Introduction
Gastric carcinoma is the second leading cause of
cancer-related mortality in the world (1), after lung cancer. Despite a sharp
worldwide decline in both the incidence and mortality of gastric
carcinoma since the second half of the 20th century, it
still continues to be a major health problem worldwide. This can be
attributed to the particularly slow decrease in the incidence of
gastric carcinoma in Asia and a high mortality rate of the
diagnosed gastric carcinoma cases in the West (2). Therefore, research efforts that
elucidate the molecular mechanisms underlying gastric
carcinogenesis and subsequent disease progression and that identify
reliable biomarkers are of critical importance for the prevention,
treatment, and prognostic evaluation of gastric cancer.
The S100 proteins are calcium-binding, acidic,
low-molecular-weight proteins (10–12 kDa), which are named
according to their solubility in 100% saturated ammonium sulfate.
In recent years, these proteins are gaining importance due to their
association with diseases such as cardiomyopathy, neurodegenerative
disorders and cancer (3). The S100
protein family includes ~25 members, with the genes clustered on
human chromosome 1q21. The encoded proteins contain two EF-hand
calcium-binding helix-loop-helix motifs. Each member contains two
EF-hands connected by a central hinge, which is S100
family-specific in the N-terminus and canonical in C-terminus
(4). Overexpression of the S100
calcium-binding protein A4 [S100A4, also known as Mts1
(metastasis-associated gene), p9Ka, 18A2, pEL98, 42A, CAPL and
calvasculin]of the S100 protein family has been shown to control
cell cycle progression and modulate intercellular adhesion, as well
as invasive and metastatic properties of cancer cells (5). The human S100A4 gene is located at
position 1q21 on chromosome 1 and encodes a polypeptide of 101
amino acids with a molecular mass of ~11.5 kDa (6). The S100A4 gene contains four exons,
and its open reading frame begins from the third exon. The S100A4
promoter contains an ErbB2 response element, and the enhancer and
silencer elements of the S100A4 gene may be strongly affected by
methylation (7,8).
The S100A4 protein has been shown to be present at
detectable limits in a subset of cells of the ovary, prostate,
spleen, thymus, bone marrow, T lymphocytes, neutrophils and
macrophages, whereas very low levels have been detected in normal
tissues of the pancreas, colon, thyroid, lungs and kidneys
(8). S100A4 overexpression has been
reported to be positively correlated with rheumatoid arthritis,
kidney fibrosis, liver fibrosis, peritoneal fibrosis, corneal
dystrophy, neural diseases, and cardiac and lung disease (7,8).
Furthermore, S100A4 overexpression is involved in
epithelial-to-mesenchymal transition (EMT), oncogenic
transformation, and aggressive progression of cancers (9,10).
Transgenic mice that overexpress S100A4 in the mammary epithelium
are phenotypically indistinguishable from wild-type mice and
exhibit increased metastasis (11).
Inhibition of S100A4 (by antisense or anti-ribozyme approaches)
suppressed the metastatic potential of tumor cells in animal models
of lung carcinoma and osteosarcoma (12,13).
Several proteins have been identified as targets for the S100A4
protein, including liprin β1 and methionine aminopeptidase
(14). S100A4 is reported to bind
to the C-terminal regulatory domain of p53 in vitro and
inhibit its phosphorylation, thus suppressing the transcription of
p53 target genes including p21/WAF, Bax, thrombospondin-1 and Mdm-2
(15). S100A4 is also known to
interact with proteins involved in cytoskeletal rearrangement and
cell motility such as F-actin, myosin-IIA, tropomyosin, and the
heavy chain of nonmuscle myosin II (16–18).
Additionally, S100A4 has been shown to exhibit its prometastatic
role through its influence on angiogenesis, cytoskeletal integrity,
matrix metalloproteinases (MMPs), tumor-related transcription
factors and stromal factors (19,20).
The aim of the present study was to analyze S100A4
expression in gastric cancer and precancerous lesions at both the
protein and mRNA levels and to further compare this S100A4
expression with the clinicopathological features of gastric
cancer.
Materials and methods
Patients and tissue specimens
Gastric cancer tissue was collected from gastrectomy
specimens and from gastritis, gastric intestinal metaplasia (IM),
and adenoma specimens by endoscopic biopsies performed between
January 1995 and January 2005 at Department of General Surgery,
Shengjing Hospital of China Medical University. The tissues were
fixed in 10% neutral formalin, embedded in paraffin, and cut into
4-μm sections. The sections were stained by hematoxylin and eosin
(H&E) to confirm histological diagnosis and other microscopic
characteristics. Portions of a few samples were frozen in liquid
nitrogen until the tissue was homogenized for RNA and protein
extraction. The tumor-node-metastasis (TNM) staging for each
gastric carcinoma specimen was performed according to the Union
Internationale Contre le Cancer (UICC) system for the extent of
tumor spread (21). Histological
architecture of the gastric carcinoma was expressed in terms of
Lauren’s classification (22,23).
Furthermore, tumor size, depth of invasion, and lymphatic and
venous invasion were determined. Lymphatic and venous invasion were
assessed first by H&E staining, followed by D2–40
immunostaining (for lymphatic invasion) and EvG staining (for
venous invasion), if necessary. None of the patients underwent
chemotherapy, radiotherapy, or adjuvant treatment before surgery.
The University Ethics Committee of China Medical University
approved the research protocol. The patients were carefully
followed up by consulting their case documents and through
telephone monitoring.
Tissue microarray (TMA) and
immunohistochemistry
Representative areas of the solid tumors were
identified in the H&E-stained sections of the selected tumor
cases, and a tissue core (2 mm in diameter) per donor block was
punched out and transferred to a recipient block with a maximum of
48 cores using a manual arraying device (MTA-1; Beecher
Instruments, Inc., Sun Prairie, WI, USA). Sections (4-μm) were
consecutively incised from the recipient block and transferred to
poly-lysine-coated glass slides. H&E staining was performed on
the TMA sections for confirmation of the tumor tissue.
Immunohistochemistry was performed on 4-μm
formalin-fixed, paraffin-embedded tissue sections. The sections
were first deparaffinized in xylene and rehydrated through graded
concentrations of alcohol. For antigen retrieval, endogenous
peroxidase activity was blocked by incubating the slides in 1.5%
hydrogen peroxide in absolute methanol at room temperature for 10
min. Next, the sections were subjected to antigen retrieval by
heating the slides in target retrieval solution (TRS; Dako) for 15
min in a microwave oven (Oriental Rotor Ltd. Co., Tokyo, Japan).
