Introduction
Arsenic trioxide (As2O3) has
shown substantial efficacy in the treatment of patients with acute
promyelocytic leukemia (APL), a specific subtype of acute myeloid
leukemia (AML) (1). Previous
studies have demonstrated that As2O3
influences various intracellular signaling pathways, which may
result in the induction of apoptosis, the inhibition of growth and
angiogenesis, and the promotion of differentiation (2). In clinical studies, the complete
remission (CR) rate of As2O3 treatment was
substantial (3–5). However, at the same time, a
significant portion of patients could not achieve CR in spite of
the treatment using As2O3 as well as
all-trans-retinoic acid (ATRA) and conventional
chemotherapy. Hence, it is necessary to investigate a novel
treatment method overcoming the resistance of current
treatments.
The 1,25-dihydroxyvitamin D3 (also known
as 1,25(OH)2D3 or calcitriol), the active
form of vitamin D, is also known to regulate cell proliferation and
differentiation as well as classical actions on calcium homeostasis
(6–8). 1,25(OH)2D3 was
found to cause differentiation of myeloid leukemic cells and to
prolong the survival of leukemic mice (9,10). In
addition, previous studies have demonstrated that
1,25(OH)2D3 causes differentiation of the
chemo-naïve APL cell line HL-60 (11,12)
and the ATRA-resistant APL cell line UF-1 (13). However, in a previous clinical study
performed in patients with myelodysplastic syndrome,
1,25(OH)2D3 single therapy resulted in
hypercalcemia in half of the patients before concentrations
necessary for sufficient anti-leukemic activity could be achieved
(14). This hypercalcemic side
effect has limited the clinical application of
1,25(OH)2D3 single therapy to hematologic
malignancies.
In this study, we aimed to elucidate the
anti-leukemic effect of 1,25(OH)2D3 combined
with As2O3 on human AML cells. However,
considering the dose-dependent hypercalcemic effect of
1,25(OH)2D3, we used a low-dose of
1,25(OH)2D3. Thus, the objective of this
study was to elucidate the anti-leukemic effect of low-dose
1,25(OH)2D3 combined with
As2O3 on HL-60 and K562 cells.
Materials and methods
Cell culture
The HL-60 and K562 cell lines were obtained from the
Korean Cell Line Bank (Seoul, Korea). HL-60 and K562 cells were
cultured in RPMI-1640 medium containing 10% fetal bovine serum
(FBS), 100 units of penicillin and 100 μg/ml of streptomycin in a
37°C incubator under 5% CO2.
Measurement of cytotoxicity with trypan
blue exclusion test
Inhibition of the proliferation rate of HL-60 and
K562 cells was measured by the trypan blue exclusion test. The
cytotoxic effect of As2O3 on HL-60 and K562
cells was examined by treating 2.4×105 cells with 0,
0.5, 1.0, 1.5, 2.0 and 3.0 μM As2O3 for 24,
48 and 72 h. The cytotoxic effect of
1,25(OH)2D3 on HL-60 and K562 cells was
examined by treating 2.5×104 cells with 0, 100, 200,
300, 400 and 500 μM As2O3 for 24, 48 and 72
h. The effect of the combination treatment on HL-60 cells was
evaluated by treating 1.2×106 cells for 24 h with the
following combinations: 0.5 μM As2O3 plus 50
nM 1,25(OH)2D3, 1.0 μM
As2O3 plus 100 nM
1,25(OH)2D3, 1.5 μM
As2O3 plus 150 nM
1,25(OH)2D3, 2.0 μM
As2O3 plus 200 nM
1,25(OH)2D3 and 3.0 μM
As2O3 plus 300 nM
1,25(OH)2D3. The effect of the combination
treatment on K562 cells was evaluated by treating
1.2×106 cells for 24 h with the following combinations:
0.25 μM As2O3 plus 25 nM
1,25(OH)2D3, 0.50 μM
As2O3 plus 50 nM
1,25(OH)2D3, 0.75 μM
As2O3 plus 75 nM
1,25(OH)2D3, 1.00 μM
As2O3 plus 100 nM
1,25(OH)2D3 and 1.50 μM
As2O3 plus 150 nM
1,25(OH)2D3.
After treatment, we diluted the cells in complete
medium, without FBS, to an approximate concentration of
1–2×105 cells/ml. Then, we added 0.1 ml of 0.4% trypan
blue solution to 0.5 ml of the cell suspensions and mixed them
thoroughly. These mixtures were incubated for 5 min at 15–30°C. The
cell count was calculated using a hemocytometer (Paul Marienfeld
GmbH & Co. KG, Lauda-Königshofen, Germany) and a microscope
(CKX41; Olympus, Tokyo, Japan). Each experiment was performed in
duplicate.
mRNA extraction and RT-PCR
HL-60 and K562 cells were treated for 24 h with the
following combinations: control; 100 nM
1,25(OH)2D3; 1.0 μM
As2O3; 100 nM
1,25(OH)2D3 plus 1.0 μM
As2O3; and 100 nM
1,25(OH)2D3 plus 3.0 μM
As2O3. Total RNA was isolated from the cells
using TRIzol reagent. The RNA pellets obtained were dissolved in
diethylpyrocarbonate (DEPC)-treated H2O at
concentrations of 0.5 to 1.0 μg/μl, and then stored at −70°C. The
quantity and quality of the RNA preparations were determined by
measuring the absorbance at 260 and 280 nm. One microgram of total
RNA was reverse-transcribed using a first strand cDNA synthesis kit
with random primer p(dN)6 and the primers listed in
Table I. The amplification
conditions used were 35 cycles at −94°C for 30 sec, 60°C for 30 sec
and 72°C for 30 sec. All data were normalized to the internal
control glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
 | Table IPrimers used for RT-PCR analysis of
Bcl-2, Bax, caspase-3 and GAPDH genes. |
Table I
Primers used for RT-PCR analysis of
Bcl-2, Bax, caspase-3 and GAPDH genes.
| Gene | Primer
sequence |
|---|
| Bcl-2 | F:
5′-TTTGAGTTCGGTGGGGTCAT-3′
R: 5′-TGACTTCACTTGTGGCCCAG-3′ |
| Bax | F:
5′-TGGCAGCTGACATGTTTTCTGAC-3′
R: 5′-TCACCCAACCACCCTGGTCTT-3′ |
| Caspase-3 | F:
5′-GCAGCAAACCTCAGGGAAAC-3′
R: 5′-TGTCGGCATACTGTTTCAGCA-3′ |
| GAPDH | F:
5′-CCACATCGCTCAGACACCAT-3′
R: 5′-TGACAAGCTTCCCGTTCTCA-3′ |
Western blot analysis
As2O3- or
1,25(OH)2D3-treated HL-60 and K562 cells were
prepared in the same way as described for the RT-PCR analysis.
