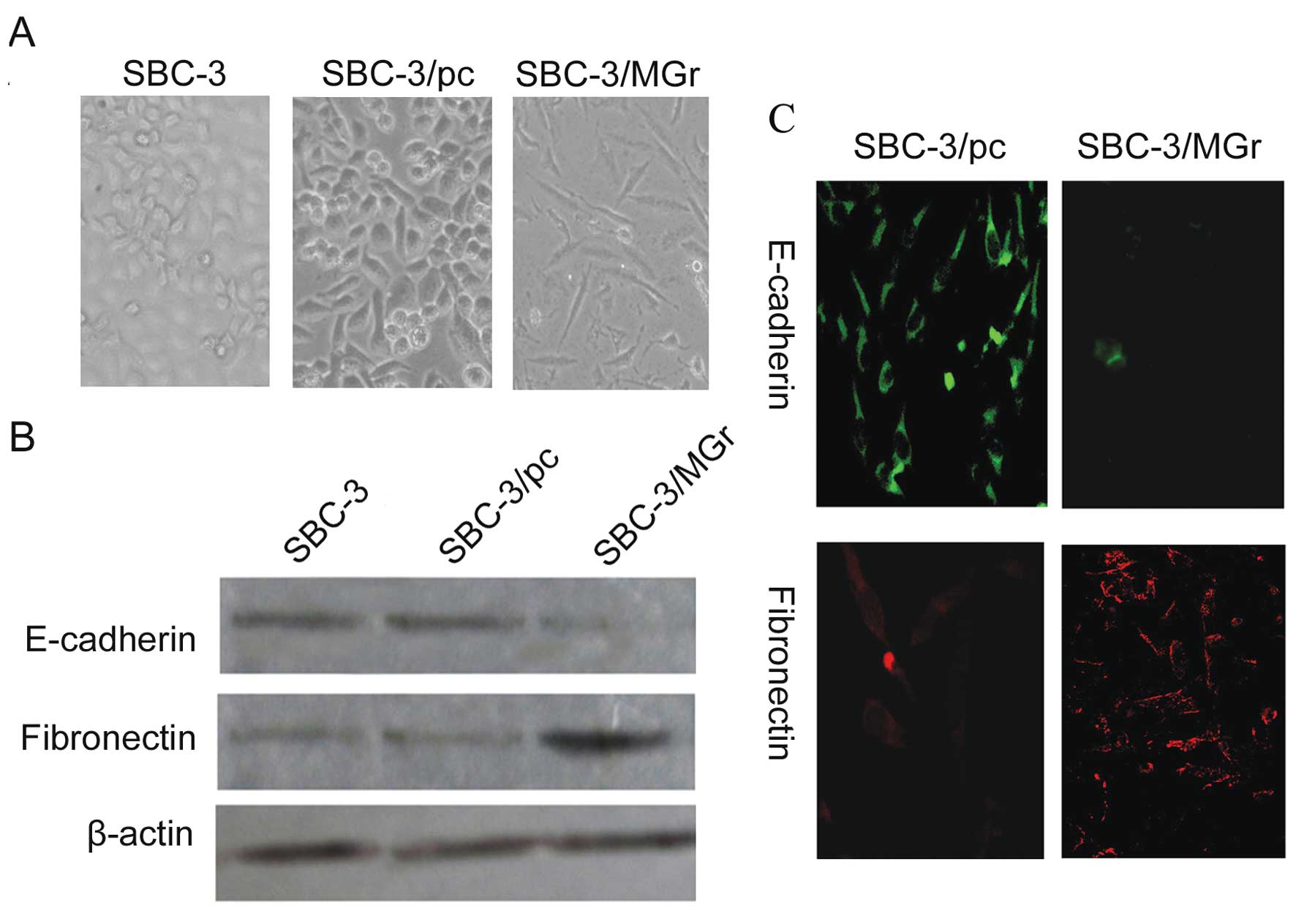
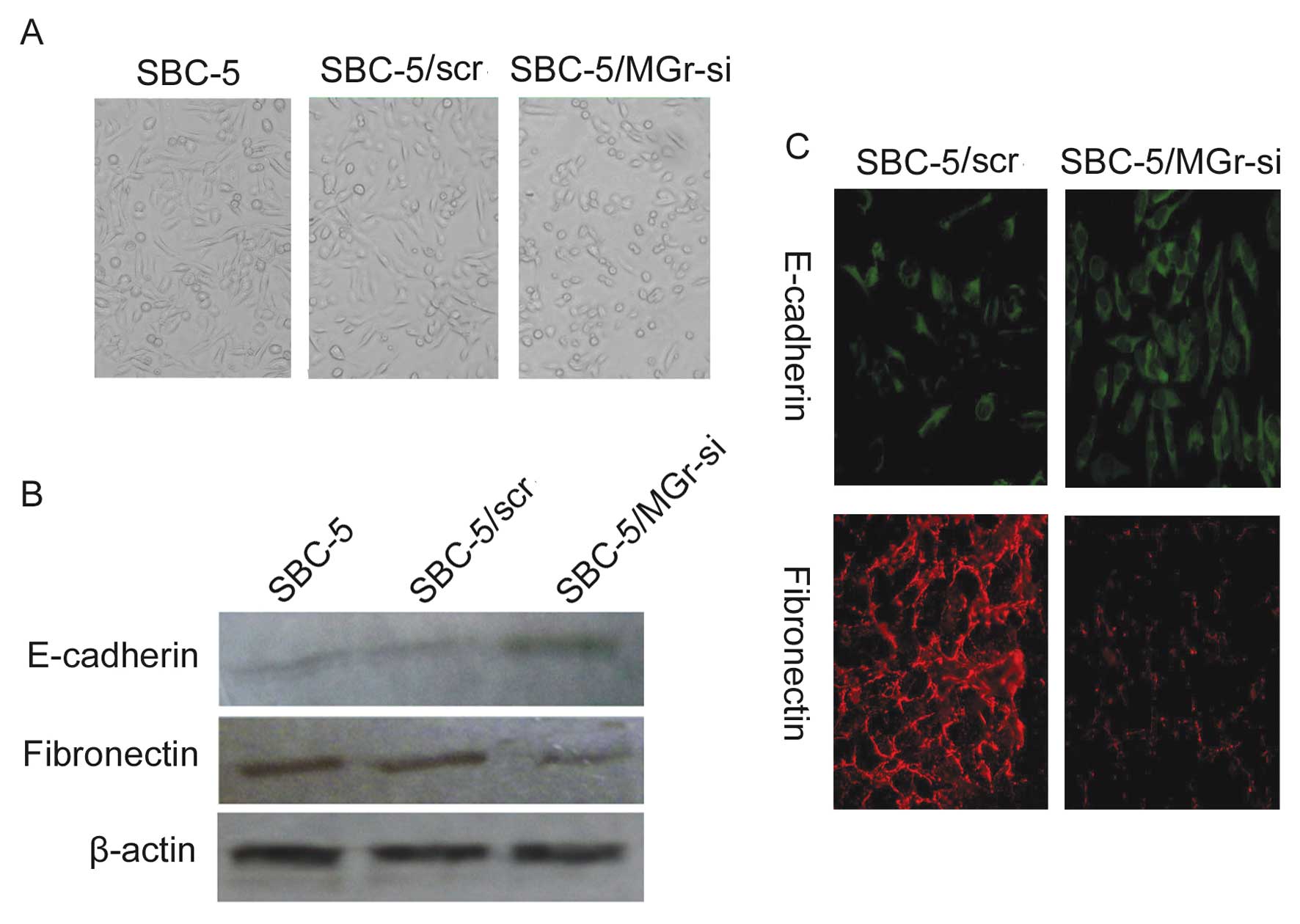
The cell morphology of most SBC-3 cells which have
no bone-metastatic ability was round, while that of SBC-5 cells
which have high bone-metastatic ability was fusiform. Apart from
this, after transfection with the MGr1-Ag overexpression vector, we
noted that the morphology of SBC-3/MGr cells changed from round to
fusiform (Fig. 4A). Yet, in the
SBC-5 cells, the cell morphology was altered from fusiform to round
after the inhibition of MGr1-Ag expression with siRNA (Fig. 5A). These differences and changes
represent one of the hallmarks of EMT and indicated that EMT was
involved in the occurrence of bone metastases in SCLC, and that
MGr1-Ag induced EMT in the SCLC cell lines. To gain insight into
the mechanism of MGr1-Ag-induced EMT, we next examined the
expression of well-characterized EMT markers. Immunofluorescence
staining showed that the epithelial marker E-cadherin had nearly
disappeared and the mesenchymal marker fibronectin was dramatically
increased in the SBC-3/MGr cells compared to the staining observed
in the control cells (Fig. 4C).
