Introduction
microRNAs (miRNA) are small non-coding RNA molecules
that inhibit gene expression at the transcriptional and
posttranscriptional level by binding to the 3′-untranslated region
(3′-UTR) of target mRNAs (1–4).
miRNAs can bind to partially complementary recognition sequences of
mRNA, subsequently causing mRNA degradation or translation
inhibition, thus effectively silencing their target genes (5–7).
Bioinformatic analysis of known miRNAs suggests that the majority
of mRNAs can be targeted by miRNAs and that a single miRNA can
regulate several hundred genes (8,9).
miRNAs have been reported to participate in many important cellular
processes, such as apoptosis, cell differentiation and
proliferation, tumor suppression, development and metabolism
(3,7–14). In
recent years, more and more miRNAs have been detected by microarray
analysis or other advanced technologies. At the same time, more
protein factors have been confirmed to affect the expression of
miRNAs, such as p53 (12,15,16).
Thus, in order to elucidate the molecular mechanisms associated
with non-small cell lung cancer (NSCLC) cell cycle arrest,
identification of the regulatory network of miRNAs and proteins is
critical.
To identify miRNAs which are differentially
expressed in NSCLC and corresponding non-tumor lung tissues, miRNA
solexa analysis was performed. Seven miRNAs were chosen for further
study. All of the candidate miRNAs which have been verified in our
laboratory play an important role in NSCLC cell cycle arrest.
Potential target genes of 7 miRNAs were predicted by TargetScan
(Table I). All proteins can
regulate the cell cycle. To further identify the miRNAs that may
regulate the expression of p53 or be regulated by p53 protein, we
performed a prediction using software and constructed two
expression vectors of p53 (with or without 3′-UTR). Only miR-150
was predicted to bind to the 3′-UTR of p53 by TargetScan software.
The p53 expression vector contained the coding sequence (cd) only
(pcDNA3.1-p53) and cds with 3′-UTR containing the binding sequences
of miR-150 (pcDNA3.1-p53-3′-UTR) were constructed, respectively.
Our results showed that miR-150 targets the 3′-UTR of p53 and
reduces G1 phase arrest in the H1299 cell line triggered by p53.
miR-34a, miR-184, miR-181a and miR-148 were significantly
upregulated in the H1299 cells transfected by pcDNA3.1-p53.
Moreover, the expression of miR-34a and miR-184 was consistent with
p53 in the NSCLC cell lines, including SPCA-1, H1299, A549 and
HCC827. These findings suggest that miR-150, p53 protein and
relevant miRNAs may be members of a regulatory network in NSCLC
tumorigenesis.
 | Table IPotential target genes of 7 miRNAs
predicted by TargetScan. |
Table I
Potential target genes of 7 miRNAs
predicted by TargetScan.
| miRNA | Target gene(s) | Published in
TargetScan |
|---|
| miR-34a | CCNE2 | 2005, 2007, 2009 |
| CDKN1C | 2009 |
| miR-184 | | |
| miR-181a | CAPRIN1 | 2005, 2007,
2009 |
| CCNJ/K | 2009 |
| CCNT2 | 2005, 2007,
2009 |
| CCNG1 | |
| CDK8 | 2009 |
| miR-148 | CDK5R1 | 2005, 2007,
2009 |
| CDK6/8/13/19 | 2003, 2005, 2007,
2009 |
| miR-10a | CNNM4 | 2005, 2007 |
| CDK6 | 2009 |
| miR-182 | CCNJ/Y | 2005, 2007,
2009 |
| GSPT1 | |
| CNNM2/3 | 2007, 2009 |
| CNGA3 | |
| CCND2/3 | |
| CDKN1C | |
| CDK6 | 2009 |
| CCNE2 | 2005, 2007,
2009 |
| miR-34c | CCNJL | 2009 |
| CCND1 | 2005, 2007,
2009 |
Materials and methods
Cell culture
Human cell lines (SPCA-1, A549, HCC827, 95-D,
HEK293T and BEAS-2B) were obtained from the Cell Bank of the China
Academy of Sciences (Shanghai, China). H1299 was from the American
Type Culture Collection (ATCC, Manassas, VA, USA). SPCA-1, A549,
HCC827 and H1299 cell lines were derived from an NSCLC cell line,
while 95-D is a small-cell lung cancer cell line with high
metastatic potential. Human bronchial epithelial (BEAS-2B) cells
were cultured in LHC-9 medium. A549 cells were cultured in F12K
medium (Gibco, Gaithersburg, MD, USA). All the other lung cancer
cells were cultured in RPMI-1640 medium (Gibco). Human embryonic
kidney cells (HEK293T) were cultivated in Dulbecco’s modified
Eagle’s medium (DMEM, Gibco). All the media were supplemented with
10% fetal bovine serum (FBS; HyClone Laboratories, Logan, UT, USA),
100 U/ml penicillin and 100 μg/ml streptomycin. Cells were cultured
at 37°C in 5% CO2.
Clinical cancer samples
Human lung cancer samples were obtained from the
Department of Oncology, Shanghai Chest Hospital affiliated to
Shanghai Jiao Tong University, Shanghai, China, under ethical
assessment.
Construction of recombinant expression
vectors
The 3′-UTR of the tumor protein TP53 (p53) gene
containing the miR-150 binding site (665 bp) was subcloned
downstream of the firefly luciferase reporter gene in the pGL3
vector (Promega, Madison, WI, USA) and designated as
pGL3-p53-3′-UTR. The plasmid pGL3-p53-3′-mUTR which contained the
mutated binding site of miRNA-150 in the 3′-UTR was also
constructed. The cds of p53 with or without the miR-150 binding
sequence were cloned into the pcDNA3.1 (−) plasmid and named
pcDNA3.1-p53 and pcDNA3.1-p53-3′-UTR, respectively. The primer
sequences used in this study are shown in Table II.
 | Table IIPrimer sequences used in this
study. |
Table II
Primer sequences used in this
study.
| Plasmid | Primer
sequences | Restriction
enzyme |
|---|
|
pGL3-p53-3′-UTR | Forward:
CTAGTCTAGATCAGTCTACCTCCCGCCATAA
Reverse: CCGGAATTCTGACAACTCCCTCTACCTAACCAG |
XbaI
EcoR1 |
|
pGL3-p53-3′-mUTR | Forward:
CTGTGAGGGATGTCTAGCATATGTAAGAAATGTTCTTGCAGTTAAGGG
Reverse: TTTCTTACATATGCTAGACATCCCTCACAGTAAAAACCTTAAAATCTAAGC | |
| pcDNA3.1-p53 | Forward:
CCGCTCGAGATGGAGGAGCCGCAGTCAGA
Reverse: CCGGAATTCCAAAACCCAAAATGGCAGGG |
XhoI
EcoR1 |
|
pcDNA3.1-p53-3′-UTR | Forward:
CCGCTCGAGATGGAGGAGCCGCAGTCAGA
Reverse: CCGGAATTCCCCTACCTAGAATGTGGCTGATTG |
XhoI
EcoR1 |
Luciferase assay
For reporter assays, HEK293T cells cultured in
24-well plates were transiently cotransfected with 400 ng
luciferase vector pGL3-p53-3′-UTR or pGL3-p53-3′-mUTR and either
miR-150 mimics or miRNA negative control (miRNA-NC). To determine
the transfection efficiency, 20 ng pRL-SV40 (Promega) was
cotransfected as the control. Reporter assays were performed at 36
h post-transfection using the Dual-luciferase assay system
(Promega).
