Introduction
Dendritic cells (DCs) are antigen-presenting cells
(APCs) that show the unique ability to activate and regulate immune
responses (1). DCs can provide
antigenic peptides to initiate naive T-cell responses, as well as
produce potent costimulatory molecules to promote T-cells
differentiation (2,3). Plenty of studies have demonstrated
that both the cell surface markers and antigen presenting function
of tumor-infiltrating DCs decreased significantly (4,5).
However, the mechanisms during this process are still to be
illustrated.
Some research has shown that DC precursors can
trans-differentiate into endothelial-like cells (ELCs) in mouse and
human ovarian carcinomas indicating a role of VEGF-A and
β-defensins (6). CD34+
progenitor cells redirected their differentiation into endothelial
cells (ECs) from developing into DCs under the influence of
conditioned medium (CM) of murine Lewis lung carcinoma cells
(7). In addition, tumor-associated
DCs incubated with the pro-angiogenic factors VEGF and oncostatin
M, trans-differentiated into ELCs, suggesting an alternative
pathway of tumor angiogenesis (8).
The research shows that DC progenitor or immature DCs (iDCs) can
trans-differentiate into ELCs, possibly contributing to
vasculogenesis. Thereby, DCs might exert some impact on the
neovascularization process in different physiopathological
conditions (9).
Esophageal cancer is one of the most frequently
diagnosed cancers in Asia and has high-incidence and high mortality
rate. Esophageal squamous cell carcinoma is the main type of
esophageal cancer, which has strong invasiveness and poor
prognosis. We previously reported that the CM from human esophageal
squamous cell carcinoma (ESCC) cell line EC9706 induced iDCs to
differentiate into ELCs (10).
However, this CM is far different from the esophageal carcinoma
tissue of patients. The potential role of peri-esophageal carcinoma
in the endothelial-like differentiation (ELD) of iDCs is also
unknown. In the present study, we examined the possibility of ELD
of iDCs in the microenvironment derived from the tissue homogenate
of esophageal carcinoma and peri-esophageal carcinoma, and
investigated the role of extracellular signal-regulated kinase
(ERK) signaling and cAMP response element binding protein (CREB) in
this process.
Materials and methods
Preparation of tissue homogenate
supernatant of human esophageal carcinoma and peri-carcinoma
Fresh tumor specimens of ESCC tissue and
peri-carcinoma tissue (>5 cm) 6 cases (without preoperative
chemotherapy and radiotherapy) were collected to prepare to the
tissue homogenate supernatant by grinding and centrifuge. The above
specimens were contributed by donors from the Henan Tumor
Hospital.
Cell culture
The culture of primary human umbilical vein
endothelial cells (HUVECs) is according to our previous report
(10). The aseptic cords were
contributed by healthy parturient donors from the Third Affiliated
Hospital, Zhengzhou University. Peripheral blood mononuclear cells
(PBMCs) were harvested from healthy adult volunteers by density
gradient centrifugation over Ficoll (8) and seeded in 24-well plates at
2×109/l for 3 h. The adherent cells (monocytes) were
induced toward DCs with rhGM-CSF 100 μg/l (Amoytop), rhIL-4 5 μg/l
(PeproTech). ESCC homogenate supernatant (40%) or peri-carcinoma
homogenate supernatant (40%) was added at the end of day 2. After 7
days induction, induced cells were harvested on day 9 (i.e. 2+7)
for study. Parallel culturing of control DCs also were harvested on
day 9.
Western blot analysis
Total protein concentration of each group of cells
was measured by the Bradford method. Cell lysates (50 μg) were
resolved to 10% SDS-PAGE gel and transferred to PVDF membrane.
CD144 Ab (1:1000; Cell Signaling Technology), vWF Ab (1:300),
phospho-p44/42 MAPK/ERK Ab (Thr202/Tyr204; 1:1000; Cell Signaling
Technology), p44/42 MAPK/ERK Ab (Thr202/Tyr204; 1:1000; Cell
Signaling Technology) were added, respectively, overnight at 4°C.
HRP-IgG secondary antibody was incubated for 2 h at room
temperature. The membranes were visualized with ECL. The Gel Doc
Imaging system was used for detecting the gray value of the protein
bands.
Immunofluorescence
Control DCs, induced cells, and the positive control
HUVECs were seeded in 96-well plate and incubated for 24 h, then
fixed with 4% paraformaldehyde for 30 min. Rabbit anti-human vWF
antibody (1:200; Santa Cruz Biotechnology) and FITC-conjugated goat
anti-rabbit IgG antibody were used. The procedure was followed by
an immunocytochemical protocol. Cells with green fluorescent
particles in the cytoplasm were counted as having positive
expression. The Biosens Digital Imaging system was used for
detecting the fluorescence intensity of the samples.