The rabbit polyclonal antibody against S100A4 (Abcam, Cambridge,
UK; 1:200) was applied for 1 h at room temperature, according to
the manufacturer’s instructions, and the sections were washed three
times with TBST. After a final rinse with TBST, the sections were
further incubated with an anti-rabbit IgG antibody conjugated to
horseradish peroxidase (Dako) at a dilution of 1:1,000 for 1 h. The
final color was visualized by exposing the sections to 0.5 mg/ml
3,3′-diaminobenzidine and 0.005% hydrogen peroxide for ~5 min.
After counterstaining with Mayer’s hematoxylin, the sections were
dehydrated, cleared and mounted. Sections without the addition of
the primary antibody were used as a negative control.
The slides were randomly allocated for analysis by
two independent experienced pathologists with no knowledge of the
clinicopathological data. For each slide, the number of
S100A4-positive cells was counted in 10 fields at ×200
magnification, and the percentage of positively stained cells was
determined. The percentage of positively stained cells was graded
semi-quantitatively according to a four-point scoring system as
follows: negative (−), 0–5%; weakly positive (+), 6–25%; moderately
positive (++), 26–50%; and strongly positive (+++), >50%.
Real-time reverse transcriptase
(RT)-polymerase chain reaction (PCR)
Total RNA was extracted using the TRIzol®
reagent (Invitrogen, Carlsbad, CA, USA), and cDNAs were reverse
transcribed by the RevertAid™ RT (Takara Bio, Inc., Otsu, Shiga,
Japan). Real-time PCR was carried out using the ABI
PRISM® 7500 Sequence detection system (Applied
Biosystems, Foster City, CA, USA) at 50°C for 2 min, 95°C for 10
min, followed by 50 cycles at 95°C for 15 sec, and at 60°C for 1
min. The primers for glyceraldehyde-3-phosphate dehydrogenase
(GAPDH; 135 bp, 201–335, NM_002046.3) were
5′-CAATGACCCCTTCATTGACC-3′ (sense) and 5′-TGGAA GATGGTGATGGGATT-3′
(antisense). The primers for S100A4 (130 bp, 248–377, NM_019554.2)
were 5′-AGCTTCT TGGGGAAAAGGAC-3′ (sense) and 5′-TACACATCATGG
CGATGCAG-3′ (antisense). Expression of GAPDH was used to normalize
expression of the target genes. Each assay was performed in
triplicate, the average was calculated, and the expression level of
S100A4 was expressed as 2−ΔΔCt, where ΔCt = Ct (S100A4)
− Ct (GAPDH). ΔΔCt = ΔCt (carcinoma) − ΔCt (mucosa).
Western blot analysis
Protein was extracted by homogenizing the tissues in
RIPA lysis buffer [50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 5 mM EDTA,
0.5% Nonidet P-40, 5 mM dithiothreitol, 10 mM NaF, protease
inhibitor cocktail] (Sigma, St. Louis, MO, USA), and the amount of
protein was concentrated by the bicinchoninic acid (BCA) method.
The denatured proteins were separated on a 15% SDS-polyacrylamide
gel and transferred to a Hybond-P PVDF membrane (Amersham plc,
Amersham, Germany), which was then blocked overnight in 5% skim
milk in TBST (10 mmol/l Tris-HCl, 150 mmol/l NaCl, 0.1% Tween-20).
For immunoblotting, the membrane was incubated for 1 h with the
rabbit antibody against S100A4 (1: 500). The membrane was rinsed
with TBST and incubated with an anti-rabbit or anti-mouse secondary
IgG conjugated to horseradish peroxidase (1:1,000; Dako,
Carpinteria, CA, USA) for 1 h. Bands were visualized on X-ray film
(Fuji, Japan) using the ECL Plus detection reagents (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). Next, the membrane was
washed with the WB Stripping Solution (pH 2.0–3.0; Nacalai, Tokyo,
Japan) for 1 h and treated as described above, except an anti-GAPDH
antibody (1:10,000; Sigma) was used as an internal loading control.
Densitometric quantification of the S100A4 protein in gastric
samples was performed using on Scion Image software (Scion
Corporation, Frederick, MD, USA) using GAPDH as a control.
Statistical analyses
All quantitative data are expressed as means ±
standard deviation (SD). The Student’s t-test and one-way analysis
of variance (ANOVA) with the Student’s-Newman-Keuls t-test were
used to compare the means of different groups. Categorical data
were analyzed by the Spearman’s correlation test. Kaplan-Meier
survival plots were generated, and comparisons between survival
curves were carried out with log-rank statistics. Cox’s
proportional hazards model was employed for multivariate analysis.
SPSS 10.0 software (SPSS Inc., Chicago, IL, USA) was used to
analyze all data, and P<0.05 was considered to indicate a
statistically significant result.
Results
Immunohistochemical analysis for S100A4
expression in gastric carcinomas
The expression of the S100A4 protein was assessed by
determining the number of positively stained tumor cells using a
four-point scoring system, as described in Materials and methods.
