Introduction
Although adenocarcinoma has replaced squamous cell
carcinoma as the most common type of esophageal cancer in Western
countries, >90–95% of esophageal cancer cases in Asian countries
are esophageal squamous cell carcinomas (ESCCs) (1–3). The
prognosis of advanced ESCC patients in China and other Asian
countries remains quite poor, despite the use of therapies that
combine surgical resection with chemotherapy and/or radiotherapy.
The use of tyrosine kinase inhibitors (TKIs) combined with standard
chemotherapeutics may provide a novel therapeutic approach for the
majority of ESCC patients (4–6).
The human epidermal growth factor receptor (EGFR)
family, also known as the HER family, includes four closely related
receptors: HER1 (EGFR), HER2, HER3 and HER4. Downstream signaling
of the HER family plays a crucial role in cell proliferation,
apoptosis, angiogenesis and metastasis (7,8). EGFR
overexpression has been identified in several types of human
cancer, including gastric, colorectal, breast, lung, prostate and
bladder cancer (9,10). The HER2 gene is amplified and
overexpressed in ~30% of human breast and ovarian cancers, as well
as other tumors, including colorectal and gastric cancer (11–13).
Notably, it was previously reported that EGFR is overexpressed in
33.3% of Japanese ESCC patients, and HER2 is overexpressed in 30.3%
of Japanese ESCC patients (14,15).
EGFR and HER2 are the main therapeutic targets of TKIs, and
oxaliplatin and 5-FU are used as standard chemotherapies (16–18).
Thus, anti-EGFR and/or anti-HER2 targeted therapy combined with
standard chemotherapies is an attractive approach for the treatment
of patients with advanced ESCC.
Lapatinib is a reversible dual TKI that targets the
tyrosine kinases in both EGFR and HER2 tyrosine kinases, which in
turn inhibits receptor phosphorylation and activation of the
downstream signaling pathways, such as extracellular-related kinase
(ERK)-1/2 and AKT in cell lines and xenografts (19–21).
Lapatinib combined with fluoropyrimidine or trastuzumab exerted
synergistic antitumor effects in vitro and in
vivo(22), and had clinical
activities in several solid tumors (6,23,24).
However, no previous studies have described the effects of this TKI
combined with standard chemotherapy using ESCC primary xenografts
derived from Chinese patients. On the basis of these earlier
observations, we explored the potential utility of lapatinib when
administered alone or in combination with oxaliplatin and 5-FU for
treating ESCC.
Materials and methods
Ethics statement
The present study was conducted according to the
principles of the Declaration of Helsinki. The efficacy study was
approved by the Ethics Committee of the Third Xiangya Hospital,
Central South University, Hunan, China. The collection and use of
the archived paraffin tissue blocks in the ESCC study were approved
by the Ethics Committee of the Second Hospital, Jilin University,
Jilin, China, with prior consent from the patients.
Immunohistochemical analysis
Esophageal cancer tissue microarrays (TMA; Shanghai
Outdo, Shanghai, China) comprising 94 malignant esophageal human
tumors in which 78 had matched adjacent normal tissues were used
for immunohistochemistry (IHC) analysis of EGFR and HER2 protein
expression. For antigen retrieval, the tissues were incubated in
EDTA buffer with heating for 20 min, treated with 3% hydrogen
peroxide, and then incubated in Background Sniper (Biocare Medical,
Concord, CA, USA) to block any non-specific binding. Sections were
then incubated with the anti-EGFR or anti-HER2 monoclonal antibody
(Cell Signaling Technology, Beverly, MA, USA) diluted in Dako
antibody diluent (DakoCytomation, Carpinteria, CA, USA) overnight
at 4°C. The sections were then incubated using the rabbit on rodent
polymer system (Biocare Medical) for 30 min at room temperature.
Slides were subsequently treated with 3,3-diaminobenzidine
chromogen (DakoCytomation) for 5 min to visualize antibody binding.
Sections were then counterstained with hematoxylin, dehydrated and
mounted. Total rabbit IgG substituted for the primary antibodies
served as a negative control.
The assessment of EGFR or HER2 staining strength and
positivity was carried out simultaneously by a pathologist and two
other observers and a consensus was reached for each core. EGFR and
HER2 staining was scored as follows: negative, no membranous
staining in any of the tumor cells; 1+, membranous staining in
<10% of the tumor cells with any intensity or in <30% of the
tumor cells with weak intensity; 2+, staining in 10–30% of the
tumor cells with moderate to strong intensity or staining in 30–50%
of the tumor cells with weak to moderate intensity; and 3+,
staining in >30% of the tumor cells with strong intensity or
>50% of the tumor cells with any intensity. Tissues scored as 2+
or 3+ were defined as showing positive expression (25).
Animals
Athymic nude mice (Vr: NU-Foxn1nu) aged 5–6 weeks
and weighing 18–21 g were purchased from Vital River Laboratories
(Beijing, China). The health of all mice was monitored daily by
gross observation and analysis of blood samples of sentinel
animals. All mice were allowed to acclimatize and recover from any
shipping-related stress for ≥72 h prior to experimental use.
Autoclaved water and irradiated food were provided, and the mice
were maintained on a 12 h light and dark cycle. Cages, bedding and
water bottles were autoclaved before use and were changed
weekly.
All animal experiments were conducted in an
Association for Assessment and Accreditation of Laboratory Animal
Care-accredited animal facility after review by the Institutional
Animal Care and Use Committees, and in accordance with the GSK
policy on the Care, Welfare and Treatment of Laboratory
Animals.
