Introduction
Protein-bound polysaccharide-K (PSK) is a
protein-bound polysaccharide K (Krestin), extracted from cultured
mycelia of Coriolus versicolor (CM101) and the average
molecular weight is approximately 100,000 (1). PSK is one of the biological response
modifiers (BRM), and is used clinically in combination therapy for
gastrointestinal cancer and small cell lung carcinoma (1–4).
The most important and widely reported property is
the immunomodulatory effect, as direct antitumor activity is weak.
A few previous reports have indicated that PSK induces cytostatic
effects on growth and invasion by modulating the expression of
major histocompatibility complex (MHC) class I and inhibition of
nuclear factor kappaB (NF-κB) (5).
Our previous study demonstrated that PSK inhibits
cellular proliferation in a cell type-specific manner and that the
inhibition is most profound for promyelomonocytic leukemia HL-60
cells (6). We have shown that the
underlying mechanism of the inhibition in cellular proliferation is
due, in part, to caspase-3-mediated apoptosis.
Apoptosis is one of the main mechanisms for
inhibition of cancer growth and proliferation. The execution of
apoptosis, or programmed cell death, is associated with
characteristic morphological and biochemical changes mediated by a
series of gene regulations and cell-signaling pathways (7). Introduction of apoptosis included
activation of caspase, change of mitochondrial signaling pathway
and regulation of Bcl-2 family members. Recently, perturbation of
mitochondrial function has been shown to be a key event in the
apoptotic cascade. Anticancer drugs may damage the mitochondria by
increasing the permeability of the outer mitochondrial membrane,
which is associated with the collapse of the mitochondrial membrane
potential (ΔΨm) (8).
Therefore, the present study investigated the
mechanism of the apoptosis induced by PSK and focused on the
mitochondrial and p38 mitogen-activated protein kinase
(MAPK)-dependent pathway in HL-60 cells.
Materials and methods
Cells
The promyelomonocytic leukemia cell line HL-60 was
obtained from the American Type Culture Collection (ATCC, Manassas,
VA, USA), and maintained in RPMI-1640 (Gibco-BRL, Rockville, MD,
USA) supplemented with 10% fetal bovine serum (Life Technologies,
Milan, Italy).
Reagents
PSK was manufactured at Kureha Corp. (Tokyo, Japan),
and was dissolved in Dulbecco’s phosphate-buffered saline (DPBS) at
a concentration of 100 mg/ml (Gibco-BRL). In each experiment, the
PSK solution was freshly prepared and further diluted with medium.
The p38 MAPK inhibitor SB203580 was purchased from LC Laboratories
(Woburn, MA, USA), and dissolved in dimethyl sulfoxide (DMSO) at
100 mmol/l to prepare a stock solution. Prior to use, the stock
solution was diluted with medium. The final concentration of
SB203580 was 30 μmol/l and the final concentration of DMSO was
<0.1%.
Western blot analysis
HL-60 cells were suspended in medium containing DPBS
(control) or PSK (100 μg/ml), and seeded into 100-mm culture dishes
at a density of 3×105 cells/dish. After the indicated
time of incubation, cells were harvested and lysed in RIPA Lysis
Buffer (Santa Cruz Biotechnology, Santa Cruz, CA, USA) by rotating
for 30 min at 4°C. Following cell lysis, a clear cell lysate was
obtained by centrifugation and the protein concentration was
determined. Twenty micrograms of protein were used for western
blotting. Antibodies against phosphorylated p38 MAPK and p38 MAPK
(Cell Signaling Technology, Danvers, MA, USA) were used to detect
phosphorylated form of p38 MAPK and total p38 MAPK, respectively.
Chemiluminescent signals generated by using the ECL Advance™
Western Blotting Detection kit (GE Healthcare, Buckinghamshire, UK)
were detected by Light-Capture II Cooled CCD Camera systems (ATTO,
Tokyo, Japan).
