Introduction
Photodynamic therapy (PDT) is an emerging treatment
for a variety of cancers and other diseases. In PDT the activation
of a photosensitizer by a specific wavelength of light in the
presence of oxygen promotes cellular damage (1,2).
Currently, several research groups are developing PDT technology. A
variety of photosensitizers such as Photofrin®, ALA and
cisplatin have shown promising results (1–3).
However, improvements in quantum efficiency, reductions in toxicity
and the ability to specifically target a highly effective dose have
not been fully attained with current PDT technologies.
Methylene blue (MB), a well-known dye with high
light absorption at 665 nm, is effective in PDT due to its ability
to generate singlet oxygen and its proven photodynamic activity in
clinical applications against several diseases (4–6).
Previous studies have documented the effectiveness of MB-PDT
against melanoma in cell culture, and MB-PDT has been used to
efficiently treat relatively large melanoma lesions not eligible
for surgery (6). In addition, some
researchers have reported that MB is more toxic to leukemia cells
than to normal PBMCs (7). This
suggests that MB is more toxic in cancer cells than normal cells, a
potential benefit in reducing unwanted toxicity due to PDT.
Although it has been documented that in MB-PDT the
derivative of MB induces apoptosis in several cell lines (8), the current understanding of the
mechanisms of apoptosis induced by MB-PDT is limited. Currently
recognized mechanisms of apoptosis associated with MB-PDT include
the induction of DNA damage, the generation of reactive oxygen
species (ROS), and possibly mitochondrial damage (9,10).
Published data have indicated mitochondrial damage in
MB-PDT-induced tumor regression because MB is likely to bind to the
negative electrochemical environment of the mitochondrial matrix in
melanoma and HeLa cells (11–13). A
better understanding of the mechanisms of apoptosis induced by
MB-PDT will help inform cancer therapeutic strategies.
In this study, we investigated the role of MB in
PDT-induced apoptosis in human lung adenocarcinoma cells. We found
that MB sensitizes A549 cells to PDT-induced apoptosis, suggesting
that MB-PDT may provide an effective therapeutic strategy for lung
adenocarcinoma. In addition, we found that caspase activation,
downregulation of anti-apoptotic proteins, reduced mitochondrial
membrane potential (MMP), activation of the mitogen-activated
protein kinase (MAPK) p38, and ROS generation critically contribute
to the anticancer effect of MB-PDT.
Materials and methods
Reagents
The methylene blue (MB) used for PDT was acquired
from Aldrich (Milwaukee, WI, USA), and RPMI-1640 medium was
purchased from Hyclone (Logan, UT, USA). Antibodies against PARP-1,
Bcl-2, Bcl-xL, Mcl-1, and actin were purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA), and anti-phospho-JNK,
anti-phospho-p38, and anti-phospho-ERK were purchased from Cell
Signaling (Beverly, MA, USA). Benzyl
carbonyl-Val-Ala-Asp-fluoromethyl ketone (z-VAD-fmk) was purchased
from Biomol (Plymouth Meeting, PA, USA),
2′7′-dichlorodihydrofluorescein diacetate (H2DCFDA) was
purchased from Molecular Probes (Eugene, OR, USA), and
N-acetylcysteine (NAC) and all other chemicals used in this study
were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture and chemical treatments
Human lung adenocarcinoma cells (A549 cells) were
obtained from the American Type Culture Collection (Rockville, MD,
USA). The A549 cells were cultured in RPMI-1640 medium supplemented
with 10% heat-inactivated fetal bovine serum (FBS), penicillin (100
U/ml) and streptomycin (100 U/ml) at 37ºC in a humidified incubator
with 5% CO2 and 95% air. When the cells were
subconfluent, the medium was replaced with fresh medium and 0.1–2.0
μg/ml of MB was added to the culture medium for 1 h. The cells were
then irradiated with 650 nm light produced by a dye laser (Red
Diode Laser) at 30–120 Joules (J).
Cellular viability assay
For the morphological evaluation of cell death,
approximately 5×105 A549 cells were plated into 60-mm
cell culture dishes overnight. For the trypan blue exclusion assay,
trypsinized cells were pelleted and resuspended in 0.2 ml of
medium, 0.5 ml of 0.4% trypan blue solution, and 0.3 ml of
phosphate-buffered saline solution (PBS). The samples were mixed
thoroughly, incubated at room temperature for 15 min, and examined
under a light microscope. At least 300 cells were counted for each
survival determination.