Tumors
Excess human esophageal tumor samples were obtained
through an institutional review board-approved centralized banking
infrastructure at the hospital. Written informed consent was
obtained from all participants. None of the samples used in the
present study were derived from minors. Biopsies were taken from
the luminal surface of resected specimens by a pathologist or
surgeon, ensuring that their potential for histopathologic
diagnosis and staging was not compromised.
Solid tumor tissues were depleted of necrotic
components, cut into 10–15 mg pieces and mixed. The mixed tumor
pieces were implanted (single flank) into male Nu/Nu nude mice; 3–5
pieces were mixed with 15–30 μl Matrigel (BD Biosciences, Bedford,
MA, USA) per mouse. For continued propagation in mice, the
xenograft tumors were excised and processed into mixed tumor
pieces. The mixed tumor pieces were re-implanted subcutaneously
into new recipient nude mice. All of the primary human esophageal
tumors used in this study had undergone 3–4 passages in
vivo, and the histologic profiles of all tumors were maintained
during serial transplantation.
In vitro ATP tumor chemosensitivity
assay
Chemosensitivity was assessed in primary esophageal
tumor tissue samples using the CellTiter-Glo®
Luminescent Cell Viability Assay kit (Promega, Madison, WI, USA).
Briefly, surgical biopsies (1–2 cm3) were obtained
during primary surgery. Tumor cells were isolated by mechanical and
enzymatic dissociation. Approximately 2×104 cells were
then seeded into each well of a 96-well polypropylene microplate.
Each concentration of the test drugs was applied in triplicate. The
initial concentrations of lapatinib, oxaliplatin and 5-FU were 10,
10 and 100 μM, respectively. Two rows on each plate were reserved
for blanks and controls. After preparing the diluted drugs, 135 μl
of the cell suspension was added to each well. The plate was
incubated for 6 days at 37°C under high humidity and 5%
CO2. The cells were observed microscopically every 24 h
to check for overgrowth or infection. At the end of the incubation
period, the cells were lysed by the addition of 75 μl of
CellTiter-Glo reagent. Luminescence measurements were made using a
FlexStation 3 (Molecular Devices, Sunnyvale, CA, USA).
Immunoblotting
Tumor tissue lysates were prepared by washing the
cells with phosphate-buffered saline and subjecting them to lysis
with radio-immunoprecipitation assay buffer supplemented with a
protease inhibitor cocktail. The protein concentrations were
quantified using the Bio-Rad protein assay kit (Bio-Rad
Laboratories, Hercules, CA, USA). Equivalent amounts of proteins
were loaded, separated on NuPAGE Novex 4–12% Bis-Tris Gels, and
then transferred to polyvinyl difluoride membranes (Invitrogen,
Carlsbad, CA, USA). The membranes were blocked for 1 h with 5%
nonfat dried milk in Tris-buffer containing 0.1% Tween and were
then probed with the diluted primary antibody overnight at 4°C. The
membranes were then washed three times and probed with horseradish
peroxidase-linked goat anti-rabbit IgG, and the immunoreactive
bands were visualized using an enhanced chemiluminescent detection
system (GE Healthcare, Piscataway, NJ, USA). All antibodies were
purchased from Cell Signaling Technology.
Test agents and efficacy of study
design
Lapatinib (Tykerb®; GlaxoSmithKline,
Research Triangle Park, NC, USA) was prepared in 0.5% hydroxypropyl
methylcellulose (HPMC) and 0.1% Tween-80 (Sigma, St. Louis, MO,
USA). Clinical-grade oxaliplatin (Eloxatin®;
Sanofi-Aventis, Bridgewater, NJ, USA) was obtained as a stock
solution of 5 mg/ml, diluted with sterile water. 5-FU (Sigma) was
freshly dissolved in saline prior to use.
Administration of lapatinib and
oxaliplatin in the primary esophageal tumor model
Forty-two mice were selected and randomized into six
groups. The mice were treated with vehicle (0.5% HPMC in 0.1%
Tween-80, orally, twice daily), lapatinib alone (105 mg/kg, orally,
twice daily), oxaliplatin alone (6 mg/kg, intraperitoneal
injection, once weekly), or lapatinib (105 mg/kg, orally, twice
daily) in combination with oxaliplatin (6 mg/kg, intraperitoneal
injection, once weekly) for 3 weeks.
Administration of lapatinib and 5-FU in
the primary esophageal tumor model
Forty two mice were selected and randomized into six
groups. The mice were treated with vehicle (0.5% HPMC in 0.1%
Tween-80, orally, twice daily), lapatinib alone (105 mg/kg, orally,
twice daily), 5-FU alone (15 mg/kg, intraperitoneal injection, once
daily for 4 days per week), or lapatinib (105 mg/kg, orally, twice
daily) in combination with 5-FU (15 mg/kg, intraperitoneal
injection, once daily for 4 days per week) for 3 weeks.
Measurement of tumor growth and body
size
Tumor volume was calculated using the following
formula: tumor volume = (length × width2)/2. The tumor
volume was then used to calculate tumor growth inhibition (TGI), as
an index of the antitumor activity of each test drug, as follows:
TGI (%) = [1 - (Ti - T0)/(Vi - V0)] × 100;
where Ti is the mean tumor volume of the treated group,
T0 is the mean tumor volume of the treated group on Day
1 of treatment, Vi is the mean tumor volume of the vehicle-treated
group, and V0 is the mean tumor volume of the
vehicle-treated group on Day 1 of treatment. Tumor weight
inhibition (TWI) was calculated at the end of the study using the
following formula: TWI (%) = (1 - TTW/VTW) ×
100; where TTW is the mean tumor weight of the treated
group on the final day of the study and VTW is the mean
tumor weight of the vehicle-treated group on the final day of the
study. Efficacy data presented as the mean tumor volume ± standard
error of the mean (SEM).