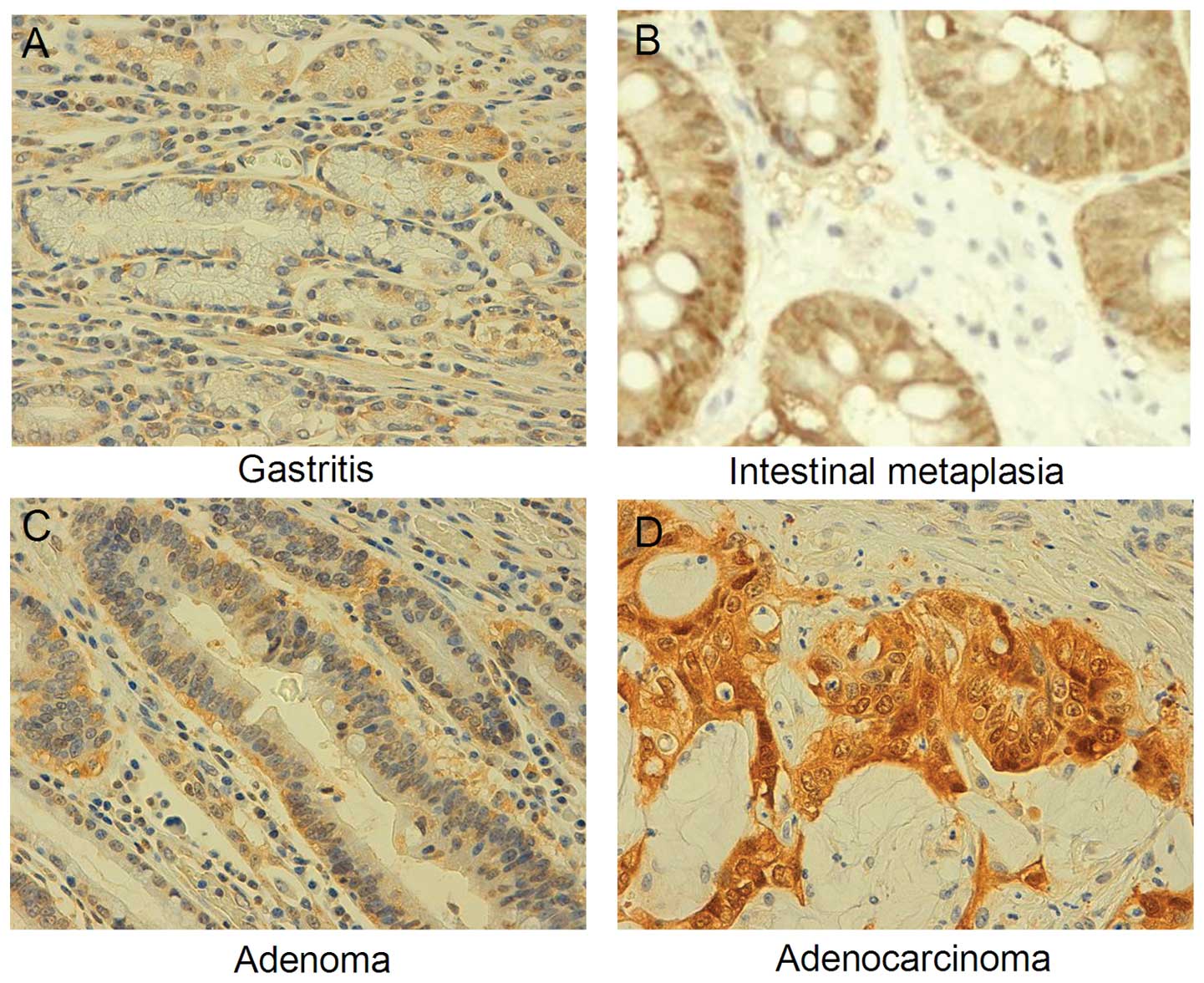
As shown in Fig. 1, S100A4 was
positively immunostained in the cytoplasm and nuclei of gastric
epithelial cells, IM, adenomatous dysplasia, and carcinoma cells.
Overall, S100A4 expression was detected in 19.2% of the gastritis
cases (14/73), 23.3% of the IM cases (20/86), 34.9% of the
adenomatous dysplasia cases (22/63), and 55.2% of the total gastric
carcinoma cases (192/348). Based on the S100A4 expression frequency
and density, we inferred that there was a gradual increase in
S100A4 expression in the following order: gastritis < IM <
dysplasia < carcinoma (P<0.001) (Table I).
 | Table IS100A4 expression in gastric
carcinogenesis. |
Table I
S100A4 expression in gastric
carcinogenesis.
| | S100A4
expression | | |
|---|
| |
| | |
|---|
| Groups | N | − | + | ++ | +++ | PR (%) | P-value |
|---|
| Gastritis | 73 | 59 | 8 | 4 | 2 | 19.2 | <0.001 |
| IM | 86 | 66 | 13 | 5 | 2 | 23.3 | |
| Adenoma | 63 | 41 | 13 | 7 | 2 | 34.9 | |
| Gastric
carcinoma | 348 | 156 | 29 | 62 | 101 | 55.2 | |
Correlation between S100A4 expression and
clinicopathological factors
Next, we analyzed the correlation between positive
S100A4 expression and various clinicopathological factors that may
affect the prognosis of patients with gastric carcinoma. As
summarized in Table II, S100A4
expression was positively correlated with tumor size, depth of
invasion, lymphatic and venous invasion, lymph node metastasis, and
TNM staging (all P<0.05) in carcinoma patients. Age and gender,
however, had no influence on S100A4 expression in the carcinoma
patients (P>0.05). S100A4 expression was higher in
intestinal-type (IT) carcinomas than that in diffuse-type (DT)
carcinomas (P<0.001).
 | Table IIRelationship between S100A4
expression and clinicopathological features of the gastric
carcinoma cases. |
Table II
Relationship between S100A4
expression and clinicopathological features of the gastric
carcinoma cases.
| | S100A4
expression | | |
|---|
| |
| | |
|---|
| Clinicopathological
features | N | − | + | ++ | +++ | PR (%) | P-value |
|---|
| Age (years) | | | | | | | 0.277 |
| <55 | 139 | 67 | 12 | 23 | 37 | 51.8 | |
| ≥55 | 209 | 89 | 17 | 39 | 64 | 57.4 | |
| Gender | | | | | | | 0.183 |
| Male | 248 | 102 | 27 | 46 | 73 | 58.9 | |
| Female | 100 | 54 | 2 | 16 | 28 | 46.0 | |
| Tumor size
(cm) | | | | | | | <0.001 |
| ≤4 | 120 | 84 | 4 | 11 | 21 | 30.0 | |
| >4 | 228 | 72 | 25 | 51 | 80 | 68.4 | |
| Depth of
invasion | | | | | | | <0.001 |
| Tis-T1 | 33 | 25 | 2 | 2 | 4 | 24.2 | |
| T2–T4 | 315 | 131 | 27 | 60 | 97 | 58.4 | |
| Lymphatic
invasion | | | | | | | <0.001 |
| − | 192 | 116 | 14 | 20 | 42 | 39.6 | |
| + | 156 | 40 | 15 | 42 | 59 | 74.4 | |
| Venous
invasion | | | | | | | 0.44 |
| − | 245 | 135 | 17 | 32 | 61 | 44.9 | |
| + | 103 | 21 | 12 | 30 | 40 | 79.6 | |
| LN | | | | | | | 0.002 |
| − | 138 | 83 | 4 | 12 | 39 | 39.8 | |
| + | 210 | 73 | 25 | 50 | 62 | 65.2 | |
| TNM staging | | | | | | | <0.001 |
| I | 6 | 6 | 0 | 0 | 0 | 0.0 | |
| II | 60 | 48 | 2 | 4 | 6 | 20.0 | |
| III | 104 | 50 | 5 | 15 | 34 | 51.9 | |
| IV | 178 | 52 | 22 | 43 | 61 | 70.8 | |
| Lauren’s
classification | | | | | | | <0.001 |
| IT | 203 | 131 | 7 | 23 | 42 | 35.5 | |
| DT | 145 | 25 | 22 | 39 | 59 | 82.8 | |
Real-time RT-PCR and western blot
analysis for S100A4 expression and its correlation with
clinicopathological features of gastric carcinoma
We designed S100A4-specific primers and performed
real-time RT-PCR to quantify the mRNA expression level of S100A4 by
using the housekeeping gene GAPDH as an internal control. The
S100A4 mRNA expression was upregulated in the following order:
gastritis < IM < dysplasia < carcinoma (P<0.001)
(Fig. 2). Higher S100A4 mRNA
expression was observed in gastric carcinomas that showed a larger
diameter (>4 cm), deeper invasion, and frequent lymph node
metastasis, and in IT carcinoma (P<0.05). These findings were
also supported by the evaluation of S100A4 protein expression as
assessed by western blot analysis (Fig.