These cells were homogenized in 10 mM Tris (pH 7.4), 1 mM sodium
vanadate (Na3VO4) and 1% sodium dodecyl
sulfate (SDS). These homogenates were boiled for 5 min at 95°C and
centrifuged at 13,000 × g for 15 min at 4°C. The pellet was
discarded and the supernatant containing the protein was
transferred to a clean tube. The total protein concentration was
measured using the micro-bicinchoninic acid (BCA) protein assay
(Pierce, Rockford, IL, USA) in accordance with manufacturer's
instructions. Samples containing 30 μg of protein, along with the
molecular weight marker (BenchMark™ Pre-Stained Protein Ladder;
Invitrogen, Grand Island, NY, USA), were subjected to 10% SDS
polyacrylamide gel electrophoresis under reducing conditions. The
proteins were then transferred to polyvinylidene difluoride (PVDF)
membranes (Millipore, Billerica, MA, USA). After nonspecific sites
were blocked with 5% powdered skim milk in 0.05% Triton
X-100/Tris-buffered saline (TBS-T) for 1 h, blots were incubated
overnight with an IgG-purified rabbit polyclonal Bcl-2, Bax, or
caspase-3 antibody (Cell Signaling Technology, Danvers, MA, USA) in
a solution containing 5% powdered skim milk and 0.05% Triton
X-100/TBS. The blots were then washed three times in TBS-T for 10
min each and incubated with a peroxidase-conjugated goat
anti-rabbit IgG at a concentration of 1 μg/ml in 5% powdered skim
milk in 0.05% TBS-T. All samples were also blotted for β-actin
(clone AC-15; Sigma-Aldrich, Dublin, Ireland) to normalize protein
amounts.
Evaluation of apoptosis using flow
cytometry
As2O3- or
1,25(OH)2D3-treated HL-60 cells were prepared
similarly as described in the RT-PCR analysis. The cells were
washed twice with cold PBS and then resuspended in 1X binding
buffer at a concentration of 2×105 cells/ml. A total of
100 μl of the mixtures was then transferred to a 5-ml culture tube
and then 5 μl of fluorescein-conjugated Annexin V (Annexin V-FITC)
and 5 μl of propidium iodide (PI) were added to the culture tube.
The cells were gently vortexed and incubated for 15 min at room
temperature in the dark, and 150 μl of binding buffer was added to
each tube. Flow cytometry was then performed within 1 h.
Statistical analysis
The combined effects of As2O3
and 1,25(OH)2D3 were analyzed by CalcuSyn
software (Biosoft, Ferguson, MO, USA) using the Chou-Talalay method
(15). This method is based on the
median-effect equation for a dose-effect relationship
fa/fu = (D/Dm)m, where
D is the dose, Dm is the
IC50, fa is the fraction
affected by dose D, fu is the unaffected
fraction (fu = 1 - fa) and m is a
coefficient of the sigmoidicity of the dose-effect curve. The
combination index (CI) was determined on the basis of the
isobologram analysis for mutually exclusive effects: CI =
(D)1/(Dx)1 +
(D)2/(Dx)2,
where (Dx)1 and
(Dx)2 are the concentrations of
As2O3 and 1,25(OH)2D3
which inhibit cell growth by x% and (D)1 and
(D)2 are the drug concentrations in the
combination treatments which inhibit cell growth by x%.
(Dx)1 and
(Dx)2 values can be determined
by a rearrangement of equation D = Dm (fa/(1-
fa))1/m. The CI values of <1, 1 and >1
indicate synergism, an additive effect, and antagonism,
respectively.
Results
Synergistic cytotoxic effect of the
combination treatment of As2O3 and low-dose
1,25(OH)2D3
As shown in Fig. 1,
the viability of HL-60 and K562 cells was in reverse proportion to
the concentration of As2O3 (0, 0.5, 1.0, 1.5,
2.0 and 3.0 μM) or 1,25(OH)2D3 (0, 100, 200,
300, 400 and 500 nM). K562 cells were more sensitive to both
As2O3 and 1,25(OH)2D3
than HL-60 cells.