Western blot analysis
For western blot analyses, A549 cells were lysed
with 1X Laemmli lysis buffer (2.4 M glycerol, 0.14 M Tris, pH 6.8,
0.21 M SDS, 0.3 mM bromophenol blue) and boiled for 10 min. Protein
content was measured with the BCA Protein Assay Reagent (Pierce,
Rockford, IL, USA). The samples were diluted with 1X Laemmli lysis
buffer containing 1.28 M β-mercaptoethanol, and equal amounts of
protein were loaded on 8–12% SDS-polyacrylamide gels. Proteins were
separated by SDS-PAGE and electrophoretically transferred to a
nitrocellulose membrane. The nitrocellulose membrane was blocked
with 5% nonfat dry milk in PBS-Tween-20 (0.1%, v/v) for 1 h. The
membrane was incubated with primary antibody (diluted according to
the manufacturer's instructions) at room temperature for 1.5 h.
Horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG was
used as the secondary antibody. Immunoreactive protein was
visualized by the chemiluminescence protocol (ECL, Amersham,
Arlington Heights, IL, USA). To ensure equal protein loading, each
nitrocellulose membrane was stripped and reprobed with an
anti-actin antibody after the experiment was completed.
DNA fragmentation and DAPI staining
assay
To assess for DNA fragmentation after MB and/or PDT
for 24 h, ~1×106 treated A549 cells were lysed for 30
min on ice in buffer containing 10 mM Tris (pH 7.4), 150 mM NaCl, 5
mM EDTA and 0.5% Triton X-100. Lysates were vortexed and cleared by
centrifugation at 12,000 × g for 30 min. Fragmented DNA in the
supernatant was extracted with an equal volume of a mixture of
neutral phenol:chloroform:isoamyl alcohol (25:24:1) and analyzed
electrophoretically on 1.5% agarose gels containing 0.1 g/ml EtBr.