The relative change in body weight (RCBW) in each
mouse was calculated using the following formula: RCBW (%) =
(BWi - BW0)/BW0 × 100; where
BWi is the mean body weight on Day i and BW0
is the mean body weight on Day 0. The mean, standard deviation and
SEM was calculated for each group.
Tolerability measures
Total body weight was used as a surrogate endpoint
for tolerability in all studies.
Statistical analyses
Statistical analyses were carried out using GraphPad
Prism 5 software (GraphPad Software Inc., San Diego, CA, USA).
Student’s t-test was used to compare EGFR and HER2 expression
levels in tumor tissues with those in adjacent normal tissues. The
survival curve was drawn by a Kaplan-Meier method, and the
statistical significance was assessed by the Gehan-Breslow-Wilcoxon
test. In efficacy studies, the treated groups were compared with
the vehicle group using repeated-measures analysis of variance and
Dunnett’s post-hoc test. Differences between groups were considered
statistically significant at P<0.05, as very significant at
P<0.01 and as highly significant at P<0.001.
Results
EGFR and HER2 overexpression in ESCCs
from Chinese patients
Previous studies have highlighted the role of the
EGFR signaling pathway in the development and progression of solid
tumors (10). Therefore, we used
IHC to examine the expression of EGFR and HER2 in serial sections
of ESCC collected from 94 patients. As illustrated by the examples
shown in Fig. 1A, the level and
distribution of EGFR and HER2 expression varied widely among the
analyzed tumors. EGFR expression was positive in 76 samples
(80.9%), while HER2 expression was positive in 23 samples (24.5%).
Twenty-one samples (22.3%) were positive for both EGFR and HER2.
The scatter plots in Fig. 1B show
the distribution of EGFR or HER2 IHC scores in ESCC tumor tissues
as compared with those in adjacent normal tissues. EGFR expression
was significantly higher in ESCC tumor tissues than in adjacent
normal tissues (P<0.0001), whereas there was no significant
difference in HER2 expression. The distribution of IHC staining of
EGFR and HER2 indicated that there was no correlation between EGFR
and HER2 expression in these ESCCs (Table I). Of note, the HER2-positive
patients were more likely to be EGFR-positive than not, although
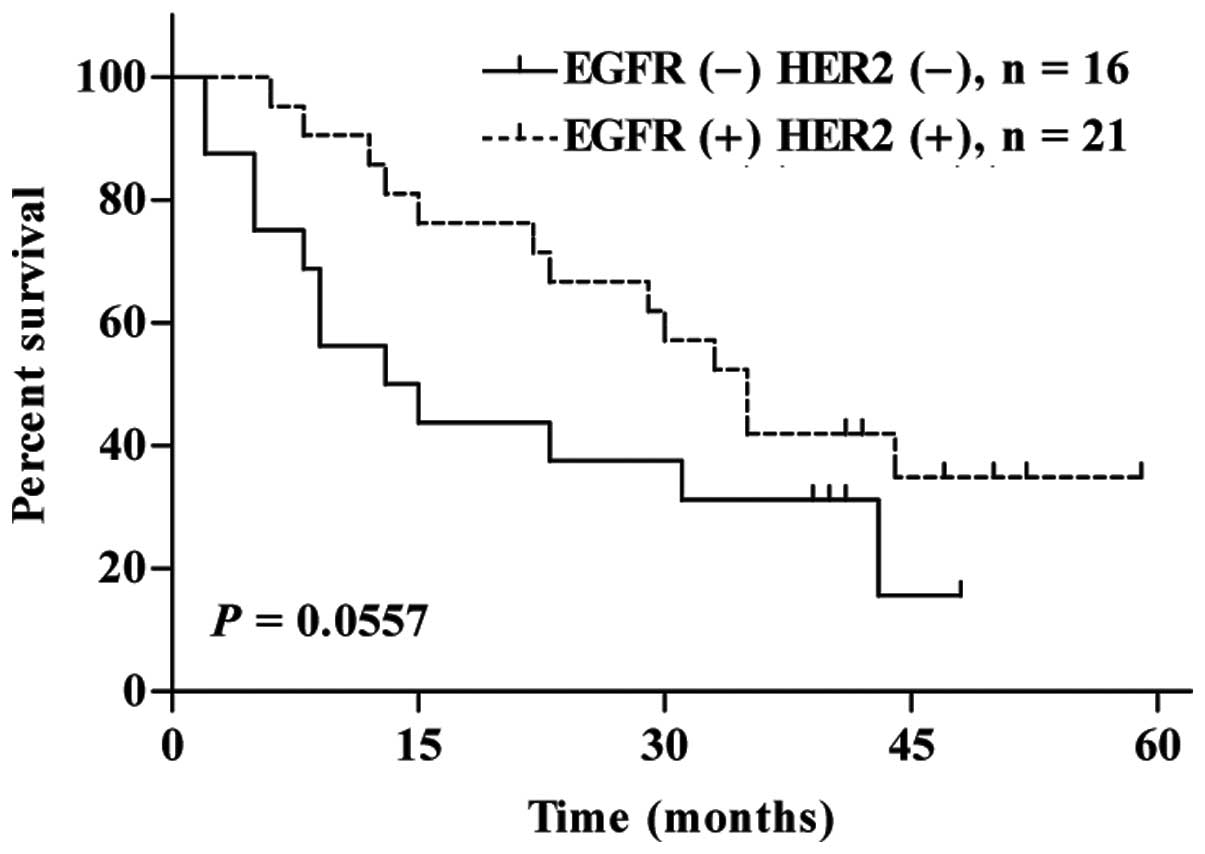
the difference was not statistically significant. The survival rate
of patients positive for both EGFR and HER2 tended to be higher
than that of patients who were negative for both EGFR and HER2
(Fig. 2). Taken together, these
results suggest that the EGFR and HER2 signaling pathway is
activated in these ESCCs obtained from Chinese patients.
