Introduction
microRNAs (miRNAs) are recognized as a class of
small (19–25 nt) non-coding RNAs that play important roles in gene
regulation by partially or fully complementary matching with the
3′-untranslated region (UTR) of target mRNAs, and trigger a
transcriptional and/or post-transcriptional suppression of the
target gene (1,2). They are involved in numerous
physiological functions such as cell differentiation, migration,
proliferation, apoptosis and senescence (3). Recently, it was reported that more
than 1,000 human miRNAs frequently target regions related to cancer
development (4), and this complex
collection could be further classified as oncogenic,
tumor-suppressive, or context-dependent miRNAs (5).
miR-330 was first discovered by Weber (6), and described as a downregulated miRNA
in tumors by Gaur et al(7),
when the authors compared the differential expression patterns of
241 miRNAs between 13 normal and 59 tumor-derived cell lines. This
finding was further confirmed by Ruike et al(8) who investigated the miRNA and mRNA
expression associations across 16 human cell lines. mir-330 is
located on chromosome 19q13, a fragile point which was reportedly
associated with tumor aggressiveness in prostate cancer (9,10). To
date, investigations concerning the biological functions of miR-330
in cancer are sparse. Recently, Qu et al(11) reported its oncogenic role in human
glioblastoma by regulating the SH3GL2 gene. In prostate cancer,
however, it was reported as a tumor-suppressor by Lee et
al(12), who demonstrated its
significantly lower expression and inverse correlation with E2F1 in
both prostate cancer cell lines and cancer specimens. They further
discovered its proapoptotic role through E2F1-mediated suppression
of Akt phosphorylation.
Specificity protein 1 (Sp1), the essential member of
the Sp/Krupel-like (KLF) transcription factor family, is regarded
as ubiquitous in cells and participates in the tumorigenesis of a
variety of cancers including prostate (13). It plays an important role in tumor
progression, including cell proliferation, angiogenesis,
differentiation, apoptosis, migration and invasion (14), representing itself as an ideal
target for cancer treatment. Here, we report the
post-transcriptional regulation of Sp1 by miR-330. Our
investigation elucidated the tumor-suppressive role of miR-330 in
prostate cancer in an anti-migratory and -invasive manner.
Materials and methods
Reagents
All the RNA duplexes were synthesized by GenePharma
Co., Ltd. (Shanghai China). The sense sequences of the duplexes
were as follows: miR-330a mimic (termed as miR-330),
5′-GCAAAGCACACGGCCUGCAGAGA-3′; negative control RNA (termed as NC),
5′-ACUACUGAGUGACA GUAGA-3′; small interfering RNA for Sp1 (termed
as siRNA), 5′-AAAGCGCUUCAUGAGGAGUGA-3′ (15). All of the primers for quantitative
real-time PCR (qPCR) were synthesized by Sangon Biotech (Shanghai
China), whose sequences were as follows: GAPDH forward,
5′-AAGGTGAAGGTCG GAGTCA-3′ and reverse, 5′-GGAAGATGGTGATGGGATT
T-3′; Sp1 forward, 5′-AGTTCCAGACCGTTGATGGG-3′ and reverse,
5′-GTTTGCACCTGGTATGATCTGT-3′; matrix metalloproteinase-2 (MMP2)
forward, 5′-TACAGGATCATT GGCTACACACC-3′ and reverse,
5′-GGTCACATCGCTCC AGACT-3′; MMP9 forward, 5′-TGTACCGCTATGGTTAC
ACTCG-3′ and reverse, 5′-GGCAGGGACAGTTGCTTCT-3′. The primary
antibodies were, anti-GAPDH (Epitomics, Burlingame, CA, USA),
anti-Sp1, anti-MMP2 (both from Santa-Cruz Biotechnology, Santa
Cruz, CA, USA) and anti-MMP9 (Epitomics).
Cell culture and transfection
The human prostate cancer cell lines DU145 and PC3
and the normal prostate epithelial cell line RWPE were purchased
from Shanghai Institute of Cell Biology, Chinese Academy of
Sciences. Cells were cultured in RPMI-1640 medium supplemented with
10% heat-inactivated fetal bovine serum (FBS) (Gibco-BRL, Grand
Island, NY, USA), penicillin (50 U/ml), and streptomycin (50 μg/ml)
in a humidified atmosphere containing 5% CO2 maintained
at 37°C. For miR-330 overexpression, we chose miR-330a mimic
transfection, which is efficient and economical. Briefly, cells
were seeded in a 6-well plate at a density of 20–40×104
cells/well in medium without antibiotics. At ~60–70% confluency,
transfection was performed using Lipofectamine™ 2000 reagent
purchased from Invitrogen Life Technologies (Carlsbad, CA, USA)
according to the manufacturer’s instructions. The transfection
efficiency was assessed by qPCR.
RNA isolation and qPCR
Cells were harvested 48 h after transfection, and
were treated with RNAiso for small RNA and RNAiso Plus for total
RNA. The extracted small RNA and total RNA were transcribed into
cDNA with the One Step PrimeScript miRNA cDNA Synthesis kit and
PrimeScript RT reagent kit (Takara, Japan), respectively. For
quantification, qPCR was performed using SYBR Premix Ex Taq
(Takara) with the 7500 Fast Sequence Detection System (Applied
Biosystems, Foster City, CA, USA). After the Ct values of the
target genes were subtracted by that of the internal control GAPDH,
the relative expression level of each target gene was obtained
using the 2−ΔΔCt method.
Apoptosis assay
Apoptosis was assessed by the Annexin V-FITC kit
(Invitrogen Life Technologies). Both cell lines were transfected
for 48 h, and were harvested and stained with Annexin V-FITC and PI
according to the manufacturer’s instructions. Cell samples were
analyzed by BD LSR II Flow Cytometry System with FACSDiva software
(BD Biosciences, Franklin Lakes, NJ, USA) and apoptotic cell
fractions were determined.
