Introduction
Nasopharyngeal carcinoma (NPC) is the most common
malignant tumor in Southeast Asia particularly in South China
(1). Chemotherapy and radiotherapy
are the main treatment strategies for NPC (2); however, radioresistance remains a
serious obstacle to successful treatment in the clinic (2,3). To
develop better approaches, it is important to seek innovative
therapeutics for NPC.
An increasing amount of attention has been paid to
the use of complementary and alternative medicine as a part of the
treatment strategy for various types of cancers (4). Curcumin, a phenolic compound from the
plant Curcuma longa, is a well-known food additive and
constituent of traditional medicine, and has been reported to
inhibit cell proliferation and induce apoptosis in many types of
human cancers (5,6). However, the exact molecular mechanisms
involved in the tumor-suppressive effects of curcumin remain to be
identified. Although curcumin is remarkably non-toxic and has
promising anticancer activities, preclinical and clinical studies
indicate that its poor bioavailability and pharmacokinetic profiles
due to its instability under physiological conditions have limited
its application in antitumor therapies (7). It has been previously reported that
curcumin exerts its pro-apoptotic effect by inducing endoplasmic
reticulum ER stress in human leukemia HL-60 cells (8). Furthermore, it has been reported that
ER stress contributes to radiosensitization (9). Therefore, curcumin analogues with
higher potency that specifically activate the ER stress pathway are
needed as effective therapeutic agents for NPC.
Recently, more effort has been paid to the chemical
modification of curcumin to identify potential analogues with
better bioavailability and antitumor activities (10,11).
We also designed and synthesized a series of mono-carbonyl
analogues of curcumin by deleting the β-diketone moiety (12,13).
Our preliminary studies revealed that several mono-carbonyl
analogues not only have enhanced stability and antitumor activities
in vitro but also have better pharmacokinetic profiles in
vivo. One such compound,
(1E,4E)-1,5-bis(2-bromophenyl)penta-1,4-dien-3-one (GL63) (Fig. 1) was synthesized in our laboratory
as part of a series of novel curcumin analogues. We demonstrated
that GL63, as a new curcumin analogue, was more active than
curcumin in the inhibition of cell proliferation and induction of
apoptosis in human hepatocellular carcinoma HepG2 cells and human
lung epithelial H460 cancer cells (14,15).
In the present study, we characterized the biologic activity of
GL63 on NPC cells. Our data indicated that GL63 inhibits cell
viability, proliferation, induces cell cycle arrest and apoptosis.
GL63 displayed a greater specificity for activating the ER stress
pathway than curcumin. Treatment of NPC with GL63 also resulted in
enhanced tumor growth suppression in vivo. Our data suggest
that GL63 represents a promising lead compound that can be
optimized further for development as a therapeutic agent for
NPC.
Materials and methods
Materials
Cell culture reagents and fetal bovine serum (FBS)
were obtained from Invitrogen (Carlsbad, CA, USA). Antibodies
against CHOP, XBP-1, ATF-4, lamin B and horseradish peroxidase
(HRP)-conjugated donkey anti-goat IgG were from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). Western Lightning
Chemiluminescence Plus reagent was from Perkin-Elmer Life Sciences
(Boston, MA, USA). Annexin V/PI kit was from BD Biosciences (Palo
Alto, CA, USA). Curcumin, dimethyl sulfoxide (DMSO) and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were from Sigma-Aldrich, Inc. (St. Louis, MO, USA). GL63 was stored
in our laboratory as previously described (15).
Cell lines and culture conditions
The NPC cell line, CNE2, was obtained from the
Experimental Animal Center of Sun Yat-Sen University.
Radioresistant NPC cell line, CNE2R, was established in our
laboratory as previously described (16). The NPC cells were cultured in
RPMI-1640 medium with 10% FBS and antibiotics (100 U/ml of
penicillin and 100 μg/ml of streptomycin) in cell culture
incubators that were set at 37°C and aired with 5%
CO2.
MTT cell viability assay
The MTT assay was used to evaluate cell viability as
previously described (17).
Briefly, NPC cells were seeded in 96-well plates (2,000 cells/well)
in RPMI-1640 medium with 10% FBS. The following day the cells were
treated with GL63 and curcumin as indicated and incubated for 48 h.
MTT (20 μl) (5 mg/ml) was added to each well and incubation was
carried out for 3.5 h. The medium was discarded and 150 μl of DMSO
was added to each well, and incubated for 10 min. The absorbance
was read at 570 nm. The half-maximal inhibitory concentration
(IC50) was used as the concentration of drug required to
obtain 50% of maximal inhibition in cell viability.
Colony formation assay
We performed a colony formation assay as previously
described (18). In brief, the NPC
cells (200 cells/well) were plated in a 6-well plate for growth
analysis in RPMI-1640 medium with 10% FBS. The following day the
cells were treated with 1 μM GL63 or 1 μM curcumin and incubated
for 24 h. The NPC cells were grow at 37°C for 14 days. The effect
of the drugs on growth was determined by colony growth. Colonies
were stained with Giemsa dye and scored by counting with an
inverted microscope, using the standard definition that a colony
consists of 50 or more cells. Numbers were normalized to the
percentage of the colonies formed in DMSO treatment.