Quantitative real-time PCR (qRT-PCR)
analysis of miRNAs and target genes
Total RNA was extracted from the cell cultures using
TRIzol reagent (Bio Basic Inc., Toronto, Canada) according to the
manufacturer’s instructions. Reverse transcription was performed
using the M-MLV Reverse Transcriptase cDNA Synthesis kit (Takara,
Dalian, China). A cDNA library of miRNAs was synthesized by the
QuantiMir cDNA kit (Takara). U6 snRNA and the housekeeping gene 18S
RNA were used as the endogenous control for miRNA and mRNA,
respectively. The target genes and controls were treated under the
same condition and analyzed by qRT-PCR using SYBR Premix Ex Taq™
(Takara) according to the manufacturer’s protocol.
Western blot analysis
Protein for western blot analysis was precipitated
according to the standard protocol (17–20).
Equal amounts of protein samples were subjected to
SDS-polyacryl-amide gel electrophoresis (SDS-PAGE) and then
transferred to a PVDF membrane. The membrane was soaked in
Tris-buffered saline (TBS)-Tween buffer containing 5% low-fat milk
for 60 min with gentle shaking and then incubated with a specific
antibody overnight followed by washing and incubating with a
secondary antibody and the final chemiluminescence ECL (Thermo
Scientific, Rockford, IL, USA) detection of the bands. Protein
bands were quantitated by densitometric analysis using Image Lab
analysis software and expressed as the fold of the control after
being normalized to GAPDH. The primary antibodies used were rabbit
anti-p53 (1:1,000) and mouse anti-GAPDH (1:1,000). The secondary
antibodies were rabbit anti-mouse (1:10,000) and mouse anti-rabbit
(1:10,000). All antibodies were purchased from Cell Signaling
Technology.
Cell cycle analysis
Cells were fixed in 70% ethanol for 12 h at 4°C.
After washing with phosphate-buffered solution (PBS), cells were
treated with RNase A (50 μg/ml) and stained with propidium iodide
(PI; 25 μg/ml) for 30 min at 37°C. Samples were analyzed using
MoFlo XDP flow cytometer (Beckman Coulter, Inc., Brea, CA, USA) and
the distribution of cell cycle phases was determined using FlowJo
software. The phase ratio (%) was calculated as the percentage of
cells in the G1/S/G2 phase.
Statistical analysis
Results are expressed as the group means ± SEM and
analyzed using GraphPad Prism 5 software, using t-tests for 2-group
comparisons and one-way ANOVA for three or more group comparisons.
A P<0.05 was considered to indicate a statistically significant
result.
Results
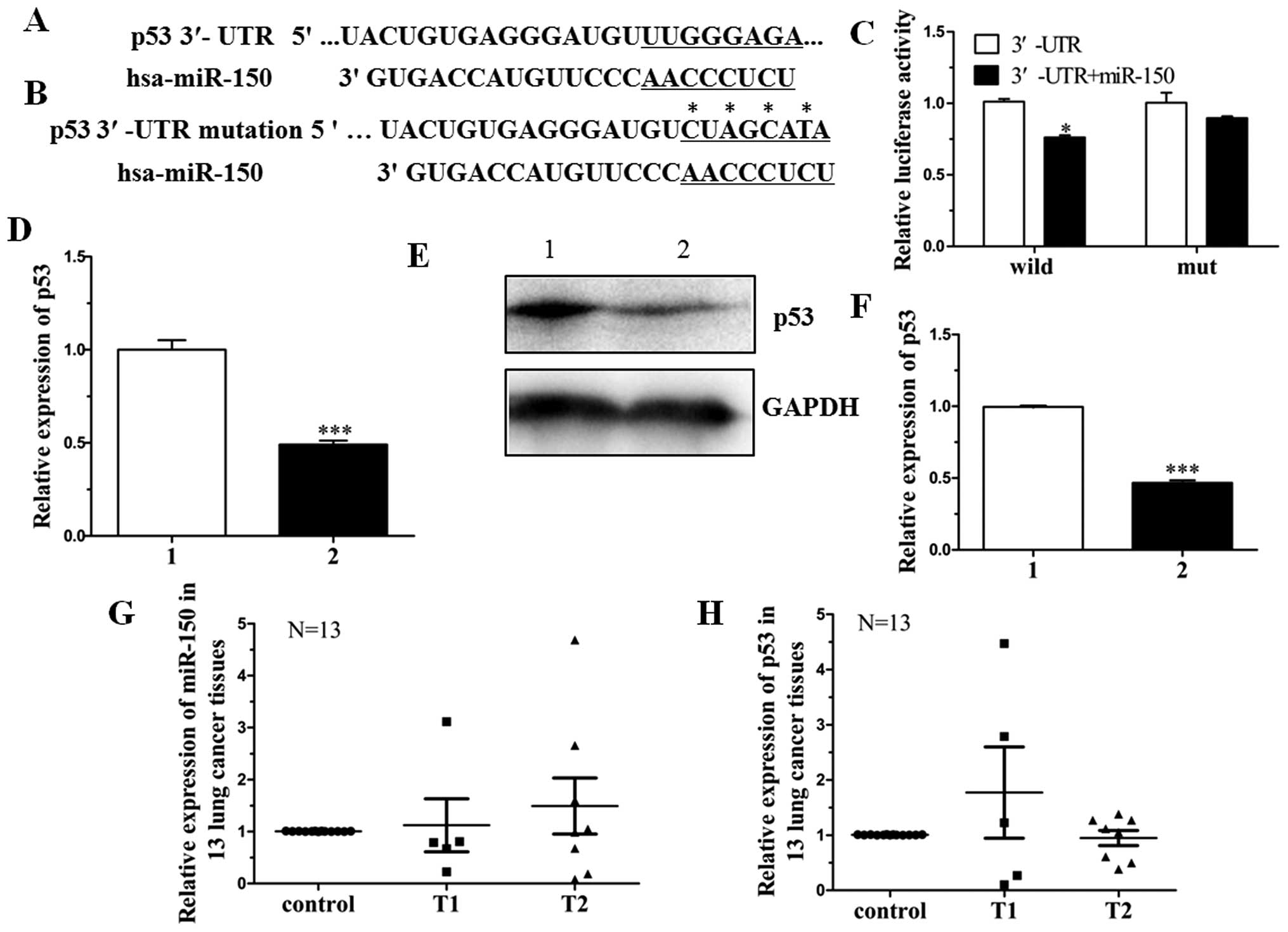
miR-150 directly targets the p53 gene by
interaction with the 3′-UTR
TargetScan and Pictar are two types of software
broadly used on-line to predict the targets of miRNAs. Generally,
the software was used to predict the targets of miRNA. In the
present study, we used it to predict the target miRNA of p53.