Western blot analysis revealed nearly a complete loss of epithelial
marker E-cadherin expression and high expression of mesenchymal
marker fibronectin (Fig. 4B). In
contrast, the same experiments were performed using SBC-5 cells.
Immunofluorescence staining demonstrated that, compared to the
SBC-5/scr cell line, the staining of E-cadherin was markedly
increased in the SBC-5/MGr-si cell line whose MGr1-Ag expression
was abolished by specific siRNA, while the staining of fibronectin
was decreased (Fig. 5C). Data from
the western blot analysis were consistent with data from the
immunofluorescence staining in SBC-5 cells (Fig. 5B). Therefore, both the cell
morphological and molecular changes observed in the SCLC cell lines
indicated that EMT was associated with the occurrence of bone
metastasis in SCLC, and Mgr1-Ag induced SCLC cells to undergo
EMT.
Discussion
SCLC exhibits aggressive behavior including rapid
growth and early spread to distant sites. Bone is one of the most
frequent targets of SCLC metastasis. Bone metastasis leads to rapid
deterioration in the quality of life of patients. However,
effective curative therapy is limited in the clinical (2). Therefore, more basic studies
concerning the mechanism of bone metastasis in SCLC are desperately
needed. Previous research has indicated that bone metastases
present as two types of lesions, osteoblastic or osteolytic, which
mainly result from an imbalance between osteoblast-mediated bone
formation and osteoclast-mediated bone resorption (26). Numerous signaling pathways and
various molecules are activated to drive this vicious imbalance in
malignant disease (27,28). In general, most of the basic
research has focused on the relationship between tumor cells and
the imbalance of osteoblasts and osteoclasts. Yet, the
understanding of the mechanisms by which tumor cells attach and
lodge in bone remains a void to be filled.
Malignant cell attachment and lodgment in bone are
complex processes involving multiple interactions of tumor cells
with host cellular and extracellular structures. One critical event
is the formation of micrometastases. The attachment of cancer cells
to basement membrane components is the first step in the metastatic
process. This step may be mediated in part by specific cell-surface
receptors which bind to laminin in the basement membrane. Notably,
MGr1-Ag demonstrates high laminin-binding activity. Additionally,
an increase in the expression of MGr1-Ag has been found in a
variety of common cancers. In many cases, a positive correlation
with the aggressiveness or metastatic potential has also been
observed (16–19). Yet, whether MGr1-Ag plays a role in
the bone metastasis of SCLC is unclear.
In the present study, we first detected the
expression of MGr1-Ag in SBC-3 and SBC-5 cell lines, which have
similar genetic background to human SCLC but different potential
for bone metastasis (SBC-5 cells demonstrate a higher capability
for promoting bone metastasis). Western blot analysis and real-time
PCR showed that the expression of MGr1-Ag was markedly higher in
SBC-5 cells than that in SBC-3 cells. Overexpression of MGr1-Ag was
found to be associated with the high rate of bone metastasis in
SCLC cells. Next, we upregulated the expression of MGr1-Ag in SBC-3
cells and observed the alterations in cellular behavior. We found
that forced overexpression of MGr1-Ag in SBC-3 cells enhanced cell
invasive and migratory abilities in vitro as well as
increased bone metastatic lesions in vivo. Meanwhile, RNA
interference targeting MGr1-Ag in SBC-5 cells partly abolished cell
invasive and migratory abilities in vitro and decreased the
number of bone metastatic lesions in vivo. Taken together,
these findings suggest that MGr1-Ag functions to promote the
invasion, migration and bone-metastatic ability of SCLC cells.