Cell cycle analysis by flow
cytometry
Following treatment with 5 μM of curcumin or GL63,
cells were collected and fixed overnight in 75% cold ethanol at
4°C. Cells were washed twice in cold PBS and labeled with propidium
iodide (PI, Sigma) as previously described (17), and analyzed immediately after
staining using Epics Elite flow cytometer (Coulter Diagnostics, Opa
Locka, FL, USA) and WinMDI29 software.
Measurement of apoptosis by Hoechst
staining and flow cytometry
To detect apoptosis, we performed nuclear staining
as previously described (18).
Cells exposed to GL63 or curcumin (5 μM) for 48 h were washed twice
with PBS and fixed with methanol for 15 min. The fixed cells were
then washed again with PBS and stained with 10 μg/ml of Hoechst
33342 for 15 min. The cells were examined under a fluorescence
microscope (Olympus, DX50).
Flow cytometric analysis, as previously described
(17), was used to differentiate
between living, early apoptotic, late apoptotic/necrotic and
necrotic cells by staining with Annexin V and PI. Briefly, after
treatment with 5 μM GL63 or curcumin for 48 h, all of the cells
were collected and resuspended in 100 μl binding buffer containing
Annexin and PI according to the manufacturer’s recommendations.
Quantification of Annexin V and PI binding was performed by a
FACScan.
Western blot analysis
Following treatment with 5, 10 and 20 μM curcumin or
GL63, the culture medium was collected and cells were washed with
ice-cold phosphate-buffered saline (PBS). The whole cell lysate was
prepared using lysis buffer containing 150 mM NaCl, 1.0% Nonidet
P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulphate (SDS),
50 mM Tris, pH 8.0 and protease and phosphatase inhibitors. Nuclear
extracts were isolated as previously described (15). The protein concentration was