The cells were fixed on slide glass through the application of 4%
paraformaldehyde for 30 min at room temperature. After washing with
PBS, 300 nM 4′, 6′-diamidino-2-phenylindole (DAPI) was added to the
fixed cells for 10 min, after which they were examined by
fluorescence microscopy. Apoptotic cells were identified by
condensation and fragmentation of nuclei. All DAPI staining
experiments were performed in duplicate.
Measurement of reactive oxygen
species
The generation of ROS was measured by staining with
2′,7′-dichlorofluorescein diacetate (DCF-DA). Briefly, A549 cells
were seeded in six-well plates (1×105 cells per well),
allowed to attach overnight and treated with MB and/or PDT. The
cells were stained with 20 μM DCF-DA for 30 min at 37ºC and
fluorescence was detected by a fluorescence microscope.
JC-1 mitochondrial membrane potential
assay
To monitor MMP, JC-1 dye was used as previously
described (14). In cells with
undamaged mitochondria, the aggregated dye appears as red
fluorescence, whereas in cells with altered MMP that are undergoing
apoptosis, the dye remains as monomers in the cytoplasm and emits
diffuse green fluorescence. The red/green fluorescence ratio is
dependent on MMP. After A549 cells were treated with MB and PDT,
they were stained with a JC-1 MMP detection kit for 10 min and
analyzed by flow cytometry. The fluorescence intensity was measured
with the FACScan flow cytometer (Beckman Coulter, Inc., Hialeah,
FL, USA).
Statistical analysis
All experiments were repeated three or more times.
The results are represented as means ± standard deviations (SDs).
The difference between two mean values was analyzed using Student's