Evaluation of apoptosis
Phosphatidylserine externalization and membrane
integrity were evaluated using TACS™ Annexin V-FITC Apoptosis
Detection kit (Trevigen, Gaithersburg, MD, USA), which contains
fluorescein isothiocyanate (FITC)-conjugated Annexin V (Annexin
V-FITC) and propidium iodide (PI). HL-60 cells were suspended in
medium containing DPBS (control) or PSK (100 μg/ml) in the presence
or absence of SB203580 (30 μmol/l) and seeded into 6-well plates at
a density of 6×104 cells/well. After the indicated time
of incubation, cells were harvested and stained using the kit,
according to the manufacturer’s instructions. Stained cells were
analyzed by flow cytometry (FACSCalibur; Becton-Dickinson, Franklin
Lakes, NJ, USA).
Detection of active caspase-3
HL-60 cells were suspended in medium containing DPBS
(control) or PSK (100 μg/ml) in the presence or absence of SB203580
(30 μmol/l) and seeded into 175-cm2 culture flasks at a
density of 1.05×106 cells/flask. After the indicated
time of incubation, cells were harvested and resuspended in PBS
containing 1% saponin at a density of 1×106 cells/ml.
The active form of caspase-3 was detected using APO ACTIVE 3™
(antibody to active caspase-3; Cell Technology Inc., Minneapolis,
MN, USA) according to the manufacturer’s instructions. Stained
cells were analyzed by flow cytometry.
Cellular proliferation assay
Cellular proliferation was determined using WST-8
(Dojindo, Kumamoto, Japan), a water-soluble form of methyl
thiazolyl tetrazolium (MTT), according to the manufacturer’s
instructions. Briefly, cells were suspended in medium containing
DPBS (control) or PSK (100 μg/ml) in the presence or absence of
SB203580 (30 μmol/l) and seeded into 96-well plates at a density of
3×103 cells/well. After 72 h of incubation, WST-8 was
