Introduction
Gastric cancer is one of the most common
malignancies throughout the world (1). Gastric cancer is difficult to be
diagnosed at an early stage and is insensitive to chemotherapy and
radiotherapy. Therefore, the prognosis of gastric cancer is poor
(2). Mechanisms underlying the
carcinogenesis and progression of gastric cancer are still poorly
understood. The development of human gastric cancer is a multistep
process which involves host-environment interactions among which
environmental contaminants such as polycyclic aromatic hydrocarbon
(PAH) and halogenated aromatic hydrocarbon (HAH) play important
roles (3). Research concerning the
molecular toxicology of these environmental pollutants has led to
the identification of an important transcription factor, aryl
hydrocarbon receptor (AhR), which mediates the toxicity of PAH and
HAH (4,5).
Aryl hydrocarbon receptor (AhR) is a
ligand-activated transcription factor. Upon binding to a ligand,
AhR translocates into the nucleus and dimerizes with the AhR
nuclear translocator (6). This
complex binds to the specific DNA region and thereby activates a
battery of gene expression. Traditional research concerning its
function has focused on the transcriptional regulation of genes
encoding xenobiotic metabolizing enzymes such as cytochrome P450
enzymes (7). A previous study found
that AhR has an important function in controlling the balance among
processes involved in cell proliferation, death and
differentiation, which contribute to events such as tumor
initiation, promotion and progression (8). Activation of the AhR by high-affinity
HAH or PAH ligands such as
2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and
benzo[a]pyrene results in a wide range of cell cycle perturbations,
including G0/G1 and G2/M cell cycle arrest, diminished capacity for
DNA replication, and inhibition of cell proliferation (9). Yet, in the absence of exogenous
ligands, the ability of this receptor to promote or to inhibit cell
proliferation depends on the phenotype of the cell. In MCF-7 human
breast cancer cells, AhR siRNAs were found to promote the G1/S
transition of the cell cycle and cell proliferation, suggesting a
growth inhibitory role of the receptor. In contrast, in HepG2 human
hepatoma cells, AhR siRNAs blocked the G1/S transition of the cell
cycle and downregulated cyclin D1, cyclin E and CDK-2/4, thus
revealing a growth promoting activity of the receptor (10). Therefore, it appears that cell
phenotype is a critical parameter in determining whether AhR
promotes (oncogenic) or inhibits (tumor suppressor) cell growth and
proliferation. AhR also has dual effects on the regulation of cell
migration and invasion, two processes highly important in the
development of cancer. TCDD-activated AhR pathway in urothelial
carcinoma T24 cells enhanced T24 cell invasion associated with the
upregulation of matrix metalloproteinase (MMP)-1 and MMP-9
(11), while in breast cancer MCF-7
cells, TCDD specifically inhibited the cell migration CXCL12
(12). Therefore, the role of AhR
in cancer invasion is cell-specific.
Our previous study found that AhR expression was
significantly increased in gastric cancer tissues and gastric
cancer cell lines (13).
Furthermore, activation of the AhR pathway enhanced the
invasiveness of gastric cancer cells (14). Yet, the role of AhR in gastric
cancer initiation and progression is still unclear. In the present
study, we investigated the effects of the inhibition of AhR
expression by RNA interference on the biological behavior of
gastric cancer cells, and further clarified the specific mechanisms
of AhR in the development of gastric cancer.
Materials and methods
Cell culture and transfection
The human gastric cancer cell line SGC7901 was
obtained from the Cancer Institute of Chinese Academy of Medical
Science (Shanghai, China). Another human gastric cancer cell line,
MKN45, was purchased from the Riken Cell Bank (Tsukuba, Japan). All
cells were grown in RMPI-1640 (Gibco) medium supplemented with 10%
fetal bovine serum (HyClone, Logan, UT, USA), penicillin G (100
U/ml) and streptomycin (100 μg/ml). Cells were maintained in a
monolayer culture at 37°C in humidified air with 5%
CO2.
In order to knock down AhR expression for subsequent
experiments, SGC7901 and MKN45 cells (30–50% confluence) were
transfected with AhR-siRNA and control siRNA for the indicated time
using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according
to the manufacturer’s instructions. siRNA pools targeting AhR,
containing 4 selected siRNA duplexes each with a modification
pattern that addresses off-target effects caused by both strands
(ON-TARGETplus SMART pool) and the non-targeting control pool
(ON-TARGETplus siCONTROL non-targeting pool) were purchased from
Dharmacon (Lafayette, CO, USA) and used at 100 nM. The experiments
involving cells were repeated at least three times.
RT-PCR
Twenty-four hours after transfection, cells were
collected, and total RNA was extracted using the RNeasy Mini kit
(Qiagen) and reverse-transcribed into cDNA with a
reverse-transcription kit (Toyobo Co., Ltd., Osaka, Japan)
according to the manufacturer’s instructions. The primer sequences
were as follows: AhR, 5′-ACT CCA CTT CAG CCA CCA TC-3′ (forward)
and 5′-ATG GGA CTC GGC ACA ATA AA-3′ (reverse); CYP1A1, 5′-CCA TGT
CGG CCA CGG AGT T-3′ (forward) and 5′-ACA GTG CCA GGT GCG GGT T-3′
(reverse); CYP1B1, 5′-AAC GTC ATG AGT GCC GTG TGT-3′ (forward) and
5′-GGC CGG TAC GTT CTC CAA ATC-3′ (reverse);
glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5′-GGG AAA CTG
TGG CGT GAT-3′ (forward) and 5′-AAA GGT GGA GGA GTG GGT-3′
(reverse). PCR conditions were the following: 95°C for 5 min, 30
cycles of 94°C for 30 sec, 55-57°C (depending on the primer set)
for 30 sec, 72°C for 30 sec and 72°C for 7 min. The resultant PCR
products were 204 bp (AhR), 174 bp (CYP1A1), 360 bp (CYP1B1) and
309 bp (GAPDH). PCR products were detected by agarose
electrophoresis.