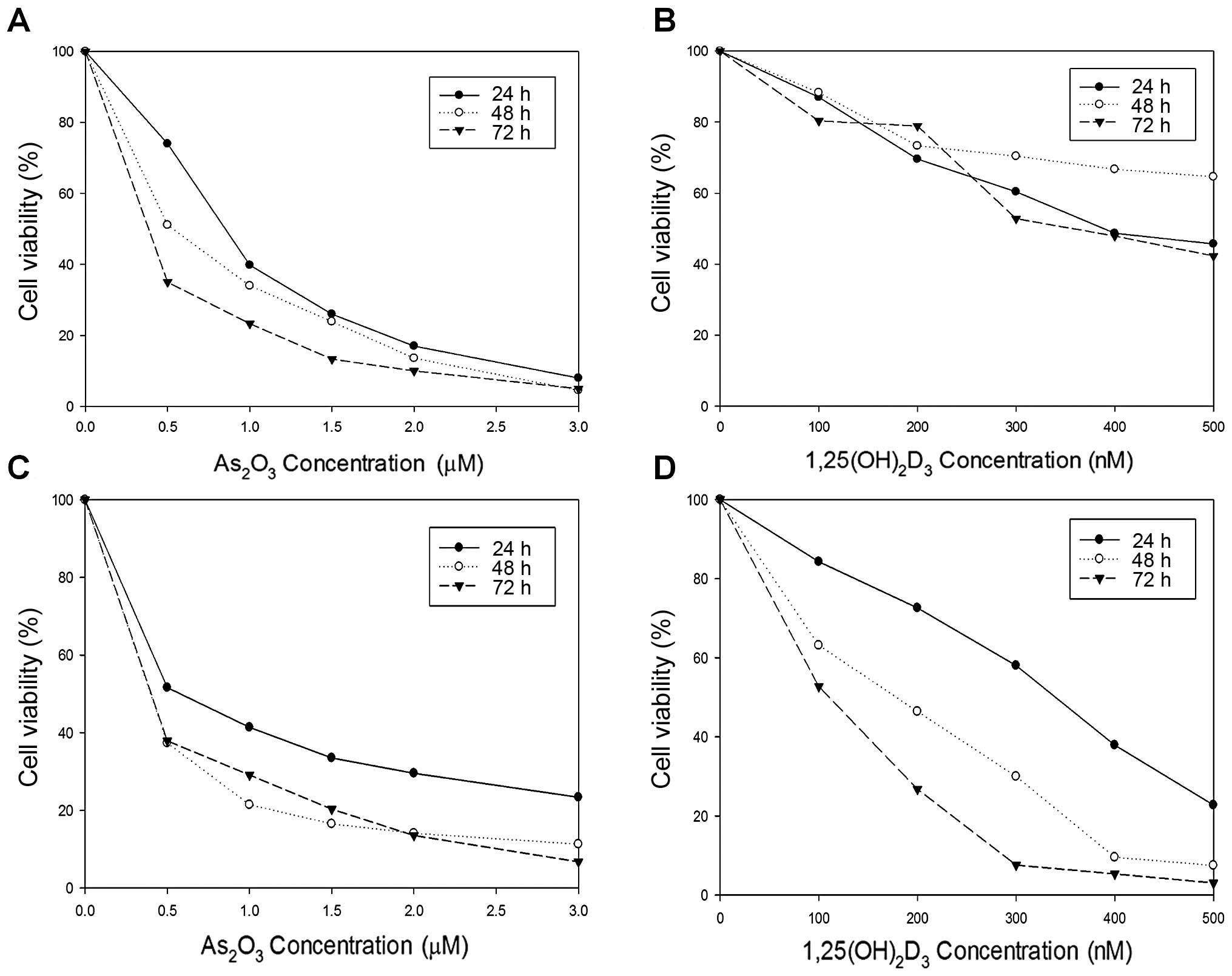 | Figure 1Cytotoxic effects of
As2O3 and 1,25(OH)2D3
on HL-60 and K562 cells. HL-60 cells were treated with (A)
As2O3 and (B)
1,25(OH)2D3 at a variety of concentrations
(0, 0.5, 1.0, 1.5, 2.0 and 3.0 μM for As2O3
and 0, 100, 200, 300, 400 and 500 nM for
1,25(OH)2D3). K562 cells were also treated
with (C) As2O3 and (D)
1,25(OH)2D3 at the same conditions. The cell
viability was in reverse proportion to the concentration of
As2O3 or 1,25(OH)2D3.
K562 cells tended to be more sensitive to both
As2O3 and 1,25(OH)2D3
than HL-60 cells. |
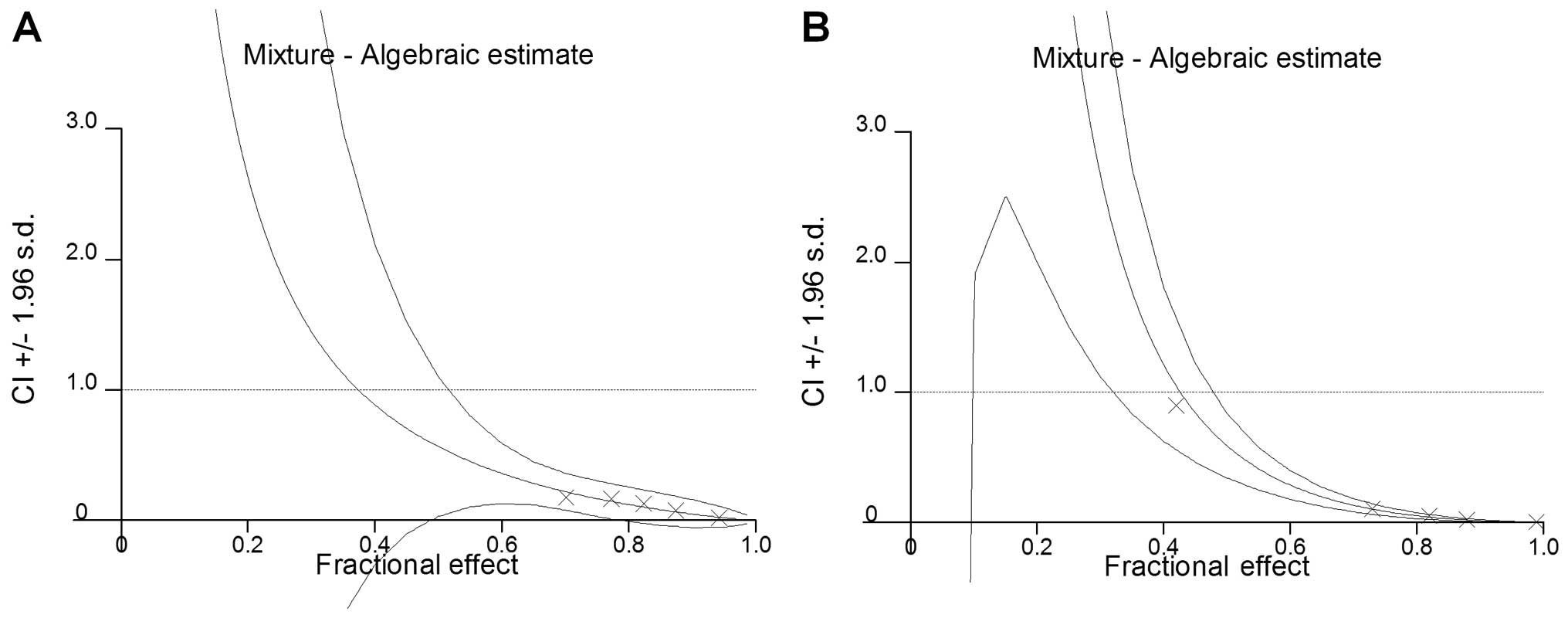
The results of the combination treatment with both
As2O3 and low-dose
1,25(OH)2D3 are summarized in Table II and III. In both HL-60 and K562 cells, the
combination treatment with As2O3 and
1,25(OH)2D3 at a 10:1 ratio revealed a more
prominent cytotoxicity compared to single treatments with either
As2O3 or 1,25(OH)2D3.