Cell cycle analysis
Briefly, cells were harvested 48 h after
transfection, washed with phosphate-buffered saline (PBS),
detrypsinized, resuspended and finally fixed in 75% ethanol at
−20°C for 24 h after removal of the supernatant. Subsequently,
cells were washed with PBS, and incubated with DNA Prep Stain
(Beckman Coulter, Fullerton, CA, USA) for 30 min. We used the BD
LSR II Flow Cytometry System with FACSDiva software to detect cell
cycle distribution, which was analyzed by ModFit LT software
package and presented as the percentage of cell counts in every
cycle for the G1, S and G2 phases.
Western blot analysis
Cells were harvested ~48 h after treatment, and
lysed in RIPA buffer; the isolated protein was quantified using the
Bradford protein assay (Bio-Rad, Hercules, CA, USA) and separated
with equivalent loading on 10% SDS-polyacrylamide gels subsequently
transferred to nitrocellulose membranes (Millipore, Bedford, MA,
USA). The membranes were blocked with 5% non-fat milk and incubated
with the primary antibody with the recommended dilution rate at 4°C
for at least 12 h, followed by incubation with the corresponding
horseradish peroxidase (HRP)-conjugated secondary antibody at an
appropriate dilution in TBS-Tween at room temperature for 1 h.
Finally, Enhanced Chemiluminescence Plus reagent (Millipore) was
used to detect the bound secondary antibody.
Transwell assay
Millicell Transwell inserts (8-μm) (Millipore) were
placed in a 24-well plate (Corning Incorporated, Corning, NY, USA)
with 600 μl serum-free RPMI-1640 medium in the lower chamber. The
oligo-treated cells were harvested, counted and seeded
(6–8×104 cells/well) to the upper chamber. For the
invasion assay, the wells were coated with Matrigel (BD
Biosciences, Canada) diluted with serum-free RPMI-1640 medium in a
1:8 ratio before seeding. After incubation at 37°C with 5%
CO2 for 48 h, the inserts were withdrawn and fixed in
cold methanol for 10 min and then stained with 0.5% crystal violet
in PBS. After 15 min of incubation, the inserts were washed
thoroughly in water and the cells on the upper surfaces of the
membranes were removed, and then dried overnight. The membranes
were excised and fixed on slides with neutral resin, and the cells
fixed to the bottom were observed using an inverted microscope.
Plasmid construction and luciferase
reporter assay
For investigating the possible target genes of
miR-330, we used the target prediction program Targetscan
(http://www.targetscan.org/). As the
program predicted, the potential target gene Sp1 has 2 putative
binding sites in its 3′-UTR. Therefore, we designed 2 pairs of
primers for amplification from the cDNA of RWPE cells: Site 1
forward, 5′-TGCAAGGTAGCATGGGTCCAAGAG-3′ and reverse,
5′-TGGTGGGAAGCCAAGACAACA-3′; Site 2 forward,
5′-GTCGCAGCAGTAGCTTTGGGGA-3′ and reverse,
5′-ACTTAGGGCAGTTGAGAGGCAGA-3′. The products were then inserted into
the pmirGLO Dual-Luciferase miRNA target expression vector (Promega
Corporation, Madison, WI, USA) between the SacI and
SalI sites (named pGL-WT1 and pGL-WT2). All the insertions
were confirmed by sequencing. HEK293T cells were plated in a
24-well plate and cultured for 24 h. Co-transfection was performed
with 50 nM of either the miR-330 mimic or NC oligo and 100 ng
wild-type reporter plasmid. The relative luciferase activity was
measured by the Dual-Luciferase reporter assay system (Promega) 24
h after transfection.
Statistical analysis
All the graphical data were constructed by GraphPad
Prism version 5 for Windows. Data were analyzed by use of SPSS 15.0
software, and are expressed as means ± standard deviation (SD) for
each group. The t-test or one-way ANOVA was used to make
comparisons; P<0.05 was considered to indicate a statistically
significant result.