Western blotting
Seventy-two hours after transfection, cells were
lysed in RIPA buffer (50 mM Tris pH 7.5, 150 mM NaCl, 1% NP-40 and
0.5% deoxycholic acid, sodium salt) supplemented with complete
protease inhibitor cocktail (Roche). Protein concentration of each
sample was assayed using BCA protein assay reagent according to the
manufacturer’s instructions (Pierce Biotechnology, Inc., Rockford,
IL, USA). Proteins of different groups (20 μg) were separated in
10% SDS-PAGE and transferred onto PVDF membranes. After blocking
with 5% non-fat dry milk in TBST buffer (10 mM Tris, pH 7.5, 150 mM
NaCl and 0.05% Tween-20) for 1 h at room temperature with
agitation, the membranes were then incubated with primary antibody
against AhR (SC-5579; Santa Cruz Biotechnology; working dilution
1:150), CYP1A1 (AB1258; Chemicon International; working dilution
1:500), CYP1B1 (ab32649; Abcam; working dilution 1:1000) or GAPDH
(#2118; Cell Signaling Technology, Beverly, MA, USA; working
dilution 1:1000) overnight at 4°C with agitation. After being
washed with 0.1% Tween-20 in Tris-saline, the membranes were
incubated with HRP-conjugated secondary antibody (#7074; Cell
Signaling Technology; working dilution 1:2,000) for 1 h at room
temperature with agitation. Reactive protein was detected using an
ECL chemiluminescence system (Pierce Biotechnology).
Proliferation assay
Cell viability was determined by the 3-(4,
5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. Briefly, a total of 5×103 trypsin-dispersed cells
in 0.1 ml of culture medium were seeded into each well of a 96-well
plate and cultured for 24, 48 or 72 h after siRNA transfection, and
20 μl of MTT (5 g/l in PBS; Sigma) was added to each well and
incubated for another 4 h. Subsequently, the supernatant was
removed and 150 μl of dimethyl sulfoxide (DMSO) was added to each
well. Finally, plates were read on an enzyme-linked immunity
implement (Bio-Rad 2550; Bio-Rad Laboratories Hercules, CA, USA) at
a wavelength of 490 nm.
Flow cytometry
Apoptosis and cell cycle distribution were analyzed
by flow cytometry. After transfection for 72 h, cells were
harvested and fixed with 70% ethanol overnight at 4°C. The cells
were then centrifuged and stained with propidium iodide (PI) (50
mg/l, Sigma) for 30 min at room temperature. The apoptotic rate and
cell cycle distribution were determined using a FACS Calibur flow
cytometer (Becton-Dickinson, San Jose, CA, USA). Data were analyzed
using CellQuest software (Becton-Dickinson).
Invasion and migration assays
We chose SGC7901 cells to perform the invasion and
migration assays. The cell invasion assay was performed using a
24-well Transwell chamber (Corning Inc., Corning, NY, USA) coated
with Matrigel (50 μl/well; BD Biosciences, Bedford, MA, USA).
Forty-eight hours after siRNA transfection, 1×105 cells
with serum-free medium were seeded into the upper chamber. Medium
supplemented with 10% fetal bovine serum was placed in the lower
chambers as chemoattractants. After 24 h of incubation, the cells
on the upper membrane surface were removed with a cotton swab, and
cells which invaded through the Matrigel and attached to the lower
membrane surface were fixed with 90% ethanol, stained with 0.5%
crystal violet and counted under a light microscope by randomly
selecting five fields per filter (magnification, ×200). Each
experiment was carried out in triplicate wells and repeated at
least three times. Cell migration assay was performed according to
the protocol described above, except that the cells were seeded
onto an uncoated filter.
Quantitative real-time RT-PCR
analysis
The PCR reactions were performed in a SYBR-Green
QPCR Master Mix (Takara, Dalian, China) according to the
manufacturer’s instructions. After 10 min at 95°C to denature the
cDNA, the cycling conditions were 95°C for 30 sec, 55–57°C
(depending on the primer set) for 30 sec and 72°C for 1 min with 40
cycles. The LightCycler software was used to construct the
calibration curve by plotting the crossing point (Cp), and the
numbers of copies in unknown samples were calculated by comparison
of their Cps with the calibration curve. To correct differences in
both RNA quality and quantity between samples, the data were
normalized to those for GAPDH.
The primer sequences were as follows: MMP-2 forward,
5′-GGA TGA CAT CAA GGG CAT TC-3′ and reverse, 5′-GTC ACA GTC CGC
CAA ATG AAC C-3′ (189 bp); MMP-9 forward, 5′-CAA GTG GGC TAC GTG
ACC TAT GAC-3′ and reverse, 5′-CCC TTT CCT CCA GAA CAG AAT ACC-3′
(156 bp); GAPDH forward, 5′-GCA CCG TCA AGG CTG AGA AC-3′ and
reverse, 5′-TGG TGA AGA CGC CAG TGG A-3′ (138 bp).
Gelatin zymography assay
After transfection with AhR siRNA or control siRNA
for 48 h, SGC7901 cells were continuously incubated in serum-free
RPMI-1640 medium at 37°C for 36 h. The conditioned media were then
collected and centrifuged to remove cells and debris, and the
protein concentrations were determined using BCA protein assay
reagent. Equal amounts of protein (20 μg) were mixed with SDS
sample buffer without reducing agents. For gelatinolytic activity
of MMP-2 and MMP-9, the assay samples were separated on 10%
polyacrylamide gels containing 1 mg/ml gelatin (type A, Sigma).