Additionally, as shown in Fig. 2,
in both HL-60 and K562 cells, the CI values of all combinations
evaluated were <1, which suggests evident synergistic cytotoxic
effects for As2O3 and low-dose
1,25(OH)2D3.
 | Table IICombined effects of
As2O3 and 1,25(OH)2D3
in HL-60 cells. |
Table II
Combined effects of
As2O3 and 1,25(OH)2D3
in HL-60 cells.
| | | | Combination
treatment | | |
|---|
| | | |
| | |
|---|
|
As2O3 (μM) | Fraction of cell
death |
1,25(OH)2D3
(nM) | Fraction of cell
death |
As2O3 (μM) |
1,25(OH)2D3
(nM) | Fraction of cell
death | CI |
|---|
| 0.5 | 0.538 | 50 | 0.097 | 0.5 | 50 | 0.702 | 0.179 |
| 1.0 | 0.570 | 100 | 0.112 | 1.0 | 100 | 0.773 | 0.167 |
| 1.5 | 0.608 | 150 | 0.124 | 1.5 | 150 | 0.824 | 0.129 |
| 2.0 | 0.637 | 200 | 0.135 | 2.0 | 200 | 0.875 | 0.075 |
| 3.0 | 0.750 | 300 | 0.157 | 3.0 | 300 | 0.943 | 0.019 |
 | Table IIICombined effects of
As2O3 and 1,25(OH)2D3
in K562 cells. |
Table III
Combined effects of
As2O3 and 1,25(OH)2D3
in K562 cells.
| | | | Combination
treatment | | |
|---|
| | | |
| | |
|---|
|
As2O3 (μM) | Fraction of cell
death |
1,25(OH)2D3
(nM) | Fraction of cell
death |
As2O3 (μM) |
1,25(OH)2D3
(nM) | Fraction of cell
death | CI |
|---|
| 0.25 | 0.409 | 25 | 0.100 | 0.25 | 25 | 0.589 | 0.901 |
| 0.50 | 0.476 | 50 | 0.143 | 0.50 | 50 | 0.622 | 0.103 |