Results showed that only miR-150 targeted the 3′-UTR of p53. To
confirm this, pGL3-p53-3′-UTR containing the miR-150 binding
sequence and pGL3-p53-3′-mUTR were constructed (Fig. 1A and B). Analysis of the luciferase
activity showed that the activity of miR-150 mimics cotransfected
with pGL3-p53-3′-UTR was obviously inhibited when compared to
miRNA-NC. However, the activity of miR-150 mimics cotransfected
with pGL3-p53-3′-mUTR exhibited no difference when compared with
miRNA-NC. Results of the luciferase activity assay indicated that
mutated 3′-UTR affected the binding of miR-150 (Fig. 1C).
To further investigate whether miR-150 affects the
expression of p53 at both the transcriptional and translational
levels, we constructed an expression vector, pcDNA3.1-p53-3′-UTR,
which contained the miR-150 binding sequence. The vector was
cotransfected into H1299 cells with miR-150 mimics or miRNA-NC. The
expression level of p53 mRNA in the miR-150 mimic-transfected H1299
cells was significantly decreased by 47% when compared with that in
the miRNA-NC-transfected cells (Fig.
1D). Moreover, the expression level of p53 protein was
significantly inhibited by 60% (Fig. 1E
and F).
Expression of miR-150 and its target p53 was also
detected in NSCLC patient tissue samples. The clinicopathological
characteristics of 13 NSCLC patients are shown in Table III. The expression of miR-150 in
stage T2 tissue samples was higher than that in T1 stage tissue
samples. The corresponding target gene p53 was correlated with
miR-150 expression (Fig. 1G and H).
These data indicate that miR-150 directly targets p53 in NSCLC by
binding to the 3′-UTR of the p53 gene.
 | Table IIIData of the NSCLC patients. |
Table III
Data of the NSCLC patients.
| No. | Gender | Age (years) | Specimen type | Histologic
type | Histology | Lymphatic
invasion | pTNM |
|---|
| 1 | M | 83 | Wedge
resection | Adenosquamous