t-test and was considered statistically significant at
p<0.05.
Results
Methylene blue promotes PDT-mediated A549
cell toxicity
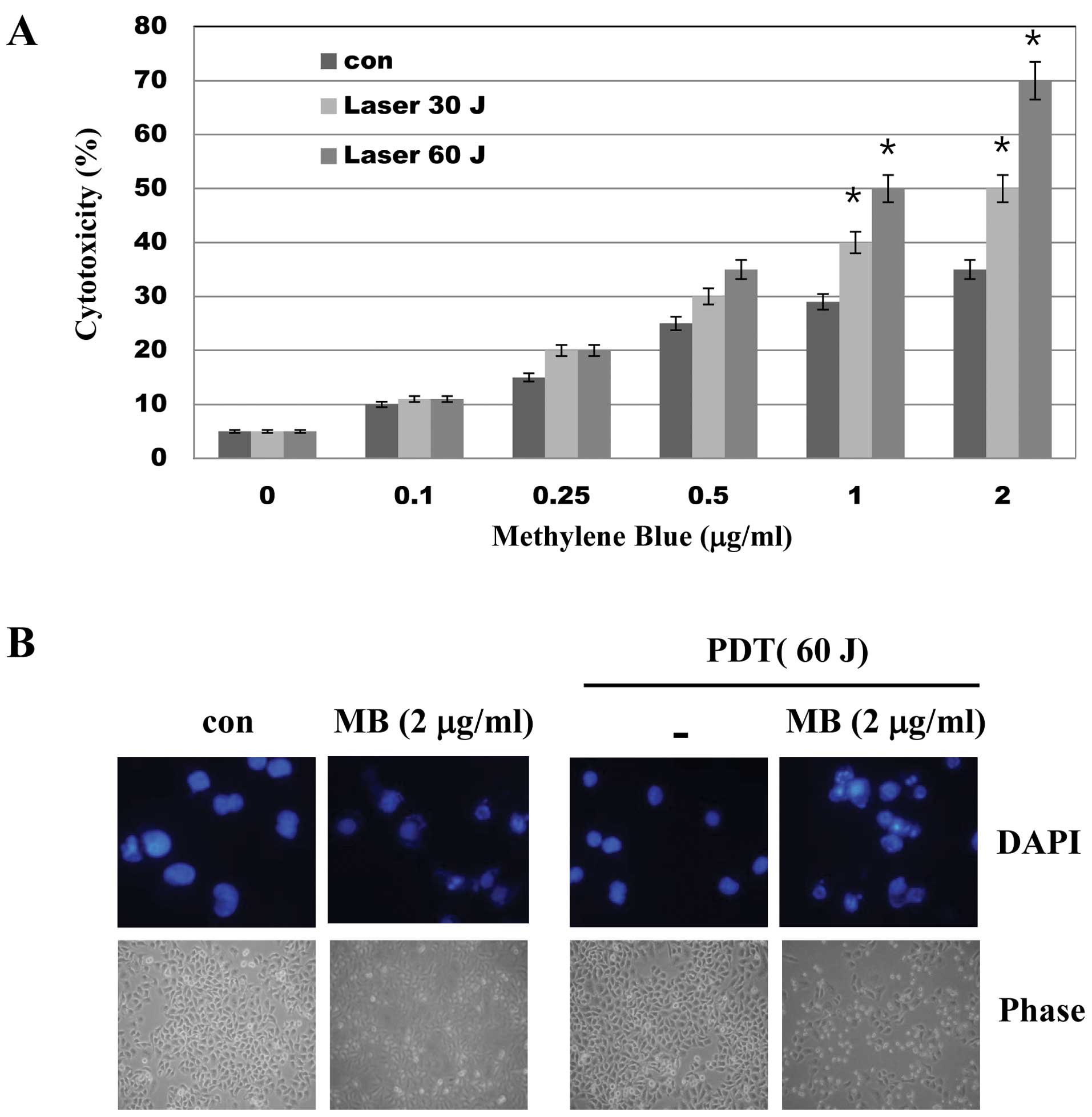
To evaluate the effects of MB-PDT on cell viability,
A549 cells were treated with various concentrations of MB and/or
PDT (30 and 60 J) for 24 h and a trypan blue assay was performed.
As shown in Fig. 1, while MB alone
had little effect on cell viability, MB treatment followed by PDT
significantly decreased cell viability as determined by trypan blue
exclusion (Fig. 1A) and DAPI
staining (Fig. 1B). These results
suggest that PDT enhances the toxicity of MB in A549 cells.
Methylene blue promotes PDT-induced
apoptosis by caspase activation
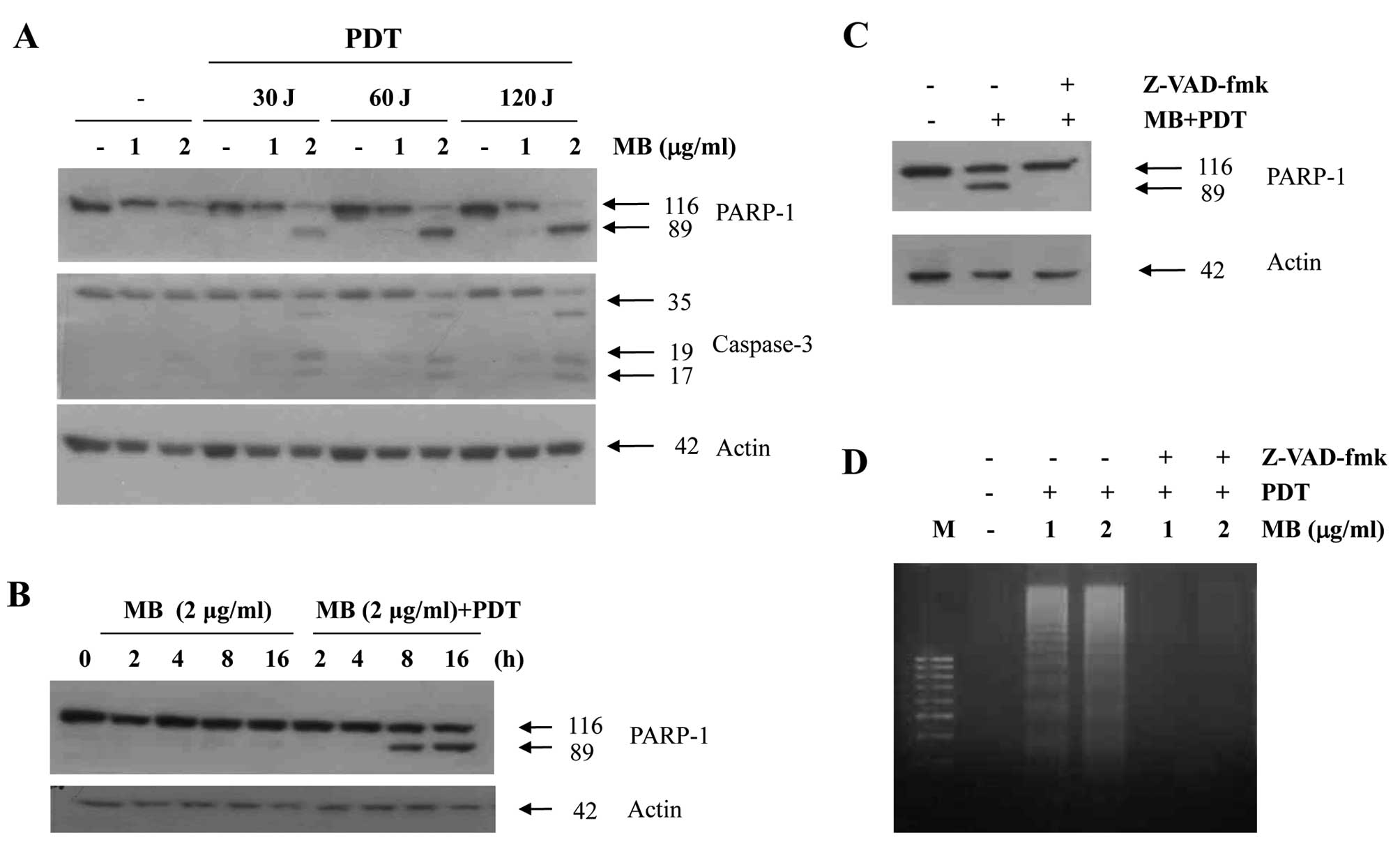
To address the significance of caspase activation in
MB-PDT-induced apoptosis, we examined caspase activity after
MB-PDT. Treatment of A549 cells with various concentrations of MB
and PDT resulted in a dramatic increase in the cleavage of PARP, a
caspase substrate, and in the cleavage of procaspase-3 (Fig. 2A) in a time-dependent manner
(Fig. 2B). In order to confirm that
the activation of caspases is a key step in MB-PDT-induced
apoptosis, the A549 cells were pretreated with z-VAD-fmk (25 μM), a
cell-permeable caspase inhibitor, followed by MB-PDT for 24 h. As
shown in Fig. 2C, MB-PDT-induced
apoptosis was prevented by pretreatment with z-VAD-fmk, as
indicated by the reduced PARP cleavage. We also found that
z-VAD-fmk prevented the dose-dependent increase in the accumulation
of apoptotic DNA after treatment with MB-PDT (Fig. 2D). These results suggest that
MB-PDT-induced cell death is associated with caspase
activation.