Results
miR-330 is downregulated in prostate
cancer cells
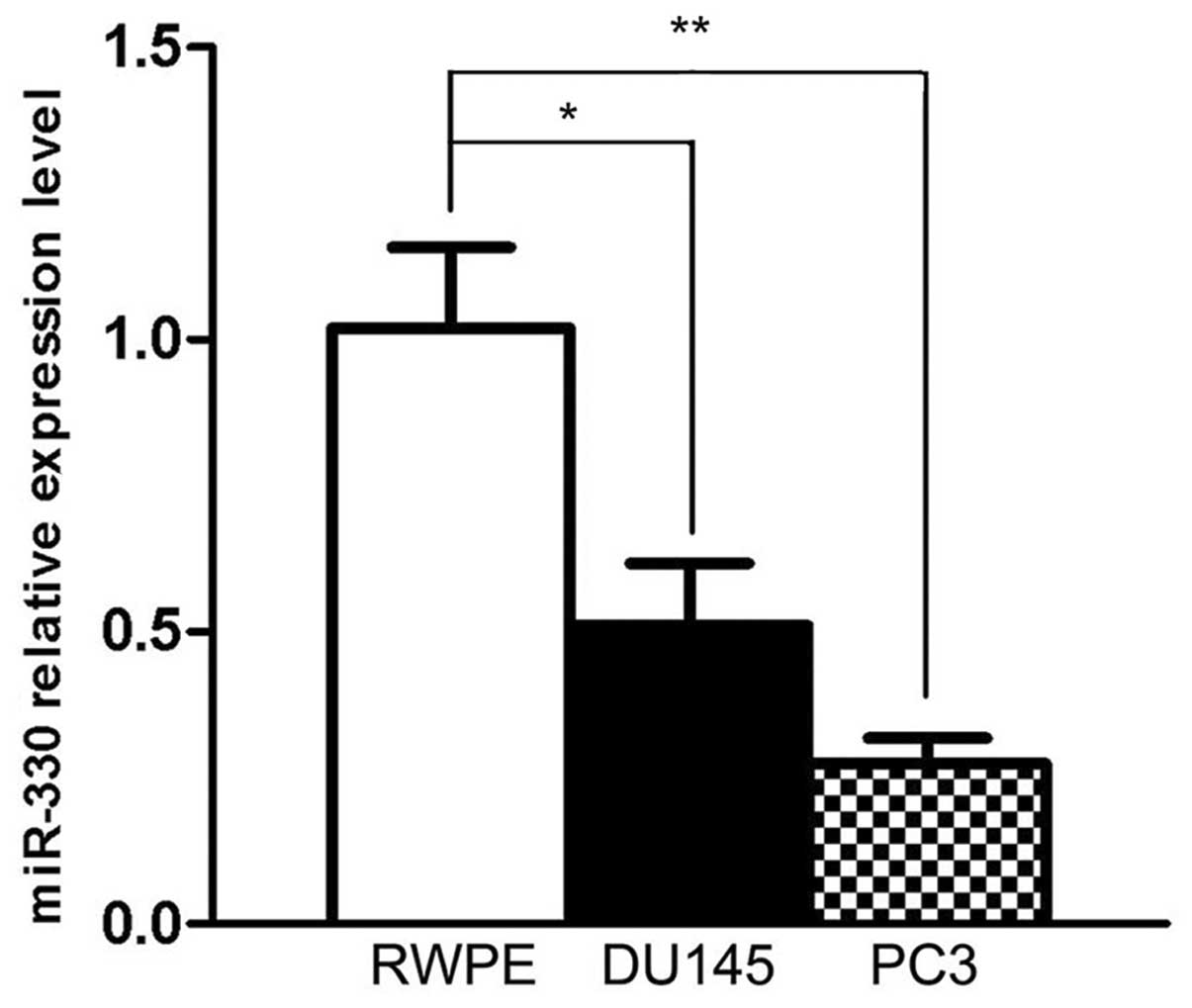
To confirm the expression levels of miR-330 in the
human prostate cancer cell lines, mRNAs were extracted from DU145,
PC3 and RWPE cells, and then transcribed into the template for qPCR
quantification analysis. We used U6 RNA level as an internal
control. Compared with the RWPE cells, both cancer cell lines
exhibited significantly decreased expression of miR-330 when
normalized to U6 [DU145 downregulated by 0.5-fold (P<0.05), and
PC3 by 0.7-fold (P<0.01)] (Fig.
1). This result indicates that miR-330 is downregulated in
prostate cancer cells.
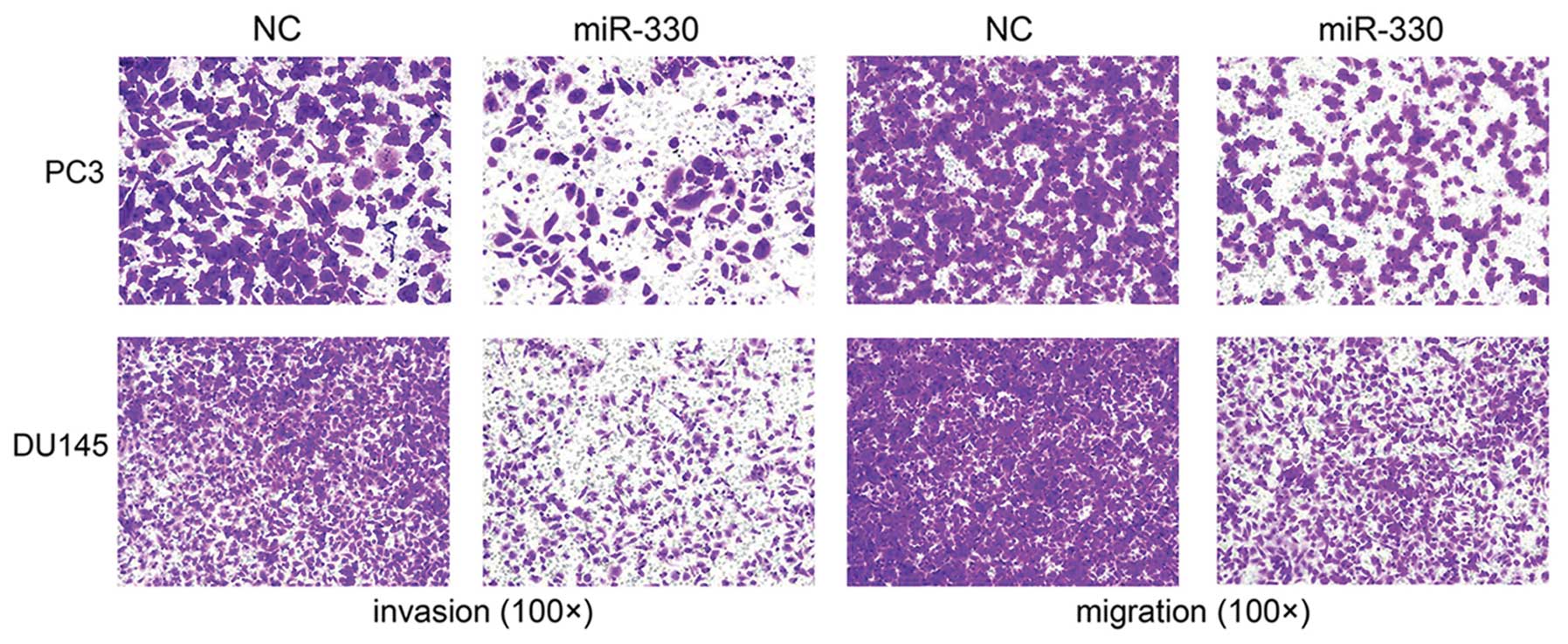
Cell migration is inhibited by miR-330 in
prostate cancer
To determine whether miR-330 affects cell migratory
capacity, cells with miR-330 overexpression were subjected to a
Transwell assay (FBS-induced migration and Matrigel invasion test).