measured using Bio-Rad protein assay reagent. The nuclear proteins
(15 μg of proteins) were resolved on 10% Bis-Tris Criterion XT gels
(Bio-Rad) and transferred onto nitrocellulose membranes.
Immunoblots were blocked with 5% non-fat milk in Tris-buffered
saline (TBS) for 1 h at room temperature, and then incubated with
primary antibodies against CHOP, XBP-1, ATF-4 and Lamin B at 4–8°C
overnight. Immunoreactive bands were detected using HRP-conjugated
secondary antibodies with the Western Lightning Chemiluminescence
Plus reagent.
Tumorigenicity assays in nude mice
The NPC xenograft model was used to evaluate tumor
growth-suppressive activity as previously described (19). Briefly, female 4- to 6-week-old nude
mice were obtained from the Animal Center of Sun Yat-Sen University
(Guangdong, China) and divided into four experimental groups (n=4
for each group). NPC cells were divided into the DMSO (control)
group, curcumin (5.0 μM) group and GL63 (2.5 and 5.0 μM) groups.
After treatment for 24 h, cells (2×106) were harvested
and injected s.c. into the right flank of each mouse. Tumor volumes
were measured every 2 days by measuring the length (L) and width
(W). The tumor volume was calculated as LW2/2. At the
end of the experiment, the mice were sacrificed, and the tumors
were removed for weighing.
Statistical analysis
Results are shown as means ± standard error (SE).
Statistical analysis for the results was carried out using the
Student’s t-test when only two groups were compared, or one-way
analysis of variance when more than two groups were compared.
Differences between groups were stated to be statistically
significant at P<0.05.
Results
GL63 inhibits NPC cell viability and
proliferation more potently than curcumin
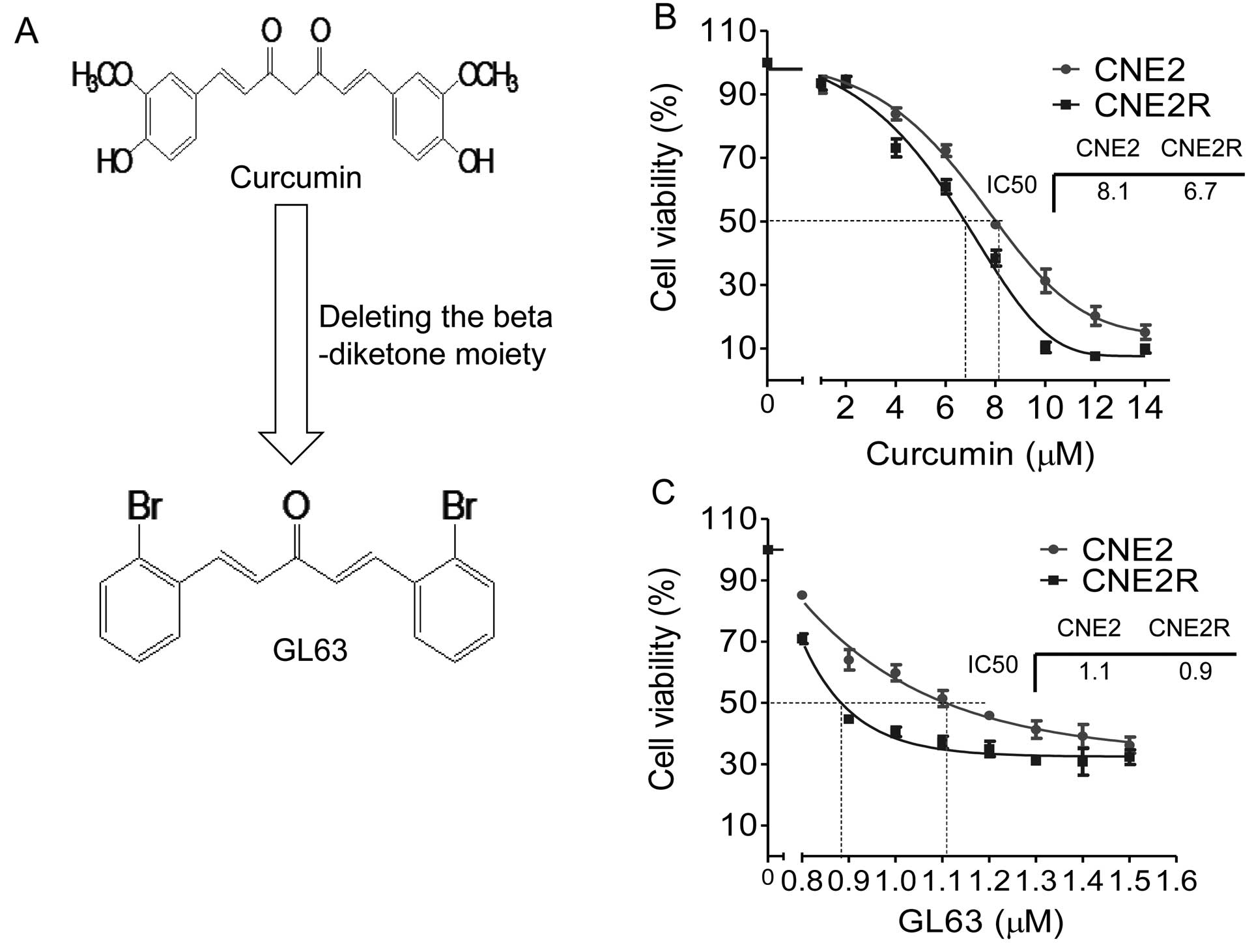
We examined the growth suppressive activities of