Dil-Ac-LDL and India ink uptake assay
(8,11)
The induced cells and control groups were seeded in
96-well plate and incubated for 36 h. Dil-Ac-LDL (10 μg/ml)
(Biomedical Technologies Inc.) was added and incubated at 37°C for
4 h. The medium containing Dil-Ac-LDL was removed, and washed 3
times with PBS. India ink (10 μl/ml) was added and the cells were
incubated at 37°C for another 4 h. The cells were washed 3 times
with PBS and observed with a fluorescence microscope.
Preparation of whole-cell antigen
EC9706 cells were collected and washed twice with
PBS. Resuspended to a concentration of 1×1010/l. The
antigen was prepared by ultrasonic crushing technique for 3 min
(pulse 4 sec, amplitude 1) and centrifuged 13,000 rpm/min for 30
min at 4°C. The supernatant was collected and filtered. Antigen
concentration was determined by Bradford method and stored at −20°C
for use.
Mixed lymphocyte reaction
EC9706 cell antigen was added into the cells on day
4 at the final concentration (100 mg/l) and these cells were
collected on day 9. Pulsed cells were regarded as stimulator cells,
and the autologous T cells were regarded as response cells, and
they were mixed in 1:20 ratios. The mixed cells were placed in
96-well plate (1×104/well) in quadruplicate for 72 h.
CCK-8 (10 μl/well) (Dojindo Laboratories) was incubated with the
mixed cells for 2 h and the absorbance was measured at 450 nm. The
proliferation rate of the T cells was defined as (%) = (A value of
experimental group - A value of quiescent T cells)/(A value of
quiescent T cells) × 100%.
Cytotoxicity assay
Autologous T cells and pulsed cells were co-cultured
in a ratio of 20:1 for 72 h. CTLs and EC9706 cells were placed in
96-well plate in quadruplicate for 72 h, according to the ratio of
30:1, in a total volume of 200 μl/well (1×104/well). CTL
killing activity was detected by CCK-8 kit (Dojindo Laboratories)
according to the protocol. The CTL killing ratio was defined as (%)
= (A value of the control group - A value of the experimental
group)/(A value of the control group) × 100%.
Statistical analysis
Data were expressed as means ± standard deviation