carcinoma | GX | | T1bN0M0 |
| 2 | M | 67 | Lobectomy | Adenocarcinoma | G3 | | T1bN0M0 |
| 3 | M | 62 | Lobectomy | Adenosquamous
carcinoma | G2 | | T2bN0M0 |
| 4 | M | 54 | Lobectomy | Squamous cell
carcinoma | G3 | | T2bN2M0 |
| 5 | F | 64 | Lobectomy | Adenocarcinoma | G2 | | T1bN0M0 |
| 6 | F | 60 | Lobectomy | Adenocarcinoma | G2 | Present | T2aN2M0 |
| 7 | F | 52 | Lobectomy | Adenocarcinoma
(some BAC) | G2 | | T2bN0M0 |
| 8 | M | 61 | Lobectomy | Adenocarcinoma | G1–G2 | | T2bN2M0 |
| 9 | M | 62 | Lobectomy | Squamous cell
carcinoma | G2 | Present | T2aN1M0 |
| 10 | F | 45 | Lobectomy | Adenocarcinoma | G2 | | T2aN0M0 |
| 11 | F | 67 | Lobectomy | Adenocarcinoma | G2 | Present | T1bN1M0 |
| 12 | M | 61 | Lobectomy | Adenocarcinoma | G2 | | T2bN0M0 |
| 13 | F | 54 | Lobectomy | Mucoepidermoid
carcinoma | G3 | Absent | T1bN0M0 |
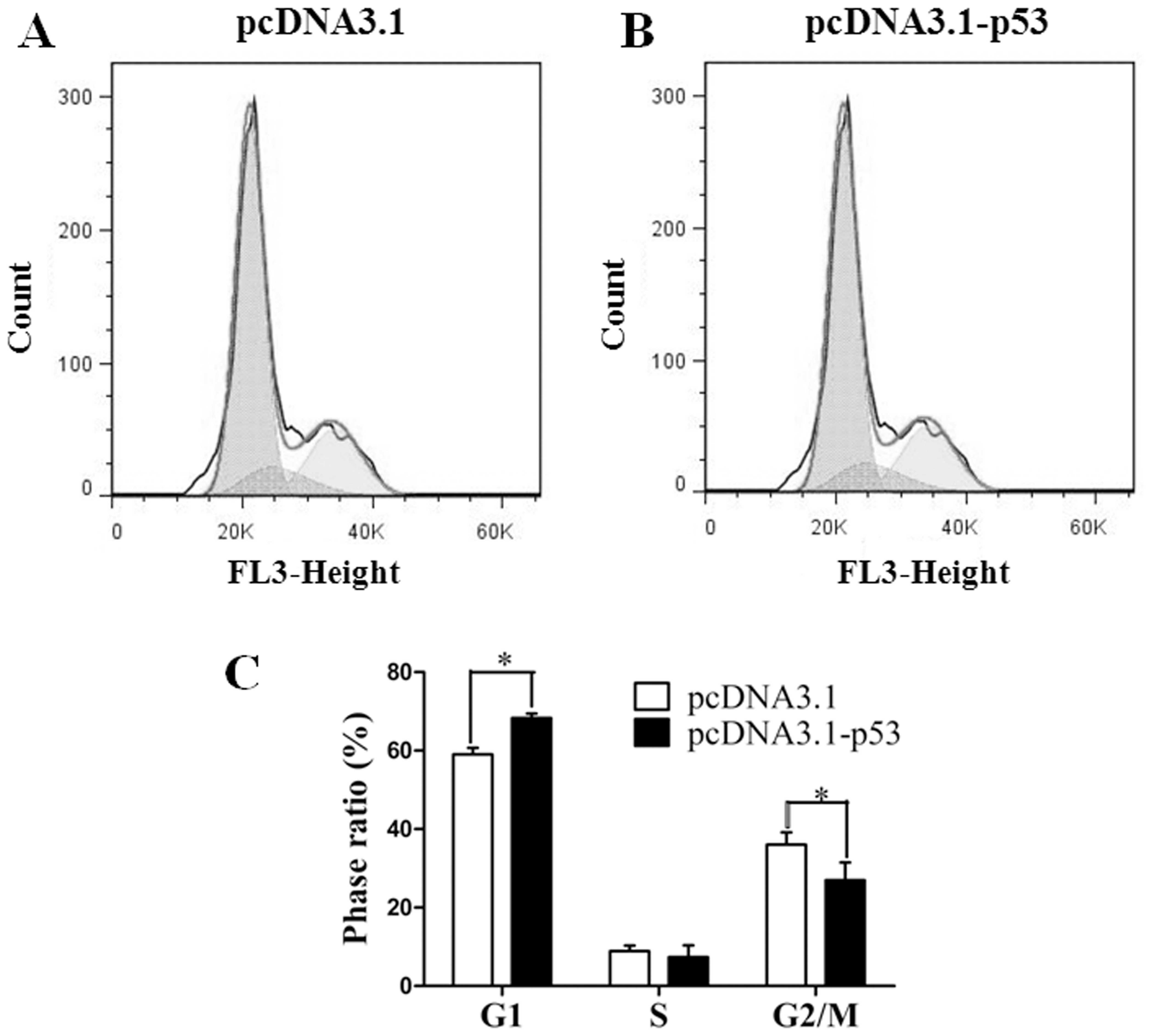
Overexpression of miR-150 inhibits the
cell cycle arrest by targeting p53
Cell cycle analysis was performed after transfection
with pcDNA3.1-p53 or pcDNA3.1 for 48 h. Results showed that the
cells transfected with pcDNA3.1-p53 were significantly arrested in
the G1 phase when compared to the control which was transfected
with empty vector pcDNA3.1 (Fig.
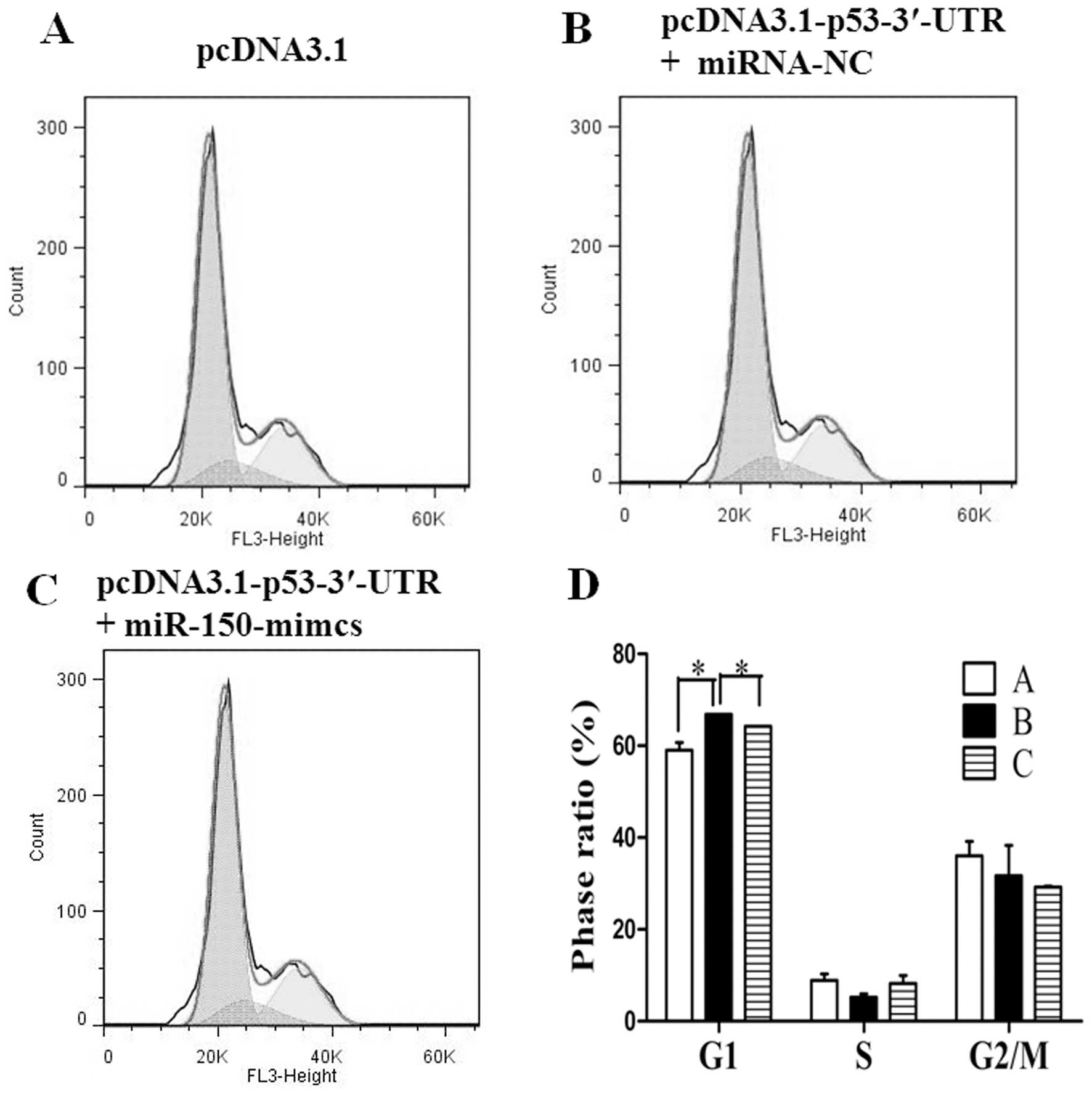
2). The expression vector pcDNA3.1-p53-3′-UTR was then
cotransfected into H1299 cells with the miR-150 mimics or miRNA-NC.
Cell cycle analysis was also performed 48 h later. Both of the
miR-150 mimics- or miRNA-NC-cotransfected samples exhibited an
obviously cell cycle arrest in the G1 phase when compared to the
control which was transfected with pcDNA3.1. However, when compared
to the pcDNA3.1-p53-3′-UTR- and miRNA-NC-cotransfected samples,
miR-150 mimics cotransfected with pcDNA3.1-p53-3′-UTR inhibited
cell cycle arrest (Fig. 3). These
results indicate that miR-150 inhibits the cell cycle arrest
triggered by p53.