curcumin and GL63 (Fig. 1A) in two
human NPC cell lines, CNE2 and CNE2R. After treatments for 48 h,
both GL63 and curcumin caused inhibition of cell viability in the
two NPC cell lines (Fig. 1B and C).
However, GL63 exhibited greater inhibition than curcumin.
IC50 values for GL63 were 1.1 μM in the CNE2 cells and
0.9 μM in the CNE2R cells, respectively, which were substantially
more potent than curcumin (IC50 values 8.1 and 6.7
μM).
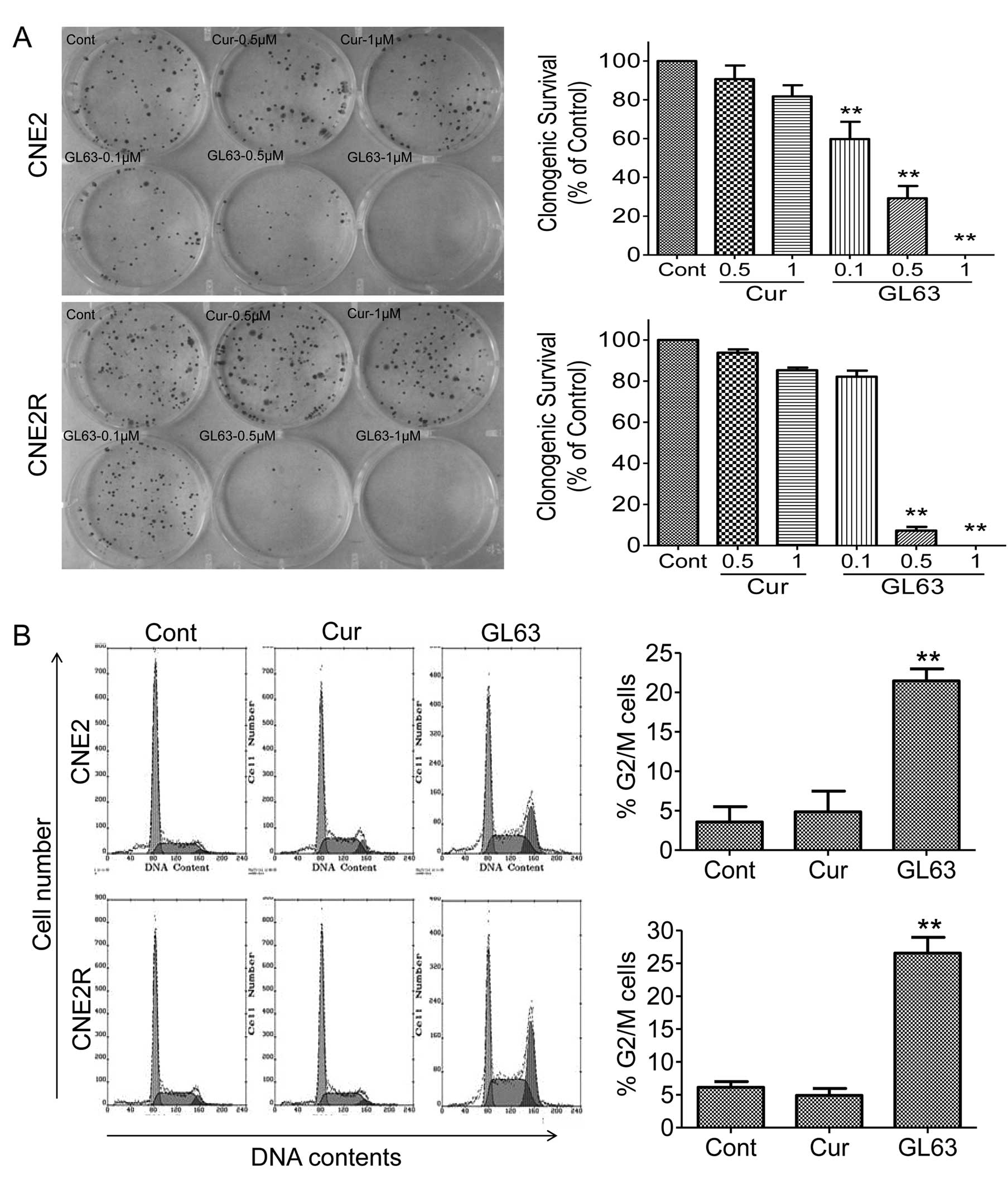
We further performed a colony formation assay to
test the effect of GL63 on NPC cell proliferation. The clonogenic
survival rate decreased 71 and 100% following treatment with 0.5
and 1 μM GL63, while the survival rate was reduced only 10 and 19%
following treatment with the same concentrations of curcumin
(Fig. 2A). We observed similar
results for the CNE2R cells; a 93 and 100% decrease in the survival
rate was noted after exposure to 0.5 and 1 μM GL63, while only a 7
and 15% reduction was noted after exposure to the same doses of
curcumin. These results demonstrated that GL63 was more potent than
curcumin in inhibiting cell viability and proliferation in NPC
cells.
GL63 is more potent than curcumin in
inducing NPC cell cycle arrest
Cell cycle distribution of CNE2 and CNE2R cells was
assessed following a 24-h treatment with 5 μM GL63 or curcumin by
flow cytometry. Treatment with GL63 resulted in an increase in the
percentage of cells in the G2/M phase from 3.6 to 21.5% in CNE2 and
from 6.1 to 26.6% in CNE2R cells, whereas 5 μM curcumin did not
cause a significant change in the cell cycle distribution,
indicating that GL63 induces G2/M phase arrest in NPC cells
(Fig. 2B). These data indicate that
GL63 exerts antitumor activity through cell cycle arrest.
GL63 promotes NPC cell apoptosis more
potently than curcumin