added to each well and incubated for another 3 h. WST-8 is reduced
to an orange colored formazan, which has maximum absorption at 460
nm. Optical density (OD) at 450 nm and 630 nm was measured. The OD
at 630 nm was then subtracted from the OD at 450 nm.
Flow cytometry for Δψm detection
The ΔΨm of HL-60 cells after treatment with PSK (100
μg/ml) in the presence or absence of SB203580 (30 μmol/l) after the
indicated time of incubation was measured using a Flow Cytometry
Mitochondrial Membrane Potential Detection kit (Mito Screen BD
Biosciences). Fluorescence emission was analyzed by flow cytometry
(JC-1 monomers, excitation wavelength 488 nm, emission filter
530/30 nm; JC-1 aggregates, excitation wavelength 488 nm, emission
filter 585/42 nm) using a flow cytometer (FACSCalibur;
Becton-Dickinson).
Statistical analysis
Statistical analyses were performed using the
Student’s t-test. All values are expressed as the means ± standard
error. P-values of ≤0.05 were considered to indicate a
statistically significant difference.
Results
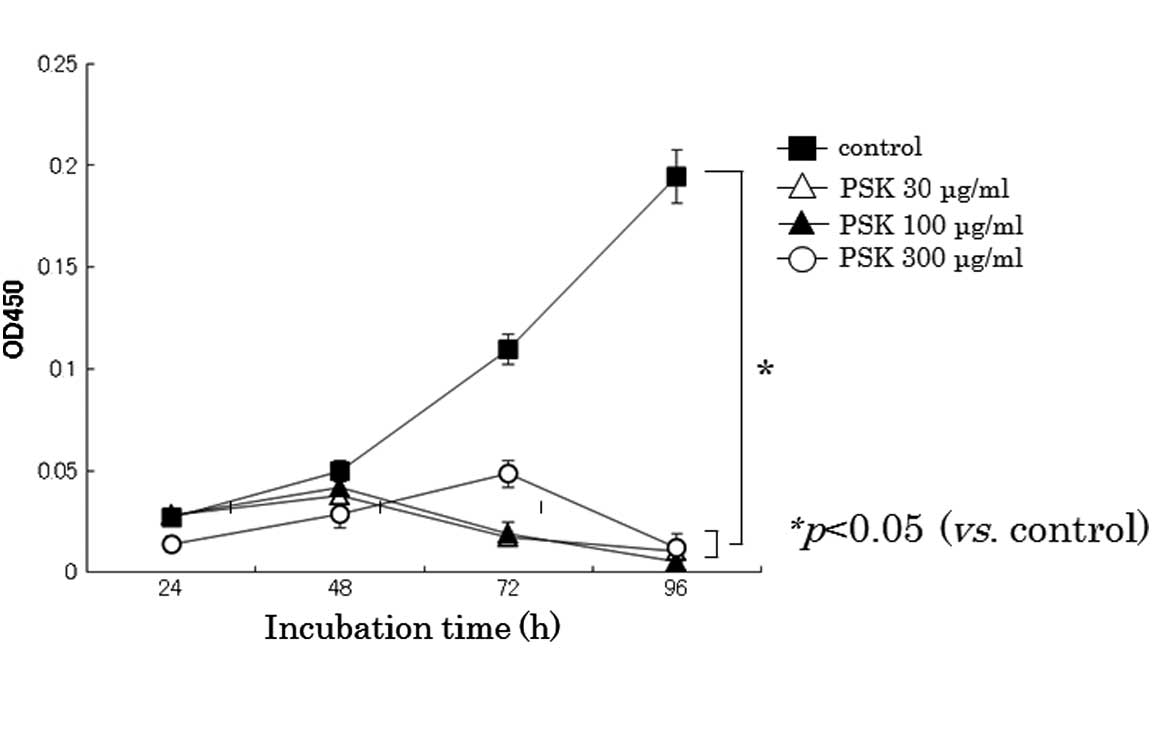
PSK inhibits the proliferation of HL-60
cells
As shown in Fig. 1,
PSK effectively inhibited cellular proliferation of HL-60 cells.
PSK significantly inhibited cellular proliferation at 30 μg/ml in
HL-60 cells.
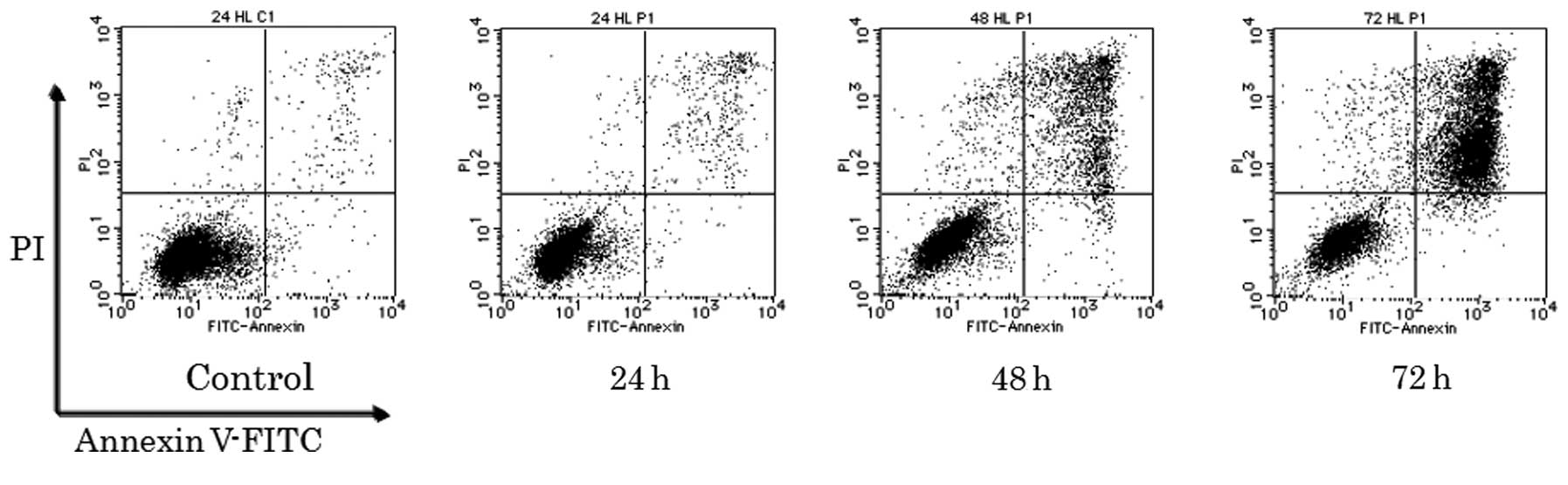
PSK induces apoptosis
Next, we analyzed whether the growth inhibitory
effect of PSK was attributable to the induction of apoptosis. To
this end, we performed three different assays. As shown in Fig. 2, Annexin V-FITC and PI stained
cells, which would be expected as late apoptotic or necrotic cells,
were observed 48 h after PSK treatment and further increased until
72 h after treatment in HL-60 cells.
