Introduction
Lung cancer is the leading cause of cancer-related
mortality in China and worldwide (1); it is a high-grade malignancy with a
poor 5 year-survival rate that remains incurable with current
therapies. The severity of the situation has led researchers to
combinative therapeutic strategies. Deregulation of cell survival
and resistance to apoptosis are regarded as crucial aspects of
tumorigenesis. Several toxic DNA-damaging agents, such as ionizing
radiation (2) and ultraviolet (UV)
radiation (3–5), are associated with cell apoptosis and
have been used for tumor radiation therapy.
The MAPK signal transduction pathway is mainly
responsible for UV-induced cell apoptosis (6). The MAPK family consists of at least
four families that include extracellular signal-regulated kinase
(ERK), c-Jun NH2-terminal kinase (JNK), p38, and ERK5/BMK1. The
MAPK family regulates a wide variety of cellular responses such as
proliferation, differentiation and apoptosis, which could be
activated by various extracellular signals including growth factors
and cellular stress such as UV irradiation, protein synthesis
inhibitors and hydrogen peroxide (7,8). The
p38 MAPK signal pathway has been shown to play a critical role in
the UV relative apoptosis (9). In
mammalian cells, p38 MAPK is strongly activated in response to
stress stimuli ranging from osmotic shock to inflammatory cytokines
to UV and ionizing radiation, resulting in CK2 activation (10,11).
Several studies have demonstrated that CK2 kinase
activity can be stimulated following UV radiation in a
p38-dependent manner (10,12). As one of the important substrates of
p38, CK2 may be activated by p38 MAPK in response to stress,
through a direct protein-protein interaction (13,14).
Significantly, casein kinase 2 (CK2) is frequently overexpressed in
various types of human cancer, including lung cancer (15), and can cause mammary tumors
(16–20) and lymphomas (21). Traditionally, CK2 has been accepted
as a constitutively active, highly conserved and ubiquitous
serine/threonine protein kinase in search of specific physiological
functions (22). However, several
studies have demonstrated that CK2 plays a key role in the
regulation of cell proliferation and apoptosis (23,24).
Based on these data, we inquired about the role of
CK2 in apoptosis induced by UV. Therefore, in the present study, we
inhibited the CK2 activation through CK2a siRNA and CK2 inhibitor
TBB, together with UV radiation, to investigate whether the
combination would enhance the cell apoptosis on two different human
lung cancer cell lines, A549 and H2030, and we explored the
possible underlying mechanism.
Materials and methods
Materials
CK2a siRNA plasmid was purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA; sc-29918).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and 4,5,6,7-tetrabromobenzotriazole (TBB) were purchased from
Sigma. Fetal bovine serum (FBS), Dulbecco’s modified Eagle’s medium
(DMEM), penicillin and 100 mg/ml streptomycin were purchased from
Gibco. The antibodies anti-cytochrome c, anti-caspase-3,
anti-cleaved caspase-3, anti-poly ADP-ribose polymerase (PARP),
anti-PML, anti-p38 and anti-p-p38 were purchased from Santa Cruz
Biotechnology.
Cell culture
The human A549 and H2030 cells were cultured in DMEM
with 10% FBS, 100 U/ml penicillin, and 100 mg/ml streptomycin
(Gibco) under standard culture conditions (37°C and 5%
CO2). The A549 and H2030 cells were irradiated at the
indicated doses with a UV lamp (254 nm).
Cell viability assays
A549 and H2030 cells were cultured in 96-well plates
at a density of 1–1.5×104 cells/well in 150 μl of
complete medium. Each group was repeated in six separate wells. MTT
reagent [15 μl, 5 mg/ml in phosphate-buffered saline (PBS)] was
added to each well for 4 h. After 4 h, each well was dissolved in
150 μl DMSO. Absorbance was recorded at a wavelength of 490 nm.
siRNA transfection
Prior to 12 h of transfection, 1×105
cells were cultured in 6-well plates in normal medium, with a
target of 40–60% confluency at the time of transfection. Cells were
transfected with 50 nmol/l of siRNA using Lipofectamine RNAiMAX
(Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s
protocol. Cells were harvested for western blotting at 48 h
post-transfection.
Western blot analysis
Following treatment, A549 and H2030 cells were
washed with cold PBS twice and then 200 μl radioimmunoprecipitation
(RIPA) buffer [50 mM Tris-HCl (pH 6.8), 0.1% SDS, 150 mM NaCl, 1 mM
EDTA, 0.1 mM Na3VO4,1 mM sodium fluoride
(NaF), 1% Triton X-100, 1% NP-40, 1 mM dithiothreitol, and 1mM
PMSF, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin A]
was added to each dish. Cell lysates were shaken at 4°C for 20 min
and then centrifuged at 13,000 × g for 15 min. Protein
concentrations in the supernatants were detected using the BCA
protein assay. For western blot analysis, 45 μg protein were
separated by 10% (w/v) SDS-polyacrylamide gel electrophoresis and
transferred onto PVDF membranes and were then blocked with 5% (w/v)
skim milk in buffer [10 mM Tris-HCl (pH 7.6), 100 mM NaCl, and 0.1%
(v/v) Tween-20] for 1 h at room temperature; the primary antibodies
were added overnight at 4°C. The following day, membranes were
incubated with secondary antibodies (Thermo Scientific) for 1 h at
room temperature. The semi-quantitation of proteins was surveyed
with a Tanon GIS gel imager system.
Statistical analysis
The data were analyzed by t-test. P<0.05 was
considered to represent a statistically significant difference.
Data are representative of three independent experiments performed
in triplicate.