| 0.75 | 0.516 | 75 | 0.154 | 0.75 | 75 | 0.675 | 0.050 |
| 1.00 | 0.565 | 100 | 0.174 | 1.00 | 100 | 0.760 | 0.024 |
| 1.50 | 0.608 | 150 | 0.264 | 1.50 | 150 | 0.848 | 0.000 |
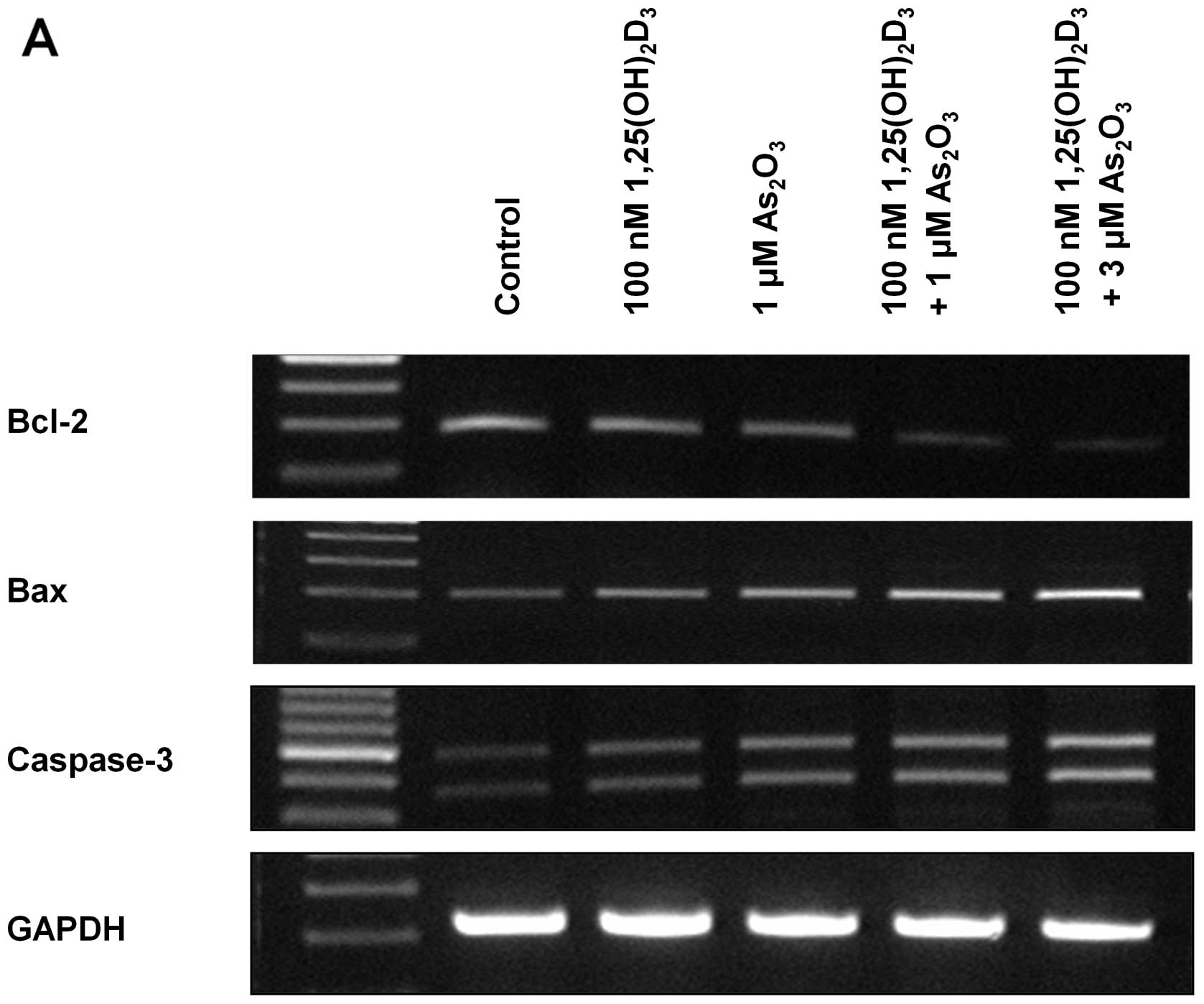
Expression of apoptosis-related genes
(mRNA)
The expression of apoptosis-related genes in HL-60
and K562 cells was analyzed using the RT-PCR method (Fig. 3). In both HL-60 and K562 cell lines,
decreased Bcl-2 and increased Bax and caspase-3 expression was
observed in either As2O3- or
1,25(OH)2D3-treated cells compared to the
control. In addition, combination treatment using both
As2O3 and 1,25(OH)2D3
more prominently decreased Bcl-2 expression and increased Bax and
caspase-3 expression. Additionally, the effect of the combination
treatment was enhanced in proportion to the increased concentration
of As2O3; 3 μM
As2O3-treated cells showed decreased Bcl-2
and increased Bax and caspase-3 expression when compared to the
expression in 1 μM As2O3-treated cells.
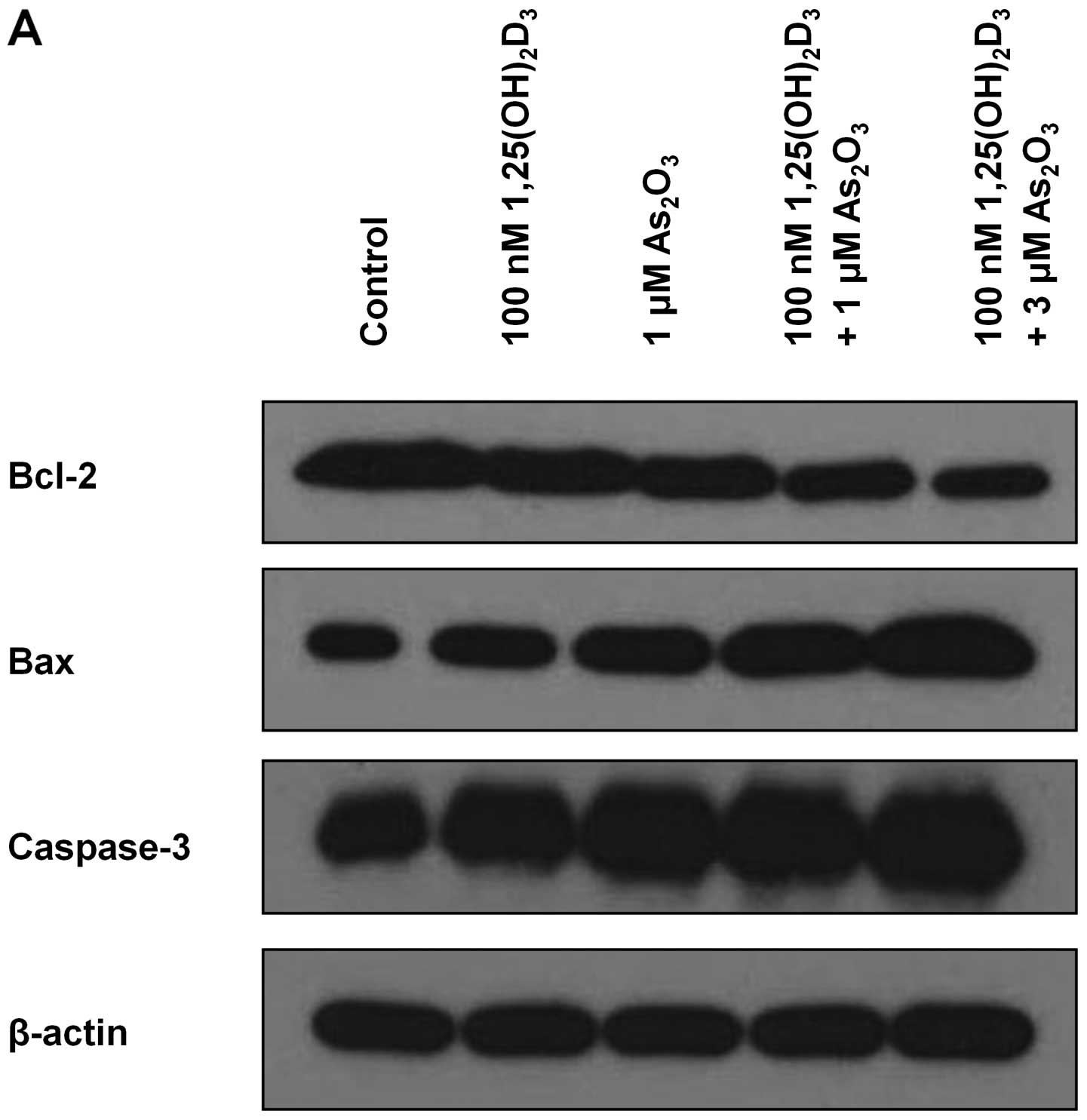
Expression of apoptosis-related
proteins
Western blot analysis was performed to analyze the
expression of apoptosis-related proteins in the HL-60 and K562
cells. As shown in Fig. 4, the
results for both HL-60 and K562 cell lines were identical to those
of the RT-PCR analysis (Fig. 3).