3).
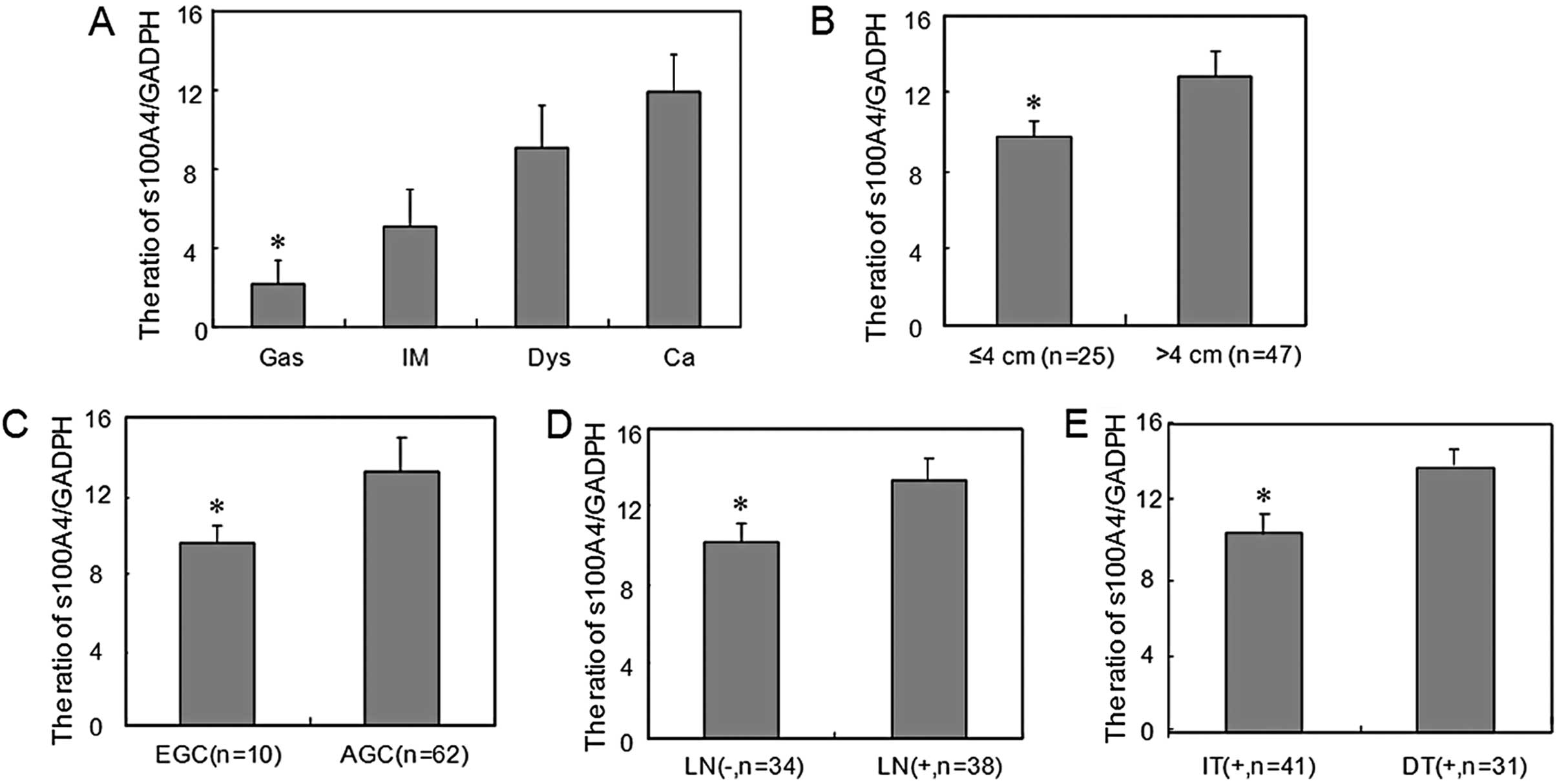 | Figure 2S100A4 mRNA expression level during
gastric carcinogenesis and its correlation with clinicopathological
features of carcinoma. (A) S100A4 mRNA was detectable in gastritis
(Gas), intestinal metaplasia (IM), dysplasia (Dys), and carcinoma
(Ca) samples, as revealed by real-time RT-PCR analysis, and it
gradually increased from Gas, IM, Dys to Ca (*P<0.05;
Ca vs. Gas). S100A4 mRNA expression was higher in gastric
carcinomas that showed (B) a larger diameter
(*P<0.05; >4 cm vs. ≤4 cm), (C) deeper invasion
(*P<0.05; AGC vs. EGC), (D) frequent lymph node
metastasis [*P<0.05; LN(+) vs. ≤LN(−)], and (E)
intestinal-type (*P<0.05; DT vs. IT). Data are
presented as means ± SD. EGC, early gastric cancer; AGC, advanced
gastric cancer; LN, lymph node metastasis; IT, intestinal-type; DT,
diffuse-type. |
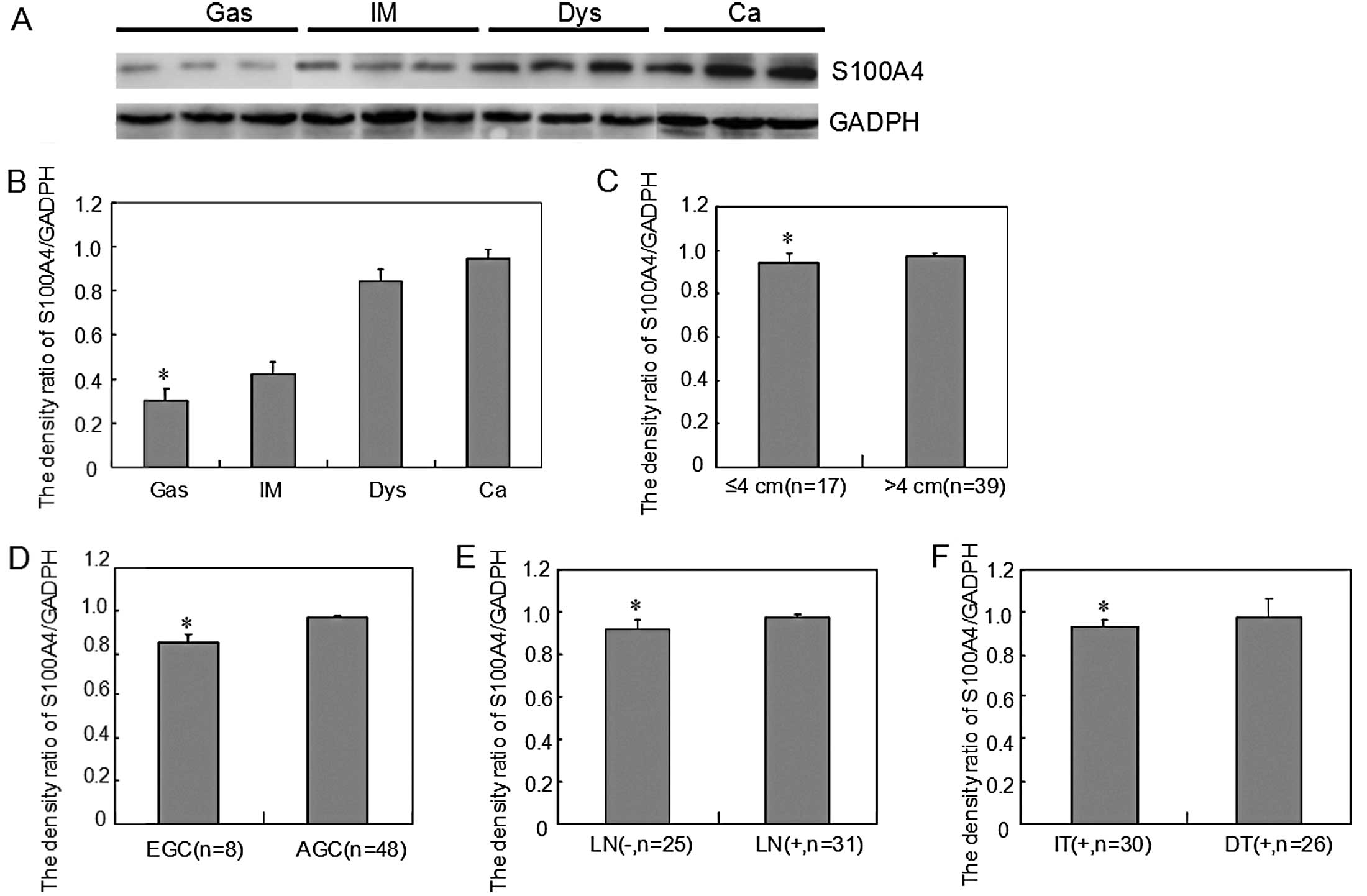 | Figure 3S100A4 protein expression level
during gastric carcinogenesis and its correlation with
clinicopathological features of carcinoma. (A) Cell lysates were
separated on a 15% SDS-polyacrylamide gel, transferred to a Hybond
membrane and probed with anti-S100A4 (29 kDa), using GADPH (37 kDa)
as an internal control. (B) Densitometric analysis demonstrated