PAGE gels were run at 120 V, washed in 2.5% Triton X-100 for 1 h,
and then incubated for 20 h at 37°C in activation buffer (50 mM
Tris-HCl, pH 7.5, 5 mM CaCl2, 0.02% Brij-35). After
staining with Coomassie Blue (10% glacial acetic acid, 30% methanol
and 0.5% Coomassie Blue) for 3 h, the gel was destained with a
solution of 10% glacial acetic acid and 50% methanol without
Coomassie Blue for 1 h. White lysis zones indicating gelatin
degradation were revealed by staining with Coomassie Blue
R-250.
Statistical analysis
Data values are expressed as mean expression levels
(± SD). All statistical analyses were carried out using the SPSS
statistical software package (version 11.0, SPSS Inc., Chicago, IL,
USA). Student’s t-test was used for statistical analysis. A P-value
<0.05 was taken as the level of statistical significance
(two-sided).
Results
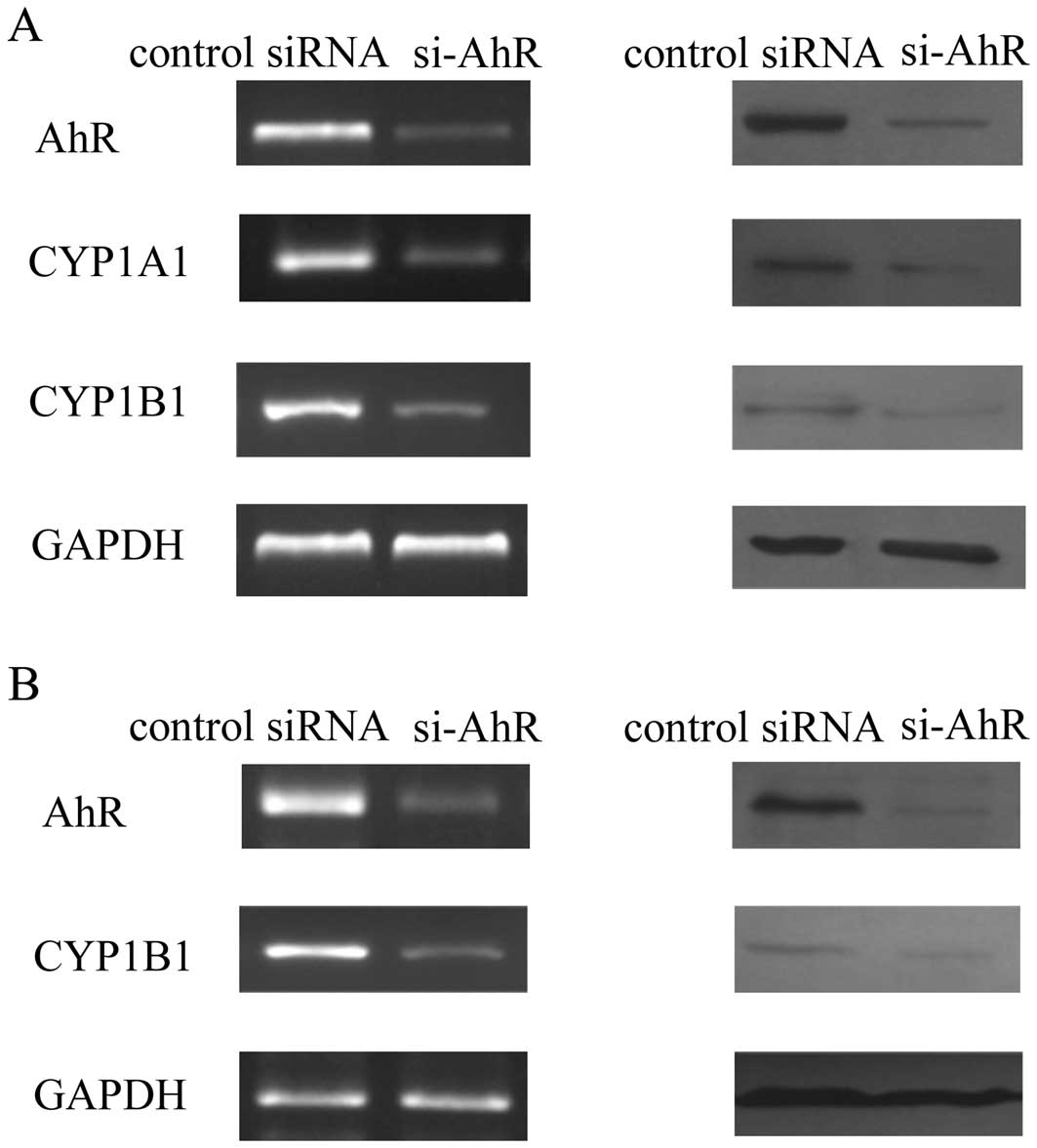
Effect of AhR siRNA on the AhR
pathway
Compared with the cells transfected with
negative-control siRNA, transfection with AhR siRNA significantly
decreased the mRNA and protein levels of AhR in MKN45 and SGC7901
cells. AhR expression was inhibited in the AhR siRNA-transfected
cells at the mRNA and protein levels (Fig. 1). In MKN45 cells, AhR siRNA
downregulated the mRNA and protein expression of CYP1A1 and CYP1B1,
which are two classic target genes of the AhR pathway (15). As the baseline level of CYP1A1
expression was not observed in SGC7901 cells, we only found that
AhR siRNA decreased the expression of CYP1B1. These results suggest
that these AhR siRNAs successfully inhibited AhR expression and the
AhR pathway.