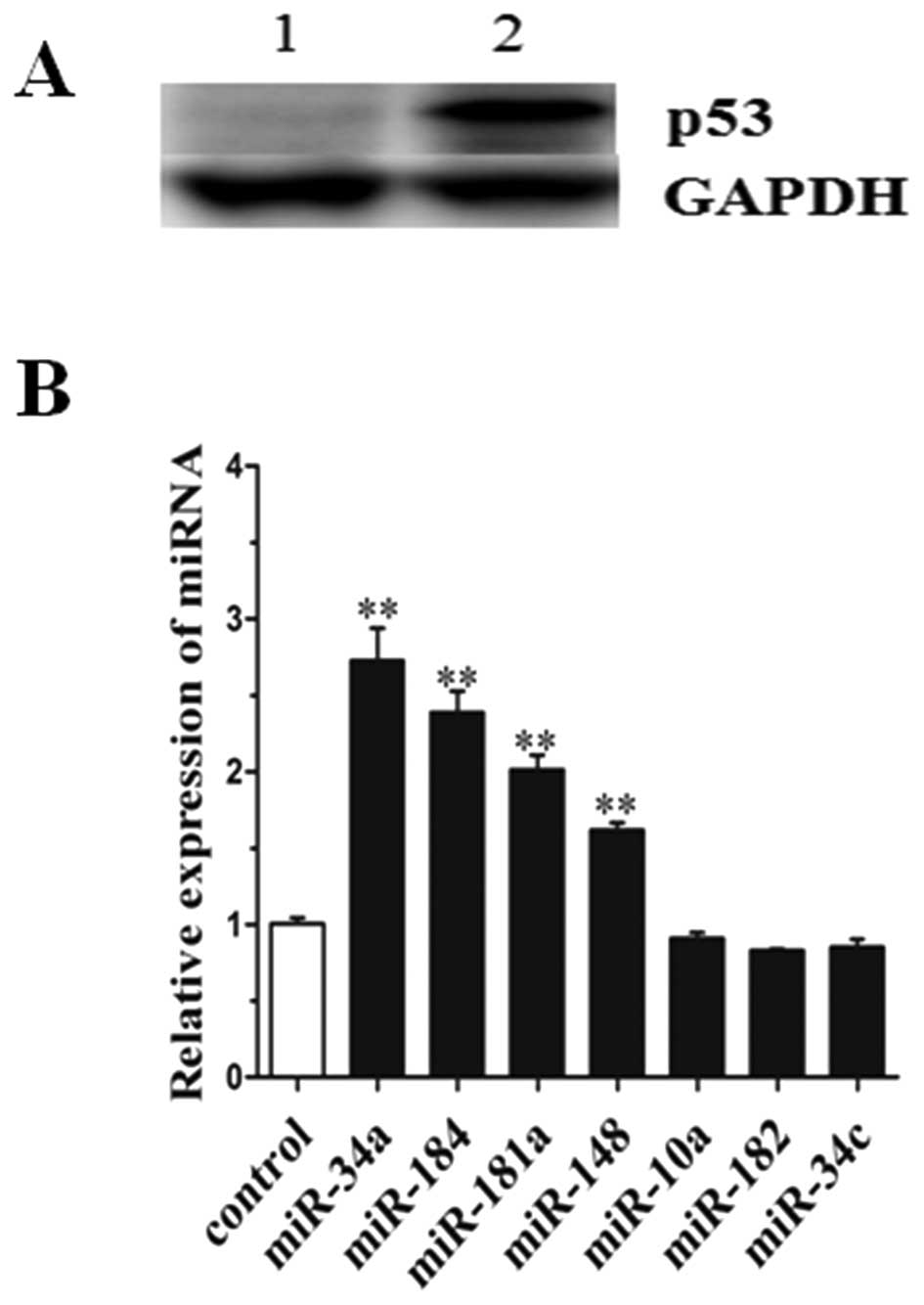
Expression level of miRNAs in the H1299
cell line transfected with pcDNA3.1-p53
H1299 cell lines have a homozygous partial deletion
of the p53 gene, and lack expression of p53 protein. To identify
miRNAs which were differentially expressed after p53 ectopic
expression in the H1299 cell line pcDNA3.1-p53 was transfected into
H1299 cells. To avoid targeting by miRNAs in the 3′-UTR, the
pcDNA3.1-p53 contained cds only. Western blot analysis was
performed to detect the expression of p53 protein in the H1299 cell
line transfected with pcDNA3.1 or pcDNA3.1-p53. The data showed
that the p53 protein was significantly expressed in the H1299 cells
transfected with pcDNA3.1-p53, but was not detectable in the
control (transfected with pcDNA3.1) (Fig. 4A). qRT-PCR was then performed to
identify miRNAs. Results showed that the level of miR-34a, miR-184,
miR-181a and miR-148 expression were significantly upregulated by
2.8-, 2.5-, 2.2- and 1.7-fold of the control (Fig. 4B). However, the expression levels of
miR-10a, miR-182 and miR-34c demonstrated no difference when
compared with the control. The expression values were normalized to
the levels of U6 RNA. In particular, all of the upregulated miRNAs
play a cancer-suppressor role in lung cancer tumorigenesis which
has been previously reported (21–27).
Thus, these data indicate that p53 protein promotes the expression
of miRNAs, particularly tumor suppressors miR-34a and miR-184.