Results
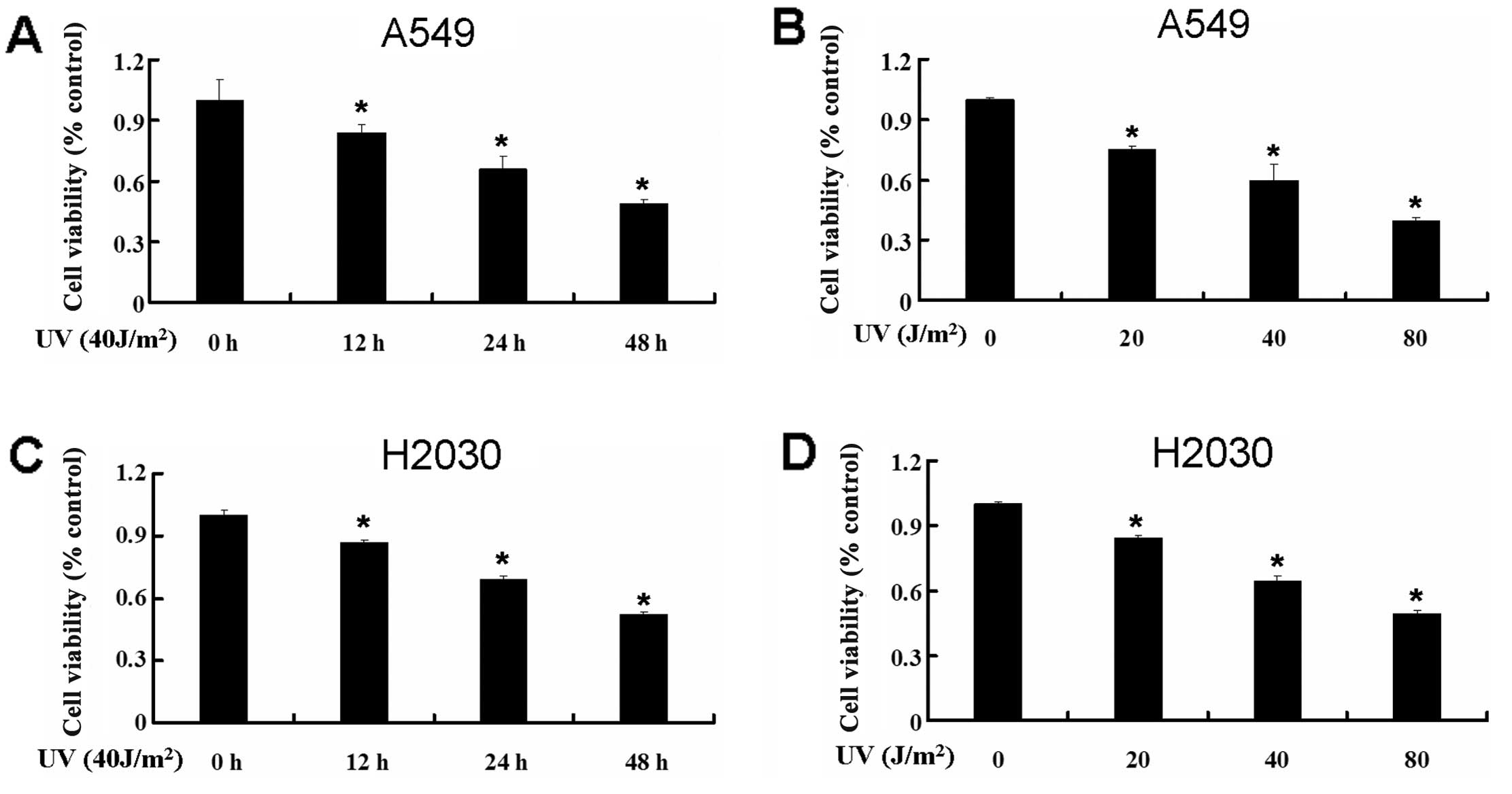
UV decreases the cell viability of A549
and H2030
To examine the effect of UV on the cell viability of
the lung cancer cell lines A549 and H2030, we used MTT to detect
the change of cell viability of the A549 and H2030 cell lines. We
treated A549 and H2030 cells with UV at different times and
intensities. UV decreased cell viability significantly in A549
cells (Fig. 1A and B). The same
phenomenon was observed in H2030 cells (Fig. 1C and D). These results revealed that
UV decreased the cell viability of the lung cancer cell lines A549
and H2030.
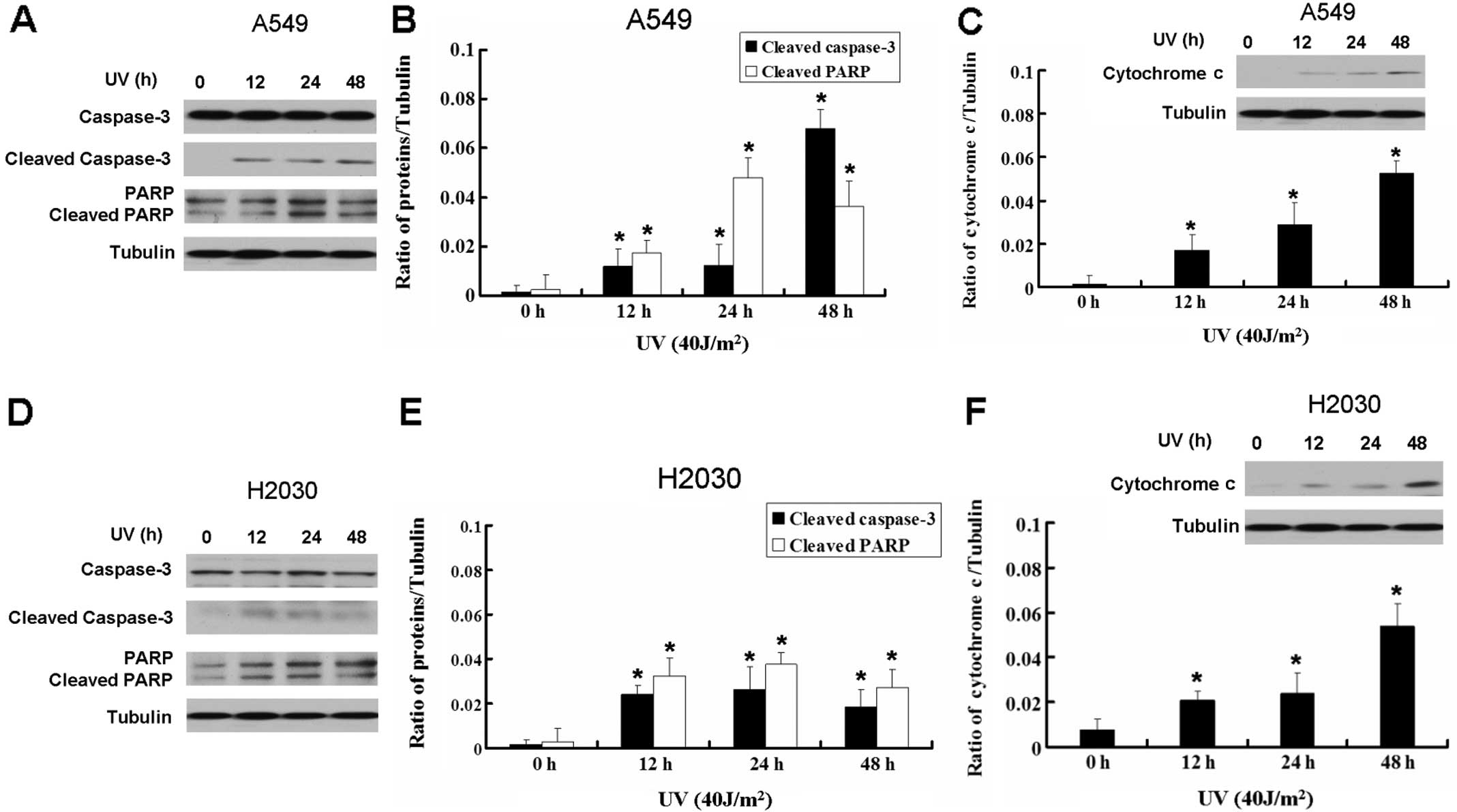
UV induces apoptosis in A549 and H2030
cells
Based on the above results, we inquired whether the
inhibition of A549 and H2030 growth by UV was due to apoptosis. We
detected the apoptotic relative proteins caspase-3 and PARP. UV
increased the expression of cleaved caspase-3 and cleaved PARP in
A549 and H2030 cells, which indicated that UV can induce apoptosis
in the lung cancer cell lines (Fig.