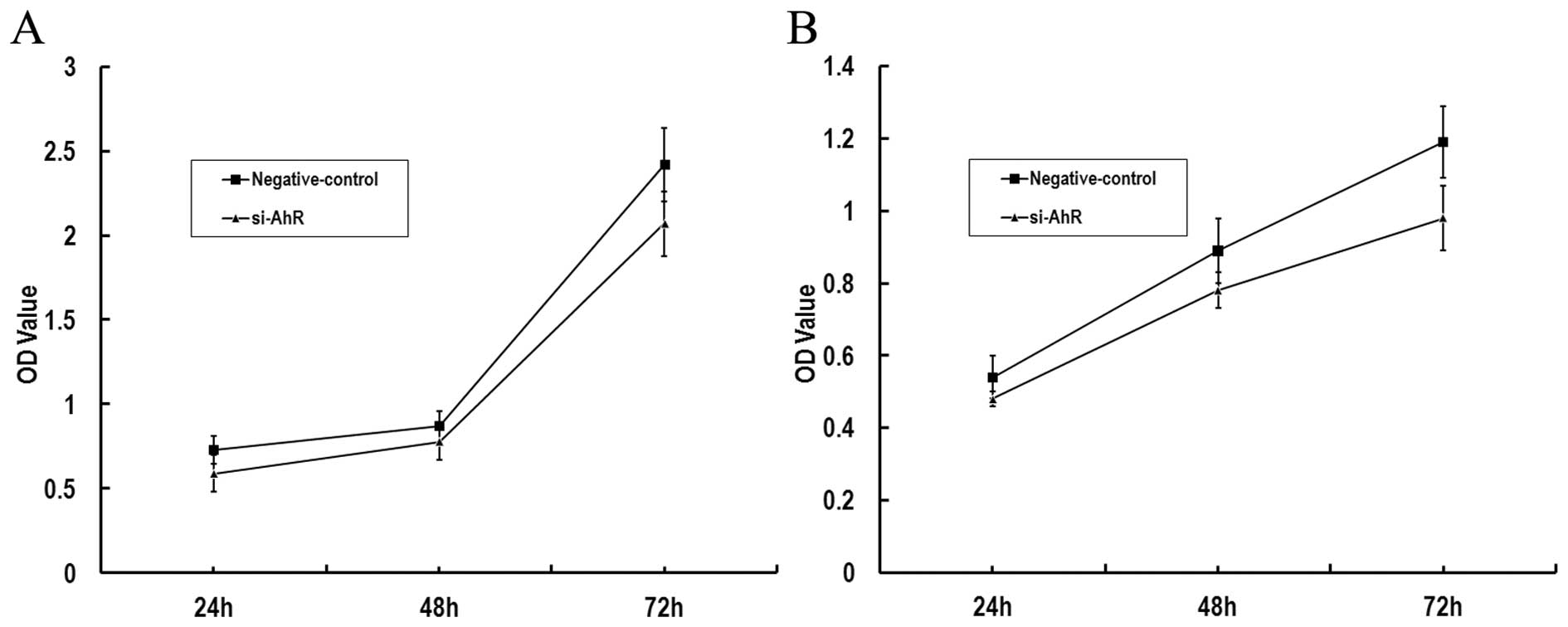
Effect of AhR siRNA on gastric cancer
cell proliferation
We investigated whether AhR siRNA decreases the
survival of gastric cancer cells by MTT assay. The results showed
that MKN45 and SGC7901 cells transfected with AhR siRNA survived at
decreased rates relative to matched cells transfected with a
non-targeting control siRNA (Fig.
2).
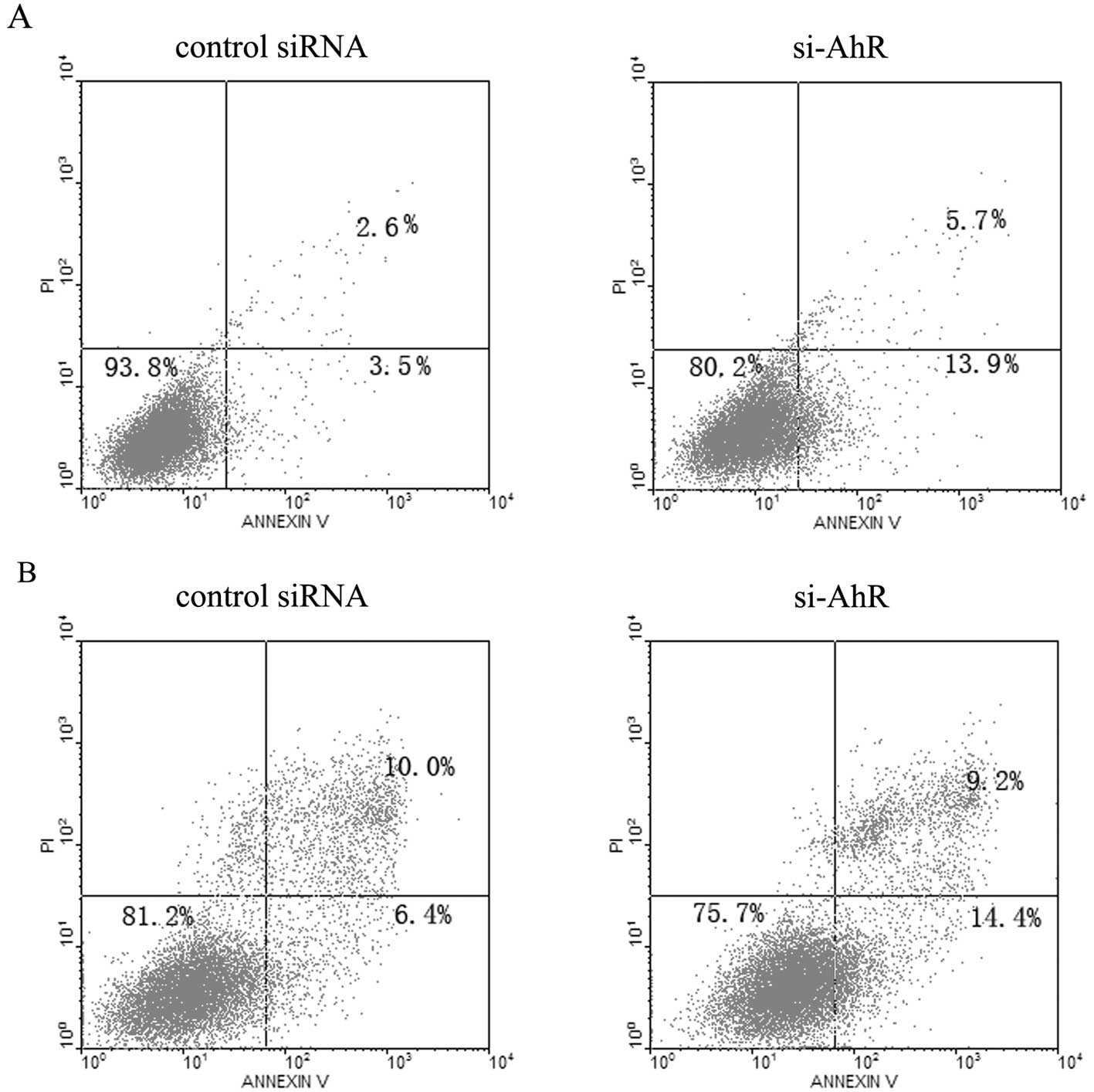
Effect of AhR siRNA on gastric cancer
cell apoptosis
To evaluate whether knockdown of AhR induces gastric
cancer cell apoptosis at 72 h after transfection, the cells were
harvested and analyzed by flow cytometry. The apoptotic rates of
MKN45 and SGC7901 cells were 13.9 and 14.4%, respectively, which
were higher than the apoptotic rates of 3.5 and 6.4% in the control
cells (Fig. 3).
Effect of AhR siRNA on the cell cycle
distribution of gastric cancer cells
We further examined the effects of AhR siRNA on cell
cycle progression by flow cytometry. As shown in Table I, silencing of AhR in MKN45 and