Expression level of miR-34a, miR-184 and
p53 was relevant in NSCLC cell lines
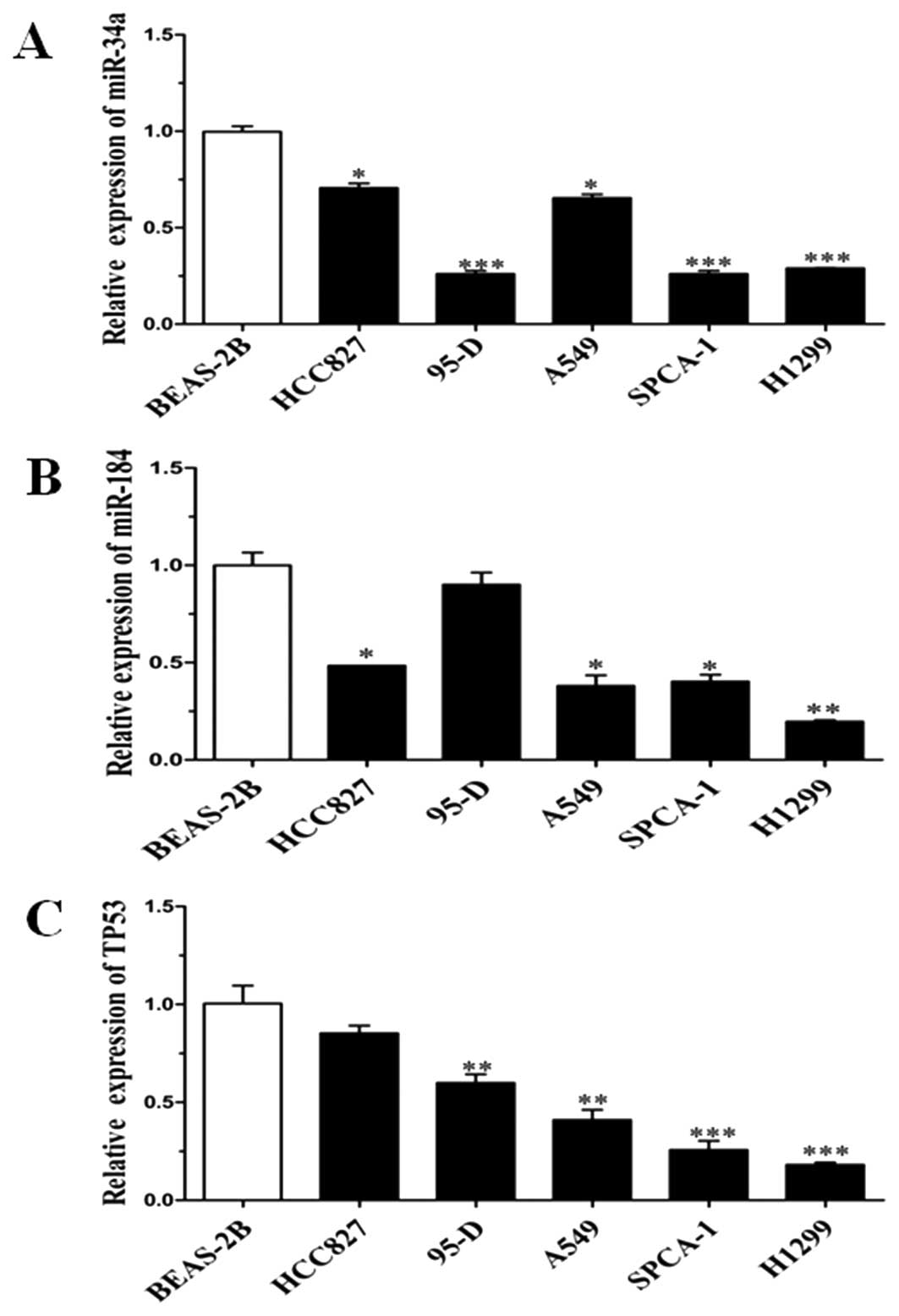
To confirm that the expression level of miR-34a,
miR-184 and p53 was relevant, 5 lung cancer cell lines (A549,
H1299, 95-D, SPCA-1 and HCC827) were chosen as the samples and
normal lung cell line (BEAS-2B) as the control. qRT-PCR analysis
was performed to detect the expression levels of miR-34a, miR-184
and p53. The data showed that the expression of miR-34a and miR-184
was consistent with p53 except for that in the 95-D cell line
(Fig. 5). Notably, all of the other
4 lung cancer cell lines originated from NSCLC. Altogether, these
results indicate that p53 protein affects the expression of miR-34a
and miR-184.
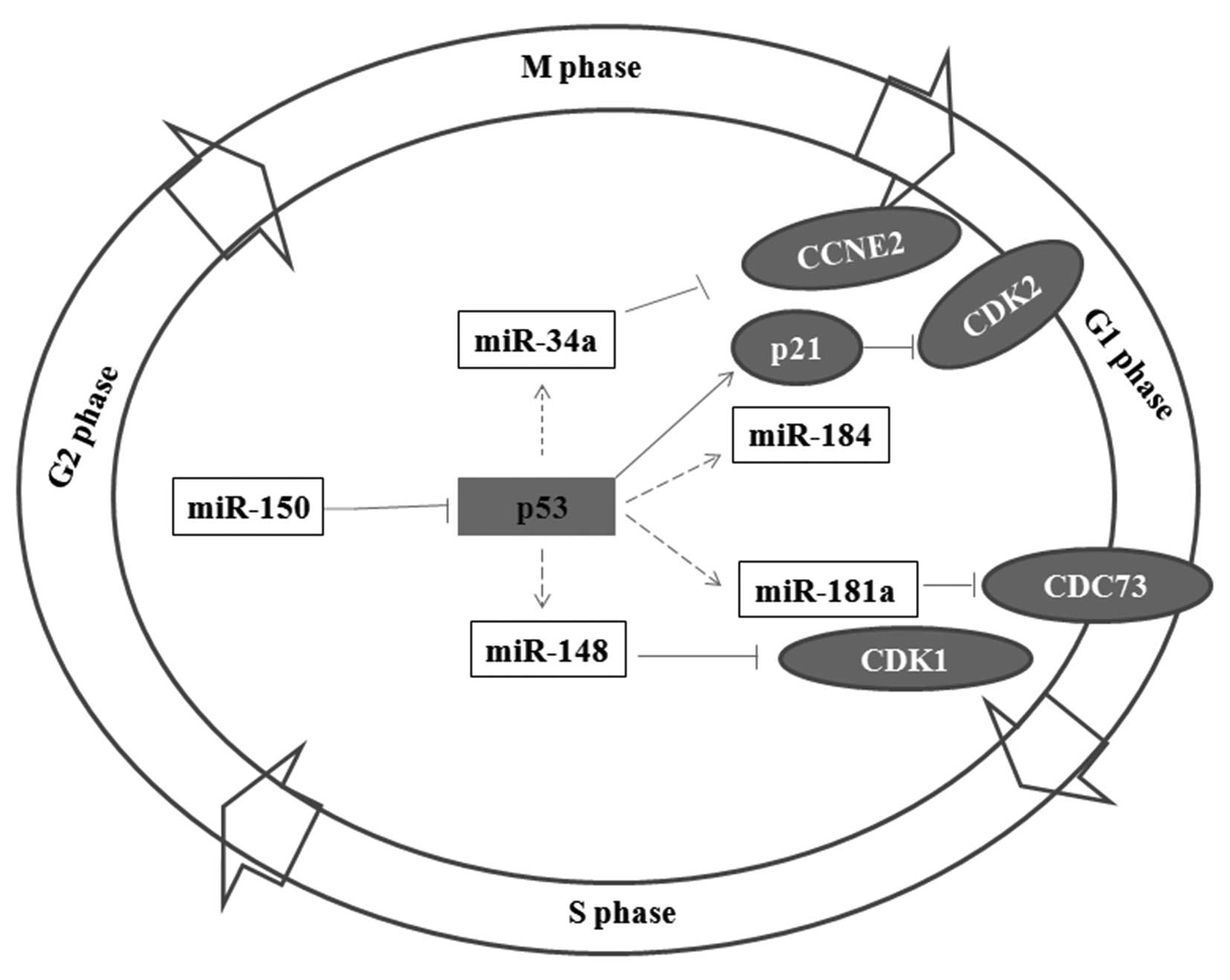
miR-150, p53 protein and miRNAs are
members of a regulatory network in NSCLC tumorigenesis
As the results confirmed, miR-150 targets the 3′-UTR
of p53. Overexpression of p53 can significantly enhance the
expression of miR-34a, miR-184, miR-181a and miR-148. In
particular, the expression of miR-34a and miR-184 was increased
higher than 2-fold of the control. The targets of miR-34a have been
previously reported (21,28,29).