(SD) with at least three separate experiments and analyzed by
one-way ANOVA and q-test. Significance was defined as
P<0.05.
Results
The changes of morphology and cell marker
of the ELD of iDCs in tissue homogenate supernatant of esophageal
carcinoma and peri-carcinoma
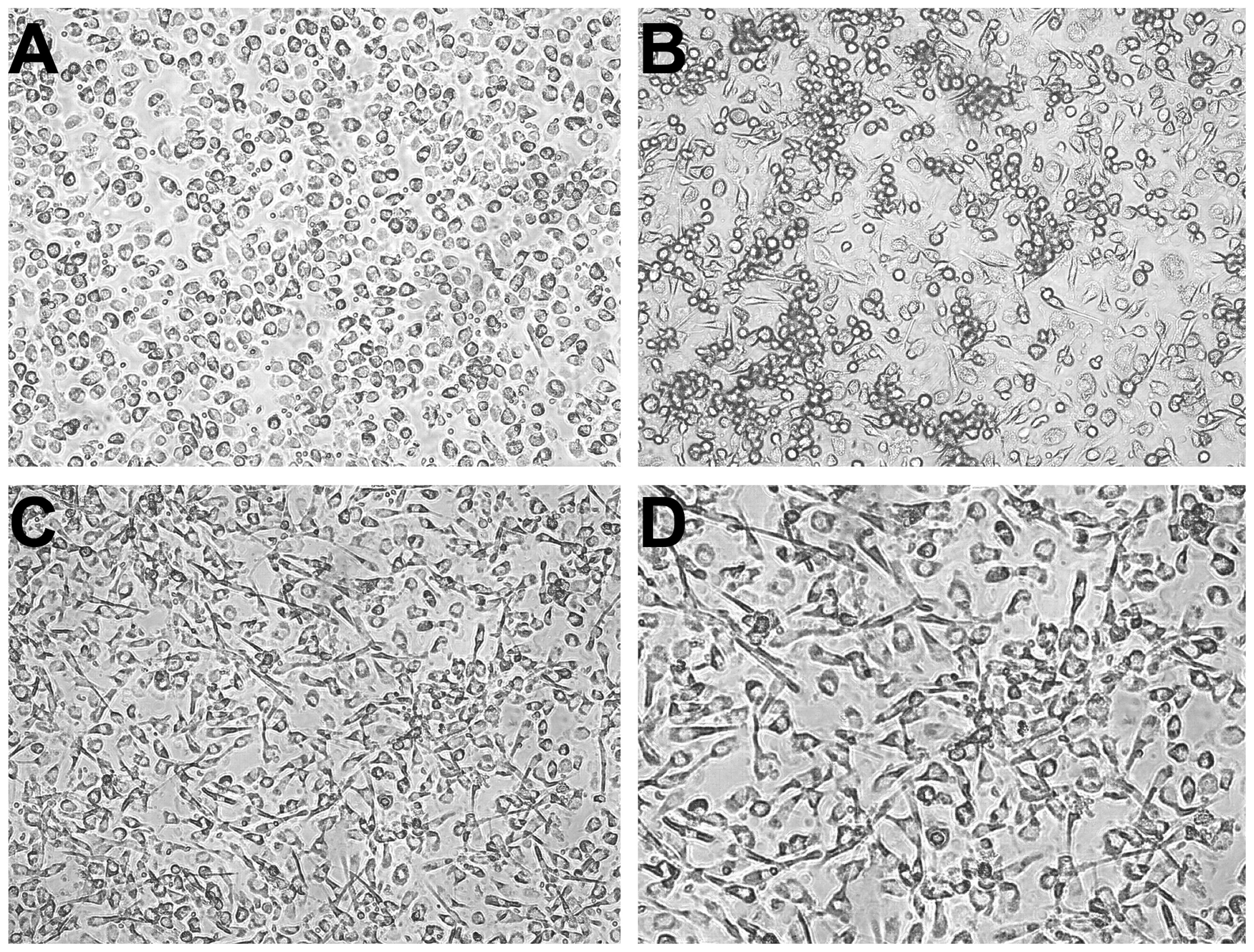
The iDCs were induced by esophageal carcinoma or
peri-carcinoma homogenate for 7 days. The cells induced by
carcinoma homogenate were slender, while most of the cells induced
by peri-carcinoma homogenate were round, similar to the appearance
of control DCs (Fig. 1).
The expression levels of endothelial cell markers
vWF and CD144 were significantly increased in cells induced by
carcinoma homogenate compared with the peri-carcinoma group (n=3,
P<0.001, P<0.01, respectively) and control DCs (n=3,
P<0.001, P<0.01, respectively). There was no obvious
difference between the peri-carcinoma group and control DCs (n=3,
P>0.05) (Fig. 2A).
Immunofluorescence analysis also revealed the enhanced expression
of vWF in carcinoma homogenate induced cells compared with the
peri-carcinoma group (n=3, P<0.01) and control DCs (n=3,
P<0.01) (Fig. 2B).
The functional changes of the ELD of iDCs
in tissue homogenate supernatant of esophageal carcinoma and
peri-carcinoma
Uptake of Dil-Ac-LDL is considered to be one of the
typical functions of ECs although shared with other cells as
macrophages and monocytes (12,13).
However, monocytes and macrophages show uptake of Dil-Ac-LDL as
well as India ink (11). In
previous research we have proved that PBMCs had weak uptake of
Dil-Ac-LDL, and strong uptake of India ink (10). In the present study, the induced
cells by esophageal carcinoma homogenate did not intake India ink
(Fig. 3 right panel), excluding the
possibility that macrophages and monocytes were mixed in with the
induced cells. The obviously increased uptake of Dil-Ac-LDL in
induced cells by carcinoma homogenate showed that the iDCs tended
to differentiate toward ECs (Fig.
3C). In contrast, there was no difference of Dil-Ac-LDL uptake
between peri-carcinoma and control DCs (Fig. 3A and B).