SGC7901 cells increased the proportion of cells in the G1 phase and
correspondingly decreased the proportion of cells in the S phase of
the cell cycle. The proportion of cells in the G2 phase showed no
significant change at 72 h post-transfection with AhR siRNA in
comparison with the negative control group.
 | Table IEffect of AhR siRNA on cell cycle
distribution. |
Table I
Effect of AhR siRNA on cell cycle
distribution.
| MKN45 cells | SGC7901 cells |
|---|
|
|
|
|---|
| NC | si-AhR | NC | si-AhR |
|---|
| G1 (%) | 48.55±3.32 | 60.27±2.78a | 55.30±1.85 | 62.13±1.59a |
| G2 (%) | 26.52±2.34 | 23.94±2.29 | 17.80±148 | 19.96±0.61 |
| S (%) | 24.39±1.85 | 15.04±2.14a | 26.93±1.52 | 17.53±0.95a |
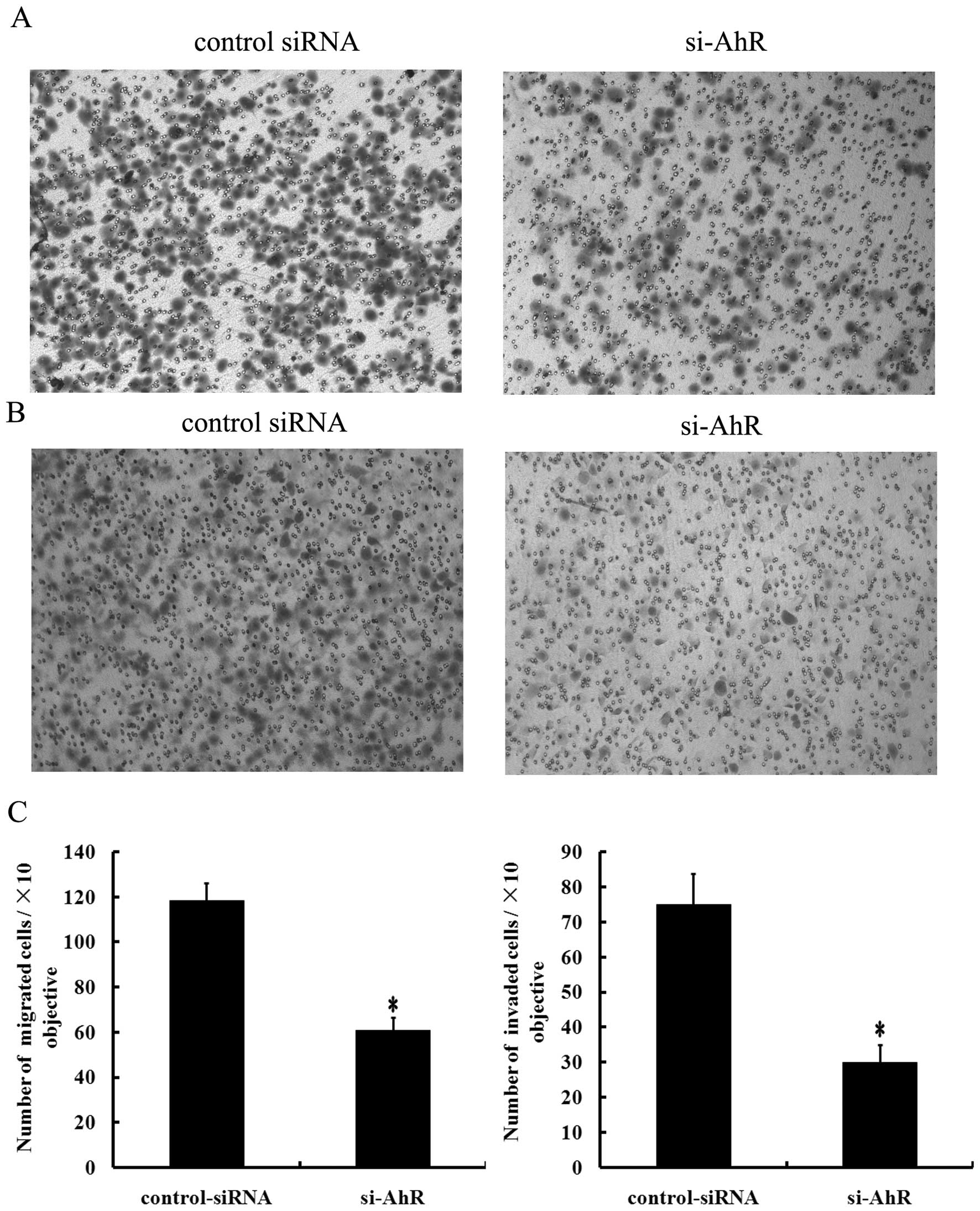
Effects of AhR siRNA on gastric cancer
cell migration and invasion
To determine the effects of decreased AhR expression
on the migratory and invasive potential of gastric cancer cells, we
transfected SGC7901 cells with AhR siRNA and performed a Transwell