The protein cyclin E2 (CCNE2) is a key regulator in the cell cycle,
and it is a potential target of miR-34a (Table I). miR-181a and miR-148 regulate the
expression of CDC73 and CDK1, respectively. Both CDC73 and CDK1 can
affect the G1 phase in the cell cycle (24,30–36).
Thus, miR-150, p53 protein, the relevant miRNAs and their targets
may consist of a complicated regulation network in NSCLC
tumorigenesis (Fig. 6).
Discussion
H1299 cells have a homozygous partial deletion of
the p53 gene, and lack expression of p53 protein (37). In the present study, we detected the
expression variation of miRNAs when ectopic expression of p53 was
present in the H1299 cell line. We found that miR-34a, miR-184,
miR-181a and miR-148 expression was significantly upregulated.
miR-34a and miR-181a have been reported as tumor-suppressor genes
in neuroblastoma cells, urothelial bladder carcinoma, human brain
glioma cells, head and neck squamous cell carcinoma and breast
cancer (21–23,38–40).
miR-34a can target many protein factors, such as the Notch-1
signaling pathway, Bcl-2, SIRT-1 and CDK1 and then promote the
process of cell apoptosis and inhibit the cell cycle and
proliferation (22,23,41,42).
miR-181a can target k-ras, a typical oncogene (39). However, miR-184 and miR-148 have not
been thoroughly studied. These results suggest that p53 protein may
regulate the expression of various miRNAs which play a
tumor-suppressor role in NSCLC cell lines. Yet, how p53 protein
affects the expression of miRNAs is still unknown.
miR-150 was the only predicted miRNA which binds to
the 3′-UTR sequence of p53. The luciferase activity analysis showed
that the activity of miR-150 mimics cotransfected with
pGL3-p53-3′-UTR was inhibited obviously compared to miRNA-NC.
Western blot analysis also showed consistent results that the
translation of p53 protein was inhibited significantly when miR-150
mimics were cotransfected with pcDNA3.1-p53-3′-UTR. miR-150 also
reduces the cell cycle arrest triggered by p53. These results
suggest that miR-150 may promote lung cancer tumorigenesis by
targeting p53.
In conclusion, we confirmed that p53 is a direct
target of miR-150, and overexpression of p53 promotes the
expression of miRNAs including miR-34a, miR-184, miR-181a and
miR-148. Our findings suggest that miR-150, p53 protein and
relevant miRNAs consist of a complicated regulatory network in
NSCLC tumorigenesis.
Acknowledgements
This study was in part supported by grants from the
Innovation Program of Shanghai Municipal Commission of Sciences and
Technology (11ZR141220), the National Natural Science Foundation
(31170750), the National Key Research and Development Program of
China (2011CB811304), the National Basic Research Program of China
(2011CBA01105) and the Program of Baoshan District Commission of
Sciences and Technology, Shanghai (CXY-2011-32).
References
|
1
|
Zabaleta J: MicroRNA: a bridge from H.
pylori infection to gastritis and gastric cancer development.
Front Genet. 3:2942012.PubMed/NCBI
|
|
2
|
Harquail J, Benzina S and Robichaud GA:
MicroRNAs and breast cancer malignancy: an overview of
miRNA-regulated cancer processes leading to metastasis. Cancer
Biomark. 11:269–280. 2012.PubMed/NCBI
|
|
3
|
Wang F, Sun GP, Zou YF, Hao JQ, Zhong F
and Ren WJ: MicroRNAs as promising biomarkers for gastric cancer.
Cancer Biomark. 11:259–267. 2012.PubMed/NCBI
|
|
4
|
Odjele A, Charest D and Morin PJ: miRNAs
as important drivers of glioblastomas: a no-brainer? Cancer
Biomark. 11:245–252. 2012.PubMed/NCBI
|
|
5
|
Eldem V, Celikkol Akcay U, Ozhuner E,
Bakir Y, Uranbey S and Unver T: Genome-wide identification of
miRNAs responsive to drought in peach (Prunus persica) by
high-throughput deep sequencing. PloS One. 7:e502982012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Endo H, Muramatsu T, Furuta M, et al:
Potential of tumor-suppressive miR-596 targeting LGALS3BP as a
therapeutic agent in oral cancer. Carcinogenesis. 34:560–569. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li KK, Pang JC, Lau KM, et al: miR-383 is
downregulated in medulloblastoma and targets peroxiredoxin 3
(PRDX3). Brain Pathol. Dec 11–2012.(Epub ahead of print).
View Article : Google Scholar
|
|
8
|
Masuda M, Miki Y, Hata S, et al: An
induction of microRNA, miR-7 through estrogen treatment in breast
carcinoma. J Transl Med. 10(Suppl 1): S22012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Novello C, Pazzaglia L, Cingolani C, et
al: miRNA expression profile in human osteosarcoma: role of miR-1
and miR-133b in proliferation and cell cycle control. Int J Oncol.
42:667–675. 2013.PubMed/NCBI
|
|
10
|
Ando H, Okamoto A, Yokota M, et al:
Development of miR-92a delivery system for antiangiogenesis-based
cancer therapy. J Gene Med. 15:20–27. 2012. View Article : Google Scholar
|
|
11
|
Teixeira AL, Gomes M and Medeiros R: EGFR
signaling pathway and related-miRNAs in age-related diseases: the
example of miR-221 and miR-222. Front Genet. 3:2862012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boldrup L, Coates PJ, Wahlgren M, Laurell
G and Nylander K: Subsite-based alterations in miR-21, miR-125b,
and miR-203 in squamous cell carcinoma of the oral cavity and
correlation to important target proteins. J Carcinog. 11:182012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hirata H, Ueno K, Shahryari V, et al:
Oncogenic miRNA-182–5p targets Smad4 and RECK in human bladder
cancer. PloS One. 7:e510562012.PubMed/NCBI
|
|
14
|
Hassan F, Nuovo GJ, Crawford M, et al:
MiR-101 and miR-144 regulate the expression of the CFTR chloride
channel in the lung. PloS One. 7:e508372012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sasaki A, Udaka Y, Tsunoda Y, et al:
Analysis of p53 and miRNA expression after irradiation of
glioblastoma cell lines. Anticancer Res. 32:4709–4713.