PSK treatment activates caspase-3
To examine which proteins are involved in
PSK-induced apoptosis, a comprehensive analysis of
apoptosis-related proteins was performed. Proteome Profiler™ Arrays
Human Apoptosis array (R&D Systems) indicated that several
proteins were implicated in PSK-induced apoptosis (data not shown).
Among these candidate proteins, we focused on caspase-3. As a
result, active form of caspase-3 was detected 48 h after PSK
treatment and further increased until 72 h after treatment
(Fig. 3). These results suggested
that caspase-3 activation is involved in PSK-induced apoptosis.
Phosphorylation of p38 MAPK
Several reports indicated that p38 MAPK plays an
important role in apoptosis of HL-60 cells induced by various
stimuli (16–19). Therefore, we examined the role of
p38 MAPK in PSK-induced apoptosis. Firstly protein expression of
the phosphorylated form of p38 MAPK, which is believed to be
activated, was increased after 48 h of PSK treatment (Fig. 4).
Effect of p38 MAPK inhibitor on
PSK-induced apoptosis
A p38 MAPK inhibitor, SB203580, was used to examine
the role of p38 MAPK in PSK-induced apoptosis. PSK-induced
apoptosis, as demonstrated by Annexin V-FITC and PI staining, was
blocked by co-treatment with SB203580 (Fig. 5). The active form of caspase-3 was
increased upon PSK treatment and was reduced by co-treatment with
SB203580 (Fig. 6). Furthermore,
PSK-induced growth inhibition was also blocked by SB203580
(Fig. 7). These results suggest
that p38 MAPK plays an important role in PSK-induced apoptosis and
growth inhibition.
PSK induces mitochondrial pathway of
apoptosis
To determine whether mitochondria were affected by
PSK, we first examined the depolarization of ΔΨm by measuring the
fluorescence emission shift (red to green) of the ΔΨm sensitive
cationic JC-1 dye. JC-1 is readily taken up by mitochondria of
healthy cells as a monomer. This uptake increases the concentration
of the JC-1 inside the mitochondria leading to the formation of
JC-1 aggregates which appear greenish red, whereas depolarized
mitochondria do not accumulate JC-1 which remains in the cytoplasm
as green colored control. Therefore, increase in green to red ratio
symbolizes depolarization of mitochondria. PSK treatment showed a
time-dependent increase in the green/red fluorescence intensity of
HL-60 cells loaded with the JC-1 dye (Fig. 8).
Effect of p38 MAPK inhibitor SB203580 on
PSK-induced mitochondrial pathway of apoptosis
A p38 MAPK inhibitor, SB203580, was used to examine
the role of p38 MAPK in PSK-induced apoptosis. The depolarization
of mitochondria induced by PSK treatment was reversed by
co-treatment with SB203580 (Fig.
8).
Discussion
To study the biological properties of protein-bound
polysaccharide-K (PSK), we investigated the effects of PSK on cell
proliferation in human leukemia HL-60 cells.
PSK-induced antiproliferative activity against HL-60
cells is caused, at least in part, by the induction of apoptosis,
which has been reported, but has yet to be fully characterized
(9–11). Comprehensive analysis of
apoptosis-related protein expression upon PSK treatment of HL-60
cells showed altered expression of several proteins. Among these
proteins, pro-caspase-3 was prominently upregulated. Pro-caspase-3
is activated by various stresses and the active form of caspase-3
plays a key role in the execution of apoptosis (3). We and other groups have found that the
active form of caspase-3 is increased upon PSK treatment (10). Therefore, caspase-3 plays an
important role in PSK-induced apoptosis.
To assess the mechanism by which PSK inhibits HL-60
cell proliferation via induction of apoptosis, we focused on cell
survival signaling in apoptosis. The MAPK signaling pathway played
important roles in apoptosis induction. One important molecular
mechanism for apoptosis might include p38- and Erk1/2-MAPK. We
found altered expression of p38 MAPK protein in
mitochondrion-dependent apoptosis.