2). Moreover, UV-induced apoptosis in A549 and H2030 cells was
associated with the release of cytochrome c, indicating that
it was mediated via the mitochondrial pathway (Fig. 2C and F). Thus, UV induced lung
cancer cell apoptosis through the mitochondrial apoptotic
pathway.
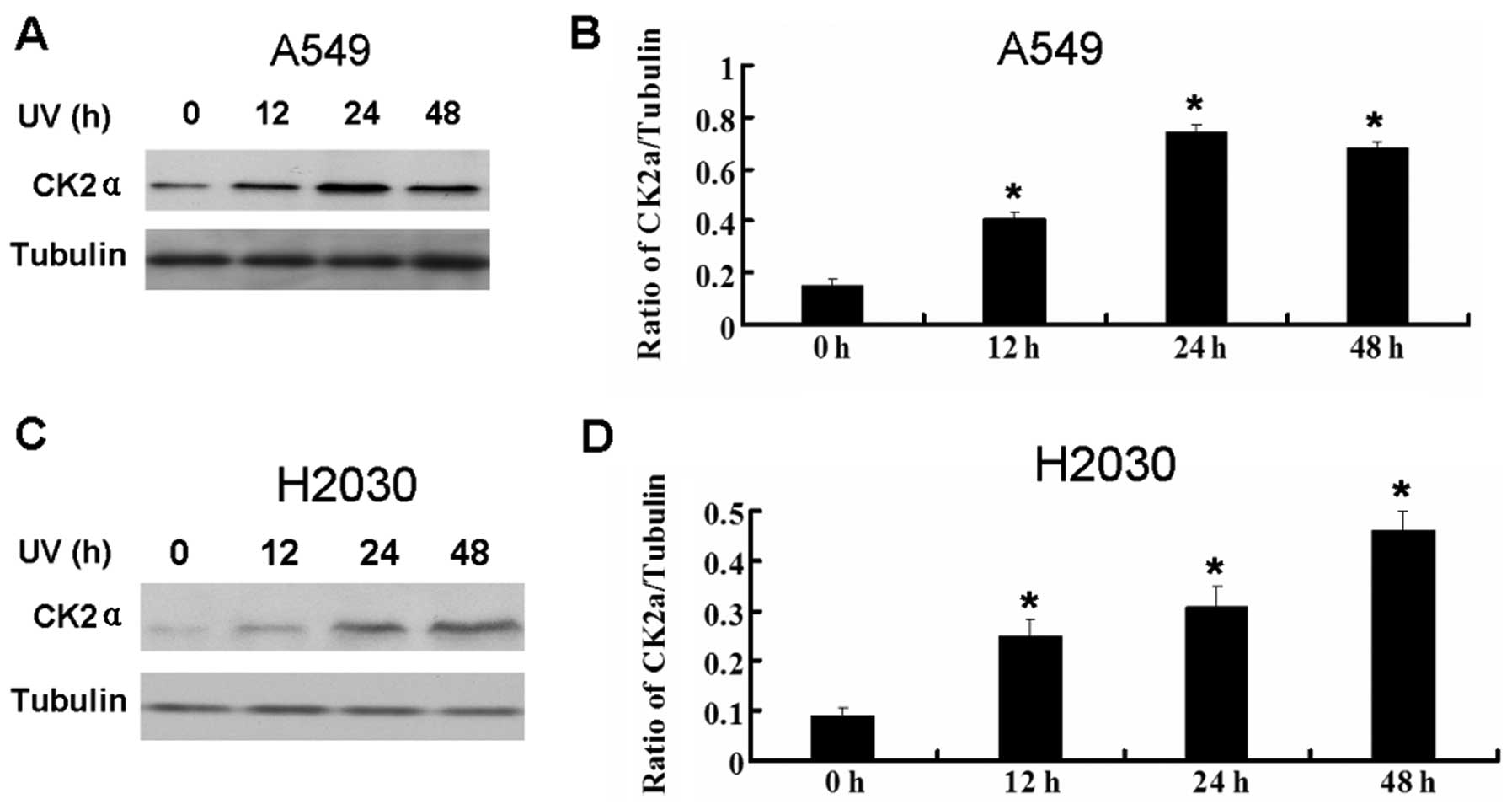
UV enhances the expression of CK2a
It was reported that UV can activate CK2 in tumor
cells (10). We detected the
expression of CK2a in A549 and H2030 cells following UV treatment.
UV also increased the expression of CK2a in the lung cancer cell
lines (Fig. 3).
Inhibition of CK2 increases apoptosis in
A549 and H2030 cells induced by UV
Aside from inducing apoptosis, UV also increases the
expression of CK2a. However, the effect of active CK2 on the growth
inhibition of tumor cells by UV is unknown. Therefore, we next used
the CK2 siRNA and CK2 inhibitor TBB to explore the relationship
between the activation of CK2 and growth inhibition induced by UV.