After 24 to 48 h, the migrating cells were fixed, stained and
observed microscopically. As the representative micrographs clearly
demonstrate, miR-330 overexpression led to potent inhibition of
cell migration and invasion (Fig.
2).
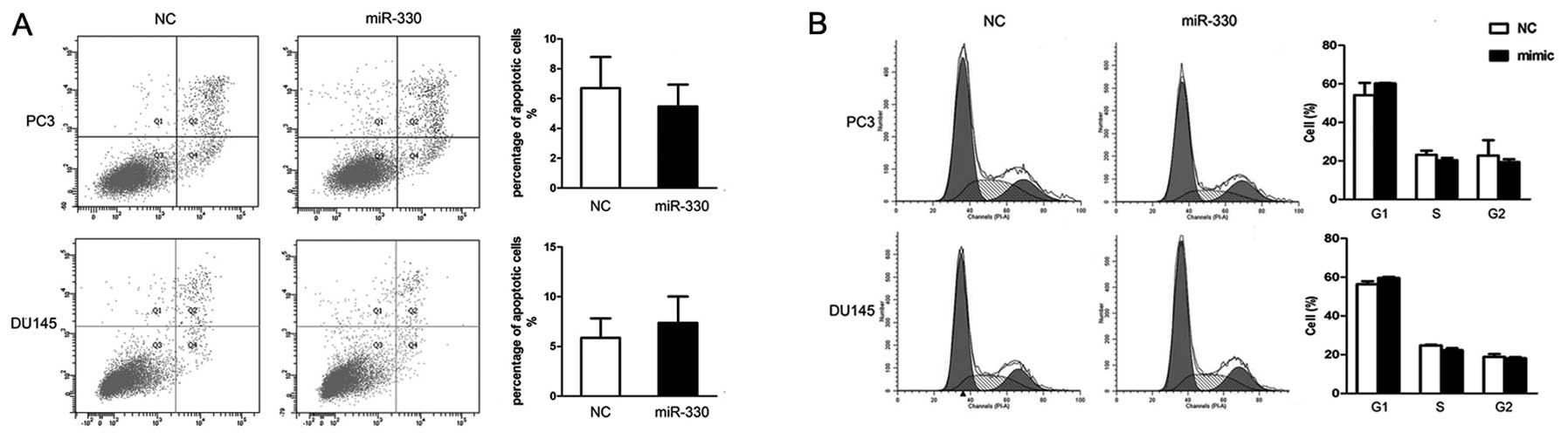
Neither apoptosis nor cell cycle arrest
is induced by miR-330 in prostate cancer cells
To investigate the biological function of miR-330 we
performed flow cytometry, as previous reports indicate a
pro-apoptotic role of miR-330. Both cell lines were treated by
either miR-330 or NC for 48 h, and flow cytometric analysis was
carried out. There was an insignificant difference in the
percentage of apoptotic cells between the miR-330 and NC
pretreatment groups (DU145, 7.37±2.65 vs. 5.87±1.95; P>0.05 and
PC3, 5.47±1.48 vs. 6.70±2.09; P>0.05) (Fig. 3A). Likewise, cell cycle analysis did
not reveal any obvious change in cell cycle distribution in G1, S,