The combination treatment enhanced the production of pro-apototic
Bax and caspase-3 proteins and reduced the production of
anti-apoptotic Bcl-2 protein. In addition, the effect of the
combination treatment was enhanced in proportion to the increased
concentration of As2O3.
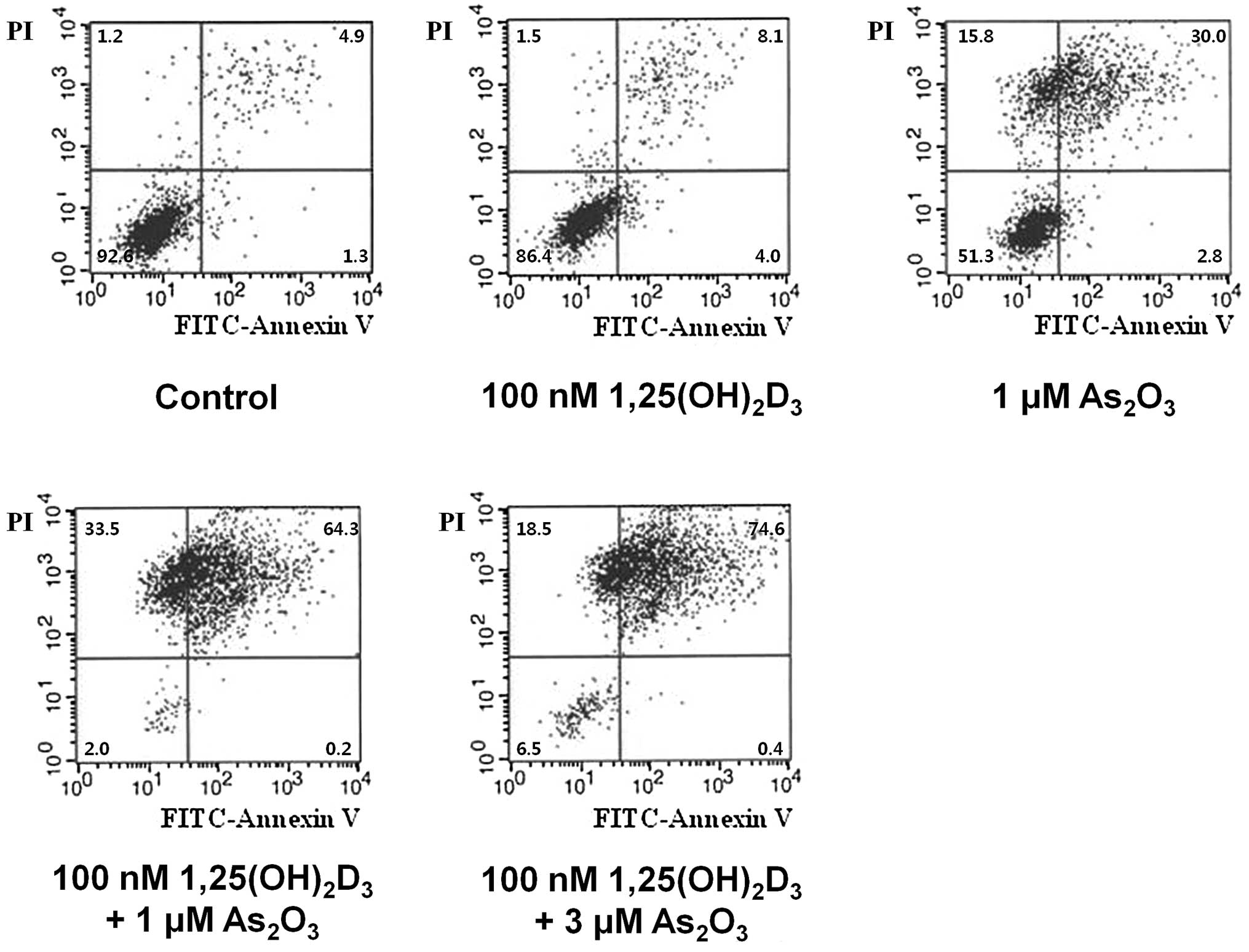
Enhancement of
As2O3 and
1,25(OH)2D3-induced apoptosis
HL-60 cells were labeled with Annexin V-FITC and
PI, and analyzed using flow cytometry to differentiate whether the
main cause of cell death was apoptosis or necrosis. As shown in
Fig. 5, cell death was
significantly increased after the combination treatment with
As2O3 and 1,25(OH)2D3
in the HL-60 cells. The proportion of living cells (Annexin V-FITC-
and PI-negative) was 92.6% in the control; 51.3% in the cells
treated with 1.0 μM As2O3; 86.4% in the cells
treated with 100 nM 1,25(OH)2D3; and 2.0% in
the cells treated with 1.0 μM As2O3 plus 100
nM 1,25(OH)2D3. The proportion of early
apoptotic cells (Annexin V-FITC-positive and PI-negative) was 1.3%
in the control; 2.8% in the cells treated with 1.0 μM
As2O3; 4.0% in the cells treated with 100 nM
1,25(OH)2D3; and 0.2% in the cells treated
with 1.0 μM As2O3 plus 100 nM
1,25(OH)2D3. The proportion of late apoptotic
cells (Annexin V-FITC- and PI-positive) was 4.9% in the control;
30.0% in the cells treated with 1.0 μM As2O3;
8.1% in the cells treated with 100 nM
1,25(OH)2D3; and 64.3% in the cells treated
with 1.0 μM As2O3 plus 100 nM
1,25(OH)2D3. The proportion of necrotic cells
(Annexin V-FITC-negative and PI-positive) was 1.2% in the control;
15.8% in the cells treated with 1.0 μM As2O3;
1.5% in the cells treated with 100 nM
1,25(OH)2D3; and 33.5% in the cells treated
with 1.0 μM As2O3 plus 100 nM
1,25(OH)2D3. In concordance with RT-PCR and
western blot analysis, the combination treatment resulted in a more
prominent apoptotic cell death compared to the single-drug
treatments. In contrast, the contribution of necrosis to cell death
was relatively smaller compared to apoptosis in all treatment
groups.