MB and PDT induce downregulation of
anti-apoptotic proteins and loss of mitochondrial membrane
potential in A549 cells
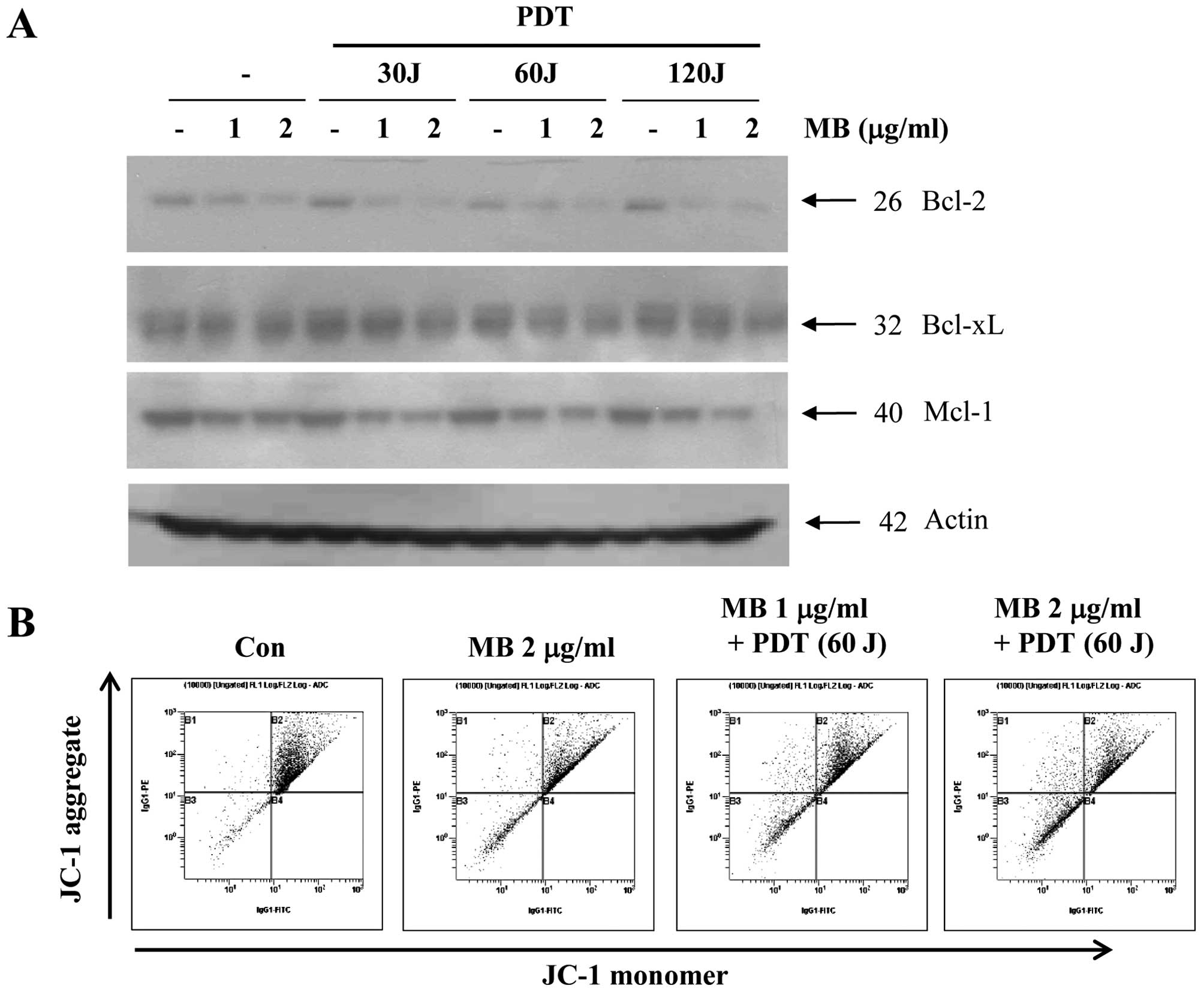
We further examined whether MB-PDT-induced apoptosis
is associated with the modulation of apoptosis regulatory proteins.
As shown in Fig. 3A, exposure of
A549 cells to MB and PDT led to a slight decrease in Bcl-xL.
However, levels of Bcl-2 and Mcl-1 in A549 cells were markedly
reduced by MB and PDT in a dose-dependent manner. We assessed MMP
in order to determine the role of mitochondrial damage in
MB-PDT-induced apoptosis of A549 cells. As shown in Fig. 3B, we found that exposure of A549
cells to MB and PDT significantly reduced MMP in a dose-dependent
manner. The proportion of cells with a loss of MMP substantially
increased with MB treatment (1 and 2 μg/ml) plus PDT (60 J).