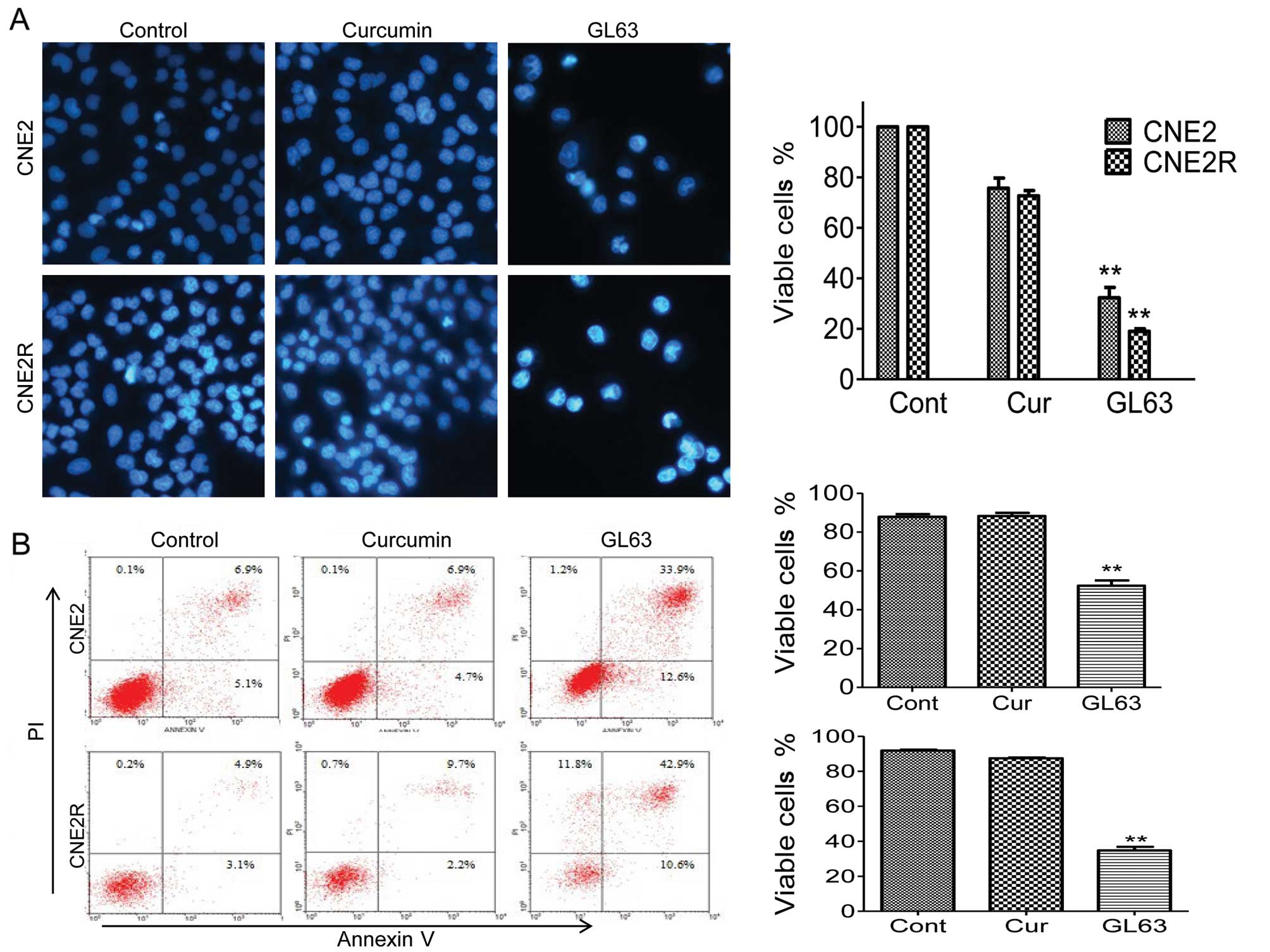
We next aimed to determine whether the GL63-induced
inhibition of cell viability occurs through increased apoptosis. We
first analyzed the effect of GL63 on the viability of cells using
Hoechst fluorescence. In agreement with our MTT data, GL63 markedly
decreased the viable cell number; there was a 68 and 81% reduction
in CNE2 and CNE2R cells, while only a 24 and 27% reduction was
noted following treatment with the same dose of curcumin (Fig. 3A). Furthermore, GL63 induced
morphological changes, which were characteristic of apoptosis in
the two NPC cell lines (Fig. 3A).
In contrast, the curcumin-treated cells displayed excellent growth
characteristics.
Flow cytometric analysis of NPC cells exposed to
GL63 confirmed the morphological observations noted above. As shown
in Fig. 3B, after treatment with 5
μM of curcumin for 48 h, the apoptosis rate exhibited no difference
when compared with the control group. However, following treatment
with the same concentration of GL63, the apoptotic ratios were
47.7% (CNE2) and 65.3% (CNE2R), respectively, which confirmed the
enhanced activity of induction of apoptosis by GL63 in NPC.
GL63 represses the tumorigenicity of NPC
cells more potently than curcumin
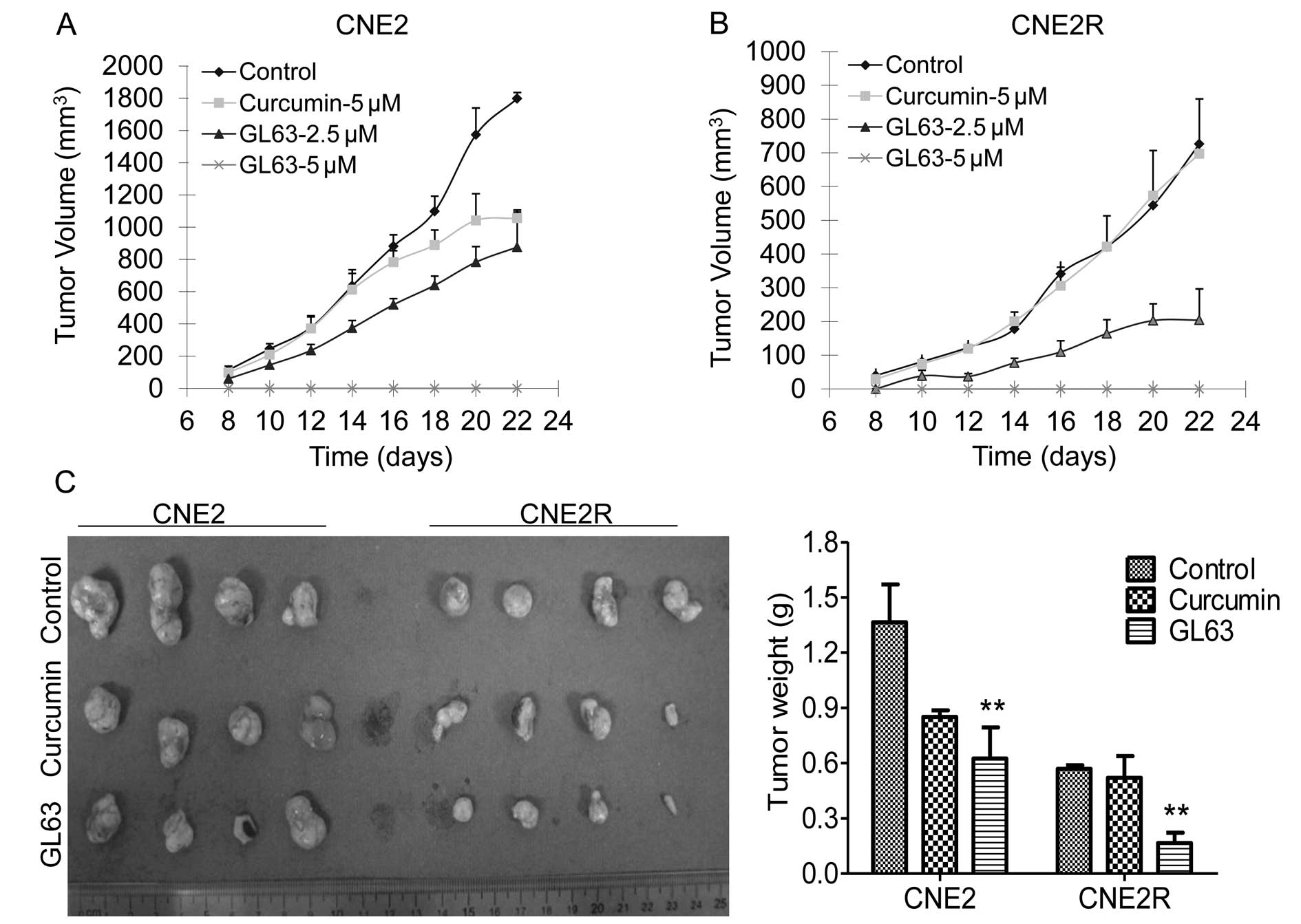
We explored the tumor-suppressive activity of the
curcumin analogue in NPC xenograft models. CNE2 and CNE2R cells
were treated with DMSO control or curcumin (5 μM) or GL63 (2.5 or 5
μM) for 24 h. The cells were then implanted into nude mice. Tumor
growth was observed in the control and the drug-treated mice. GL63
effectively suppressed the growth of tumors in mice bearing the
CNE2 (Fig. 4A) and CNE2R cells
(Fig. 4B), reducing the tumor
weight by 54.2% (CNE2) and 70.8% (CNE2R), respectively, compared
with the control group (Fig. 4C).