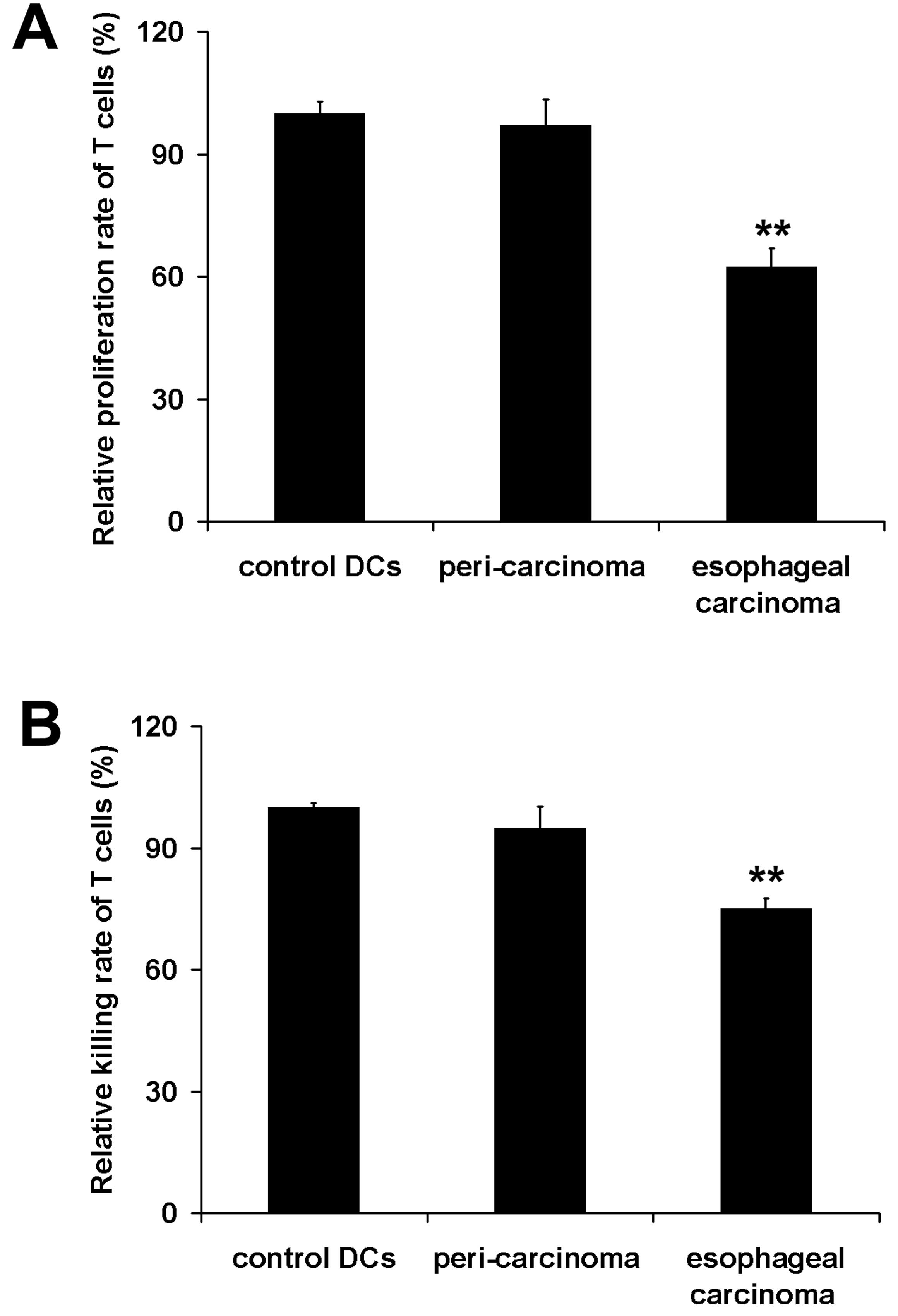
To determine the change of antigen presentation
function of iDCs induced by esophageal carcinoma homogenate, we
measured their ability to stimulate T cell proliferation and CTL
activity for killing EC9706 cells in vitro. The results
showed that the microenviroment produced by esophageal carcinoma
homogenate significantly disabled the antigen presenting function
of the cells (P<0.01), however, the peri-carcinoma homogenate
did not have this effect on the cells (Fig. 4).
Thus, the above results showed that esophageal
carcinoma homogenate, not peri-esophageal carcinoma homogenate,
induced iDCs to differentiate into endothelial-like cells, instead
of differentiation into mature DCs.
ERK signaling and CREB is involved in ELD
of iDCs induced by the esophageal carcinoma tissue homogenate
supernatant
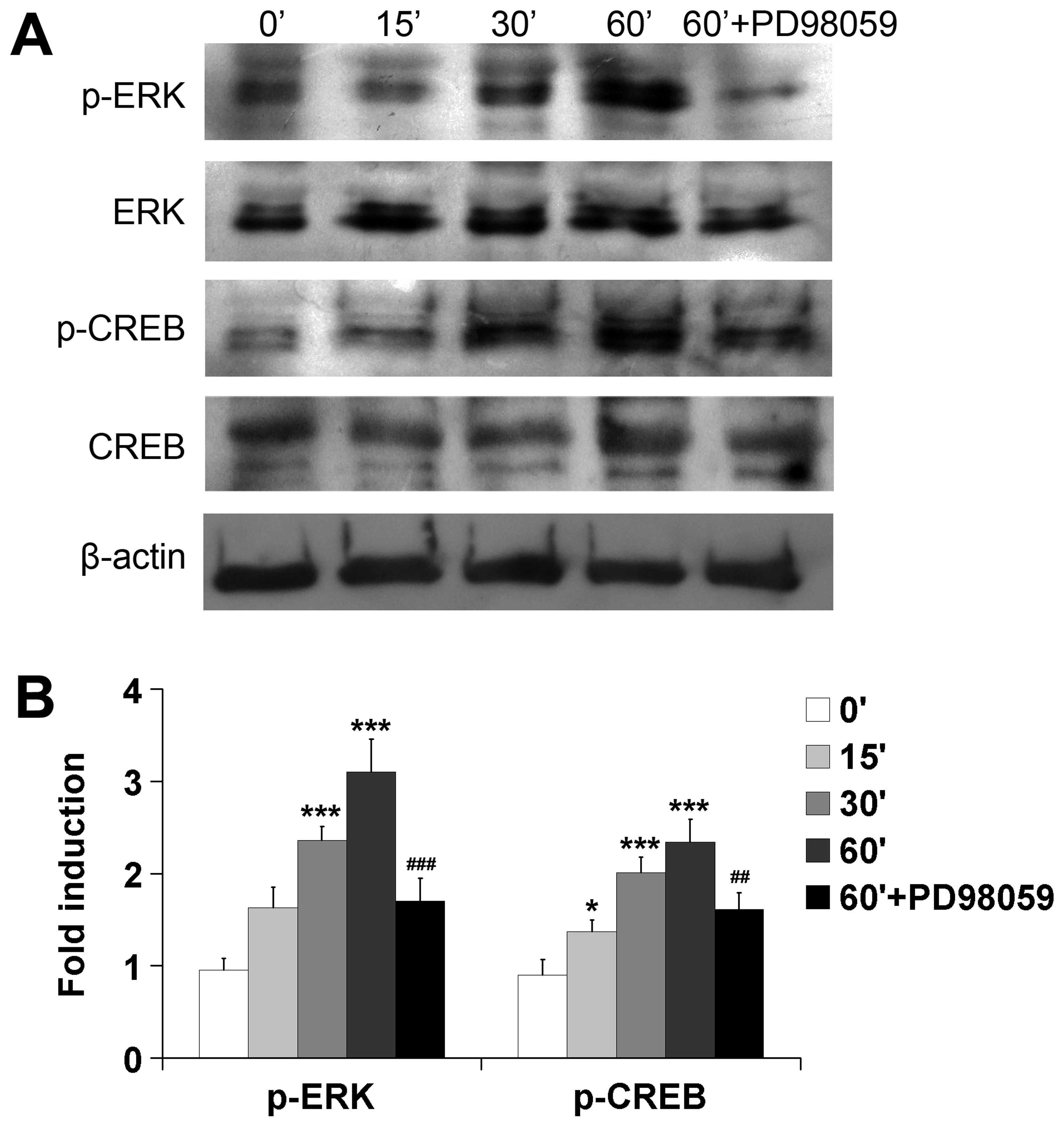
In previous research, we found that EC9706 CM
activated ERK signaling pathway and CREB to mediate ELD of iDCs
(10). Thus, we asked if ERK
signaling and CREB are also involved in ELD of iDCs induced by
esophageal carcinoma homogenate. We found that the phosphorylation
levels of ERK and CREB increased in a time-dependent manner in the
differentiating iDCs. Esophageal carcinoma homogenate stimulated
strong phosphorylation of ERK after 30 min of incubation and CREB
after 15 min of incubation. PD98059 is a selective inhibitor of
MEK, the upstream regulator of phosphorylation of ERK. Therefore,