 | Table IEGFR and HER2 expression pattern in
ESCCs obtained from 94 Chinese patients. |
Table I
EGFR and HER2 expression pattern in
ESCCs obtained from 94 Chinese patients.
| HER2 (+) | HER2 (−) | |
|---|
|
|
| |
|---|
| No. | % | No. | % | P-value |
|---|
| EGFR (+) | 21 | 22.3 | 55 | 58.5 | 0.1093 |
| EGFR (−) | 2 | 2.1 | 16 | 17 | |
EGFR and HER2 expression in primary
esophageal tumor models
We next examined the total protein expression levels
and phosphorylation levels of EGFR and HER2 in primary tumor cells
derived from ESCCs from Chinese patients (n=7). The levels of
unphosphorylated and phosphorylated EGFR and HER2 proteins were
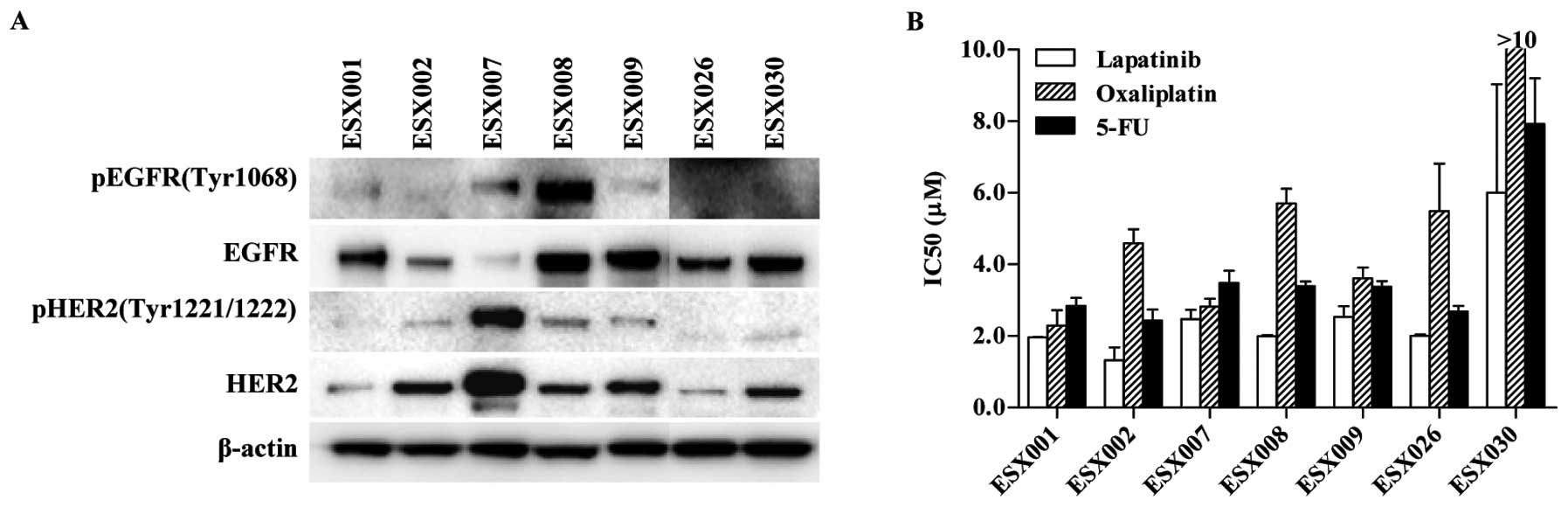
highly variable among the primary ESCC cells. As shown in Fig. 3A, five out of seven primary tumor
cells (ESX001, ESX008, ESX009, ESX026 and ESX030) expressed high
levels of EGFR. Five tumor cells (ESX002, ESX007, ESX008, ESX009
and ESX030), particularly ESX007, expressed high levels of total
HER2 protein. The level of phosphorylated EGFR or HER2 expression
also varied widely. Of the seven primary ESCC tumor cells, most of
the cells expressed phosphorylated EGFR or HER2. Very high levels
of phosphorylated EGFR (ESX008) or phosphorylated HER2 (ESX007)
were observed in one cell line each. These data indicate that EGFR
and HER2 are highly expressed and their signaling pathways are
activated in ESCCs obtained from Chinese patients.
Inhibitory effects of lapatinib in
combination with oxaliplatin or 5-FU on the growth of primary ESCC
cells
It is well established that lapatinib inhibits
protein phosphorylation in cells overexpressing HER2 (19–21).
Therefore, we assessed the inhibitory effects of lapatinib,
oxaliplatin, and 5-FU alone or lapatinib in combination with either
oxaliplatin or 5-FU on the growth of seven primary ESCC cells. The
cells were observed microscopically to assess morphologic changes
after 7 days of treatment. In the seven primary ESCC cells,
lapatinib and 5-FU both inhibited cell proliferation in
concentration-dependent manners, with a calculated IC50
of 1–10 μM. Oxaliplatin also inhibited proliferation with similar
IC50 values for six of the seven primary cells; the
exception was ESX030, which was resistant to oxaliplatin in
vitro (Fig. 3B).