CK2 siRNA decreased the expression of CK2a (Fig. 4A and B). Inhibition of CK2 increased
the growth inhibition in A549 and H2030 cells induced by UV
(Fig 4C and D).
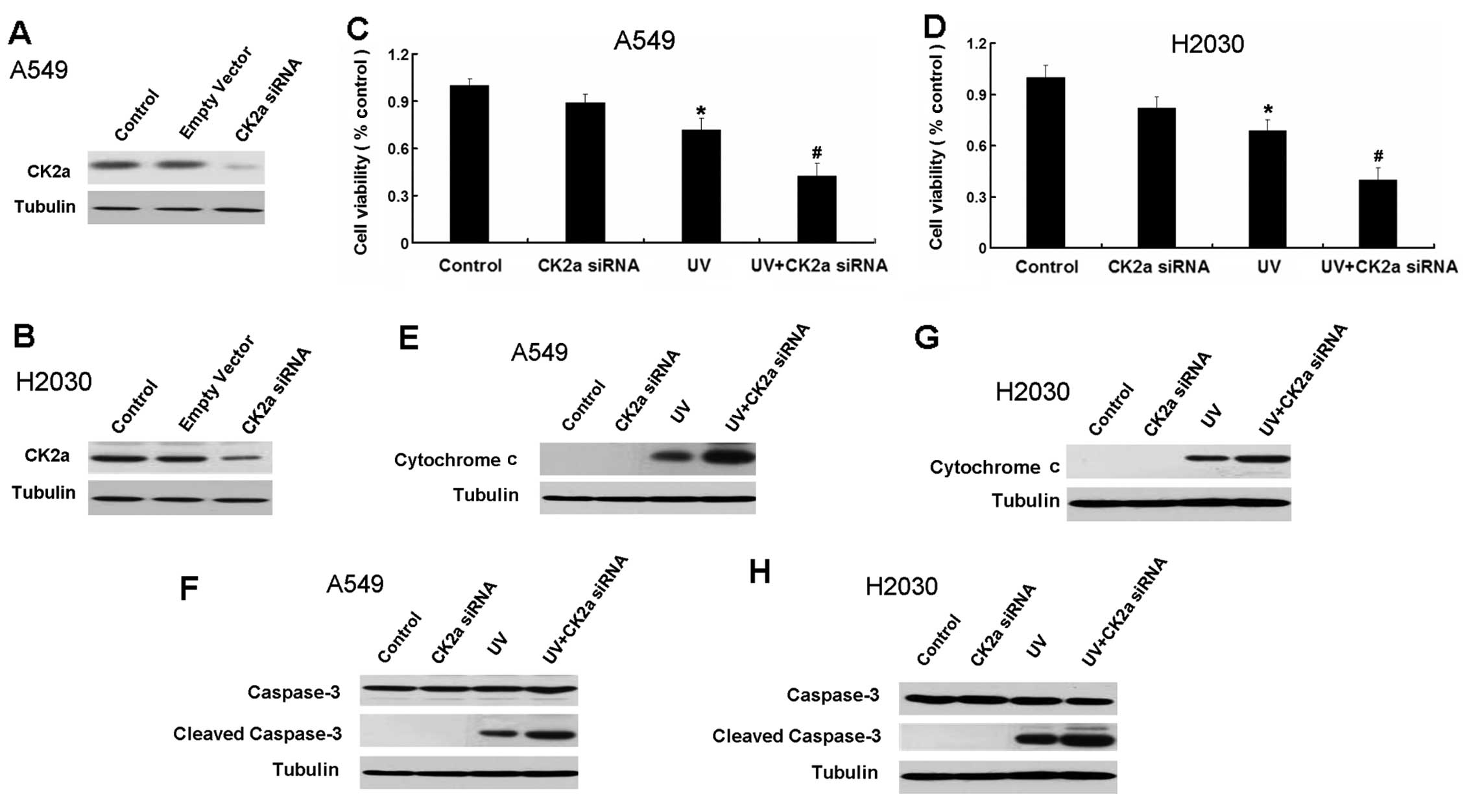 | Figure 4Inhibition of CK2 by CK2a siRNA
increases apoptosis in A549 and H2030 cells induced by UV. (A)
Western blot analysis for the expression of CK2a in A549 cells
transfected with empty vector and CK2a siRNA for 24 h. (B) Western
blot analysis for the expression of CK2a in H2030 cells transfected
with empty vector and CK2a siRNA for 24 h. (C) A549 cells were
treated with CK2a siRNA, UV and a combination of CK2a siRNA and UV
for 24 h. Cell viability was determined by MTT assay. Data are
presented as the means ± SD, n=6. (D) H2030 cells were treated with
CK2a siRNA, UV and a combination of CK2a siRNA and UV for 24 h.
Cell viability was determined by MTT assay. Data are presented as
the means ± SD, n=6. (E) Western blot analysis for the expression
of cytochrome c in A549 cells treated with CK2a siRNA, UV
and a combination of CK2a siRNA and UV for 24 h. (F) Western blot
analysis for the expression of caspase-3, activated caspase-3 in
A549 cells treated with CK2a siRNA, UV and a combination of CK2a
siRNA and UV for 24 h. (G) Western blot analysis for the expression
of cytochrome c in H2030 cells treated with CK2a siRNA, UV
and a combination of CK2a siRNA and UV for 24 h. (H) Western blot
analysis for the expression of caspase-3, activated caspase-3 in
H2030 cells treated with CK2a siRNA, UV and a combination of CK2a
siRNA and UV for 24 h. All UV treatment mentioned in this figure is
40 J/m2. Data are presented as the means ± SD, n=3.
*P<0.05 vs. control group; #P<0.05 vs.
UV group. |
Subsequently, we detected the apoptotic relative
proteins cytochrome c and caspase-3. CK2 siRNA increased the
expression of cytochrome c and cleaved caspase-3 in A549 and
H2030 cells induced by UV (Fig.
4E–H).
We also used the CK2 inhibitor TBB to explore the
role of CK2 in apoptosis in A549 and H2030 cells induced by UV. TBB
also enhanced the growth inhibition induced by UV in A549 and H2030
cells (Fig. 5A and B). UV increased
the expression of the apoptotic relative proteins caspase-3, PARP
and cytochrome c in A549 and H2030 cells (Fig. 5C–H). Thus, inhibition of CK2
increases the apoptosis induced by UV.