assay. Results showed that the number of migrated cells
(60.89±5.78) decreased significantly in the AhR knockdown SGC7901
cells, compared to that in the control cells (118.43±7.83,
P<0.05; Fig. 4A and C).
Moreover, we found that SGC7901 cells transfected with AhR siRNA
exhibited decreased invasive activity (30.11±4.865) in comparison
with the cells that were transfected with the control siRNA
(75.14±8.684, P<0.05; Fig. 4B and
C). Taken together, these results clearly indicate that
suppression of AhR inhibits the migratory and invasive ability of
SGC7901 cells.
Effects of AhR siRNA on the expression
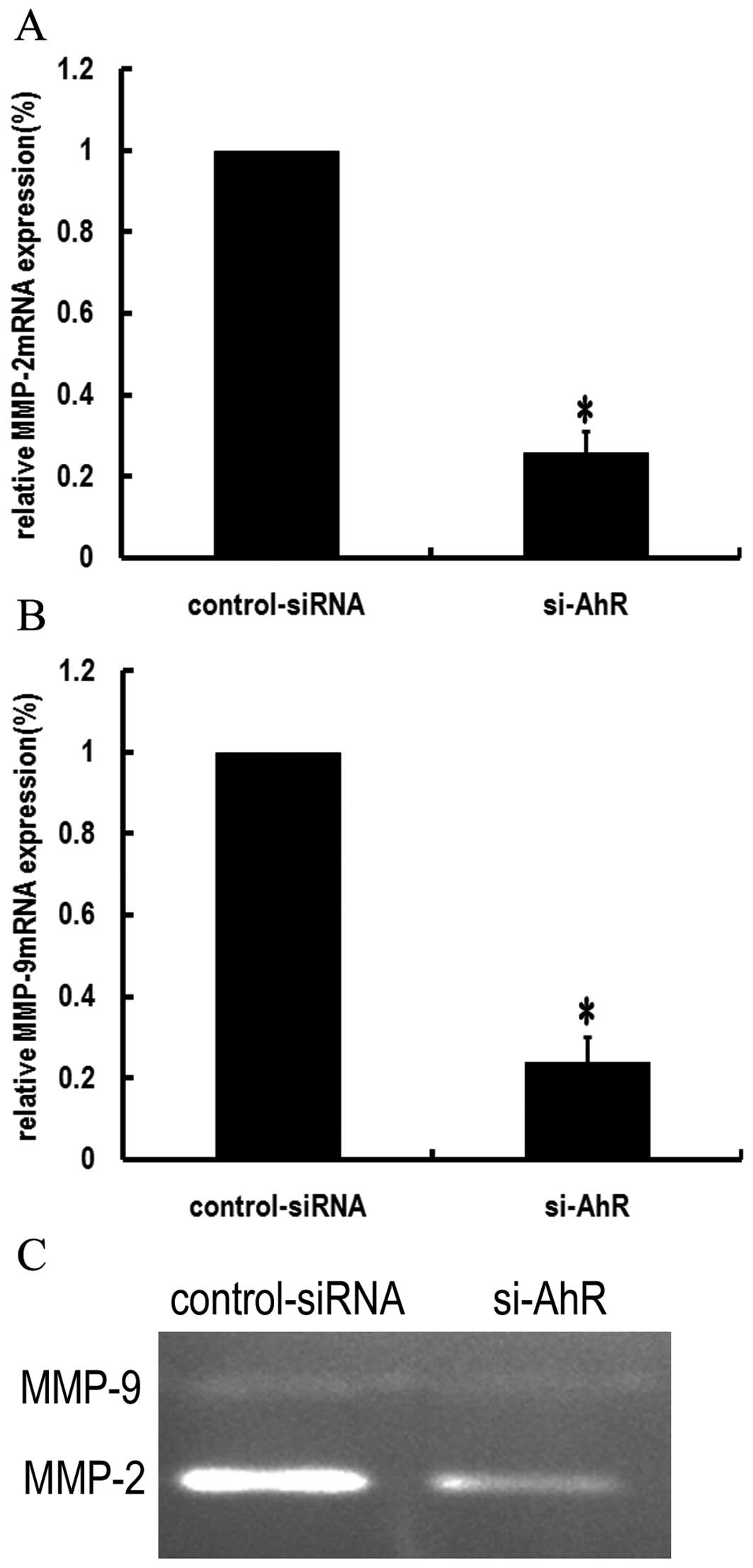
and activity of MMP-2 and MMP-9 in SGC7901 cells
Since MMP-2 and MMP-9 play critical roles in tumor
cell invasion (16), we examined
the effects of AhR siRNA on the expression and enzyme activities of
MMP-2 and MMP-9. Real-time RT-PCR results showed that AhR siRNA
downregulated the mRNA expression of MMP-2 and MMP-9 in SGC7901
cells (P<0.05; Fig. 5A and
B).