Equal concentrations of curcumin showed only a tumor growth
inhibition of 37.6% in the CNE2 tumors and 8.5% in the CNE2R
tumors, respectively (Fig. 4C).
Furthermore, no tumor development was noted in mice of the 5 μM
GL63 group, suggesting that GL63 is more effective than curcumin in
inhibiting NPC tumorigenicity.
Specific activation of the ER stress
pathway by GL63
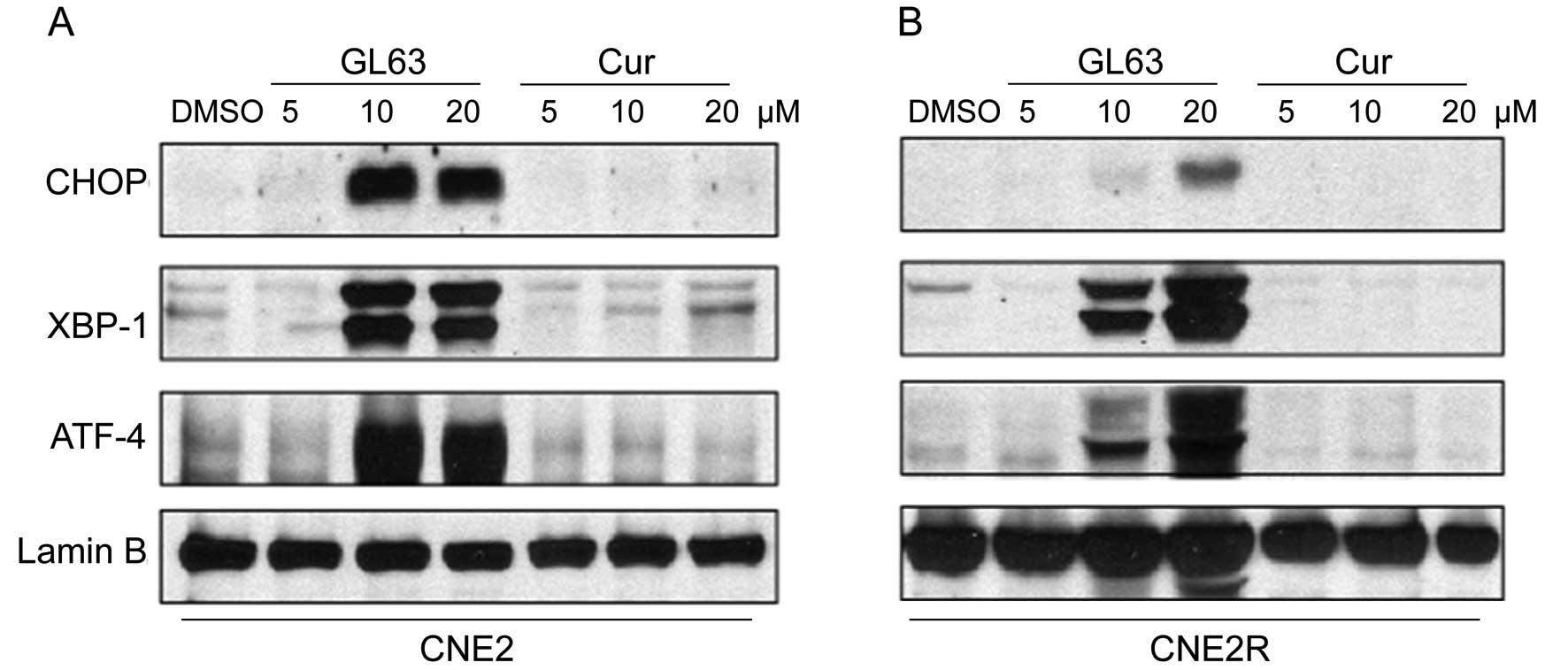
Induction of downstream transcription factors,
GRP78, XBP-1, ATF-4 and CHOP, are markers for the activation of ER
stress (20,21). We compared the effects of GL63 and
curcumin on the ER stress pathway in NPC cells. GL63 significantly
increased the levels of CHOP, XBP-1, ATF-4 protein at 10 and 20 μM
in CNE2 (Fig. 5A) and CNE2R cells
(Fig. 5B), whereas curcumin
treatment did not cause any change in the expression levels of
these proteins, even at 20 μM (Fig.