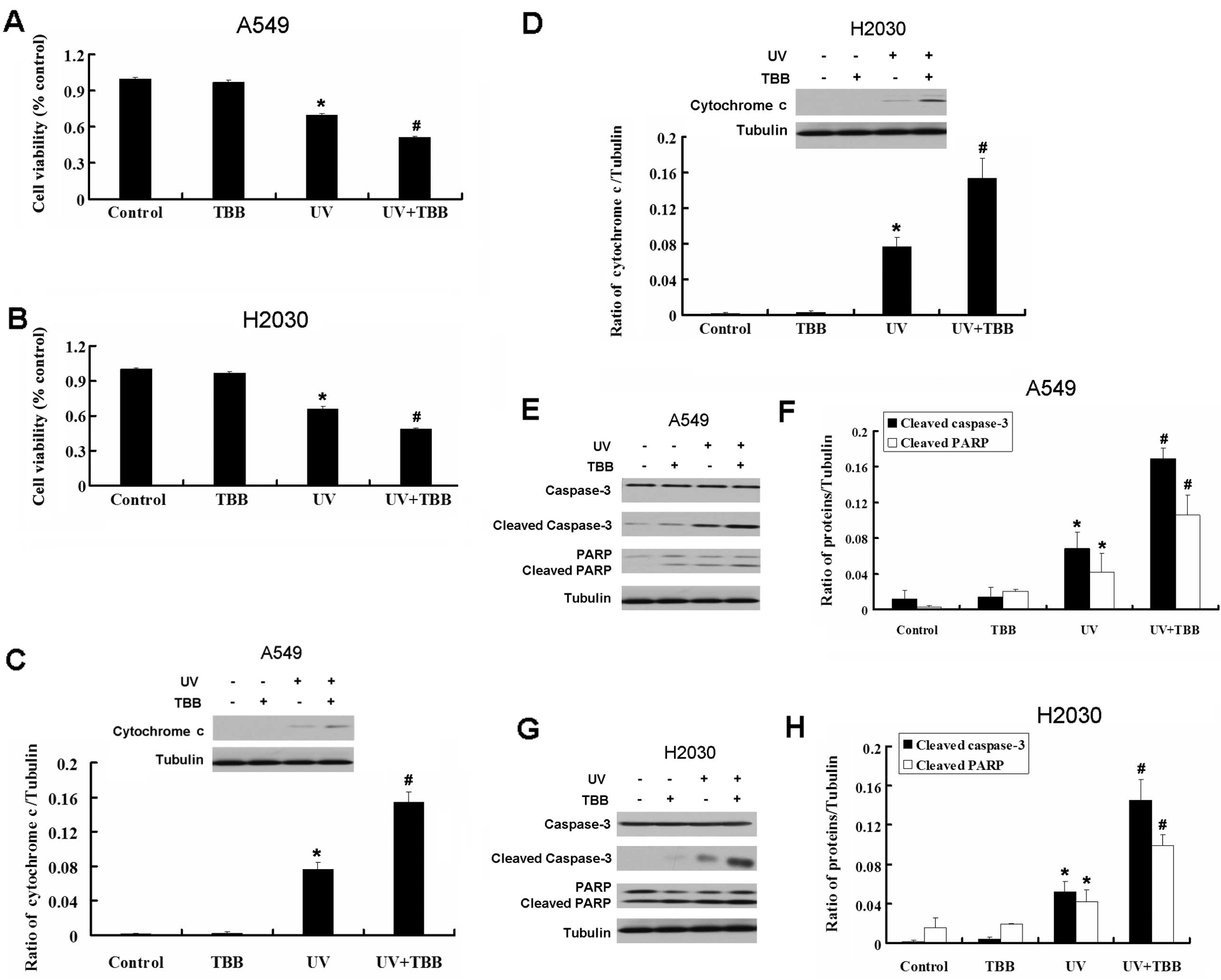 | Figure 5CK2 inhibitor TBB increases apoptosis
in A549 and H2030 cells induced by UV. (A) A549 cells were treated
with TBB (20 μM, 30 min pretreatment), UV and a combination of TBB
and UV for 24 h. Cell viability was determined by MTT assay. Data
are presented as the means ± SD, n=6. (B) H2030 cells were treated
with TBB (20 μM, 30 min pretreatment), UV and a combination of TBB
and UV for 24 h. Cell viability was determined by MTT assay. Data
are presented as the means ± SD, n=6. (C) Western blot analysis for
the expression of cytochrome c in A549 cells treated with
TBB (20 μM, 30 min pretreatment), UV and a combination of TBB and
UV for 24 h. (D) Western blot analysis for the expression of
cytochrome c in H2030 cells treated with TBB (20 μM, 30 min
pretreatment), UV and a combination of TBB and UV for 24 h. (E)
Western blot analysis for the expression of caspase-3, activated
caspase-3 and cleaved PARP in A549 cells treated with TBB (20 μM,
30 min pretreatment), UV and a combination of TBB and UV for 24 h.
(F) Quantitation of activated caspase-3 and cleaved PARP protein
levels. (G) Western blot analysis for the expression of caspase-3,
activated caspase-3 and cleaved PARP in H2030 cells treated with
TBB (20 μM, 30 min pretreatment), UV and a combination of TBB and
UV for 24 h. (H) Quantitation of activated caspase-3 and cleaved
PARP protein levels. All UV treatment mentioned in this figure is
40 J/m2. Data are presented as the means ± SD, n=3,
*P<0.05 vs. control group. #P<0.05 vs.