G2 phases between both cell groups (P>0.05) (Fig. 3B). Therefore, miR-330 did not affect
apoptosis or cell cycle arrest in the prostate cancer cells, at
least during our observation period.
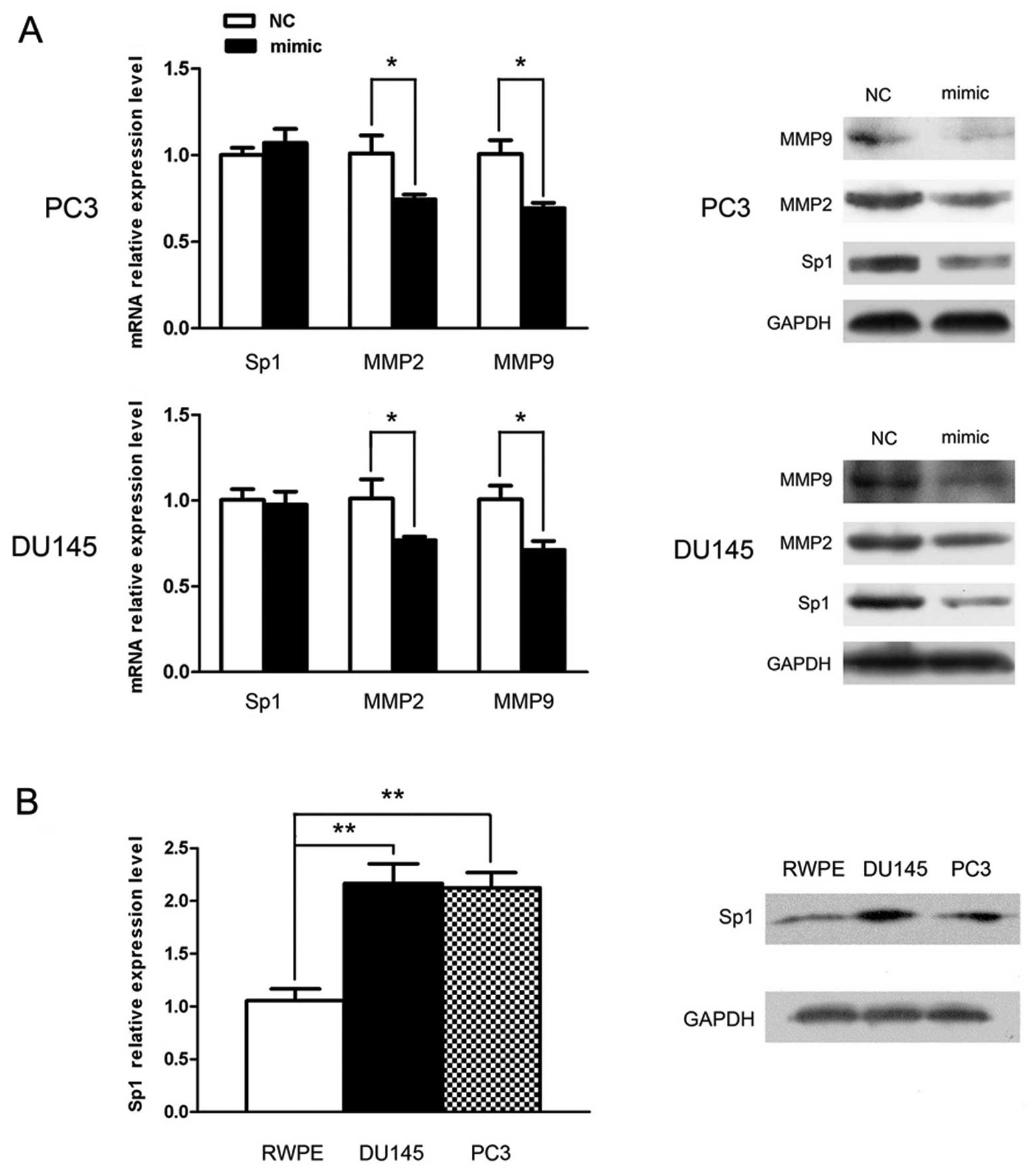
Sp1 is post-transcriptionally
downregulated by miR-330
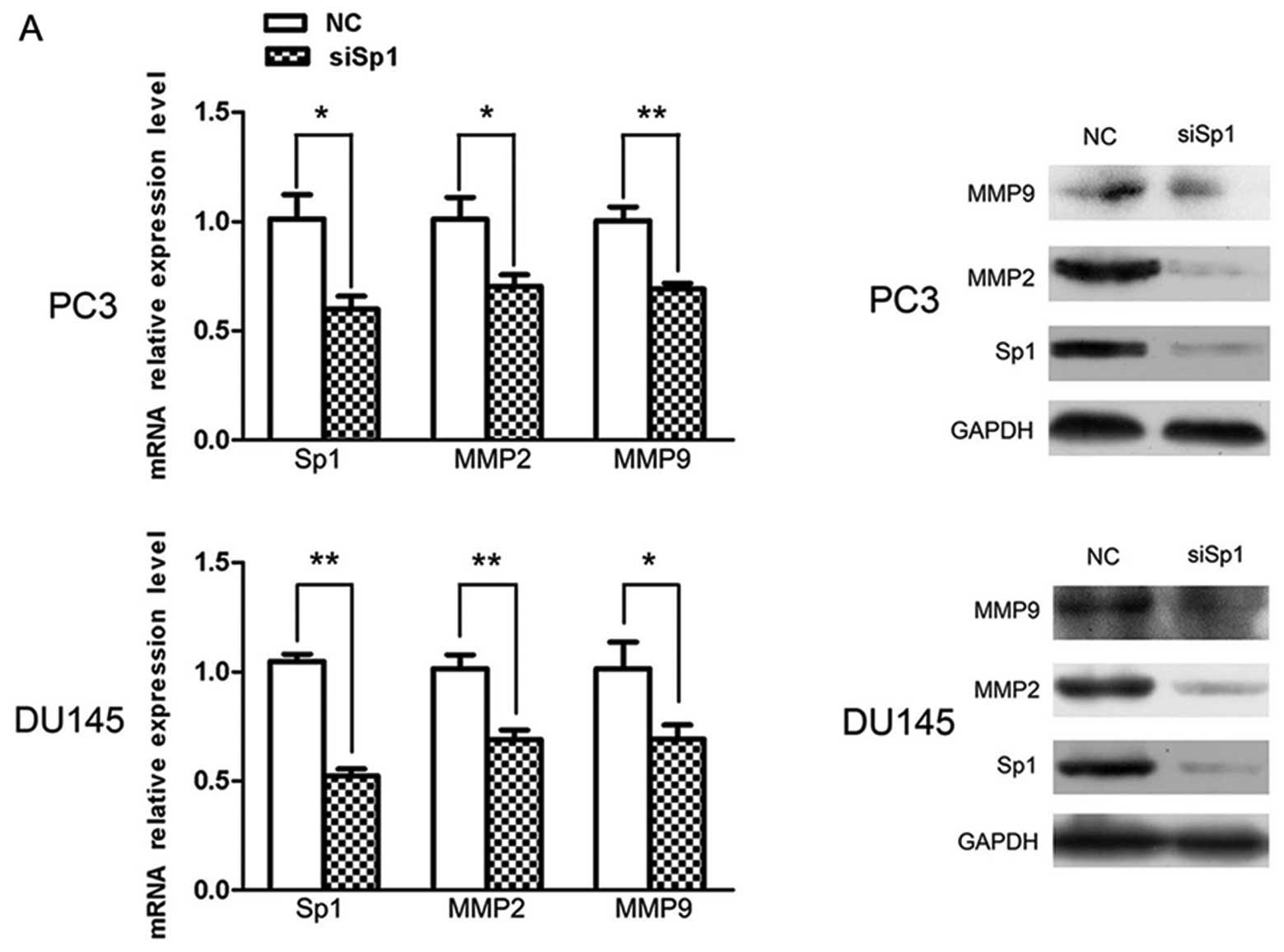
To further investigate the underlying mechanism of
miR-330-induced metastatic inhibition, Sp1 was selected for qPCR
and western blot analysis, by the aid of a bioinformatic tool. The
result showed that following miR-330 overexpression, Sp1 expression
was inhibited only at the protein level rather than at the mRNA
level, which suggests a post-transcriptional regulation. Expression
of other metastasis-related molecules such as MMP2 and MMP9 was
also markedly downregulated at the mRNA and protein levels
(Fig. 4A) (P<0.05), which
verified the involvement of miR-330 in the regulation of
metastasis.