Recent research has shown a novel view relating
metastasis with the heterogeneity of SCLC (23). This study found that tumors were
often composed of phenotypically different cells with either a
neuroendocrine marker or mesenchymal marker profile. Moreover, the
crosstalk between mesenchymal and neuroendocrine cells strongly
influenced their behavior. When engrafted as a mixed population,
the mesenchymal cells endowed the neuroendocrine cells with
metastatic capacity. These findings provide evidence that the poor
prognosis of SCLC is due to the presence of cells with a
mesenchymal character. Furthermore, in our research, variations in
the cell morphology between SBC-3 and SBC-5 cells were observed.
After transfection with the MGr1-Ag overexpression vector, the
morphology of the SBC-3 cells changed from a round to a fusiform
shape. Moreover, in the SBC-5 cells, the cell morphology was
altered from fusiform to a round shape after the inhibition of
MGr1-Ag expression with siRNA. These findings suggest that EMT was
involved in the MGr1-Ag-mediated promotion of invasion, migration
and bone-metastasis in SCLC cells. Thus, we detected the expression
of epithelial marker (E-cadherin) and mesenchymal marker
(fibronectin) in cells with different bone-metastatic potential and
MGr1-Ag expression level. The results indicated that, in cells with
high bone-metastatic potential and high MGr1-Ag expression level,
the expression of the epithelial marker was decreased, and the
expression of the mesenchymal marker was increased. In contrast, in
cells with low bone-metastatic potential and low MGr1-Ag expression
level, the levels of expression of the EMT markers were reversed.
Thus, Mgr1-Ag likely promotes SCLC invasion and bone metastasis via
the EMT pathway, but the precise role of EMT and its heterogeneity
character in SCLC with bone metastasis still requires further
study.
In conclusion, our present data strongly
demonstrated that MGr1-Ag promoted invasion and bone metastasis in
SCLC via the EMT pathway both in vitro and in vivo.
MGr1-Ag is a promising therapeutic target for SCLC with bone
metastasis. In regards to the inducers of EMT, microenvironmental
factors such as hypoxia and growth factors such as epidermal growth
factor and transforming growth factor-β may induce the expression
of certain EMT regulators, such as ZEB-1, directly or indirectly to
prompt tumor invasion. The extent of the upregualation and
downregulation of the epithelial and mesenchymal markers varies in
different types of cancer cells and under different stimuli. The
mechanisms underlying the role of MGr1-Ag in these processes
require further study.
Acknowledgements
This study was supported by grants from the National
Nature Science Foundation of China (nos. 81101765 and
81172011).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Rodriguez E and Lilenbaum RC: Small cell
lung cancer: past, present, and future. Curr Oncol Rep. 12:327–334.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jackman DM and Johnson BE: Small-cell lung
cancer. Lancet. 366:1385–1396. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van Meerbeeck JP, Fennell DA and De
Ruysscher DK: Small-cell lung cancer. Lancet. 378:1741–1755.
2011.
|
|
5
|
Mundy GR: Metastasis to bone: causes,
consequences and therapeutic opportunities. Nat Rev Cancer.
2:584–593. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Uy HL, Mundy GR, Boyce BF, et al: Tumor
necrosis factor enhances parathyroid hormone-related
protein-induced hypercalcemia and bone resorption without
inhibiting bone formation in vivo. Cancer Res. 57:3194–3199.
1997.
|
|
7
|
Cai Z, Chen Q, Chen J, et al: Monocyte
chemotactic protein 1 promotes lung cancer-induced bone resorptive
lesions in vivo. Neoplasia. 11:228–236. 2009.PubMed/NCBI
|
|
8
|
Han JH, Choi SJ, Kurihara N, Koide M, Oba
Y and Roodman GD: Macrophage inflammatory protein-1alpha is an
osteoclastogenic factor in myeloma that is independent of receptor
activator of nuclear factor kappaB ligand. Blood. 97:3349–3353.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miki T, Yano S, Hanibuchi M, Kanematsu T,
Muguruma H and Sone S: Parathyroid hormone-related protein (PTHrP)
is responsible for production of bone metastasis, but not visceral
metastasis, by human small cell lung cancer SBC-5 cells in natural
killer cell-depleted SCID mice. Int J Cancer. 108:511–515. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li M, Zhou M, Gong M, et al: A novel
animal model for bone metastasis in human lung cancer. Oncol Lett.