S100A4 expression in gastritis (Gas), intestinal metaplasia (IM),
dysplasia (Dys), and carcinoma (Ca) specimens, and the S1004A
protein expression level gradually increased from Gas, IM, Dys to
Ca (*P<0.05, Ca vs. Gas). S100A4 protein expression
was higher in gastric carcinomas that showed (C) a larger diameter
(*P<0.05, >4 cm vs. ≤4 cm), (D) deeper invasion
(*P<0.05, AGC vs. EGC), (E) frequent lymph node
metastasis [*P<0.05, LN(+) vs ≤LN(−)], and (F)
intestinal-type (*P<0.05, DT vs. IT). Data are
presented as means ± SD. EGC, early gastric cancer; AGC, advanced
gastric cancer; LN, lymph node metastasis; IT, intestinal-type; DT,
diffuse-type. |
Correlation between S100A4 expression and
survival of gastric carcinoma patients
Follow-up information was available on 348 gastric
carcinoma patients for periods ranging from 1 month to 10.1 years
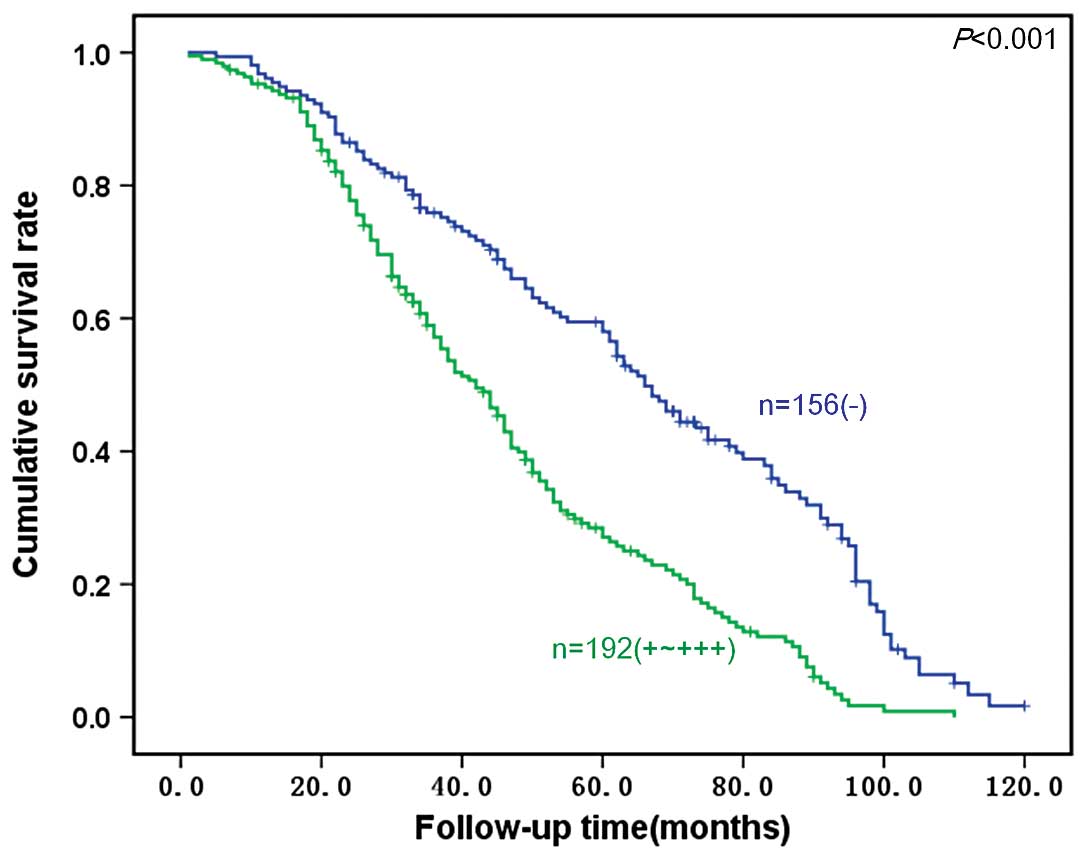
(median, 65.1 months). Fig. 4 shows
survival curves stratified according to S100A4 protein expression
in the gastric carcinomas. Univariate analysis using the
Kaplan-Meier method indicated the cumulative survival rate of
patients with weak or moderate S100A4 expression (n=192) to be
clearly lower than that of patients not expressing S100A4 (n=156;
P<0.05).