UV group. |
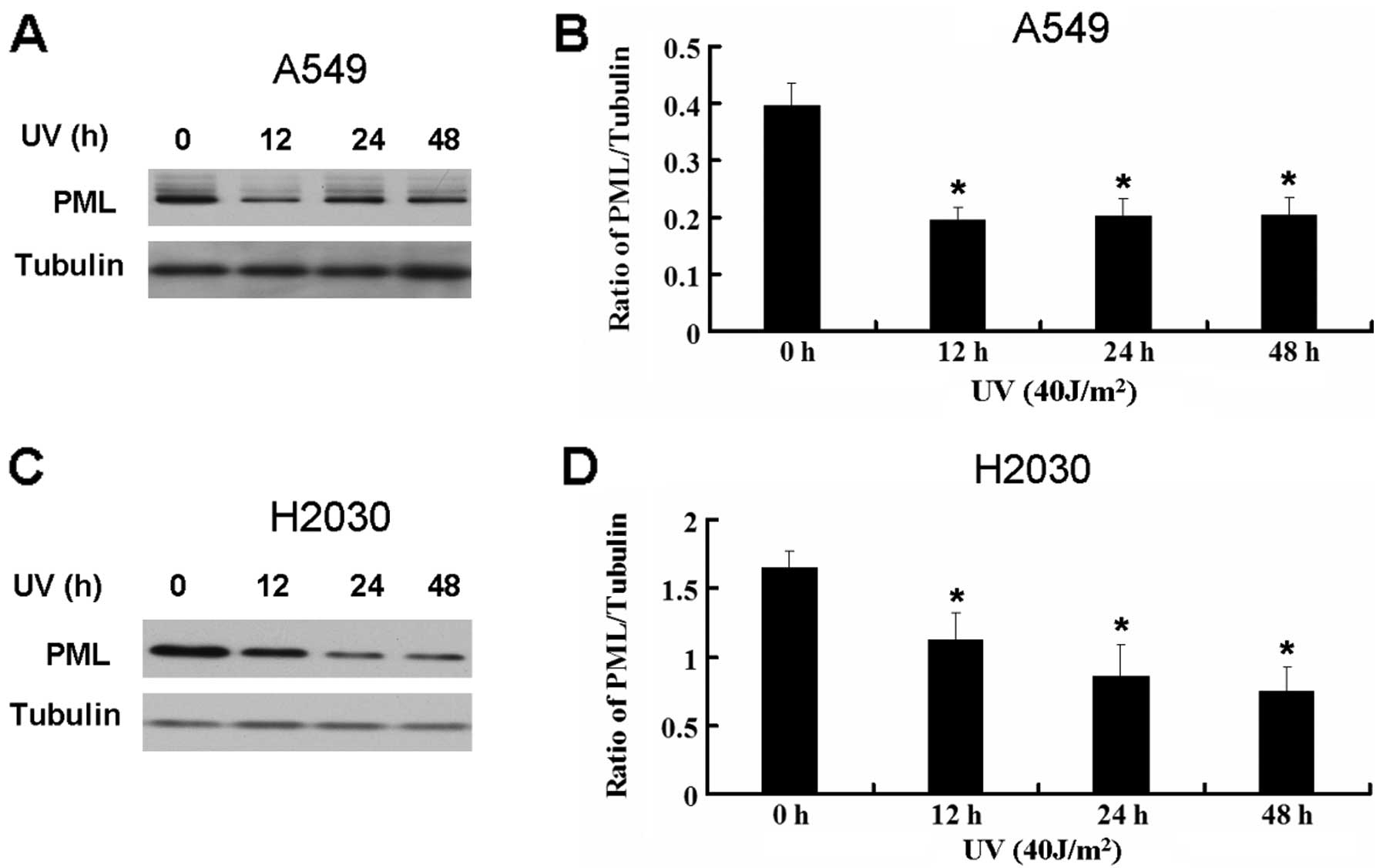
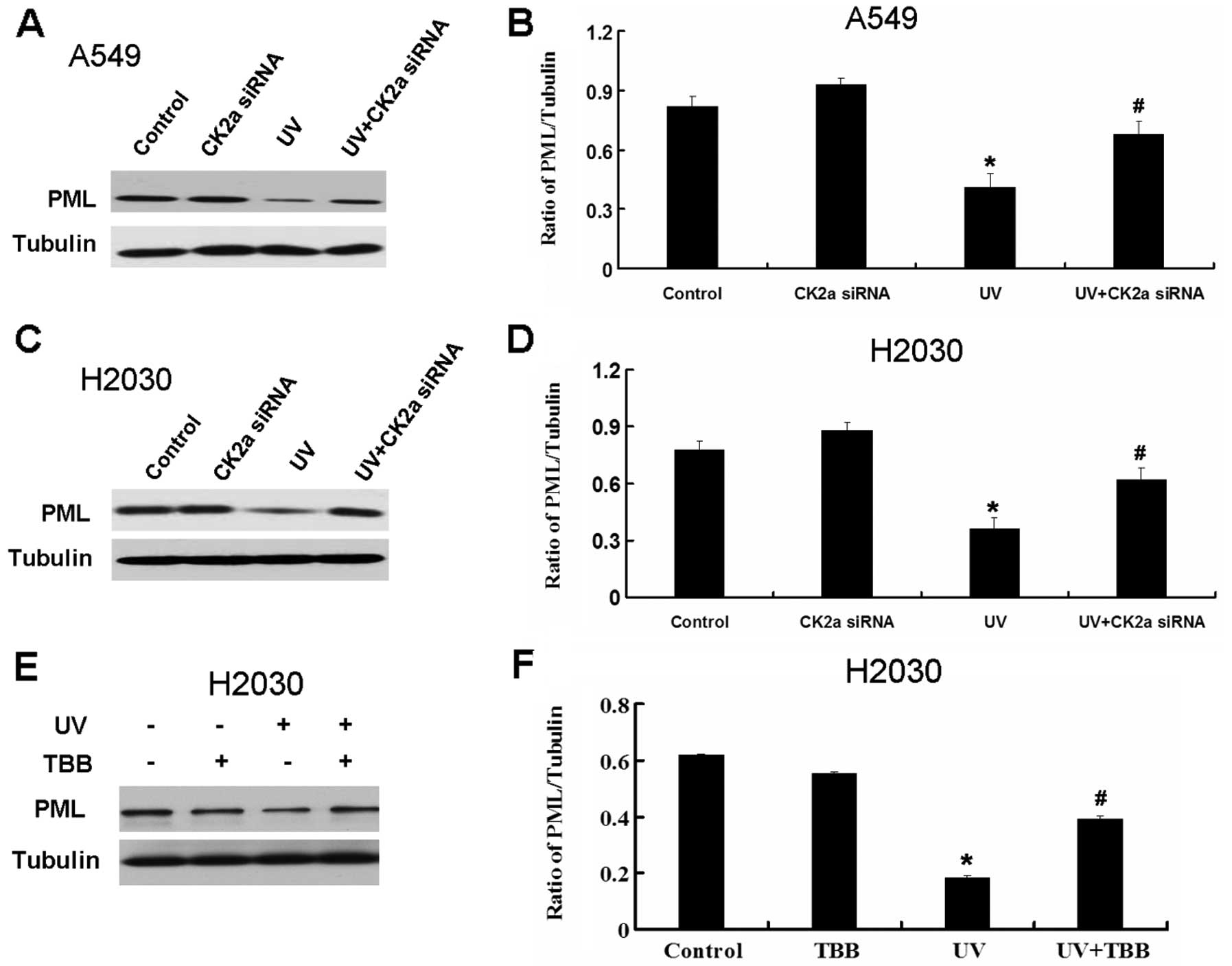
The action of CK2 in the UV-induced
apoptosis may be through the recovery of the PML expression in A549
and H2030 cells
It is reported that active CK2 can promote the
degradation of PML which is a tumor suppressor (2). As seen above, UV activates CK2 in lung
cancer cell lines. Thus, we further detected the expression PML
following UV treatment. UV decreased the expression of PML in A549
and H2030 cells (Fig. 6). Based on
these results, UV may decrease the expression of PML through the
activation of CK2. We next detected the effect of CK2 on the
expression of PML in A549 and H2030 cells treated by UV. CK2 siRNA
and TBB recover the decrease of PML induced by UV (Fig. 7).