The effect of lapatinib in combination with standard
chemotherapy was examined to determine the nature of the
interaction (i.e., synergistic, additive or antagonistic). As
compared with lapatinib in combination with oxaliplatin, lapatinib
in combination with 5-FU showed some evidence of synergy in primary
tumor cells, although this was not significant (Table II).
 | Table IIAntitumor effects of lapatinib,
oxaliplatin and 5-FU alone or lapatinib in combination with
oxaliplatin or 5-FU in primary ESCC cells. |
Table II
Antitumor effects of lapatinib,
oxaliplatin and 5-FU alone or lapatinib in combination with
oxaliplatin or 5-FU in primary ESCC cells.
| ESCC primary
cells | Single agent
IC50 (mean ± SD, μM) | Combination 1
IC50 (mean ± SD, μM) | Combination 2
IC50 (mean ± SD, μM) |
|---|
|
|
|
|---|
| Lapatinib | Oxaliplatin | 5-FU | Lapatinib | Oxaliplatin | Lapatinib | 5-FU |
|---|
| ESX001 | 1.96±0.01 | 2.29±0.43 | 2.84±0.22 | 1.01±0.13 | 1.01±0.13 | 0.33±0.02 | 3.32±0.19 |
| ESX002 | 1.32±0.36 | 4.59±0.39 | 2.43±0.31 | 0.95±0.08 | 0.95±0.08 | 0.24±0.03 | 2.39±0.30 |
| ESX007 | 2.47±0.26 | 2.82±0.22 | 3.48±0.34 | 1.28±0.08 | 1.28±0.08 | 0.41±0.03 | 4.03±0.29 |
| ESX008 | 1.99±0.03 | 5.70±0.41 | 3.39±0.13 | 1.29±0.09 | 1.29±0.09 | 0.29±0.03 | 2.93±0.26 |
| ESX009 | 2.53±0.30 | 3.61±0.30 | 3.37±0.16 | 1.99±0.29 | 1.99±0.29 | 0.35±0.00 | 3.47±0.04 |
| ESX026 | 2.00±0.04 | 5.49±1.32 | 2.68±0.16 | 1.51±0.20 | 1.51±0.20 | 0.32±0.02 | 3.21±0.24 |
| ESX030 | 6.00±3.03 | >10 | 7.92±1.28 | 2.80±0.32 | 2.80±0.32 | 0.92±0.08 | 9.18±0.85 |
Effects of lapatinib, oxaliplatin and
5-FU on the primary ESCC model
Lapatinib in combination with
oxaliplatin
After 21 days of treatment, lapatinib and
oxaliplatin administered alone at doses of 105 and 6 mg/kg,
respectively, did not appear to inhibit tumor cell growth in the
primary ESCC model. The TGIs for lapatinib and oxaliplatin alone
were 29.65 and 26.22%, respectively, and the TWIs were 21.95 and
22.88%, respectively. However, administration of lapatinib in
combination with oxaliplatin at the same doses had significantly
greater inhibitory effects compared with vehicle (P<0.001;
Fig. 4A), with a TGI and TWI of
51.42 and 44.55%, respectively (Fig. 4E
and F).
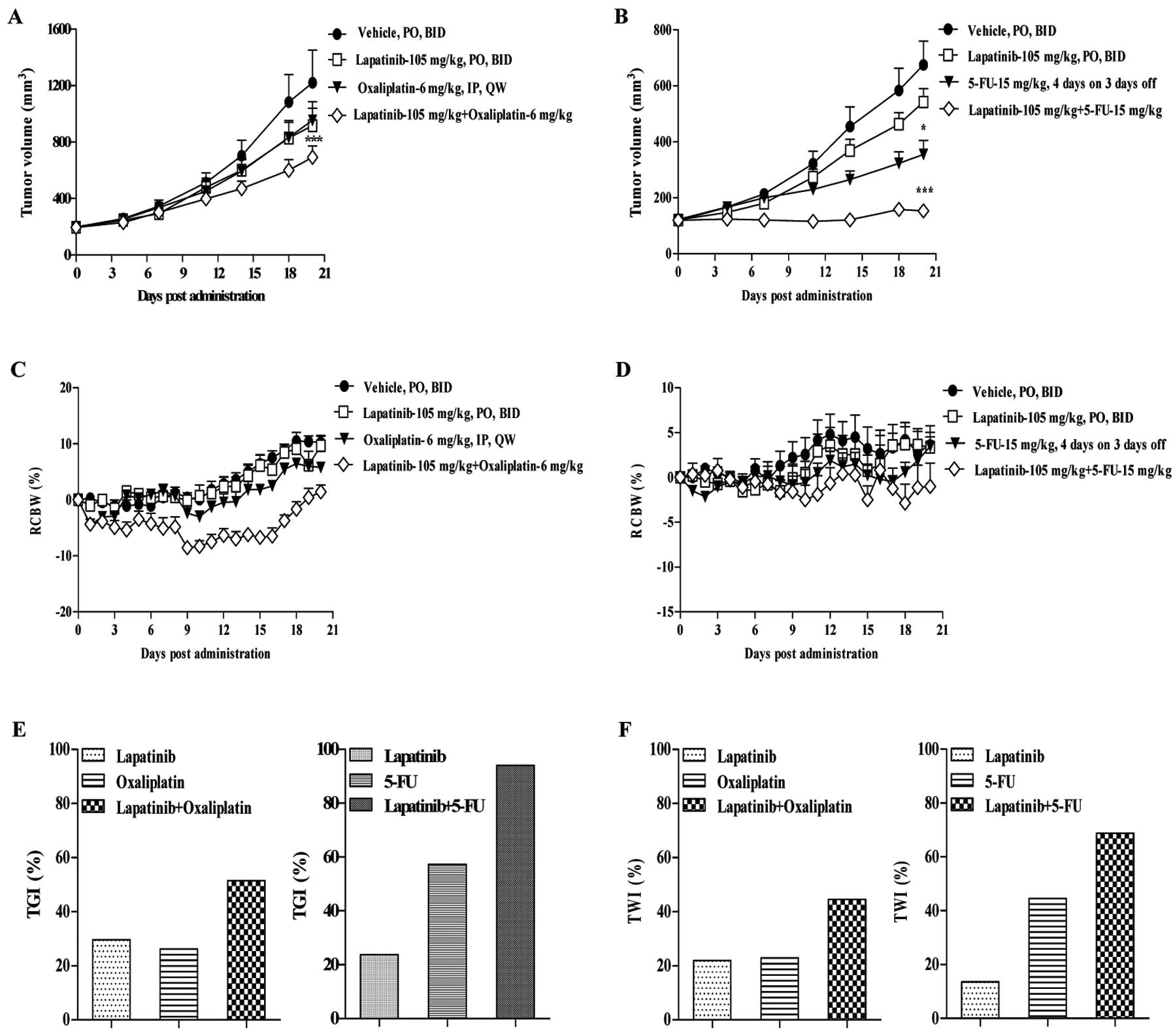 | Figure 4Antitumor activity of lapatinib,