3:802–806. 2012.PubMed/NCBI
|
|
11
|
Miki T, Yano S, Hanibuchi M and Sone S:
Bone metastasis model with multiorgan dissemination of human
small-cell lung cancer (SBC-5) cells in natural killer
cell-depleted SCID mice. Oncol Res. 12:209–217. 2000.PubMed/NCBI
|
|
12
|
Zhang H, Yano S, Miki T, et al: A novel
bisphosphonate minodronate (YM529) specifically inhibits osteolytic
bone metastasis produced by human small-cell lung cancer cells in
NK-cell depleted SCID mice. Clin Exp Metastasis. 20:153–159. 2003.
View Article : Google Scholar
|
|
13
|
Liu Y, Zhang N, Wang Y, et al: Zinc finger
E-box binding homeobox 1 promotes invasion and bone metastasis of
small cell lung cancer in vitro and in vivo. Cancer Sci.
103:1420–1428. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Y, Zhang Y, Min J, et al: Calcineurin
promotes proliferation, migration, and invasion of small cell lung
cancer. Tumour Biol. 31:199–207. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma NQ, Liu LL, Min J, et al: The effect of
down regulation of calcineurin Aα by lentiviral vector-mediated
RNAi on the biological behavior of small-cell lung cancer and its
bone metastasis. Clin Exp Metastasis. 28:765–778. 2011.
|
|
16
|
Jaseja M, Mergen L, Gillette K, Forbes K,
Sehgal I and Copié V: Structure-function studies of the functional
and binding epitope of the human 37 kDa laminin receptor precursor
protein. J Pept Res. 66:9–18. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi Y, Zhai H, Wang X, et al:
Multidrug-resistance-associated protein MGr1-Ag is identical to the
human 37-kDa laminin receptor precursor. Cell Mol Life Sci.
59:1577–1583. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Annabi B, Currie JC, Bouzeghrane M, et al:
Contribution of the 37-kDa laminin receptor precursor in the
anti-metastatic PSP94-derived peptide PCK3145 cell surface binding.
Biochem Biophys Res Commun. 346:358–366. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu L, Ning X, Sun L, et al: Involvement
of MGr1-Ag/37LRP in the vincristine-induced HIF-1 expression in
gastric cancer cells. Mol Cell Biochem. 303:151–160. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu L, Sun L, Zhang H, et al:
Hypoxia-mediated up-regulation of MGr1-Ag/37LRP in gastric cancers
occurs via hypoxia-inducible-factor 1-dependent mechanism and
contributes to drug resistance. Int J Cancer. 124:1707–1715. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Micalizzi DS, Farabaugh SM and Ford HL:
Epithelial-mesenchymal transition in cancer: parallels between
normal development and tumor progression. J Mammary Gland Biol
Neoplasia. 15:117–134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Voulgari A and Pintzas A:
Epithelial-mesenchymal transition in cancer metastasis: mechanisms,
markers and strategies to overcome drug resistance in the clinic.
Biochim Biophys Acta. 1796:75–90. 2009.PubMed/NCBI
|
|
23
|
Calbo J, van Montfort E, Proost N, et al:
A functional role for tumor cell heterogeneity in a mouse model of
small cell lung cancer. Cancer Cell. 19:244–256. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lois C, Hong EJ, Pease S, Brown EJ and
Baltimore D: Germline transmission and tissue-specific expression
of transgenes delivered by lentiviral vectors. Science.
295:868–872. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang F, Wang Q, Ye L, Feng Y and Zhang X:
Hepatitis B virus X protein upregulates expression of calpain small
subunit 1 via nuclear factor-kappaB/p65 in hepatoma cells. J Med
Virol. 82:920–928. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bogenrieder T and Herlyn M: Axis of evil:
molecular mechanisms of cancer metastasis. Oncogene. 22:6524–6536.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Roodman GD: Biology of osteoclast
activation in cancer. J Clin Oncol. 19:3562–3571. 2001.PubMed/NCBI
|
|
28
|
Sone S and Yano S: Molecular pathogenesis
and its therapeutic modalities of lung cancer metastasis to bone.
Cancer Metastasis Rev. 26:685–689. 2007. View Article : Google Scholar : PubMed/NCBI
|