Activation of p38 signaling plays an
important role in MB-induced photosensitization in A549 cells
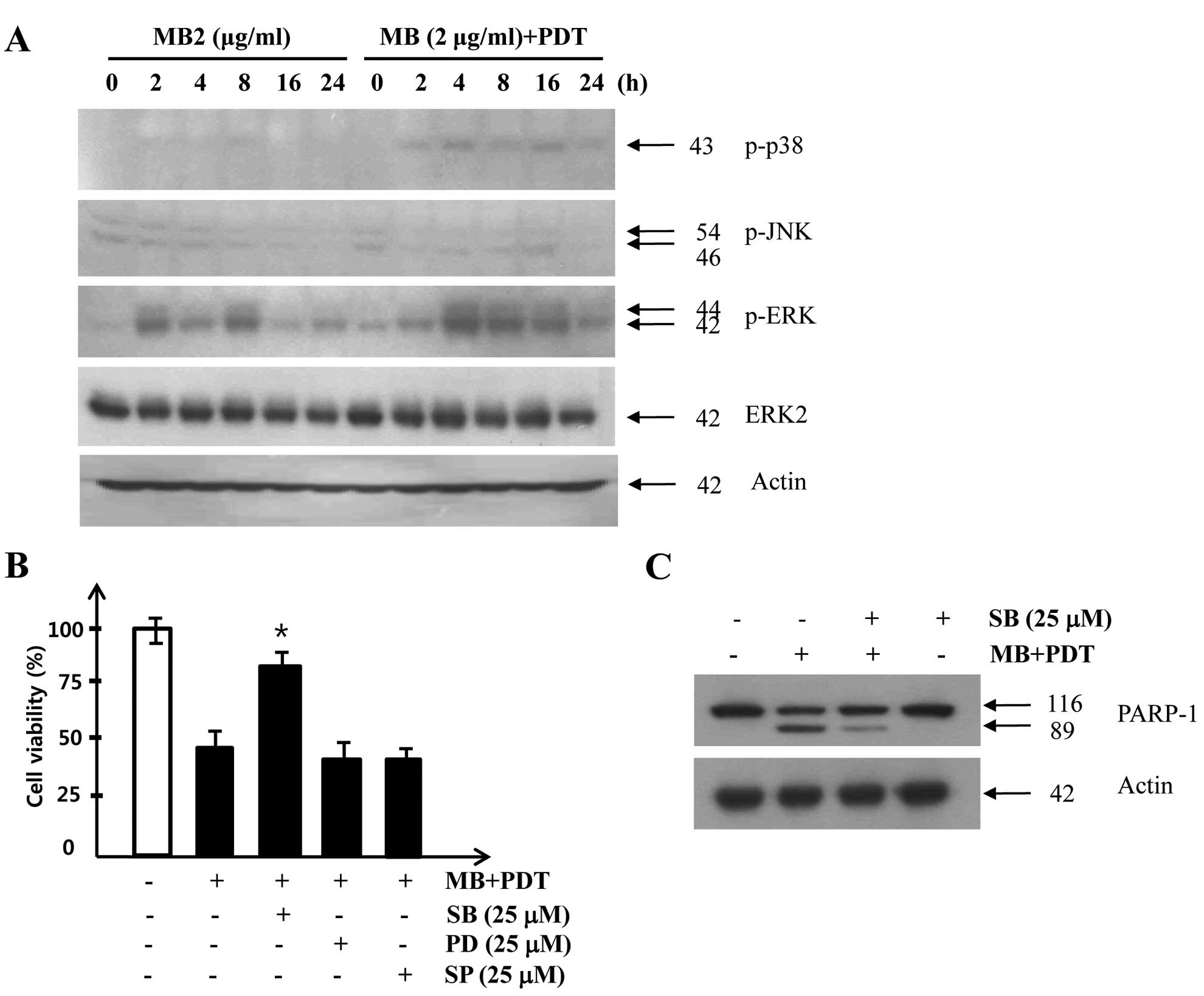
In order to investigate whether a MAPK signaling
pathway was involved in cell death in A549 cells treated with
MB-PDT, we examined the phosphorylation of p38, JNK and ERK in A549
cells after MB and/or PDT. As shown in Fig. 4A, p38 activity was increased
slightly at 2 h after MB application (2 μg/ml) and the increased
p38 activity was sustained after 8 h of treatment with MB alone,
whereas a much stronger and prolonged activation of p38 was
observed after combined treatment with MB and PDT. To further
confirm the involvement of the p38 signaling pathway in
MB-PDT-induced apoptosis, we applied specific inhibitors of JNK
(SP600125), p38 (SB203589), and ERK (PD98059). Pretreatment of A549
cells with the p38 inhibitor SB203589 before MB-PDT increased cell
viability (Fig. 4B) and reduced
proteolytic cleavage of PARP (Fig.
4C). Taken together, these results suggest that the prolonged
activation of the MAPK p38 pathway plays an important role in
MB-induced photosensitization.
MB potentiates PDT-induced ROS
generation, which is attenuated by the addition of antioxidants to
A549 cells
ROS are byproducts of the normal metabolism of
oxygen, and they are generated by various environmental stresses
such as drugs, UV light and heat shock. ROS play an important role
in apoptosis under both physiologic and pathologic conditions
(15,16). Therefore, we examined whether ROS
generation was involved in MB-induced photosensitization of A549
cells by measuring the intracellular hydrogen peroxide level in
A549 cells after MB-PDT. As shown in Fig. 5A, the combination of MB and PDT
markedly increased intracellular hydrogen peroxide levels in A549
cells. We next investigated whether ROS generation was directly
associated with MB-PDT-induced apoptosis. Pretreatment with NAC, a
well-known antioxidant, markedly increased cell viability after
MB-PDT (Fig. 5B). Moreover,
apoptosis induced by MB-PDT was markedly attenuated by pretreatment
with NAC (Fig. 5C). Taken together,
these results indicate that MB-induced photosensitization and
MB-PDT-induced apoptosis are mediated by the generation of ROS.