UV decreases the expression of PML through
activation of CK2. Inhibition of CK2 by CK2 siRNA and TBB can
recover the decrease of PML induced by UV. Therefore, the
combination of UV and CK2 inhibitor may be an efficient strategy
for the treatment of lung cancer.
Discussion
It is well known that high intensity UV radiation
can lead to skin cancer, while proper intensity of UV radiation has
been used to induce cell apoptosis in cancer therapy and basic
experiment research (25–28). Aside from inducing apoptosis, it is
reported that UV can activate the protein kinase CK2 (10). CK2, a Ser/Thr protein kinase, is
associated with various types of human cancer, including lung
cancer. In this study, we gained insight into the role of CK2
inhibition in cancer cell apoptosis induced by UV.
We first observed the effect of different
irradiation times (from 0 to 48 h) and different exposure doses of
UV radiation (from 0 to 80 J/m2) on two human lung
cancer cell lines, A549 and H2030, via MTT assay. We found that the
cells treated by UV radiation displayed lower cell growth ability
in a time- and dose-dependent manner, compared with the non-treated
group.
Apoptosis frequently involves the activation of
caspase-3, accompanied by cleavage of substrates such as PARP and
the release of cytochrome c from mitochondria. Therefore, we
investigated the cell apoptosis treated by UV by detecting these
apoptotic protein marker expressions by western blotting. The
results showed that the cleavage of caspase-3 PARP expressions and
cytochrome c release levels were upregulated respectively
following UV treatment in a time-dependent manner. In the
subsequent experiment, we chose the UV radiation intensity of 40
J/m2 and in conjunction with the CK2 inhibitor TBB, to
analyze the combination effect on cancer cell apoptosis.
These results showed that UV can induce apoptosis at
the same time as activating CK2 in lung cancer cell lines. However,
the role of CK2 in apoptosis induced by UV is unknown. We used CK2
siRNA and CK2 inhibitor to inhibit CK2 activation in lung cancer
cell lines. Compared with the single-treatment group (UV radiation
group), the MTT assay showed that the combination of UV and CK2a
siRNA displayed the lowest viability. To further clarify the
molecular events underlying apoptosis, alterations of several
executive components in apoptotic machinery were examined. One of
them is cytochrome c release. The results showed that
cytochrome c release was increased heavily in the
combination treatment group compared with that in each
single-treatment group and the non-treated group. Consistent with
these results, the cleaved caspase-3 also increased markedly in the
co-treatment group compared with that in other groups.
The observation that TBB, a very selective
cell-permeant inhibitor of protein kinase CK2 (29), displays a striking selectivity for
this enzyme only amid a panel of more than 30 protein kinases,
provided a new tool for investigating the biological functions of
this pleiotropic and, in some respects, still enigmatic kinase. In
this study, we further used the CK2 inhibitor TBB to explore the
role of CK2 in apoptosis induced by UV. Similar to CK2 siRNA, the
CK2 inhibitor TBB also enhanced the growth inhibition by UV in the
lung cancer cell lines A549 and H2030. Moreover, the combination of
UV and TBB increased the apoptotic relative proteins caspase-3,
PARP and cytochrome c in A549 and H2030 cells
A previous study defined the negative relationship
between CK2 and PML, a tumor suppressor protein which is capable of
inducing growth and apoptosis (30). When CK2 kinase activity is
upregulated (as often happens in human cancer), PML is
polyubiquitinylated and degraded (31). Scaglioni et al(15) demonstrated that CK2, a kinase
associated with cancer promotion, phosphorylates PML and targets it
for degradation by the proteasome. Loss of the critical CK2
phosphorylation site in PML results in stabilization of this
protein, enhancement of PML-induced apoptosis and senescence, and
abrogation of sensitivity to CK2 inhibitors. In conditions of
oncogenic stress, such as the ones triggered by oncogenic Ras, PML
is activated and exerts its tumor suppressive function. PML has
been the focus of extensive investigations due to its multiple
tumor-suppressive functions and its ability to regulate key
tumor-suppressive pathways (32,33).
PML degradation upon CK2 activation could account for the frequent
loss of PML expression observed in multiple human tumors (2). Our results showed that the tumor
suppressor protein PML expression was decreased in the UV group,
while use of CK2a siRNA or TBB significantly recovered the
expression of PML.
Collectively, our results revealed that the
alterations of diverse apoptotic factors, including cytochrome
c, caspase-3 and PARP, may contribute to the enhancement of
apoptosis in human lung cancer cells via combination treatment of
CK2a siRNA or TBB with UV radiation. The recovered PML expression
by CK2 inhibitor TBB is part of a cellular circuitry that enhances
apoptosis. Further studies including generating a lung cancer cell
xenograft mouse model should be conducted to confirm our results.
We therefore propose that therapy with specific CK2 inhibitors,
such as TBB, combined with radiation or other DNA-damaging agents
may generate effective results as an anticancer therapy tool.
References
|
1
|
Greenlee RT, Hill-Harmon MB, Murray T and
Thun M: Cancer statistics, 2001. CA Cancer J Clin. 51:15–36. 2001.
View Article : Google Scholar
|
|
2
|
Riley PA: Free radicals in biology:
oxidative stress and the effects of ionizing radiation. Int J
Radiat Biol. 65:27–33. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lentine B, Antonucci L, Hunce R, Edwards
J, Marallano V and Krucher NA: Dephosphorylation of threonine-821
of the retinoblastoma tumor suppressor protein (Rb) is required for
apoptosis induced by UV and Cdk inhibition. Cell Cycle.