The basic expression levels of Sp1 were also
examined and compared between the DU145 and PC3 cells and normal
epithelial RWPE cells. Sp1 expression was obviously elevated in the
cancer cells and was ~2-fold increased when compared with the level
in the RWPE cells (P<0.05) (Fig
4B). Considering the above data and the inverse correlation
with miR-330 (Fig. 1), we surmised
that Sp1 is very likely the target of miR-330.
Inhibition of cell motility by miP-330 is
associated with downregulation of Sp1
For further investigation of the possible novel
target Sp1, knockdown technique was employed to evaluate its
biological function in vitro. A small interfering RNA
(siSp1) designed to knock down the Sp1 gene was used to treat the
cancer cells compared with NC. The Transwell assay clearly
demonstrated that Sp1 knockdown caused significant suppression of
the migratory and invasive capability in much the same pattern as
miR-330 overexpression (Fig. 5B).
In the mechanistic study, Sp1 knockdown, as anticipated, had a
similar effect as miR-330 overexpression; MMP2 and MMP9 expression
was prominently decreased at both the mRNA and protein levels; a
drastic decrease in Sp1 expression indicated efficiency of
knockdown (Fig. 5A).
Sp1 is a novel target of miR-330
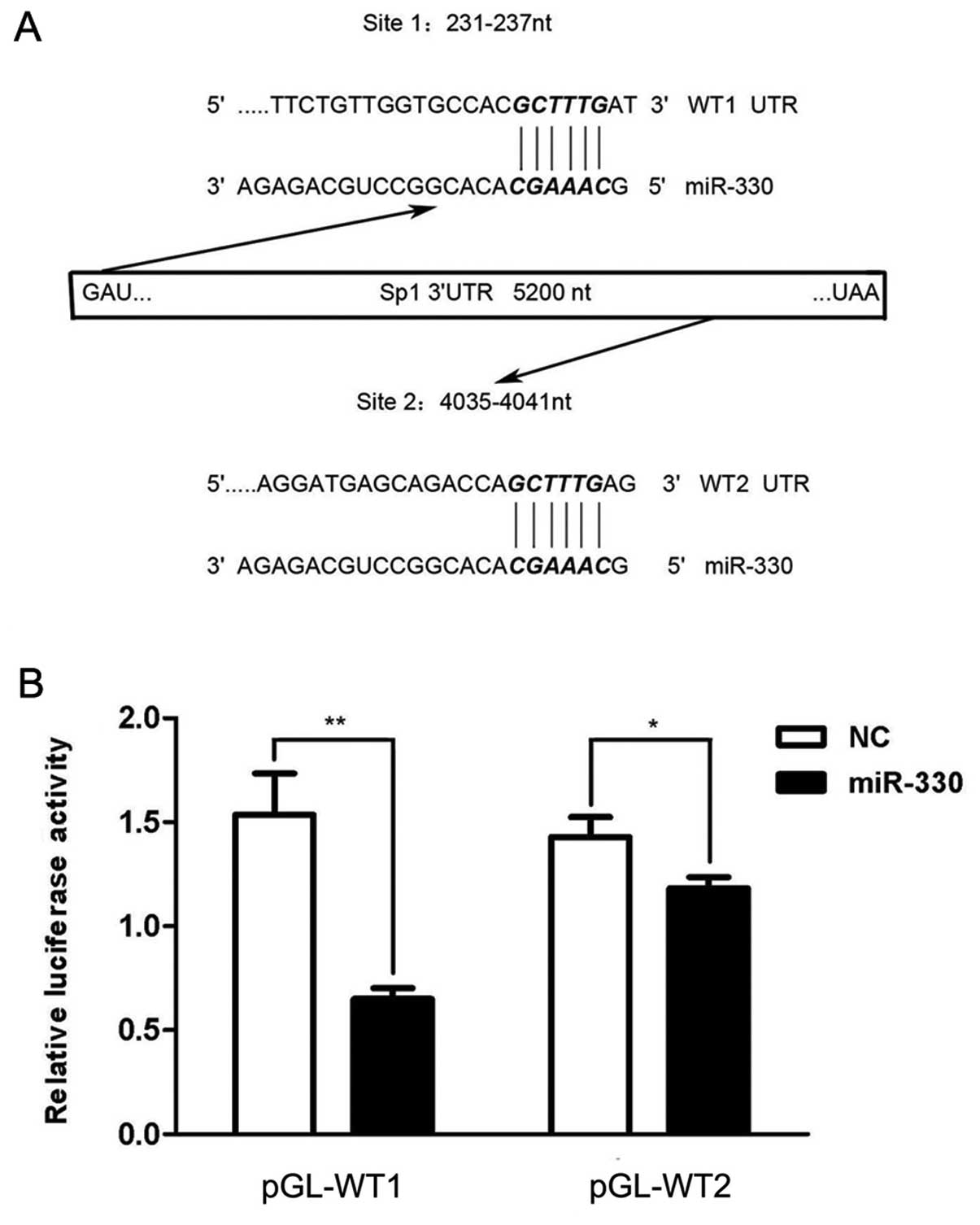
We further determined the relationship between
miR-330 and Sp1. As the bioinformatic tool revealed, two putative
target sites were identified, which starts from the 231 base
position and the 4035 base position on the 3′UTR sequence
respectively (Fig. 6A). Two 400
bp-long fragments around these target sites were cloned downstream
of the firefly luciferase of pmirGLO Dual-Luciferase miRNA target
expression vector, and termed pGL-WT1 and pGL-WT2, respectively. An
individual plasmid with either miR-330 or the NC duplex was used to
co-transfect HEK293T cells. The luciferase activity in the groups
treated with miR-330 was deceased, when compared with the control;
the combination of pGL-WT1 and miR-330 resulted in a more prominent
change with the activity value ~40% (P<0.01) of the NC group
than pGL-WT2 (P<0.05) (Fig. 6B).