Discussion
Although PDT is a commonly applied cancer therapy,
its efficacy is limited by injury to healthy tissue, varying tumor
sensitivity and various other side effects. Therefore, novel
therapeutic strategies are needed to selectively induce the death
of cancer cells while sparing normal cells. Recently, many attempts
to improve the therapeutic effects of PDT have been reported
(17) and several photosensitizers
such as porfimer sodium (Photofrin), ALA and cisplatin have been
used to enhance tumor cell death in PDT (18,19).
In this study, we found that MB effectively sensitizes A549 human
lung adenocarcinoma cells to PDT-induced apoptosis through caspase
activation, downregulation of anti-apoptotic proteins, reduced MMP,
activation of p38 signaling pathways and increased ROS.
MB is already approved for systemic administration
by intravenous injection for clinical use in patients with other
diseases. It has various biological applications, it is used as a
staining dye for parathyroid tissue (20), an antifungal agent in goldfish, and
an antimalarial agent (21). In the
present study, we showed that compared with MB or PDT alone, the
combination of MB and PDT resulted in significantly enhanced A549
cell death (Fig. 1). This
enhancement of PDT-induced apoptosis by MB was dependent on caspase
activation, since z-VAD-fmk, a caspase inhibitor, reduced the cell
death induced by MB-PDT (Fig.
2).
Mitochondria play an important role in apoptotic
pathways that result from a variety of intracellular events
including the release of caspase activators, changes in MMP, ROS
generation, and changes in the Bcl-2 family of proteins (22). In the current study, loss of MMP was
observed in A549 cells co-treated with MB and PDT (Fig. 3). Levels of Bcl-2 in A549 cells were
also markedly decreased by MB-PDT. Taken together, these results
suggest that the mitochondrial pathway is involved in the
enhancement of PDT-induced apoptosis by MB.
The activation of MAPK pathways plays an essential
role in apoptosis induced by many cellular stresses. The p38 MAPK
is activated by ROS in many cells treated with radiation,
anticancer drugs, and chemopreventive agents (23). In our study, MB-PDT-treated A549
cells showed remarkably activated p38, but not JNK or ERK MAPK
(Fig. 4). Pretreatment of A549
cells with the p38 inhibitor SB203589 prevented MB-PDT-induced
apoptosis. These results suggest that activation of the p38 MAPK
pathway contributes to MB-PDT-induced apoptosis in A549 cells.
ROS are generated by many environmental stresses
(e.g., UV light and heat shock), and they are produced as a normal
product of cellular metabolism (e.g., phagocytosis). ROS play an
important role in apoptosis induction under both physiologic and
pathologic conditions (16). In the
current study, we show that co-treatment with MB and PDT induced
ROS generation in A549 cells. Furthermore, antioxidant (NAC)
pretreatment attenuated ROS generation and MB-PDT-induced apoptosis
(Fig. 4), suggesting that the
elevation of ROS levels by combined treatment with MB and PDT plays
an important role in enhancing the apoptotic susceptibility of A549
cells.
In conclusion, the present study shows that MB
enhances PDT-induced apoptosis in human lung adenocarcinoma cells
via caspase activation, reductions in anti-apoptotic protein
levels, loss of MMP, activation of the p38 MAPK signaling pathway
and ROS generation. Therefore, a combined regimen of MB and PDT may
offer a better therapeutic strategy to enhance photosensitivity in
tumor cells.
Acknowledgements
This work was supported by Basic Science Research
Program through the NRF funded by the Ministry of Education,
Science and Technology (2011-0004206) and a grant from Kosin
University College of Medicine (2011) and a grant from Institute
for Medical Sciences (2011).
References
|
1
|
Dougherty TJ, Gomer CJ, Henderson BW, Jori
G, Kessel D, Korbelik M, Moan J and Peng Q: Photodynamic therapy. J
Natl Cancer Inst. 90:889–905. 1998. View Article : Google Scholar
|
|
2
|
Dolmans DE, Fukumura D and Jain RK:
Photodynamic therapy for cancer. Nat Rev Cancer. 3:380–387. 2003.
View Article : Google Scholar
|
|
3
|
Sharman WM, Allen CM and van Lier JE:
Photodynamic therapeutics: basic principles and clinical
applications. Drug Discov Today. 4:507–517. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bisland SK, Chien C, Wilson BC and Burch
S: Pre-clinical in vitro and in vivo studies to examine the
potential use of photodynamic therapy in the treatment of
osteomyelitis. Photochem Photobiol Sci. 5:31–38. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tardivo JP, Del Giglio A, Paschoal LH and
Baptista MS: New photodynamic therapy protocol to treat
AIDS-related Kaposi's sarcoma. Photomed Laser Surg. 24:528–531.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wagner M, Suarez ER, Theodoro TR, Machado
Filho CD, Gama MF, Tardivo JP, Paschoal FM and Pinhal MA: Methylene
blue photodynamic therapy in malignant melanoma decreases
expression of proliferating cell nuclear antigen and heparanases.