we used PD98059 50 μM to block MEK to investigate the
phosphorylation levels of ERK and CREB. Results showed that the
phosphorylation levels of both ERK and CREB were significantly
inhibited in the presence of PD98059 (n=3, P<0.001 and
P<0.01, respectively) (Fig.
5).
Blocking ERK signaling inhibits ELD of
iDCs in the esophageal carcinoma tissue homogenate
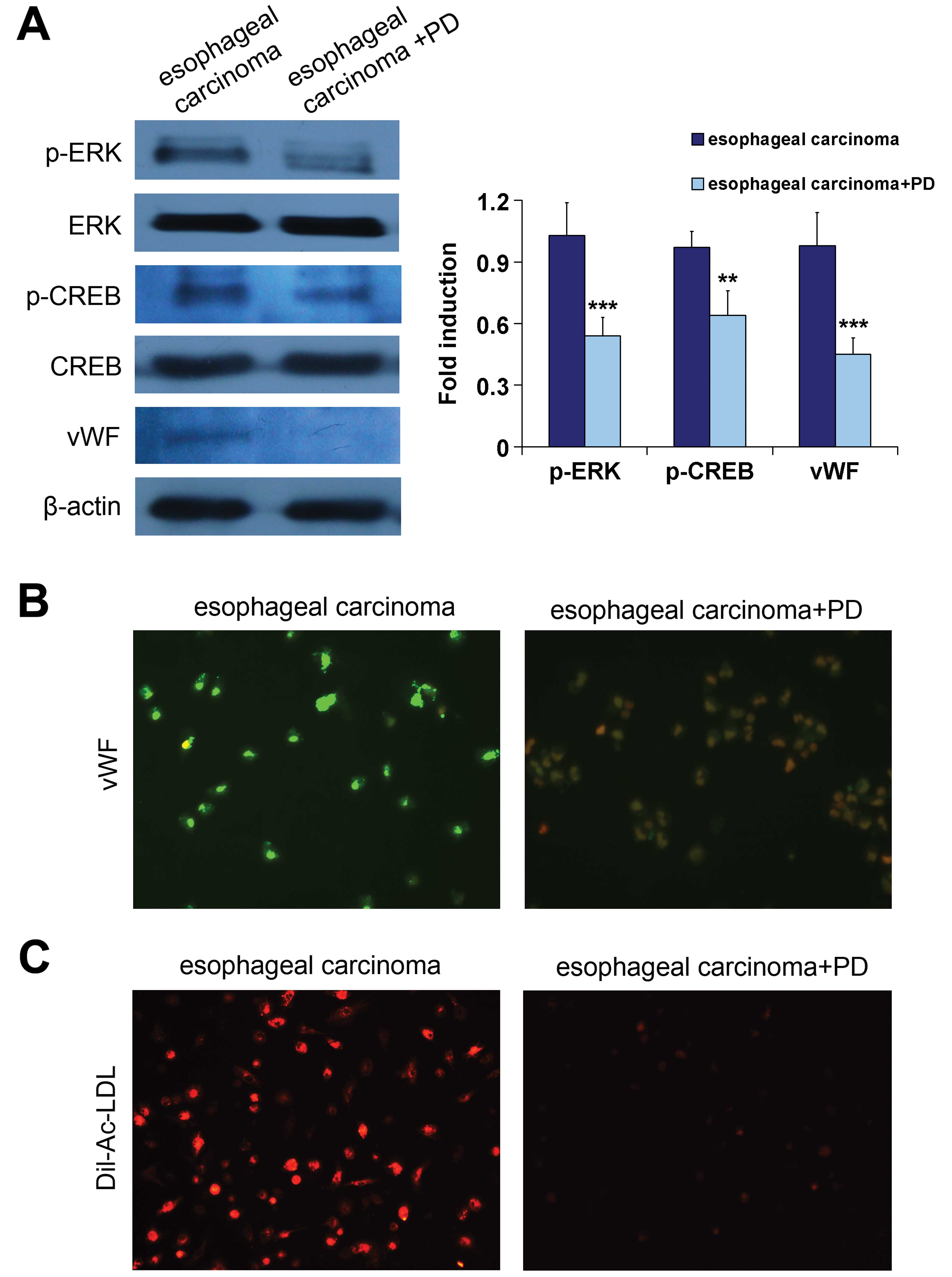
After 7 days induction by esophageal carcinoma
homogenate, the expression levels of phospho-ERK and CREB were
still strong in the differentiating iDCs. In order to clarify that
the ERK signaling pathway is essential for the differentiation, the
effect of PD98059 10 μM on the ELD of iDCs was investigated. We
found that both ERK and CREB phosphorylation was significantly
inhibited after a 7-day induction by esophageal carcinoma
homogenate in the presence of PD98059 (n=3, P<0.001 and
P<0.01, respectively) (Fig. 6A).
Inhibition of ERK phosphorylation by PD98059 was accompanied by a
significantly decreased expression of vWF in induced cells (n=3,
P<0.001) (Fig. 6A and B).
Furthermore, Dil-Ac-LDL uptake was reduced in the cells of
iDC-induced group in the presence of PD98059 (Fig. 6C). Thus, inhibition of ERK
phosphorylation by PD98059 results in the inhibition of ELD of
iDCs.
Discussion
It is traditionally believed that tumor
vascularization is ascribed to the sprouting of ECs from existing
vessels. However, recent studies have indicated that the
recruitment of endothelial progenitors that differentiate into ECs
plays an important role in tumor neovessel formation (14,15).
Research has shown that DCs and their precursors might contribute
to vasculogenesis by trans-differentiation into ELCs (6–8,14,16).
The redirection of differentiation from the DC pathway to ECs might
limit the antigen presentation function of DCs, and thereby
facilitate tumor growth and immune escape. However, till now the
related research has been limited. Therefore, this phenomenon and
its mechanisms are worthy of further investigation.
It has been shown that simulating the tumor
microenvironment by the presence of cytokines and lactate induced
monocytes to transdifferentiate into ELCs in the presence of
pro-angiogenic mediators (8,16). CM
from murine Lewis lung carcinoma cells was used to demonstrate the
CD34+ cell differentiation (7). Our previous research also showed that
the CM from EC9706 cell line induced iDCs to differentiate into
ELCs (10). However, this kind of
CM is far different from the esophageal carcinoma tissue of
patients. The potential role of peri-esophageal carcinoma in the
ELD of iDCs is also unknown. The growth and development of tumors
rely on a continuous cross-talk among cancer cells, together with
intracellular and extracellular microenvironments, including
complicated and mutual interactions among tumor cells, various
stromal and other cells (17,18).