Discussion
Previous studies have demonstrated that
anti-leukemic activity of As2O3 is mainly due
to its ability to induce apoptosis via various mechanisms including
downregulation of Bcl-2, upregulation of caspases, and generation
of reactive oxygen species (ROS) (16–18).
In addition, 1,25(OH)2D3 was also found to
induce apoptosis of various types of cells including colon cancer,
breast cancer, prostate cancer and normal adipocytes through the
activation of the apoptosis pathway (19–23).
In concordance with these results, our study found that treatment
with As2O3 and
1,25(OH)2D3 each inhibited the proliferation
of HL-60 and K562 cells, increasing the production of pro-apoptotic
Bax and caspase-3 proteins and decreasing the production of
anti-apoptotic Bcl-2 protein. In addition, in the flow cytometric
analysis using Annexin V-FITC and PI, the main cause of cell death
induced by As2O3 and
1,25(OH)2D3 was apoptosis, which suggests an
evident pro-apoptotic effect of these drugs on AML cells.
In order to overcome the resistance of
As2O3, which is an active drug against APL,
we aimed to evaluate the additional benefit of
1,25(OH)2D3 in combination with
As2O3 on HL-60 and K562 cells. Due to the
serious hypercalcemic side effect, the clinical application of
1,25(OH)2D3 single therapy to hematologic
malignancies is limited in spite of its potent in vitro
anti-leukemic activity (11,24).
Considering this side effect, we chose a relatively low
concentration of 1,25(OH)2D3, which alone
could not show sufficient anti-leukemic activity. Despite its low
concentration, 1,25(OH)2D3 combined with
As2O3 showed an evident synergistic
anti-leukemic effect on both HL-60 and K562 cells. To the best of
our knowledge, this is the first study demonstrating the
synergistic anti-leukemic effect of this combination treatment
against AML cells. This combination treatment activated the
apoptosis pathway of cells more prominently than the single
treatments. Moreover, flow cytometric analysis showed that the
combination treatment resulted in a more prominent apoptotic cell
death compared to the single-drug treatments. The results of this
study may provide evidence that low-dose
1,25(OH)2D3 may be used for improving the
therapeutic efficacy of As2O3 for the
treatment of patients with AML.
Since apoptosis was the dominant cause of cell
death in this study, we mainly focused on the activation of the
apoptosis pathway induced by 1,25(OH)2D3 and
As2O3. However, As2O3
is also known to induce caspase-independent necrotic cell death via
the mitochondrial death pathway (25). In this study, the effect of necrosis
was relatively small when compared to apoptosis, but it was also
not negligible. The proportion of necrosis in the
1,25(OH)2D3-treated cells (1.5%) did not
appear to be different from that in the control group (1.2%).
However, the proportion of As2O3-induced
necrosis (15.8%) was substantial. In addition,
1,25(OH)2D3 profoundly increased the
proportion of necrosis (33.5%) as well as apoptosis when combined
with As2O3. This novel finding regarding the
effect of 1,25(OH)2D3 on
As2O3-induced necrosis also warrants further
investigation.
It is known that both As2O3
and 1,25(OH)2D3 influence intracellular
calcium homeostasis of cells, resulting in induction of apoptosis
(20,23,26–28).
Since these drugs share a common pathway, it is speculated that the
synergistic cytotoxicity of these agents would be attributed to
intracellular calcium homeostasis and their association with
apoptosis induction. Although we did not investigate the calcium
signaling pathway in this study, this hypothesis should be
validated in subsequent studies.
In summary, low-dose
1,25(OH)2D3 in combination with
As2O3 synergistically inhibited proliferation
of the HL-60 and K562 cell lines. In addition, low-dose
1,25(OH)2D3 combined with
As2O3 more prominently activated the
apoptosis pathway than a single treatment using either
1,25(OH)2D3 or As2O3.
The main cause of cell death was also apoptosis. Our results
suggest that low-dose 1,25(OH)2D3 could be
applied to improving the therapeutic efficacy of
As2O3 against AML.
Acknowledgements
This study was supported by grant no. 2010-1153
from the Seoul National University Hospital Research Fund.
References
|
1
|
Sanz MA and Lo-Coco F: Modern approaches
to treating acute promyelocytic leukemia. J Clin Oncol. 29:495–503.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miller WH Jr, Schipper HM, Lee JS, Singer
J and Waxman S: Mechanisms of action of arsenic trioxide. Cancer
Res. 62:3893–3903. 2002.PubMed/NCBI
|
|
3
|
Shen ZX, Chen GQ, Ni JH, et al: Use of
arsenic trioxide (As2O3) in the treatment of
acute promyelocytic leukemia (APL): II. Clinical efficacy and
pharmacokinetics in relapsed patients. Blood. 89:3354–3360.
1997.PubMed/NCBI
|
|
4
|
Niu C, Yan H, Yu T, et al: Studies on
treatment of acute promyelocytic leukemia with arsenic trioxide:
remission induction, follow-up, and molecular monitoring in 11
newly diagnosed and 47 relapsed acute promyelocytic leukemia
patients. Blood. 94:3315–3324. 1999.
|
|
5
|
Soignet SL, Maslak P, Wang ZG, et al:
Complete remission after treatment of acute promyelocytic leukemia
with arsenic trioxide. N Engl J Med. 339:1341–1348. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nagpal S, Na S and Rathnachalam R:
Noncalcemic actions of vitamin D receptor ligands. Endocr Rev.