Clin Exp Dermatol. 37:527–533. 2012. View Article : Google Scholar
|
|
7
|
Kirszberg C, Rumjanek VM and Capella MA:
Methylene blue is more toxic to erythroleukemic cells than to
normal peripheral blood mononuclear cells: a possible use in
chemotherapy. Cancer Chemother Pharmacol. 56:659–665. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ball DJ, Luo Y, Kessel D, Griffiths J,
Brown SB and Vernon DI: The induction of apoptosis by a positively
charged methylene blue derivative. J Photochem Photobiol B.
42:159–163. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Noodt BB, Rodal GH, Wainwright M, Peng Q,
Horobin R, Nesland JM and Berg K: Apoptosis induction by different
pathways with methylene blue derivative and light from
mitochondrial sites in V79 cells. Int J Cancer. 75:941–948. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rodrigues T, de França LP, Kawai C, de
Faria PA, Mugnol KC, Braga FM, Tersariol IL, Smaili SS and Nantes
IL: Protective role of mitochondrial unsaturated lipids on the
preservation of the apoptotic ability of cytochrome C exposed to
singlet oxygen. J Biol Chem. 282:25577–25587. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gabrielli D, Belisle E, Severino D,
Kowaltowski AJ and Baptista MS: Binding, aggregation and
photochemical properties of methylene blue in mitochondrial
suspensions. Photochem Photobiol. 79:227–232. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen Y, Zheng W, Li Y, Zhong J, Ji J and
Shen P: Apoptosis induced by methylene-blue-mediated photodynamic
therapy in melanomas and the involvement of mitochondrial
dysfunction revealed by proteomics. Cancer Sci. 99:2019–2027.
2008.PubMed/NCBI
|
|
13
|
Lu Y, Jiao R, Chen X, Zhong J, Ji J and
Shen P: Methylene blue-mediated photodynamic therapy induces
mitochondria-dependent apoptosis in HeLa cell. J Cell Biochem.
105:1451–1460. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim MJ, Lee TH, Kim SH, Choi YJ, Heo J and
Kim YH: Triptolide inactivates Akt and induces caspase-dependent
death in cervical cancer cells via the mitochondrial pathway. Int J
Oncol. 37:1177–1185. 2010.PubMed/NCBI
|
|
15
|
Szatrowski TP and Nathan CF: Production of
large amounts of hydrogen peroxide by human tumor cells. Cancer
Res. 51:794–798. 1991.PubMed/NCBI
|
|
16
|
Simon HU, Haj-Yehia A and Levi-Schaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Firczuk M, Winiarska M, Szokalska A,
Jodlowska M, Swiech M, Bojarczuk K, Salwa P and Nowis D: Approaches
to improve photodynamic therapy of cancer. Front Biosci.
16:208–224. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nahabedian MY, Cohen RA, Contino MF, Terem
TM, Wright WH, Berns MW and Wile AG: Combination cytotoxic
chemotherapy with cisplatin or doxorubicin and photodynamic therapy
in murine tumors. J Natl Cancer Inst. 80:739–743. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peng Q, Warloe T, Moan J, Godal A,
Apricena F, Giercksky KE and Nesland JM: Antitumor effect of
5-aminolevulinic acid-mediated photodynamic therapy can be enhanced
by the use of a low dose of photofrin in human tumor xenografts.
Cancer Res. 61:5824–5832. 2001.PubMed/NCBI
|
|
20
|
Sherlock DJ and Holl-Allen RT: Intravital
methylene blue staining of parathyroid glands and tumours. Ann R
Coll Surg Engl. 66:396–398. 1984.PubMed/NCBI
|
|
21
|
Meissner PE, Mandi G, Coulibaly B, Witte
S, Tapsoba T, Mansmann U, Rengelshausen J, Schiek W, Jahn A,
Walter-Sack I, Mikus G, Burhenne J, Riedel KD, Schirmer RH, Kouyaté
B and Müller O: Methylene blue for malaria in Africa: results from
a dose-finding study in combination with chloroquine. Malar J.
5:842006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang ES, Choi MJ, Kim JH, Choi KS and Kwon
TK: Combination of withaferin A and X-ray irradiation enhances
apoptosis in U937 cells. Toxicol In Vitro. 25:1803–1810. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen YR and Tan TH: The c-Jun N-terminal
kinase pathway and apoptotic signaling. Int J Oncol. 16:651–662.
2000.PubMed/NCBI
|