These findings indicate that miR-330 inhibited Sp1 expression
through direct binding of the 3′-UTR of its transcript.
Discussion
As a major health concern among males, prostate
cancer is currently the second leading cause of cancer-related
death in Western countries. Although reports of improved survival
rates of localized or organ-confined tumors are increasingly
common, confusion still lies in the problem of dealing with
metastasis. Recent studies of metastasis-associated miRNAs have
provided us with more insights into cancer pathogenesis (16,17).
Watahiki et al(18) compared
the miRNA profiling of a transplantable metastatic vs. a
non-metastatic prostate cancer xenograft, and discovered 53
pro-metastatic miRNAs and 68 anti-metastatic miRNAs, some of which
have been widely accepted as metastic miRs including miR-21,
miR-331-3p, miR-205 and miR-203.
miR-330, a recently identified miRNA, has been
rarely investigated to date. Although it has been regarded as a
downregulated gene in many types of cancer cells, the biological
functions are reportedly diverse and contradictory (11,12).
Here, we first reported the anti-migratory and anti-invasive role
of miR-330 in prostate cancer. The relatively lower expression
level of miR-330 in the cancer cell lines PC3 and DU145 when
compared with the normal epithelial cell line RWPE was consistent
with Lee et al(12). For
forced expression, we used miR-330 mimics, the analog of miR-330,
via transfectant Lipofectamine 2000 for the sake of accessibility,
and qPCR validated the high efficiency of transfection which
favorably persisted for 4–5 days (data not shown). As for the
functional study, the anti-migration and invasion capacity of
miR-330 was clearly demonstrated by the Transwell assay, while its
association with apoptosis or cell cycle arrest was not observed in
our study. For further mechanistic investigation, we screened Sp1
as the metastasis-associated gene possibly targeted by miR-330,
based upon in silico analysis and the inverse relationship
between them. qPCR and western blotting revealed
post-transcriptional inhibition of Sp1 and a significant decrease
in downstream genes MMP2 and MMP9 by miR-330 overexpression; these
phenomena were mimicked by Sp1 knockdown, which further
strengthened the hypothesis that miR-330 plays an anti-migratory
and -invasive role via Sp1 in prostate cancer. Finally, the
luciferase reporter assay was performed to determined Sp1 as the
target gene, and even pinpoint the pairing site ‘231–237’ in the
Sp1 3′UTR as the major target region.
The implication of Sp proteins in tumorigenesis has
been widely accepted, based on the increasing number of reports
revealing ubiquitous overexpression of these transcription factors
in various neoplastic tissues (19–22).
Sp1 regulates thousands of genes including oncogenes and
tumor-suppressor genes, via its direct interaction with the
components of the basal transcription machinery, TAF4, TAF7 and the
recruitment of TBP/TFIID (23–26).
In prostate cancer, Sp1 was reported to regulate key genes
including the androgen receptor (AR), c-Met, FAS, FLIP and TGF.
MMP2 and MMP9 are two intensively studied members of the
extracellular matrix-degrading gelatinase family, whose regulation
reportedly depends on Sp1 via interaction of their GC-rich
sequences in promoters with specific domains of Sp1 (27–29).
Here, we confirmed the pivotal role of Sp1 in cell migration and
invasion, and demonstrated its close relationship with these matrix
metalloproteinases.
It should be noted that Lee et al(12) reported the anti-proliferative and
pro-apoptotic role of miR-330 in prostate cancer cells through MTT
and plate colony formation assays. We performed an apoptosis assay
for fear that proliferation-related factors may interfere with our
invasion assay. As the results indicated, both apoptosis and cell
cycle analyses yielded an insignificant difference within our
observation period. Considering the complex signaling network of
miRNAs, we proposed that the pro-apoptotic and anti-proliferative
roles of miR-330 may manifest themselves in a latent and moderate
manner.
In summary, we identified miR-330 as an
anti-metastatic miRNA in prostate cancer. By targeting the 3′UTR of
Sp1, miR-330 post-transcriptionally suppressed the expression of
Sp1 protein, and further decreased the levels of MMPs, and finally
inhibited migration and invasion of prostate cancer cells.
Admittedly, our conclusions were derived solely from an in
vitro study. Thus, our findings require further confirmation
using clinical specimens and in vivo experiments. Yet, they
suggest that miR-330 may be a therapeutic target for prostate
cancer metastasis.
Acknowledgements
This study was supported by a grant from the
Zhejiang Provincial Natural Science Foundation of China
(LY12H05006), the National Key Clinical Speciality Construction
Project of China, the Key Medical Disciplines of Zhejiang Province
and Zhejiang Provincial Natural Science Foundation of China
(Z2090356).
References
|
1
|
Ambros V and Chen X: The regulation of
genes and genomes by small RNAs. Development. 134:1635–1641. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hutvágner G and Zamore PD: A microRNA in a
multiple-turnover RNAi enzyme complex. Science. 297:2056–2060.