oxaliplatin, or 5-FU administered alone or in combination against
primary ESCC models. (A) Tumor growth curves for ESCC primary
models after treatment with lapatinib (105 mg/kg, twice daily),
oxaliplatin (6 mg/kg, once weekly), or lapatinib in combination
with oxaliplatin for 3 weeks. (B) Tumor growth curves for ESCC
primary models after treatment with lapatinib (105 mg/kg, twice
daily), 5-FU (15 mg/kg, 4 days per week), or lapatinib in
combination with 5-FU. (C and D) Body weight was measured daily in
each mouse to determine the relative change in body weight (RCBW).
Error bars represent standard error of the mean. Repeated-measures
analysis of variance showed statistically significant effects
(*P<0.05 and ***P<0.001) in both
combination groups compared with the individual drugs. (E) Tumor
growth inhibition and (F) tumor weight inhibition following
treatment with lapatinib, oxaliplatin, or 5-FU alone or lapatinib
in combination with either oxaliplatin or 5-FU. |
Lapatinib in combination with
5-FU
Consistent with the above experiment, lapatinib
administered at a dose of 105 mg/kg had no obvious antitumor
effects in the ESCC primary model, with a TGI and TWI of 23.71 and
13.51%, respectively. The antitumor effect of lapatinib alone was
not significantly different from that of vehicle (P>0.05). By
contrast, 5-FU administered at 15 mg/kg four times per week
moderately inhibited tumor growth; the TGI and TWI for 5-FU were
57.25 and 44.54%, respectively (Fig. 4E
and F). The antitumor effect of 5-FU alone was significantly
greater than that of vehicle (P<0.05). Combining lapatinib with
5-FU at the same doses resulted in greater tumor growth inhibition
compared with either drug alone (Fig.
4B). The antitumor effect of lapatinib in combination with 5-FU
(TGI=94.05%) was significantly greater than that of vehicle
(P<0.001) and compared with lapatinib alone (P<0.05). The TWI
for lapatinib in combination with 5-FU (68.82%) was also greater
than that of lapatinib or 5-FU alone.
Tolerability
There was no evidence for toxicities with lapatinib
alone or in combination with oxaliplatin or 5-FU in athymic mice
bearing human primary ESCC tumors. There were no marked changes in
RCBW during the study, or any significant differences in RCBW
between each treatment group (Fig. 4C
and D).