5). These results suggest that GL63-induced ER stress
represents a major cellular mechanism of its antitumor
activity.
Discussion
Since curcumin is poorly absorbed, more potent and
soluble curcumin analogues have been developed (7). In our previous studies (14,15), a
series of curcumin analogues were designed by the deletion of the
highly reactive β-diketone moiety in the structure of curcumin
which is considered to be responsible for the in vitro
instability and the in vivo pharmacokinetic disadvantages.
In the present study, we demonstrated that the novel curcumin
analogue GL63 significantly inhibited NPC cell viability,
proliferation, and induced cell cycle arrest, while curcumin at the
same dose did not have the same therapeutic effects.
Further study confirmed that GL63 was directly
involved in inhibiting tumor growth in vivo. To the best of
our knowledge, this is the first study to demonstrate that a
curcumin analogue has the capability for enhanced antitumor
activity in radioresistant NPC cells.
In addition to GL63, other curcumin analogues have
been reported (22,23). However, none of the current curcumin
analogues have been reported to be able to induce cell cycle arrest
and apoptosis in NPC. Our results revealed that GL63 induces G2/M
phase arrest and apoptosis in CNE2 and CNE2R cells. Mammalian cells
exhibit significant variation in radiosensitivity as the cell cycle
progresses. Cells in the G2/M phase are the most radiosensitive
(24). These findings show that
accumulation of cells in the most radiosensitive G2/M phase caused
by GL63 may enhance the radiosensitization effect in NPC cells.
A recent study found that intracellular organelles,
including the ER, promote cell apoptosis signals. The unfolded
protein response (UPR) is an intracellular signaling pathway, which
regulates the accumulation of unfolded or misfolded proteins in the
ER and plays an important role in regulating cell growth,
differentiation and apoptosis (25,26).
Therefore, the possibility that GL63 induces apoptosis via ER
stress was examined. CHOP is a typical ER stress-regulated protein
involved in ER stress-induced apoptosis (27). Our results concerning CHOP induction
by GL63 suggest that GL63 may trigger ER stress. GL63 also
increased the levels of XBP-1 and ATF-4, both of which are proteins
increased during ER stress. However, curcumin at the same
concentration had no effect on these ER stress markers, suggesting
that the enhanced antitumor effect of GL63 involves ER stress.
Furthermore, ER stress signaling appears to play an important role
in proliferation, chemoresistance, and radioresistance in cancer
cells (28,29). We found that GL63 not only affected
the CNE2 cells, but also had a markedly suppressive effect on
radioresistant CNE2R cells, which may have resulted from the
induction of the ER stress pathway.
Taken together, our results indicate that GL63
exhibits enhanced antitumor activity in NPC cells. Our findings
demonstrated that GL63 activates ER stress and supports GL63 as an
anticancer agent with important clinical relevance. The
administration of GL63 is a potential therapeutic regimen for
NPC.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (81071837 and 30670627); the
Natural Science Foundation of Guangdong Province, China
(9251008901000005 and 06021210) and the Scientific and
Technological Project of Guangdong, China (2008A030201009 and
2010B050700016) (H.Y.).
References
|
1
|
Lo KW, Chung GT and To KF: Deciphering the
molecular genetic basis of NPC through molecular, cytogenetic, and
epigenetic approaches. Semin Cancer Biol. 22:79–86. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hui EP, Ma BB, Leung SF, et al: Randomized
phase II trial of concurrent cisplatin-radiotherapy with or without
neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal
carcinoma. J Clin Oncol. 27:242–249. 2009. View Article : Google Scholar
|
|
3
|
Yip KW, Mocanu JD, Au PY, et al:
Combination Bcl-2 antisense and radiation therapy for
nasopharyngeal cancer. Clin Cancer Res. 11:8131–8144. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lev-Ari S, Lichtenberg D and Arber N:
Compositions for treatment of cancer and inflammation. Recent Pat
Anticancer Drug Discov. 3:55–62. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gandhy SU, Kim K, Larsen L, Rosengren RJ
and Safe S: Curcumin and synthetic analogs induce reactive oxygen
species and decrease specificity protein (Sp) transcription factors
by targeting microRNAs. BMC Cancer. 12:5642012. View Article : Google Scholar
|
|
6
|
Binion DG, Otterson MF and Rafiee P:
Curcumin inhibits VEGF-mediated angiogenesis in human intestinal
microvascular endothelial cells through COX-2 and MAPK inhibition.
Gut. 57:1509–1517. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Anand P, Kunnumakkara AB, Newman RA and
Aggarwal BB: Bioavailability of curcumin: problems and promises.
Mol Pharm. 4:807–818. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pae HO, Jeong SO, Jeong GS, et al:
Curcumin induces pro-apoptotic endoplasmic reticulum stress in
human leukemia HL-60 cells. Biochem Biophys Res Commun.