26:662–687. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Deeb KK, Trump DL and Johnson CS: Vitamin
D signalling pathways in cancer: potential for anticancer
therapeutics. Nat Rev Cancer. 7:684–700. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Krishnan AV, Moreno J, Nonn L, et al:
Novel pathways that contribute to the anti-proliferative and
chemopreventive activities of calcitriol in prostate cancer. J
Steroid Biochem Mol Biol. 103:694–702. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abe E, Miyaura C, Sakagami H, et al:
Differentiation of mouse myeloid leukemia cells induced by 1
alpha,25-dihydroxyvitamin D3. Proc Natl Acad Sci USA.
78:4990–4994. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou JY, Norman AW, Chen DL, Sun GW,
Uskokovic M and Koeffler HP: 1,25-Dihydroxy-16-ene-23-yne-vitamin
D3 prolongs survival time of leukemic mice. Proc Natl
Acad Sci USA. 87:3929–3932. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McCarthy DM, San Miguel JF, Freake HC, et
al: 1,25-dihydroxyvitamin D3 inhibits proliferation of
human promyelocytic leukaemia (HL60) cells and induces
monocyte-macrophage differentiation in HL60 and normal human bone
marrow cells. Leuk Res. 7:51–55. 1983.
|
|
12
|
Studzinski GP, Bhandal AK and Brelvi ZS:
Cell cycle sensitivity of HL-60 cells to the
differentiation-inducing effects of 1-alpha,25-dihydroxyvitamin
D3. Cancer Res. 45:3898–3905. 1985.PubMed/NCBI
|
|
13
|
Muto A, Kizaki M, Yamato K, et al:
1,25-Dihydroxyvitamin D3 induces differentiation of a
retinoic acid-resistant acute promyelocytic leukemia cell line
(UF-1) associated with expression of p21(WAF1/CIP1) and p27(KIP1).
Blood. 93:2225–2233. 1999.PubMed/NCBI
|
|
14
|
Koeffler HP, Hirji K and Itri L:
1,25-Dihydroxyvitamin D3: in vivo and in vitro effects
on human preleukemic and leukemic cells. Cancer Treat Rep.
69:1399–1407. 1985.
|
|
15
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: the combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen GQ, Zhu J, Shi XG, et al: In vitro
studies on cellular and molecular mechanisms of arsenic trioxide
(As2O3) in the treatment of acute
promyelocytic leukemia: As2O3 induces NB4
cell apoptosis with downregulation of Bcl-2 expression and
modulation of PML-RAR alpha/PML proteins. Blood. 88:1052–1061.
1996.PubMed/NCBI
|
|
17
|
Jing Y, Dai J, Chalmers-Redman RM, Tatton
WG and Waxman S: Arsenic trioxide selectively induces acute
promyelocytic leukemia cell apoptosis via a hydrogen
peroxide-dependent pathway. Blood. 94:2102–2111. 1999.PubMed/NCBI
|
|
18
|
Yedjou C, Tchounwou P, Jenkins J and
McMurray R: Basic mechanisms of arsenic trioxide (ATO)-induced
apoptosis in human leukemia (HL-60) cells. J Hematol Oncol.
3:282010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vandewalle B, Wattez N and Lefebvre J:
Effects of vitamin D3 derivatives on growth,
differentiation and apoptosis in tumoral colonic HT 29 cells:
possible implication of intracellular calcium. Cancer Lett.
97:99–106. 1995.
|
|
20
|
Mathiasen IS, Sergeev IN, Bastholm L,
Elling F, Norman AW and Jäättelä M: Calcium and calpain as key
mediators of apoptosis-like death induced by vitamin D compounds in
breast cancer cells. J Biol Chem. 277:30738–30745. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guzey M, Kitada S and Reed JC: Apoptosis
induction by 1alpha,25-dihydroxyvitamin D3 in prostate
cancer. Mol Cancer Ther. 1:667–677. 2002.PubMed/NCBI
|
|
22
|
Sergeev IN: Calcium as a mediator of
1,25-dihydroxyvitamin D3-induced apoptosis. J Steroid
Biochem Mol Biol. 89–90:419–425. 2004.PubMed/NCBI
|
|
23
|
Sergeev IN: 1,25-Dihydroxyvitamin
D3 induces Ca2+-mediated apoptosis in
adipocytes via activation of calpain and caspase-12. Biochem
Biophys Res Commun. 384:18–21. 2009.
|
|
24
|
Richard C, Mazo E, Cuadrado MA, et al:
Treatment of myelodysplastic syndrome with 1.25-dihydroxy-vitamin
D3. Am J Hematol. 23:175–178. 1986. View Article : Google Scholar
|
|
25
|
Scholz C, Wieder T, Stärck L, et al:
Arsenic trioxide triggers a regulated form of caspase-independent
necrotic cell death via the mitochondrial death pathway. Oncogene.
24:1904–1913. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Florea AM, Splettstoesser F and Busselberg
D: Arsenic trioxide (As2O3) induced calcium
signals and cytotoxicity in two human cell lines: SY-5Y
neuroblastoma and 293 embryonic kidney (HEK). Toxicol Appl
Pharmacol. 220:292–301. 2007.
|
|
27
|
Günes DA, Florea AM, Splettstoesser F and
Büsselberg D: Co-application of arsenic trioxide
(As2O3) and cisplatin (CDDP) on human SY-5Y
neuroblastoma cells has differential effects on the intracellular
calcium concentration ([Ca2+]i) and cytotoxicity.
Neurotoxicology. 30:194–202. 2009.
|
|
28
|
Cai BZ, Meng FY, Zhu SL, et al: Arsenic
trioxide induces the apoptosis in bone marrow mesenchymal stem
cells by intracellular calcium signal and caspase-3 pathways.
Toxicol Lett. 193:173–178. 2010. View Article : Google Scholar : PubMed/NCBI
|