Multivariate analysis using Cox’s proportional
hazard model was conducted to determine the independent prognostic
effects of the various clinicopathological parameters. Our results
demonstrated that depth of invasion (P=0.048), lymphatic and venous
invasion (P=0.049 and 0.041, respectively), lymph node metastasis
(P=0.027), TNM staging (P<0.001), and S100A4 expression
(P=0.045) were independent factors for the poor prognosis of
gastric carcinoma (all P<0.05) (Table III).
 | Table IIIMultivariate analysis of
clinicopathological variables to determine the survival of gastric
carcinoma cases. |
Table III
Multivariate analysis of
clinicopathological variables to determine the survival of gastric
carcinoma cases.
| Clinicopathological
parameters | Relative risk (95%
CI) | P-value |
|---|
| Age (≥55
years) | 1.399
(0.992–1.898) | 0.057 |
| Gender
(female) | 0.729
(0.519–1.241) | 0.087 |
| Depth of invasion
(T2–4) | 2.288
(1.689–6.234) | 0.048 |
| Lymphatic invasion
(+) | 1.528
(1.055–2.367) | 0.049 |
| Venous invasion
(+) | 1.672
(1.063–2.723) | 0.041 |
| Lymph node
metastasis (+) | 2.042
(1.114–3.931) | 0.027 |
| TNM staging
(III–IV) | 5.584
(2.126–12.045) | <0.001 |
| Lauren’s
classification (intestinal/diffuse) | 1.119
(0.886–1.393) | 0.287 |
| S100A4 expression
(+− +++) | 1.522
(1.325–4.236) | 0.045 |
Discussion
In the present study, the S100A4 protein was
detected in the cytoplasm and nuclei of gastric lesions, which is
in agreement with a previous report (24,25).
S100A4 is a transcriptional factor that can translocate from the
nucleus to the cytosol under certain physiological or pathological
conditions. Here, we found gradually increased expression of S100A4
at both the mRNA and protein level in the following ascending
order: gastritis < IM < dysplasia < carcinoma. These
results are in line with other reports regarding clear cell renal
cell carcinoma (26), esophageal
squamous cell carcinoma (ESCC) (27,28),
colorectal carcinoma (29), breast
cancer (30) and bladder cancer
(31). IM is believed to be an
adaptive condition for an injured and inflamed gastric epithelium
and can develop into globoid dysplasia, which is closely linked to
signet ring cell carcinoma, as revealed by its morphological
appearance and biological characteristics (32). Pathological and genetic observations
demonstrated that gastric dysplasia precedes the majority of
carcinomas, can undergo malignant transformation, and is classified
into cryptal, globoid, regenerative, and adenomatous subtypes
(33). Our study is the first to
suggest that higher S100A4 expression may contribute to
carcinogenesis. Based on previous literature reports (34,35),
S100A4 overexpression may result in hypomethylation of the CpG
sites in the first intron of the S100A4 gene or simply a result of
S100A4 stimulation under a hypoxic microenvironment.
To elucidate the role of the S100A4 protein in the
progression of gastric carcinoma, we tested whether the expression
status of S100A4 was associated with the aggressive behavior of
carcinomas. We found that the S100A4 mRNA and protein expression
was positively linked to tumor size, depth of invasion, lymphatic
and venous invasion, lymph node metastasis, and TNM staging in
gastric carcinomas, which supported the previous findings of Feng
et al(36). It has been
reported that cytoplasmic expression of the S100A4 protein was
positively correlated with depth of invasion and lymph node
metastasis, whereas the nuclear expression of S100A4 protein was
positively correlated with the depth of invasion and perineural
invasion of colorectal cancer (37). The positive relationship between
S100A4 expression and aggressiveness was also observed in
advanced-stage endometrial cancer (38) and bladder cancer (31). These findings suggest that S100A4
overexpression is involved in the growth, invasion, and metastasis
of gastric carcinoma and may serve as an indicator of the
aggressiveness of gastric carcinoma tumors in clinicopathological
practice. In the light of our present findings, we speculate that
S100A4 upregulation may participate in the progression of gastric
cancer by reversing the aggressive phenotype of cancer cells.
S100A4 is known to regulate migratory and invasive
behavior of human ESCC cells through the downregulation by
E-cadherin expression and modulation of the AKT/Slug pathway
(28,39). Sack et al(40) reported that S100A4-induced cell
motility and metastasis are inhibited by the Wnt/β-catenin pathway
inhibitor calcimycin in colon cancer cells. Zhang et
al(27) reported that S100A4
mediates the cell invasion and metastasis of ESCC via the
regulation of MMP-2 and E-cadherin activity. Shen et
al(41) found that S100A4
protects gastric cancer cells from anoikis through regulation of αv
and α5 integrins. Hua et al(42) demonstrated that inhibition of S100A4
promotes apoptosis and suppresses proliferation of BGC823 gastric
cancer cells in vitro and in vivo. Xie et
al(43) documented that S100A4
mediates endometrial cancer invasion, and induction of S100A4 was
associated with the activation of Smads in tumor growth factor
(TGF)-β1 signaling. Based on these data and our current findings,
we hypothesize that S100A4 upregulation enhances the aggressive
phenotypes and behavior of malignant cancers through the
Wnt/β-catenin or TGF-β signaling pathways.