We further examined the effect of AhR siRNA on the
activity of MMP-2 and MMP-9 by gelatin zymography. The results
showed that MMP-9 and MMP-2 activity in media decreased after
silencing of AhR (Fig. 5C).
Discussion
Previous studies have shown that overexpression and
abnormal activation of AhR are closely related to tumor development
(17–22). Our previous study found that AhR
expression and nuclear translocation were significant higher in
gastric cancer than in premalignant lesions and normal gastric
mucosa (13), suggesting that AhR
may be involved in gastric cancer development.
The impact of AhR on cell growth is complicated.
Whether it promotes or inhibits cell growth depends on the cell
type and the dominant pathway. Our previous study found that TCDD,
a potent AhR agonist, inhibited proliferation of gastric cancer AGS
cells via induction of growth arrest at the G1-S phase, but in the
absence of exogenous ligands, the role of AhR in the development of
gastric cancer is still unclear. In the present study, RNA
interference method was used to silence expression of AhR in
gastric cancer cell lines, and the changes in cell proliferation,
cell cycle distribution and apoptosis were noted.
RT-PCR and western blotting results showed that AhR
siRNA effectively inhibited the expression of AhR at both the mRNA
and protein levels, and downregulated the expression of CYP1A1 and
CYP1B1, two classic target genes of the AhR pathway. MTT results
showed that AhR siRNA inhibited the proliferation of SGC7901 and
MKN45 cells, suggesting that in the absence of exogenous ligands,
AhR plays a promoting role in gastric cancer cell growth. To
further explore the specific mechanisms of gastric cancer cell
growth inhibition by AhR siRNA, changes in the cell cycle
distribution and cell apoptotic rate were evaluated by flow
cytometric analysis. The results showed that AhR siRNA induced
apoptosis and delayed cell cycle G1-S progression.
In addition to the regulation of cell proliferation,
the AhR pathway is also involved in the process of tumor invasion
and metastasis. Recent evidence suggests that AhR signaling also
alters the expression of genes involved in matrix metabolism,
particularly the MMPs (23). TCDD
induces MMP expression and invasion in A2058 melanoma cells
(24), lung adenocarcinoma A549
cells (25) and prostate cancer
cell lines (26). Similarly, our
previous study demonstrated that AhR pathway activation induced
MMP-9 expression and enzymatic activity, and promoted the migratory
and invasive ability of gastric cancer AGS cells (14).
In the present study, we demonstrated that
chemically synthesized siRNAs specifically targeting AhR
successfully knocked down the expression of AhR at both the protein
and mRNA levels in human gastric cancer SGC7901 cells. Using
Transwell assays, AhR-siRNA attenuated the potential of invasion
and metastasis in gastric cancer cells. According to these results,
AhR participates in gastric cancer initiation, and also plays a key
role in cell metastasis of gastric cancer.
Furthermore, the expression and activity of MMP-2
and MMP-9 were also downregulated in AhR siRNA-transfected cells,
and this may suggest that MMP-2 and MMP-9 are the downstream
products of the AhR complex-induced cell signaling. MMPs are a
family of enzymes that degrade proteins in tissue extracellular
matrices, and are clearly involved in cancer progression, including
tumor cell degradation of basement membranes and stroma and blood
vessel penetration (27).
Expression of MMP-2 and MMP-9 is closely linked to growth,
invasion, metastasis and angiogenesis of gastric cancer (28).
In summary, we provide in vitro evidence to
support the oncogenic role of AhR in gastric cancer development.
Knockdown of AhR inhibited proliferation in the in vitro
study. Our in vitro study also revealed that knockdown of
AhR expression inhibited the ability of migration and invasion in
gastric cancer cells. These results provide preliminary direct
evidence for the potential of AhR to regulate the metastasis of
gastric cancer. These results suggest that AhR not only plays an
important role in tumorigenesis, but may also be involved in the
progression and metastasis of gastric cancer. Therfore, the
mechanisms of AhR in gastric cancer warrant further study.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (nos. 30871145 and 81072048),
the Junior Teacher Cultivation Project of Sun Yat-sen University
(no. 09ykpy22), and grants for major projects and emerging
interdisciplinary studies of Sun Yat-sen University (no. 10ykjc23)
supported by the Fundamental Research Funds for the Central
Universities.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Crew KD and Neugut AI: Epidemiology of
gastric cancer. World J Gastroenterol. 12:354–362. 2006.
|
|
3
|
Belpomme D, Irigaray P, Hardell L, Clapp
R, Montagnier L, Epstein S and Sasco AJ: The multitude and
diversity of environmental carcinogens. Environ Res. 105:414–429.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mandal PK: Dioxin: a review of its
environmental effects and its aryl hydrocarbon receptor biology. J
Comp Physiol B. 175:221–230. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schwarz M and Appel KE: Carcinogenic risks
of dioxin: mechanistic considerations. Regul Toxicol Pharmacol.