MAPKs are a family of serine/threonine protein
kinases that mediate signal transduction in mammals. MAPKs, which
can be subdivided into ERK1/2, c-Jun N-terminal kinase (JNK), and
p38 protein, are activated by modulation of important cellular
functions, including proliferation, differentiation, or responses
to environmental stimuli such as apoptosis (13). Apoptosis can be initiated via 2
alternative signaling pathways: the death receptor-mediated
extrinsic apoptotic pathway and the mitochondrion-mediated
intrinsic apoptotic pathway (14,15).
Mitochondria play a critical role in the regulation of various
apoptotic processes, including drug-induced apoptosis. The
mitochondrial apoptotic pathway is controlled by members of the
Bcl-2 family, which decide cell fate via the interaction between
pro- and antiapoptotic factors (16). The Bcl-2 family consists of pro- and
antiapoptotic members (17).
In HL-60 cells, p38 MAPK is involved in the
induction of apoptosis by various stimuli (18,19).
During apoptosis, p38 MAPK phosphorylates BCl-2 to inhibit its
antiapoptotic properties, such as prevention of cytochrome c
release from the mitochondria (20,21).
The loss of ΔΨm may be a consequence of massive cytochrome c
release. Release of cytochrome c from the mitochondria to
the cytosol is a key event in mitochondrion-dependent apoptotic
processes, leading to activation of caspase-9, which, in turn,
activates caspase-3/7 (22). Park
and Kim reported that auranofin induces p38 MAPK activation and
apoptosis in HL-60 cells and that co-treatment with SB203580
prevents cytochrome c release, caspase activation, and
apoptosis (19). Therefore,
PSK-induced p38 MAPK activation might trigger
mitochondrion-dependent apoptotic processes. We observed the loss
of ΔΨm following PSK treatment; the p38 MAPK inhibitor SB203580
restored the PSK effect, suggesting the possibility that loss of
ΔΨm causes apoptotic cell death. However, we cannot rule out the
possibility that loss of ΔΨm is the result of apoptotic cell death,
rather than its cause.
We have not examined the association between
intracellular reactive oxygen species (ROS) and cytochrome
c. Intracellular ROS act as secondary messengers in
apoptosis induced by anticancer and chemopreventive agents
(23). The generation of ROS can
cause loss of ΔΨm and induce apoptosis by releasing pro-apoptotic
proteins such as apoptosis-inducing factor and cytochrome c
from the mitochondria to the cytosol. The generation of ROS may
contribute to mitochondrial damage and may lead to cell death by
acting as an apoptosis-signaling molecule (24).
In conclusion, PSK induces apoptosis in HL-60 cells
through the activation of mitochondrial and caspase-dependent
pathways involving activation of p38 MAPK signaling cascades. Other
regulatory mechanisms that might be involved in PSK-induced
signaling events remain to be identified in future studies.
Acknowledgements
This study was supported by Kureha Corporation,
Tokyo, Japan.
References
|
1
|
Tsukagoshi S, Hashimoto Y, Fujii G,
Kobayashi H, Nomoto K and Orita K: Krestin (PSK). Cancer Treat Rev.
11:131–155. 1984. View Article : Google Scholar
|
|
2
|
Nakazato H, Koike A, Saji S, Ogawa N and
Sakamoto J: Efficacy of immunochemotherapy as adjuvant treatment
after curative resection of gastric cancer. Lancet. 343:1122–1126.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ohwada S, Ikeya T, Yokomori T, et al:
Adjuvant immunochemotherapy with oral tegafur/uracil plus PSK in
patients with stage II or III colorectal cancer: a randomized
controlled study. Br J Cancer. 90:1003–1010. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Konno K, Motomiya M, Oizumi K, et al:
Effects of protein-bound polysaccharide preparation (PSK) in small
cell carcinoma of the lung. Haigan. 28:19–28. 1988. View Article : Google Scholar
|
|
5
|
Iguchi C, Nio Y, Takeda H, et al: Plant
polysaccharide PSK: cytostatic effects on growth and invasion;
modulating effect on the expression of HLA and adhesion molecules
on human gastric and colonic tumor cell surface. Anticancer Res.