Therefore, to some extent, tumor tissue homogenate can reflect the
tumor microenvironment better than the CM from cell culture
supernatant. Thus, in the present study, we used the homogenate
supernatant of human esophageal carcinoma and peri-carcinoma to
simulate the tumor microenvironment, to demonstrate iDCs effect on
ELD. We found that similar to the CM from EC9706 cell line,
esophageal carcinoma homogenate could induce ELD of iDCs. In
contrast, peri-carcinoma homogenate did not have this effect.
Furthermore, during iDC differentiation into ELCs, the intake of
Dil-Ac-LDL, but not India ink, strongly increased in most of the
induced cells, distinguishing them from mononuclear cells and
macrophages. The activity of APCs obviously decreased, as assessed
by checking the capacity to stimulate autologous T cells in mixed
lymphocyte culture and the CTL killing activity. As is known, tumor
cells can secrete certain immune factors which can inhibit the
development and maturation of DCs (19,20).
Some studies have shown that tumor patients have defects in DC
maturation, and the reduced function of DCs leads to immune
suppression (21,22). The redirection of differentiation
from DC pathway to ECs might demonstrate one of the mechanisms of
the reduced number of tumor-infiltrating DCs.
Many growth factors, cytokines and vasoactive
substances can lead to cell differentiation via cellular signal
transduction. The MAPK/ERK signaling pathway is involved in cell
growth, differentiation, proliferation and apoptosis in multiple
physiological processes (23,24).
Activated MAPK can phosphorylate specific substrates on serine
and/or threonine residues, leading to the activation of various
transcription factors to control certain important physiological
activities (25). CREB is one of
the downstream signal molecules of ERK signaling pathway (26,27).
The phosphorylation of CREB mediated by ERK signaling responds to a
variety of external signals to regulate cell differentiation and
neurite outgrowth (28,29). In the present study, our results
demonstrated that ERK phosphorylation was required for ELD of iDCs.
PD98059, an inhibitor of MEK, not only inhibited the activation of
ERK, but also inhibited the ELD of iDCs, and CREB is involved in
this process.
In summary, our results demonstrated that iDCs could
differentiate into ELCs under the influence of tissue homogenate of
human esophageal carcinoma, while the peri-esophageal carcinoma
homogenate does not have this function. During the course of this
differentiation, ERK signaling pathway and CREB were activated to
mediate ELD of iDCs. These data suggest that esophageal carcinoma
tissue can drive iDCs to differentiate into ELCs, instead of
differentiation into mature DCs, thereby losing its ability of
antigen presentation.
Acknowledgements
This study was supported by the Natural Science
Foundation of China (no. 81101731).
References
|
1
|
Xue G, Cheng Y, Ran F, et al: SLC
gene-modified dendritic cells mediate T cell-dependent anti-gastric
cancer immune responses in vitro. Oncol Rep. 29:595–604.
2013.PubMed/NCBI
|
|
2
|
Wu H, Han Y, Qin Y, et al: Whole-cell
vaccine coated with recombinant calreticulin enhances activation of
dendritic cells and induces tumour-specific immune responses. Oncol
Rep. 29:529–534. 2013.PubMed/NCBI
|
|
3
|
Giardino Torchia ML, Ciaglia E, Masci AM,
et al: Dendritic cells/natural killer cross-talk: a novel target
for human immunodeficiency virus type-1 protease inhibitors. PLoS
One. 5:e110522010.PubMed/NCBI
|
|
4
|
Demoulin S, Herfs M, Delvenne P and Hubert
P: Tumor microenvironment converts plasmacytoid dendritic cells
into immunosuppressive/tolerogenic cells: insight into the
molecular mechanisms. J Leukoc Biol. 93:343–352. 2012. View Article : Google Scholar
|
|
5
|
Benencia F, Sprague L, McGinty J, Pate M
and Muccioli M: Dendritic cells the tumor microenvironment and the
challenges for an effective antitumor vaccination. J Biomed
Biotechnol. 2012:4254762012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Conejo-Garcia JR, Benencia F, Courreges
MC, et al: Tumor-infiltrating dendritic cell precursors recruited
by a β-defensin contribute to vasculogenesis under the influence of
Vegf-A. Nat Med. 10:950–958. 2004.
|
|
7
|
Young MR and Cigal M: Tumor skewing of
CD34+ cell differentiation from a dendritic cell pathway
into endothelial cells. Cancer Immunol Immunother. 55:558–568.