Although gastric cancer is a malignant tumor
originating from the same type of gastric epithelium, its
morphological features vary substantially among individual
patients. According to Lauren’s classification, IT carcinomas are
characterized by cohesive carcinoma cells forming gland-like
tubular structures with expanding or infiltrative growth patterns,
and include well- and moderately differentiated adenocarcinomas. DT
carcinomas display less apparent or no cell adhesion and contain
poorly differentiated signet-ring cell carcinoma (22,23).
In the present study, we noted that the protein and mRNA expression
of S100A4 was lower in IT carcinoma compared to that in DT,
indicating that S100A4 may play an important role in IT
carcinogenesis, but less so in the de novo carcinogenic
pathway, and may serve as the molecular basis for distinction
between the two types of carcinomas.
In the present study, we found that S100A4
expression was inversely correlated with the favorable prognosis of
patients with gastric carcinoma, which was consistent with previous
reports (24,25). Cox’s proportional risk analysis
indicated that the depth of invasion, lymphatic or venous invasion,
lymph node metastasis, TNM staging, and S100A4 expression were
independent factors for the poor prognosis of gastric carcinoma
patients. These findings suggest that S100A4 expression is an
independent prognostic factor for gastric carcinoma patients. Kho
et al(44) found that only
cytoplasmic S100A4 staining, but not nuclear staining, of the
frontal tissue was associated with poor survival of patients with
stage C colonic cancer. In contrast, Kang et al(37) showed that nuclear expression of the
S100A4 protein was found to be an independent prognostic factor for
poor survival of colorectal cancer patients. Similar results were
also obtained in pancreatic cancer (45), renal cell carcinoma (46), bladder cancer (31) and lung squamous cell carcinoma
(47). These findings suggest that
S100A4 expression is an independent and reliable indicator for a
less favorable prognosis of gastric cancer patients.
In summary, upregulation of S100A4 may play an
important role in the malignant transformation of gastric
epithelial cells and is positively related to growth, invasion,
metastasis, and prognosis of gastric carcinomas. Thus, S100A4 may
be considered as a promising marker to indicate the aggressive
behavior of gastric carcinomas. The distinct expression of S100A4
in DT gastric carcinoma can be employed to differentiate between
the intestinal- and diffuse-type carcinomas and may serve as a
molecular feature that distinguishes the two types of
carcinomas.
Acknowledgements
The study was supported by the Nature Science
Foundation of Liaoning Province (201202279).
References
|
1
|
Crew KD and Neugut AI: Epidemiology of
upper gastrointestinal malignancies. Semin Oncol. 31:450–464. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu M, Zheng HC, Xia P, et al: Comparison
in pathological behaviours and prognosis of gastric cancers from
general hospitals between China and Japan. Indian J Med Res.
132:295–302. 2010.PubMed/NCBI
|
|
3
|
Heizmann CW, Fritz G and Schafer BW: S100
proteins: structure, functions and pathology. Front Biosci.
7:d1356–d1368. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsai WC, Lin YC, Tsai ST, et al: Lack of
modulatory function of coding nucleotide polymorphism
S100A2_185G>A in oral squamous cell carcinoma. Oral Dis.
17:283–290. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sherbet GV and Lakshmi MS: S100A4 (MTS1)
calcium binding protein in cancer growth, invasion and metastasis.
Anticancer Res. 18:2415–2421. 1998.PubMed/NCBI
|
|
6
|
Kwak JM, Lee HJ, Kim SH, et al: Expression
of protein S100A4 is a predictor of recurrence in colorectal
cancer. World J Gastroenterol. 16:3897–3904. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boye K and Maelandsmo GM: S100A4 and
metastasis: a small actor playing many roles. Am J Pathol.
176:528–535. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mishra SK, Siddique HR and Saleem M:
S100A4 calcium-binding protein is key player in tumor progression
and metastasis: preclinical and clinical evidence. Cancer
Metastasis Rev. 31:163–172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng X, Yao Y, Xu Q, Tu K and Liu Q:
Evaluation of glioma-associated oncogene 1 expression and its
correlation with the expression of sonic hedgehog, E-cadherin and
S100a4 in human hepatocellular carcinoma. Mol Med Rep. 3:965–970.
2010.
|
|
10
|
Lo JF, Yu CC, Chiou SH, et al: The
epithelial-mesenchymal transition mediator S100A4 maintains
cancer-initiating cells in head and neck cancers. Cancer Res.
71:1912–1923. 2011. View Article : Google Scholar
|
|
11
|
Jenkinson SR, Barraclough R, West CR and
Rudland PS: S100A4 regulates cell motility and invasion in an in
vitro model for breast cancer metastasis. Br J Cancer. 90:253–262.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takenaga K, Nakamura Y and Sakiyama S:
Expression of antisense RNA to S100A4 gene encoding an S100-related
calcium-binding protein suppresses metastatic potential of
high-metastatic Lewis lung carcinoma cells. Oncogene. 14:331–337.
1997. View Article : Google Scholar
|
|
13
|
Maelandsmo GM, Hovig E, Skrede M, et al:
Reversal of the in vivo metastatic phenotype of human tumor cells
by an anti-CAPL (mts1) ribozyme. Cancer Res. 56:5490–5498.
1996.PubMed/NCBI
|
|
14
|
Garrett SC, Varney KM, Weber DJ and
Bresnick AR: S100A4, a mediator of metastasis. J Biol Chem.
281:677–680. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Helfman DM, Kim EJ, Lukanidin E and
Grigorian M: The metastasis associated protein S100A4: role in
tumour progression and metastasis. Br J Cancer. 92:1955–1958. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takenaga K, Nakamura Y, Sakiyama S,
Hasegawa Y, Sato K and Endo H: Binding of pEL98 protein, an
S100-related calcium-binding protein, to nonmuscle tropomyosin. J
Cell Biol. 124:757–768. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Watanabe Y, Usada N, Minami H, et al:
Calvasculin, as a factor affecting the microfilament assemblies in
rat fibroblasts transfected by src gene. FEBS Lett. 324:51–55.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kriajevska MV, Cardenas MN, Grigorian MS,
Ambartsumian NS, Georgiev GP and Lukanidin EM: Non-muscle myosin
heavy chain as a possible target for protein encoded by
metastasis-related mts-1 gene. J Biol Chem. 269:19679–19682.