2002.PubMed/NCBI
|
|
3
|
Hwang HW and Mendell JT: microRNAs in cell
proliferation, cell death, and tumorigenesis. Br J Cancer.
96(Suppl): R40–R44. 2007.PubMed/NCBI
|
|
4
|
Ryan BM, Robles AI and Harris CC: Genetic
variation in microRNA networks: the implications for cancer
research. Nat Rev Cancer. 10:389–402. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kasinski AL and Slack FJ: Epigenetics and
genetics. microRNAs en route to the clinic: progress in validating
and targeting microRNAs for cancer therapy. Nat Rev Cancer.
11:849–864. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weber MJ: New human and mouse microRNA
genes found by homology search. FEBS J. 272:59–73. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gaur A, Jewell DA, Liang Y, et al:
Characterization of microRNA expression levels and their biological
correlates in human cancer cell lines. Cancer Res. 67:2456–2468.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ruike Y, Ichimura A, Tsuchiya S, et al:
Global correlation analysis for micro-RNA and mRNA expression
profiles in human cell lines. J Hum Genet. 53:515–523. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Neville PJ, Conti DV, Krumroy LM, et al:
Prostate cancer aggressiveness locus on chromosome segment
19q12-q13. 1 identified by linkage and allelic imbalance studies.
Genes Chromosomes Cancer. 36:332–339. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Slager SL, Schaid DJ, Cunningham JM, et
al: Confirmation of linkage of prostate cancer aggressiveness with
chromosome 19q. Am J Hum Genet. 72:759–762. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qu S, Yao Y, Shang C, et al: microRNA-330
is an oncogenic factor in glioblastoma cells by regulating SH3GL2
gene. PLoS One. 7:e460102012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee KH, Chen YL, Yeh SD, et al:
microRNA-330 acts as tumorsuppressor and induces apoptosis of
prostate cancer cells through E2F1-mediated suppression of Akt
phosphorylation. Oncogene. 28:3360–3370. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chintharlapalli S, Papineni S, Ramaiah SK
and Safe S: Betulinic acid inhibits prostate cancer growth through
inhibition of specificity protein transcription factors. Cancer
Res. 67:2816–2823. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sankpal UT, Goodison S, Abdelrahim M and
Basha R: Targeting Sp1 transcription factors in prostate cancer
therapy. Med Chem. 7:518–525. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pore N, Liu S, Shu HK, et al: Sp1 is
involved in Akt-mediated induction of VEGF expression through an
HIF-1-independent mechanism. Mol Biol Cell. 15:4841–4853. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baranwal S and Alahari SK: miRNA control
of tumor cell invasion and metastasis. Int J Cancer. 126:1283–1290.
2010.PubMed/NCBI
|
|
17
|
Iorio MV and Croce CM: microRNAs in
cancer: small molecules with a huge impact. J Clin Oncol.
27:5848–5856. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Watahiki A, Wang Y, Morris J, et al:
microRNAs associated with metastatic prostate cancer. PLoS One.
6:e249502011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chiefari E, Brunetti A, Arturi F, et al:
Increased expression of AP2 and Sp1 transcription factors in human
thyroid tumors: a role in NIS expression regulation? BMC Cancer.
2:352002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shi Q, Le X, Abbruzzese JL, et al:
Constitutive Sp1 activity is essential for differential
constitutive expression of vascular endothelial growth factor in
human pancreatic adenocarcinoma. Cancer Res. 61:4143–4154.
2001.
|
|
21
|
Yao JC, Wang L, Wei D, et al: Association
between expression of transcription factor Sp1 and increased
vascular endothelial growth factor expression, advanced stage, and
poor survival in patients with resected gastric cancer. Clin Cancer
Res. 10:4109–4117. 2004. View Article : Google Scholar
|
|
22
|
Zannetti A, Del Vecchio S, Carriero MV, et
al: Coordinate up-regulation of Sp1 DNA-binding activity and
urokinase receptor expression in breast carcinoma. Cancer Res.
60:1546–1551. 2000.PubMed/NCBI
|
|
23
|
Chen JL, Attardi LD, Verrijzer CP,
Yokomori K and Tjian R: Assembly of recombinant TFIID reveals
differential coactivator requirements for distinct transcriptional
activators. Cell. 79:93–105. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Furukawa T and Tanese N: Assembly of
partial TFIID complexes in mammalian cells reveals distinct
activities associated with individual TATA box-binding
protein-associated factors. J Biol Chem. 275:29847–29856. 2000.
View Article : Google Scholar
|
|
25
|
Majello B, Napolitano G, De Luca P and
Lania L: Recruitment of human TBP selectively activates RNA
polymerase II TATA-dependent promoters. J Biol Chem.
273:16509–16516. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sadovsky Y, Webb P, Lopez G, et al:
Transcriptional activators differ in their responses to
overexpression of TATA-box-binding protein. Mol Cell Biol.
15:1554–1563. 1995.PubMed/NCBI
|
|
27
|
Murthy S, Ryan AJ and Carter AB: SP-1
regulation of MMP-9 expression requires Ser586 in the PEST domain.
Biochem J. 445:229–236. 2012.PubMed/NCBI
|
|
28
|
Qin H, Sun Y and Benveniste EN: The
transcription factors Sp1, Sp3, and AP-2 are required for
constitutive matrix metalloproteinase-2 gene expression in
astroglioma cells. J Biol Chem. 274:29130–29137. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang CH, Chang HC and Hung WC: p16
inhibits matrix metalloproteinase-2 expression via suppression of
Sp1-mediated gene transcription. J Cell Physiol. 208:246–252. 2006.
View Article : Google Scholar : PubMed/NCBI
|