2012.PubMed/NCBI
|
|
16
|
Suzuki HI and Miyazono K: p53 actions on
microRNA expression and maturation pathway. Methods Mol Biol.
962:165–181. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gurtler A, Kunz N, Gomolka M, et al:
Stain-Free technology as a normalization tool in Western blot
analysis. Anal Biochem. 433:105–111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hirano S: Western blot analysis. Methods
Mol Biol. 926:87–97. 2012. View Article : Google Scholar
|
|
19
|
Kao CH, Cheng CM, Chuang KH, et al: A
regularly spaced and self-revealing protein ladder for anti-tag
Western blot analysis. Anal Biochem. 431:1–3. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nybo K: Molecular biology techniques
Q&A. Western blot: protein migration. Biotechniques. 53:23–24.
2012.
|
|
21
|
Li XJ, Ji MH, Zhong SL, et al:
MicroRNA-34a modulates chemosensitivity of breast cancer cells to
adriamycin by targeting Notch1. Arch Med Res. 43:514–521. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Siemens H, Neumann J, Jackstadt R, et al:
Detection of miR-34a promoter methylation in combination with
elevated expression of c-Met and β-catenin predicts distant
metastasis of colon cancer. Clin Cancer Res. 9:710–720.
2012.PubMed/NCBI
|
|
23
|
Zhang HS, Chen XY, Wu TC, Sang WW and Ruan
Z: MiR-34a is involved in Tat-induced HIV-1 long terminal repeat
(LTR) transactivation through the SIRT1/NFkappaB pathway. FEBS
Lett. 586:4203–4207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Candas D, Fan M, Nantajit D, et al:
CyclinB1/Cdk1 phosphorylates mitochondrial antioxidant MnSOD in
cell adaptive response to radiation stress. J Mol Cell Biol. Jan
31–2013.(Epub ahead of print).
|
|
25
|
Haflidadottir BS, Bergsteinsdottir K,
Praetorius C and Steingrimsson E: miR-148 regulates Mitf in
melanoma cells. PloS One. 5:e115742010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Iovino N, Pane A and Gaul U: miR-184 has
multiple roles in Drosophila female germline development.
Dev Cell. 17:123–133. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu C, Teng ZQ, Santistevan NJ, et al:
Epigenetic regulation of miR-184 by MBD1 governs neural stem cell
proliferation and differentiation. Cell Stem Cell. 6:433–444. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen Y, Tsai YH and Tseng SH: Inhibition
of cyclin-dependent kinase 1-induced cell death in neuroblastoma
cells through the microRNA-34a-MYCN-survivin pathway. Surgery.
153:4–16. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang C, Yao Z, Zhu M, et al: Inhibitory
effects of microRNA-34a on cell migration and invasion of invasive
urothelial bladder carcinoma by targeting Notch1. J Huazhong Univ
Sci Technolog Med Sci. 32:375–382. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Venta R, Valk E, Koivomagi M and Loog M:
Double-negative feedback between S-phase cyclin-CDK and CKI
generates abruptness in the G1/S switch. Front Physiol. 3:4592012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nagle AA, Gan FF, Jones G, So CL, Wells G
and Chew EH: Induction of tumor cell death through targeting
tubulin and evoking dysregulation of cell cycle regulatory proteins
by multifunctional cinnamaldehydes. PloS One. 7:e501252012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chow JP and Poon RY: The CDK1 inhibitory
kinase MYT1 in DNA damage checkpoint recovery. Oncogene. Nov
12–2012.(Epub ahead of print).
|
|
33
|
Guarnieri V, Battista C, Muscarella LA, et
al: CDC73 mutations and parafibromin immunohistochemistry in
parathyroid tumors: clinical correlations in a single-centre
patient cohort. Cell Oncol (Dordr). 35:411–422. 2012. View Article : Google Scholar
|
|
34
|
Amrich CG, Davis CP, Rogal WP, et al:
Cdc73 subunit of Paf1 complex contains C-terminal Ras-like domain
that promotes association of Paf1 complex with chromatin. J Biol
Chem. 287:10863–10875. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang JH, Seigneur EM, Pandey M, et al:
The EIF4EBP3 translational repressor is a marker of CDC73 tumor
suppressor haploinsufficiency in a parathyroid cancer syndrome.
Cell Death Dis. 3:2662012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Masi G, Barzon L, Iacobone M, et al:
Clinical, genetic, and histopathologic investigation of
CDC73-related familial hyperparathyroidism. Endocr Relat Cancer.
15:1115–1126. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jiang Y, Zhang XY, Sun L, et al: Methyl
methanesulfonate induces apoptosis in p53-deficient H1299 and Hep3B
cells through a caspase 2- and mitochondria-associated pathway.
Environ Toxicol Pharmacol. 34:694–704. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Debernardi S, Skoulakis S, Molloy G,
Chaplin T, Dixon-McIver A and Young BD: MicroRNA miR-181a
correlates with morphological sub-class of acute myeloid leukaemia
and the expression of its target genes in global genome-wide
analysis. Leukemia. 21:912–916. 2007.PubMed/NCBI
|
|
39
|
Shin KH, Bae SD, Hong HS, Kim RH, Kang MK
and Park NH: miR-181a shows tumor suppressive effect against oral
squamous cell carcinoma cells by downregulating K-ras. Biochem
Biophys Res Commun. 404:896–902. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xie W, Li Z, Li M, Xu N and Zhang Y:
miR-181a and inflammation: miRNA homeostasis response to
inflammatory stimuli in vivo. Biochem Biophys Res Commun.
430:647–652. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kashat M, Azzouz L, Sarkar SH, Kong D, Li
Y and Sarkar FH: Inactivation of AR and Notch-1 signaling by
miR-34a attenuates prostate cancer aggressiveness. Am J Transl Res.
4:432–442. 2012.PubMed/NCBI
|
|
42
|
Xia J, Duan Q, Ahmad A, et al: Genistein
inhibits cell growth and induces apoptosis through up-regulation of
miR-34a in pancreatic cancer cells. Curr Drug Targets.
13:1750–1756. 2012. View Article : Google Scholar : PubMed/NCBI
|