1994.PubMed/NCBI
|
|
19
|
Saleem M, Kweon MH, Johnson JJ, et al:
S100A4 accelerates tumorigenesis and invasion of human prostate
cancer through the transcriptional regulation of matrix
metalloproteinase 9. Proc Natl Acad Sci USA. 103:14825–14830. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bjørnland K, Winberg JO, Odegaard OT, et
al: S100A4 involvement in metastasis: deregulation of matrix
metalloproteinases and tissue inhibitors of matrix
metalloproteinases in osteosarcoma cells transfected with an
anti-S100A4 ribozyme. Cancer Res. 59:4702–4708. 1999.PubMed/NCBI
|
|
21
|
Sobin LH and Wittekind CH: TNM
Classification of Malignant Tumors. 6th edition. John Wiley &
Sons; Hoboken, NJ: 2002
|
|
22
|
Zheng H, Takahashi H, Murai Y, et al:
Pathobiological characteristics of intestinal and diffuse-type
gastric carcinoma in Japan: an immunostaining study on the tissue
microarray. J Clin Pathol. 60:273–277. 2007. View Article : Google Scholar
|
|
23
|
Zheng HC, Li XH, Hara T, et al: Mixed-type
gastric carcinomas exhibit more aggressive features and indicate
the histogenesis of carcinomas. Virchows Arch. 452:525–534. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang YY, Ye ZY, Zhao ZS, Tao HQ and Chu
YQ: High-level expression of S100A4 correlates with lymph node
metastasis and poor prognosis in patients with gastric cancer. Ann
Surg Oncol. 17:89–97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim YJ, Kim MA, Im SA, et al:
Metastasis-associated protein S100A4 and p53 predict relapse in
curatively resected stage III and IV (M0) gastric cancer. Cancer
Invest. 26:152–158. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang H, Zhao K, Yu Q, Wang X, Song Y and
Li R: Evaluation of plasma and tissue S100A4 protein and mRNA
levels as potential markers of metastasis and prognosis in clear
cell renal cell carcinoma. J Int Med Res. 40:475–485. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang HY, Zheng XZ, Wang XH, Xuan XY, Wang
F and Li SS: S100A4 mediated cell invasion and metastasis of
esophageal squamous cell carcinoma via the regulation of MMP-2 and
E-cadherin activity. Mol Biol Rep. 39:199–208. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen D, Zheng XF, Yang ZY, et al: S100A4
silencing blocks invasive ability of esophageal squamous cell
carcinoma cells. World J Gastroenterol. 18:915–922. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim JH, Kim CN, Kim SY, et al: Enhanced
S100A4 protein expression is clinicopathologically significant to
metastatic potential and p53 dysfunction in colorectal cancer.
Oncol Rep. 22:41–47. 2009.PubMed/NCBI
|
|
30
|
Ismail NI, Kaur G, Hashim H and Hassan MS:
S100A4 overexpression proves to be independent marker for breast
cancer progression. Cancer Cell Int. 8:122008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Matsumoto K, Irie A, Satoh T, et al:
Expression of S100A2 and S100A4 predicts for disease progression
and patient survival in bladder cancer. Urology. 70:602–607. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zheng HC, Xu XY, Yu M, Takahashi H, Masuda
S and Takano Y: The role of Reg IV gene and its encoding product in
gastric carcinogenesis. Hum Pathol. 41:59–69. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang YC: Geographic pathology of gastric
dysplasia in China. Semin Surg Oncol. 10:100–106. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Horiuchi A, Hayashi T, Kikuchi N, et al:
Hypoxia upregulates ovarian cancer invasiveness via the binding of
HIF-1α to a hypoxia-induced, methylation-free hypoxia response
element of S100A4 gene. Int J Cancer. 131:1755–1767.
2012.PubMed/NCBI
|
|
35
|
Liu J, Guo Y, Fu S, Yang M, Sun KL and Fu
WN: Hypo-methylation-induced expression of S100A4 increases the
invasiveness of laryngeal squamous cell carcinoma. Oncol Rep.
23:1101–1107. 2010.PubMed/NCBI
|
|
36
|
Feng LZ, Zheng XY, Zhou LX, et al:
Correlation between expression of S100A4 and VEGF-C, and lymph node
metastasis and prognosis in gastric carcinoma. J Int Med Res.
39:1333–1343. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kang YG, Jung CK, Lee A, Kang WK, Oh ST
and Kang CS: Prognostic significance of S100A4 mRNA and protein
expression in colorectal cancer. J Surg Oncol. 105:119–124. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xie R, Loose DS, Shipley GL, Xie S,
Bassett RL Jr and Broaddus RR: Hypomethylation-induced expression
of S100A4 in endometrial carcinoma. Mod Pathol. 20:1045–1054. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang K, Zhang M, Zhao H, Yan B, Zhang D
and Liang J: S100A4 regulates motility and invasiveness of human
esophageal squamous cell carcinoma through modulating the AKT/Slug
signal pathway. Dis Esophagus. 25:731–739. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sack U, Walther W, Scudiero D, et al:
S100A4-induced cell motility and metastasis is restricted by the
Wnt/β-catenin pathway inhibitor calcimycin in colon cancer cells.
Mol Biol Cell. 22:3344–3354. 2011.PubMed/NCBI
|
|
41
|
Shen W, Chen D, Fu H, Liu S, Sun K and Sun
X: S100A4 protects gastric cancer cells from anoikis through
regulation of αv and α5 integrin. Cancer Sci. 102:1014–1018.
2011.PubMed/NCBI
|
|
42
|
Hua J, Chen D, Fu H, et al: Short hairpin
RNA-mediated inhibition of S100A4 promotes apoptosis and suppresses
proliferation of BGC823 gastric cancer cells in vitro and in vivo.
Cancer Lett. 292:41–47. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xie R, Schlumbrecht MP, Shipley GL, Xie S,
Bassett RL Jr and Broaddus RR: S100A4 mediates endometrial cancer
invasion and is a target of TGF-beta1 signaling. Lab Invest.
89:937–947. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kho PS, Jankova L, Fung CL, et al:
Overexpression of protein S100A4 is independently associated with
overall survival in stage C colonic cancer but only in cytoplasm at
the advancing tumour front. Int J Colorectal Dis. 27:1409–1417.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ikenaga N, Ohuchida K, Mizumoto K, et al:
S100A4 mRNA is a diagnostic and prognostic marker in pancreatic
carcinoma. J Gastrointest Surg. 13:1852–1858. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bandiera A, Melloni G, Freschi M, et al:
Prognostic factors and analysis of S100a4 protein in resected
pulmonary metastases from renal cell carcinoma. World J Surg.
33:1414–1420. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tsuna M, Kageyama S, Fukuoka J, et al:
Significance of S100A4 as a prognostic marker of lung squamous cell
carcinoma. Anticancer Res. 29:2547–2554. 2009.PubMed/NCBI
|