21:1007–1013. 2001.
|
|
6
|
Hirahara N, Fujioka M, Edamatsu T, et al:
Protein-bound polysaccharide-K (PSK) induces apoptosis and inhibits
proliferation of promyelocytic leukemia HL-60 cells. Anticancer
Res. 31:2733–2738. 2011.PubMed/NCBI
|
|
7
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu W and Kavanagh JJ: Anticancer therapy
targeting the apoptosis pathway. Lancet Oncol. 4:721–729. 2003.
View Article : Google Scholar
|
|
9
|
Yefenof E, Gafanovitch I, Oron E, Bar M
and Klein E: Prophylactic intervention in
radiation-leukemia-virus-induced murine lymphoma by the biological
response modifier polysaccharide K. Cancer Immunol Immunother.
41:389–396. 1995. View Article : Google Scholar
|
|
10
|
Hattori ST, Komatsu N, Shichijo S and Itoh
K: Protein-bound polysaccharide K induced apoptosis of the human
Burkitt lymphoma cell line, Namalwa. Biomed Pharmacother.
58:226–230. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiménez-Medina E, Berruguilla E, Romero I,
et al: The immunomodulator PSK induces in vitro cytotoxic activity
in tumor cell lines via arrest of cell cycle and induction of
apoptosis. BMC Cancer. 8:782008.PubMed/NCBI
|
|
12
|
Thornberry NA: Caspases: key mediators of
apoptosis. Chem Biol. 5:R97–R103. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen X, Lan X, Mo S, et al: p38 and ERK,
but not JNK, are involved in copper-induced apoptosis in cultured
cerebellar granule neurons. Biochem Res Commun. 379:944–948. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reuter S, Eifes S, Dicato M, Aggarwal BB
and Diederich M: Modulation of anti-apoptotic and survival pathways
by curcumin as a strategy to induce apoptosis in cancer cells.
Biochem Pharmacol. 76:1340–1351. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cory S and Adams JM: The Bcl-2 family:
regulators of the cellular life-or-death switch. Nat Res Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Leibowitz B and Yu J: Mitochondrial
signaling in cell death via the Bcl-2 family. Cancer Biol Ther.
9:417–422. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiao G, Fang H, Xing C and Xu W:
Structure, function and inhibition of Bcl-2 family proteins: a new
target for anti-tumor agents. Mini Rev Med Chem. 9:1596–1604. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ahn YH, Jung JM and Hong SH:
8-chloro-cyclic AMP-induced growth inhibition and apoptosis is
mediated by p38 mitogen-activated protein kinase activation in HL60
cells. Cancer Res. 65:4896–4901. 2005. View Article : Google Scholar
|
|
19
|
Park SJ and Kim IS: The role of p38 MAPK
activation in auranofin-induced apoptosis of human promyelocytic
leukemia HL-60 cells. Br J Pharmacol. 146:506–513. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Torcia M, De Chiara G, Nencioni L, et al:
Nerve growth factor inhibits apoptosis in memory B lymphocytes via
inactivation of p38 MAPK, prevention of BCL-2 phosphorylation, and
cytochrome release. J Biol Chem. 276:39027–39036. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
De Chiara G, Marcocci ME, Torcia M, et al:
BCL-2 phosphorylation by p38 MAPK. J Biol Chem. 281:21353–21361.
2006.
|
|
22
|
Riedl SJ and Salvesen GS: The apoptosome:
signalling platform of cell death. Nat Rev Mol Cell Biol.
8:405–413. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Garcia A, Morales P, Arranz N, Delgado ME,
Rafter J and Haza AI: Antiapoptotic effects of dietary antioxidants
towards N-nitrosopiperidine and N-nitrosodibutylamine-induced
apoptosis in HL-60 and HepG2 cells. J Appl Toxicol. 29:403–413.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang R, Humphreys I, Sahu RP, Shi Y and
Srivastava SK: In vitro and in vivo induction of apoptosis by
capsaicin in pancreatic cancer cells is mediated through ROS
generation and mitochondrial death pathway. Apoptosis.
13:1465–1478. 2008. View Article : Google Scholar : PubMed/NCBI
|