353:1040–1045. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Isohashi F, Endo H, Mukai M, Inoue T and
Inoue M: Insulin-like growth factor stimulation increases
radiosensitivity of a pancreatic cancer cell line through
endoplasmic reticulum stress under hypoxic conditions. Cancer Sci.
99:2395–2401. 2008. View Article : Google Scholar
|
|
10
|
Yogosawa S, Yamada Y, Yasuda S, Sun Q,
Takizawa K and Sakai T: Dehydrozingerone, a structural analogue of
curcumin, induces cell-cycle arrest at the G2/M phase and
accumulates intracellular ROS in HT-29 human colon cancer cells. J
Nat Prod. 75:2088–2093. 2012. View Article : Google Scholar
|
|
11
|
Subramaniam D, May R, Sureban SM, et al:
Diphenyl difluoroketone: a curcumin derivative with potent in vivo
anticancer activity. Cancer Res. 68:1962–1969. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liang G, Yang S, Zhou H, et al: Synthesis,
crystal structure and anti-inflammatory properties of curcumin
analogues. Eur J Med Chem. 44:915–919. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liang G, Shao L, Wang Y, et al:
Exploration and synthesis of curcumin analogues with improved
structural stability both in vitro and in vivo as cytotoxic agents.
Bioorg Med Chem. 17:2623–2631. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiao J, Chu Y, Hu K, et al: Synthesis and
biological analysis of a new curcumin analogue for enhanced
anti-tumor activity in HepG 2 cells. Oncol Rep. 23:1435–1441.
2010.PubMed/NCBI
|
|
15
|
Xiao J, Tan Y, Pan Y, et al: A new
cyclooxygenase-2 inhibitor,
(1E,4E)-1,5-bis(2-bromophenyl)penta-1,4-dien-3-one (GL63)
suppresses cyclooxygenase-2 gene expression in human lung
epithelial cancer cells: coupled mRNA stabilization and
posttranscriptional inhibition. Biol Pharm Bull. 33:1170–1175.
2010. View Article : Google Scholar
|
|
16
|
Qu C, Liang Z, Huang J, et al: miR-205
determines the radioresistance of human nasopharyngeal carcinoma by
directly targeting PTEN. Cell Cycle. 11:785–796. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pan Y, Zhang Q, Tian L, et al: Jab1/CSN5
negatively regulates p27 and plays a role in the pathogenesis of
nasopharyngeal carcinoma. Cancer Res. 72:1890–1900. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pan Y, Zhang Q, Atsaves V, Yang H and
Claret FX: Suppression of Jab1/CSN5 induces radio- and
chemo-sensitivity in nasopharyngeal carcinoma through changes to
the DNA damage and repair pathways. Oncogene. Jul 16–2012.(Epub
ahead of print).
|
|
19
|
Yang H, Wen YY, Zhao R, et al: DNA
damage-induced protein 14-3-3 sigma inhibits protein kinase B/Akt
activation and suppresses Akt-activated cancer. Cancer Res.
66:3096–3105. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsuo K, Gray MJ, Yang DY, et al: The
endoplasmic reticulum stress marker, glucose-regulated protein-78
(GRP78) in visceral adipocytes predicts endometrial cancer
progression and patient survival. Gynecol Oncol. 128:552–559. 2012.
View Article : Google Scholar
|
|
21
|
Eizirik DL, Miani M and Cardozo AK:
Signalling danger: endoplasmic reticulum stress and the unfolded
protein response in pancreatic islet inflammation. Diabetologia.
56:234–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hutzen B, Friedman L, Sobo M, et al:
Curcumin analogue GO-Y030 inhibits STAT3 activity and cell growth
in breast and pancreatic carcinomas. Int J Oncol. 35:867–872.
2009.PubMed/NCBI
|
|
23
|
Friedman L, Lin L, Ball S, et al: Curcumin
analogues exhibit enhanced growth suppressive activity in human
pancreatic cancer cells. Anticancer Drugs. 20:444–449. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Naidu MD, Mason JM, Pica RV, Fung H and
Pena LA: Radiation resistance in glioma cells determined by DNA
damage repair activity of Ape1/Ref-1. J Radiat Res. 51:393–404.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zou W, Yue P, Khuri FR and Sun SY:
Coupling of endoplasmic reticulum stress to CDDO-Me-induced
up-regulation of death receptor 5 via a CHOP-dependent mechanism
involving JNK activation. Cancer Res. 68:7484–7492. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang K and Kaufman RJ: Signaling the
unfolded protein response from the endoplasmic reticulum. J Biol
Chem. 279:25935–25938. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu D, Yin H and Zhang M: Signaling
pathways involved in endoplasmic reticulum stress-induced neuronal
apoptosis. Int J Neurosci. 123:155–162. 2012. View Article : Google Scholar
|
|
28
|
Bobrovnikova-Marjon E, Grigoriadou C,
Pytel D, et al: PERK promotes cancer cell proliferation and tumor
growth by limiting oxidative DNA damage. Oncogene. 29:3881–3895.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yamazaki T, Sasaki N, Nishi M and
Takeshima H: Facilitation of DNA damage-induced apoptosis by
endoplasmic reticulum protein mitsugumin23. Biochem Biophys Res
Commun. 392:196–200. 2010. View Article : Google Scholar : PubMed/NCBI
|