Synergistic antitumor effects of
lapatinib and 5-FU against ESCCs ex vivo
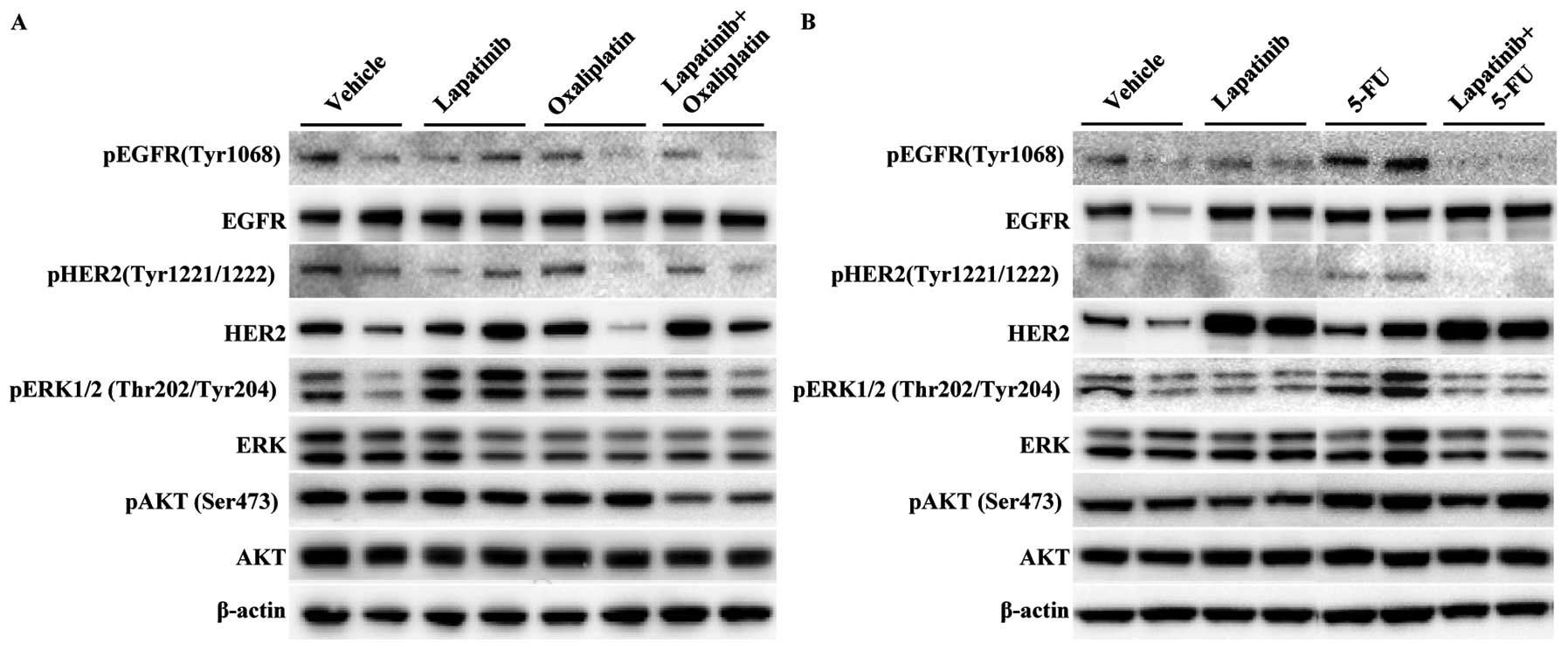
The phosphorylated and total protein levels of
several downstream markers were measured in mice treated with a
single agent or lapatinib in combination with oxaliplatin (Fig. 5A) or 5-FU (Fig. 5B). Lapatinib in combination with
5-FU induced a greatest decrease in the phosphorylation of EGFR and
HER2 in primary ESCC models as compared with each drug alone or
lapatinib in combination with oxaliplatin. The expression levels of
pERK were slightly downregulated by lapatinib in combination with
either oxaliplatin or 5-FU. There were no considerable increases in
the blocking of pAKT expression in mice treated with lapatinib in
combination with 5-FU, as compared with each drug alone or
lapatinib in combination with oxaliplatin. These results suggest
that, in primary ESCC models, blockade of pEGFR and pHER2 is
beneficial for the synergistic inhibition of tumor growth following
treatment with lapatinib in combination with 5-FU.
Discussion
Lapatinib is currently approved for the treatment of
patients with HER2-positive metastatic breast cancer whose disease
has progressed following trastuzumab-based therapy, and is licensed
for use in these patients in combination with capecitabine
(26). Based on these data and the
increasing interest in the roles of EGFR and HER2 in gastric and
esophageal cancer, we sought to evaluate the therapeutic potential
of this dual TKI for the treatment of esophageal cancer. We also
sought to compare the antitumor effects of lapatinib with standard
chemotherapeutic drugs alone or in combination, and to understand
the mechanism of action of these drugs.
In seven primary ESCC cells, the expression levels
of EGFR and HER2 (total protein and phosphorylated protein) varied
greatly. As previously reported in breast as well as in gastric
cancer, there was no correlation between lapatinib activity and
EGFR protein expression (27–29).
Similarly, no correlation was found between lapatinib activity and
HER2 protein expression. Several recent studies have examined the
roles of lapatinib administered alone or in combination with
standard chemotherapies in esophageal cancer cell lines in
vitro(21,30). Our study provides further insight
into the role of the HER family in esophageal cancer. First, our
panel of primary tumor cells was directly derived from ESCC tissue
samples obtained from Chinese patients. The physiologic condition
differs greatly between primary tumor cells and long-established
tumor cell lines. For example, primary tumor cells better reflect
the in vivo situation (31–33).
Our primary tumor cells were directly derived from ESCCs, and are
therefore more clinically relevant than cell lines. Indeed, we
found that the activity of ESCC tumor cells can be modulated by
blocking the EGFR and HER2 signaling pathways.
The doses of lapatinib, oxaliplatin, and 5-FU used
in the present study were determined based on the results of
maximum tolerated dose studies performed in nude mice (data not
shown). Efficacy studies in primary ESCC xenograft models showed
that lapatinib (105 mg/kg) in combination with 5-FU induced
near-complete tumor regression in all the mice. The effects of this
combination were much greater than those achieved using either drug
alone or with lapatinib combined with oxaliplatin. We also found
that the synergistic antitumor effects of lapatinib in combination
with 5-FU were probably mediated by changes in cell signaling. The
levels of phosphorylated EGFR and HER2 were much lower following
treatment with lapatinib in combination with 5-FU compared with
either agent alone or with lapatinib in combination with
oxaliplatin. Therefore, our data suggest that inhibition of the
EGFR/HER2 signaling pathway by combining a chemotherapeutic drug
and a TKI may augment the effects of both agents on the downstream
signaling pathways. The synergy observed for this combination may
have important clinical implications. For example, recent studies
using breast cancer models have revealed that the accumulation of
inactive HER2 receptor, as induced by lapatinib, enhances
trastuzumab activity through antibody-dependent cellular
cytotoxicity (34). Although our
results are preliminary, they support the ongoing investigation of
lapatinib in esophageal cancer as well as its possible combination
with 5-FU in tumors overexpressing EGFR and HER2. The results also
suggest that the addition of anti-EGFR/anti-HER2 therapy to
standard chemotherapeutic drugs could have direct clinical
benefits, and makes the investigation of additional
anti-EGFR/anti-HER2 therapies in esophageal cancer particularly
timely.