43:19–34. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Whitelaw M, Pongratz I, Wilhelmsson A,
Gustafsson JA and Poellinger L: Ligand-dependent recruitment of the
Arnt coregulator determines DNA recognition by the dioxin receptor.
Mol Cell Biol. 13:2504–2514. 1993.PubMed/NCBI
|
|
7
|
Rowlands JC and Gustafsson JA: Aryl
hydrocarbon receptor-mediated signal transduction. Crit Rev
Toxicol. 27:109–134. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gasiewicz TA: Expression and activity of
aryl hydrocarbon receptors in development and cancer. Crit Rev
Eukaryot Gene Expr. 18:279–321. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Puga A, Xia Y and Elferink C: Role of the
aryl hydrocarbon receptor in cell cycle regulation. Chem Biol
Interact. 141:117–130. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abdelrahim M, Smith R III and Safe S: Aryl
hydrocarbon receptor gene silencing with small inhibitory RNA
differentially modulates Ah-responsiveness in MCF-7 and HepG2
cancer cells. Mol Pharmacol. 63:1373–1381. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ishida M, Mikami S, Kikuchi E, Kosaka T,
Miyajima A, Nakagawa K, Mukai M, Okada Y and Oya M: Activation of
the aryl hydrocarbon receptor pathway enhances cancer cell invasion
by up-regulating the MMP expression and is associated with poor
prognosis in upper urinary tract urothelial cancer. Carcinogenesis.
31:287–295. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hsu EL, Yoon D, Choi HH, et al: A proposed
mechanism for the protective effect of dioxin against breast
cancer. Toxicol Sci. 98:436–444. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peng TL, Chen J, Mao W, Liu X, Tao Y, Chen
LZ and Chen MH: Potential therapeutic significance of increased
expression of aryl hydrocarbon receptor in human gastric cancer.
World J Gastroenterol. 15:1719–1729. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peng TL, Chen J, Mao W, Song X and Chen
MH: Aryl hydrocarbon receptor pathway activation enhances gastric
cancer cell invasiveness likely through a c-Jun-dependent induction
of matrix metalloproteinase-9. BMC Cell Biol. 10:272009. View Article : Google Scholar
|
|
15
|
Nebert DW, Puga A and Vasiliou V: Role of
the Ah receptor and the dioxin-inducible [Ah] gene battery in
toxicity, cancer, and signal transduction. Ann NY Acad Sci.
685:624–640. 1993.PubMed/NCBI
|
|
16
|
Zheng H, Takahashi H, Murai Y, et al:
Expressions of MMP-2, MMP-9 and VEGF are closely linked to growth,
invasion, metastasis and angiogenesis of gastric carcinoma.
Anticancer Res. 26:3579–3583. 2006.PubMed/NCBI
|
|
17
|
Lin P, Chang H, Tsai WT, Wu MH, Liao YS,
Chen JT and Su JM: Overexpression of aryl hydrocarbon receptor in
human lung carcinomas. Toxicol Pathol. 31:22–30. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim JH, Kim H, Lee KY, et al: Aryl
hydrocarbon receptor gene polymorphisms affect lung cancer risk.
Lung Cancer. 56:9–15. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schlezinger JJ, Liu D, Farago M, Seldin
DC, Belguise K, Sonenshein GE and Sherr DH: A role for the aryl
hydrocarbon receptor in mammary gland tumorigenesis. Biol Chem.
387:1175–1187. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Long JR, Egan KM, Dunning L, et al:
Population-based case-control study of AhR (aryl hydrocarbon
receptor) and CYP1A2 polymorphisms and breast cancer risk.
Pharmacogenet Genomics. 16:237–243. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Koliopanos A, Kleeff J, Xiao Y, Safe S,
Zimmermann A, Büchler MW and Friess H: Increased arylhydrocarbon
receptor expression offers a potential therapeutic target for
pancreatic cancer. Oncogene. 21:6059–6070. 2002. View Article : Google Scholar
|
|
22
|
Moennikes O, Loeppen S, Buchmann A,
Andersson P, Ittrich C, Poellinger L and Schwarz M: A
constitutively active dioxin/aryl hydrocarbon receptor promotes
hepatocarcinogenesis in mice. Cancer Res. 64:4707–4710. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hillegass JM, Murphy KA, Villano CM and
White LA: The impact of aryl hydrocarbon receptor signaling on
matrix metabolism: implications for development and disease.
Biochem Pharmacol. 387:1159–1173. 2006.PubMed/NCBI
|
|
24
|
Villano CM, Murphy KA, Akintobi A and
White LA: 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) induces
matrix metalloproteinase (MMP) expression and invasion in A2058
melanoma cells. Toxicol Appl Pharmacol. 210:212–224. 2006.
|
|
25
|
Martinez JM, Afshari CA, Bushel PR, Masuda
A, Takahashi T and Walker NJ: Differential toxicogenomic responses
to 2,3,7,8-tetrachlorodibenzo-p-dioxin in malignant and
nonmalignant human airway epithelial cells. Toxicol Sci.
69:409–423. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Haque M, Francis J and Sehgal I: Aryl
hydrocarbon exposure induces expression of MMP-9 in human prostate
cancer cell lines. Cancer Lett. 225:159–166. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar
|
|
28
|
Sier CF, Kubben FJ, Ganesh S, et al:
Tissue levels of matrix metalloproteinases MMP-2 and MMP-9 are
related to the overall survival of patients with gastric carcinoma.
Br J Cancer. 74:413–417. 1996. View Article : Google Scholar : PubMed/NCBI
|