2006.
|
|
8
|
Gottfried E, Kreutz M, Haffner S, et al:
Differentiation of human tumour-associated dendritic cells into
endothelial-like cells: an alternative pathway of tumour
angiogenesis. Scand J Immunol. 65:329–335. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sozzani S, Rusnati M, Riboldi E, Mitola S
and Presta M: Dendritic cell-endothelial cell cross-talk in
angiogenesis. Trends Immunol. 28:385–392. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu J, Zhao J, Liu K, et al: MAPK/ERK1/2
signaling mediates endothelial-like differentiation of immature DCs
in the microenvironment of esophageal squamous cell carcinoma. Cell
Mol Life Sci. 67:2091–2106. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Muftuoglu TM, Koksal N and Ozkutlu D:
Evaluation of phagocytic function of macrophages in rats after
partial splenectomy. J Am Coll Surg. 191:668–671. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aranguren XL, Luttun A, Clavel C, et al:
In vitro and in vivo arterial differentiation of human multipotent
adult progenitor cells. Blood. 109:2634–2642. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Z, Wu JC, Sheikh AY, et al:
Differentiation, survival, and function of embryonic stem cell
derived endothelial cells for ischemic heart disease. Circulation.
116:I46–I54. 2007.PubMed/NCBI
|
|
14
|
Conejo-Garcia JR, Buckanovich RJ, Benencia
F, et al: Vascular leukocytes contribute to tumor vascularization.
Blood. 105:679–681. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rehman J, Li J, Orschell CM and March KL:
Peripheral blood ‘endothelial progenitor cells’ are derived from
monocyte/macrophages and secrete angiogenic growth factors.
Circulation. 107:1164–1169. 2003.
|
|
16
|
Fernandez Pujol B, Lucibello FC, Zuzarte
M, Lutjens P, Muller R and Havemann K: Dendritic cells derived from
peripheral monocytes express endothelial markers and in the
presence of angiogenic growth factors differentiate into
endothelial-like cells. Eur J Cell Biol. 80:99–110. 2001.
|
|
17
|
Said N and Theodorescu D: RhoGDI2
suppresses bladder cancer metastasis via reduction of inflammation
in the tumor microenvironment. Oncoimmunology. 1:1175–1177. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fessler E, Dijkgraaf FE, De Sousa EMF and
Medema JP: Cancer stem cell dynamics in tumor progression and
metastasis: is the microenvironment to blame? Cancer Lett.
12:00603–00609. 2012.PubMed/NCBI
|
|
19
|
Kusmartsev S and Gabrilovich DI: Effect of
tumor-derived cytokines and growth factors on differentiation and
immune suppressive features of myeloid cells in cancer. Cancer
Metastasis Rev. 25:323–331. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Muthana M, Fairburn B, Mirza S, Slack LK,
Hopkinson K and Pockley AG: Identification of a rat bone
marrow-derived dendritic cell population which secretes both IL-10
and IL-12: evidence against a reciprocal relationship between IL-10
and IL-12 secretion. Immunobiology. 211:391–402. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Michielsen AJ, O’Sullivan JN and Ryan EJ:
Tumor conditioned media from colorectal cancer patients inhibits
dendritic cell maturation. Oncoimmunology. 1:751–753. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hurwitz AA and Watkins SK: Immune
suppression in the tumor microenvironment: a role for dendritic
cell-mediated tolerization of T cells. Cancer Immunol Immunother.
61:289–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tamminen JA, Myllarniemi M, Hyytiainen M,
Keski-Oja J and Koli K: Asbestos exposure induces alveolar
epithelial cell plasticity through MAPK/Erk signaling. J Cell
Biochem. 113:2234–2247. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shi Y, Xia YY, Wang L, Liu R, Khoo KS and
Feng ZW: Neural cell adhesion molecule modulates mesenchymal
stromal cell migration via activation of MAPK/ERK signaling. Exp
Cell Res. 318:2257–2267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu W, Sun Z, Wu J, et al: Trihydrophobin 1
phosphorylation by c-Src regulates MAPK/ERK signaling and cell
migration. PLoS One. 7:e299202012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gomez M, Manzano A, Figueras A, et al:
Sertoli-secreted FGF-2 induces PFKFB4 isozyme expression in mouse
spermatogenic cells by activation of the MEK/ERK/CREB pathway. Am J
Physiol Endocrinol Metab. 303:E695–E707. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo Y and Feng P: OX2R activation induces
PKC-mediated ERK and CREB phosphorylation. Exp Cell Res.
318:2004–2013. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kido S, Kuriwaka-Kido R, Imamura T, Ito Y,
Inoue D and Matsumoto T: Mechanical stress induces Interleukin-11
expression to stimulate osteoblast differentiation. Bone.
45:1125–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Choi HJ, Park YG and Kim CH:
Lactosylceramide alpha2,3-sialyltransferase is induced via a
PKC/ERK/CREB-dependent pathway in K562 human leukemia cells. Mol
Cells. 23:138–144. 2007.PubMed/NCBI
|