11:3324–3330. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Choi YJ, Kim YJ, Lee JW, Lee Y, Lim YB and
Chung HW: Cyto-/genotoxic effect of CdSe/ZnS quantum dots in human
lung adenocarcinoma cells for potential photodynamic UV therapy
applications. J Nanosci Nanotechnol. 12:2160–2168. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang J, Zhang L, Liu W, et al: CCDC134
interacts with hADA2a and functions as a regulator of hADA2a in
acetyltransferase activity, DNA damage-induced apoptosis and cell
cycle arrest. Histochem Cell Biol. 138:41–55. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cao C, Lu S, Kivlin R, et al:
AMP-activated protein kinase contributes to UV- and
H2O2-induced apoptosis in human skin
keratinocytes. J Biol Chem. 283:28897–28908. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kyriakis JM and Avruch J: Protein kinase
cascades activated by stress and inflammatory cytokines. Bioessays.
18:567–577. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK, and
p38 protein kinases. Science. 298:1911–1912. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Seo M, Cho CH, Lee YI, et al:
Cdc42-dependent mediation of UV-induced p38 activation by G protein
betagamma subunits. J Biol Chem. 279:17366–17375. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kato T Jr, Delhase M, Hoffmann A and Karin
M: CK2 is a C-terminal IkappaB kinase responsible for NF-kappaB
activation during the UV response. Mol Cell. 12:829–839. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Roux PP and Blenis J: ERK and p38
MAPK-activated protein kinases: a family of protein kinases with
diverse biological functions. Microbiol Mol Biol Rev. 68:320–344.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Keller DM, Zeng X, Wang Y, et al: A DNA
damage-induced p53 serine 392 kinase complex contains CK2, hSpt16,
and SSRP1. Mol Cell. 7:283–292. 2001. View Article : Google Scholar
|
|
13
|
Jacks KA and Koch CA: Differential
regulation of mitogen- and stress-activated protein kinase-1 and -2
(MSK1 and MSK2) by CK2 following UV radiation. J Biol Chem.
285:1661–1670. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sayed M, Kim SO, Salh BS, Issinger OG and
Pelech SL: Stress-induced activation of protein kinase CK2 by
direct interaction with p38 mitogen-activated protein kinase. J
Biol Chem. 275:16569–16573. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Scaglioni PP, Yung TM, Cai LF, et al: A
CK2-dependent mechanism for degradation of the PML tumor
suppressor. Cell. 126:269–283. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Landesman-Bollag E, Song DH, Romieu-Mourez
R, et al: Protein kinase CK2: signaling and tumorigenesis in the
mammary gland. Mol Cell Biochem. 227:153–165. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Giroux V, Dagorn JC and Iovanna JL: A
review of kinases implicated in pancreatic cancer. Pancreatology.
9:738–754. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ruzzene M and Pinna LA: Addiction to
protein kinase CK2: a common denominator of diverse cancer cells?
Biochim Biophys Acta. 1804:499–504. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Trembley JH, Wang G, Unger G, Slaton J and
Ahmed K: Protein kinase CK2 in health and disease: CK2: a key
player in cancer biology. Cell Mol Life Sci. 66:1858–1867. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ahmed K, Davis AT, Wang H, et al:
Significance of protein kinase CK2 nuclear signaling in neoplasia.
J Cell Biochem (Suppl). 35:130–135. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Piazza F, Manni S, Ruzzene M, et al:
Protein kinase CK2 in hematologic malignancies: reliance on a
pivotal cell survival regulator by oncogenic signaling pathways.
Leukemia. 26:1174–1179. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pinna LA: Protein kinase CK2: a challenge
to canons. J Cell Sci. 115:3873–3878. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ahmed K, Gerber DA and Cochet C: Joining
the cell survival squad: an emerging role for protein kinase CK2.
Trends Cell Biol. 12:226–230. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Manni S, Brancalion A, Tubi LQ, et al:
Protein kinase CK2 protects multiple myeloma cells from ER
stress-induced apoptosis and from the cytotoxic effect of HSP90
inhibition through regulation of the unfolded protein response.
Clin Cancer Res. 18:1888–1900. 2012. View Article : Google Scholar
|
|
25
|
Pustisek N and Situm M: UV-radiation,
apoptosis and skin. Coll Antropol. 35(Suppl 2): 339–341. 2011.
|
|
26
|
Zandi S, Kalia S and Lui H: UVA1
phototherapy: a concise and practical review. Skin Therapy Lett.
17:1–4. 2012.PubMed/NCBI
|
|
27
|
Tomas D: Apoptosis, UV-radiation,
precancerosis and skin tumors. Acta Med Croatica. 63(Suppl 2):
53–58. 2009.(In Croatian).
|
|
28
|
Lee WR, Shen SC, Lin HY, et al: Wogonin
and fisetin induce apoptosis in human promyeloleukemic cells,
accompanied by a decrease of reactive oxygen species, and
activation of caspase 3 and Ca(2+)-dependent endonuclease. Biochem
Pharmacol. 63:225–236. 2002.PubMed/NCBI
|
|
29
|
Sarno S, Reddy H, Meggio F, et al:
Selectivity of 4,5,6,7-tetrabromobenzotriazole, an ATP
site-directed inhibitor of protein kinase CK2 (‘casein kinase-2’).
FEBS Lett. 496:44–48. 2001.
|
|
30
|
Bernardi R and Pandolfi PP: Structure,
dynamics and functions of promyelocytic leukaemia nuclear bodies.
Nat Rev Mol Cell Biol. 8:1006–1016. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Scaglioni PP, Yung TM, Choi S, Baldini C,
et al: CK2 mediates phosphorylation and ubiquitin-mediated
degradation of the PML tumor suppressor. Mol Cell Biochem.
316:149–154. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
de Stanchina E, Querido E, Narita M, et
al: PML is a direct p53 target that modulates p53 effector
functions. Mol Cell. 13:523–535. 2004.PubMed/NCBI
|
|
33
|
Lavau C, Marchio A, Fagioli M, et al: The
acute promyelocytic leukaemia-associated PML gene is induced by
interferon. Oncogene. 11:871–876. 1995.PubMed/NCBI
|