In the present study, we focused on the effects of
lapatinib alone or in combination with standard chemotherapeutic
drugs for esophageal cancer. Several clinical trials have suggested
that the activity of anti-EGFR drugs seems to be limited to tumors
of the gastroesophageal junction, with the response to both
erlotinib and gefitinib being approximately 10% (35,36).
In addition, in a clinical study of lapatinib in upper
gastrointestinal cancer, disease control (prolonged stable disease)
was only detected in patients with HER2-amplified disease. Our
in vitro and in vivo observations support these
clinical findings and the ongoing development of lapatinib in
patients with tumors overexpressing HER2 (37,38).
Esophageal carcinoma is a highly malignant and
prevalent cancer in China, for which the existing treatments have
limited potential in reducing morbidity and mortality. Therefore,
there is an urgent need to develop and refine new combinatorial
therapies for this cancer. We have shown that lapatinib in
combination with 5-FU has a significant synergistic therapeutic
effect against ESCCs overexpressing EGFR and HER2. Lapatinib not
only had a direct biological effect in terms of inhibiting the
growth of ESCC primary cells in vitro, but also augmented
the antitumor effects of 5-FU in primary ESCC models in
vivo. Therefore, a regimen in which lapatinib is combined with
an established chemotherapeutic drug represents a promising
strategy for most patients with ESCC.
Acknowledgements
The present study was supported by grants from the
One Hundred Talents Program of the Chinese Academy of Sciences, the
National Natural Science Foundation (31070802), and the Ministry of
Science and Technology of China (2009CB919000 and
2011BAK10B00).
References
|
1
|
Demeester SR: Epidemiology and biology of
esophageal cancer. Gastrointest Cancer Res. 3:S2–S5.
2009.PubMed/NCBI
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
3
|
Blot W, McLaughlin J and Fraumeni J:
Esophageal cancer. New Jersey Comprehensive Cancer Control Plan,
2008–2012. Schottenfeld D and Fraumeni J: Oxford University Press;
New York: pp. 697–706. 2006
|
|
4
|
Ishikura S, Nihei K, Ohtsu A, et al:
Long-term toxicity after definitive chemoradiotherapy for squamous
cell carcinoma of the thoracic esophagus. J Clin Oncol.
21:2697–2702. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tepper J, Krasna MJ, Niedzwiecki D, et al:
Phase III trial of trimodality therapy with cisplatin,
fluorouracil, radiotherapy, and surgery compared with surgery alone
for esophageal cancer: CALGB 9781. J Clin Oncol. 26:1086–1092.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Del Campo JM, Hitt R, Sebastian P, et al:
Effects of lapatinib monotherapy: results of a randomised phase II
study in therapy-naive patients with locally advanced squamous cell
carcinoma of the head and neck. Br J Cancer. 105:618–627.
2011.PubMed/NCBI
|
|
7
|
Scaltriti M and Baselga J: The epidermal
growth factor receptor pathway: a model for targeted therapy. Clin
Cancer Res. 12:5268–5272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Klapper LN, Kirschbaum MH, Sela M and
Yarden Y: Biochemical and clinical implications of the ErbB/HER
signaling network of growth factor receptors. Adv Cancer Res.
77:25–79. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baselga J and Arteaga CL: Critical update
and emerging trends in epidermal growth factor receptor targeting
in cancer. J Clin Oncol. 23:2445–2459. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Roskoski R Jr: The ErbB/HER receptor
protein-tyrosine kinases and cancer. Biochem Biophys Res Commun.
319:1–11. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Witton CJ, Reeves JR, Going JJ, Cooke TG
and Bartlett JM: Expression of the HER1–4 family of receptor
tyrosine kinases in breast cancer. J Pathol. 200:290–297. 2003.
|
|
12
|
Slamon DJ, Godolphin W, Jones LA, et al:
Studies of the HER-2/neu proto-oncogene in human breast and ovarian
cancer. Science. 244:707–712. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moasser MM: The oncogene HER2: its
signaling and transforming functions and its role in human cancer
pathogenesis. Oncogene. 26:6469–6487. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hanawa M, Suzuki S, Dobashi Y, et al: EGFR
protein overexpression and gene amplification in squamous cell
carcinomas of the esophagus. Int J Cancer. 118:1173–1180. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mimura K, Kono K, Hanawa M, et al:
Frequencies of HER-2/neu expression and gene amplification in
patients with oesophageal squamous cell carcinoma. Br J Cancer.
92:1253–1260. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kawaguchi Y, Kono K, Mimura K, et al:
Targeting EGFR and HER-2 with cetuximab- and trastuzumab-mediated
immunotherapy in oesophageal squamous cell carcinoma. Br J Cancer.
97:494–501. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Higuchi K, Koizumi W, Tanabe S, et al:
Current management of esophageal squamous-cell carcinoma in Japan
and other countries. Gastrointest Cancer Res. 3:153–161.
2009.PubMed/NCBI
|
|
18
|
Ilson DH: Esophageal cancer chemotherapy:
recent advances. Gastrointest Cancer Res. 2:85–92. 2008.
|
|
19
|
Rusnak DW, Alligood KJ, Mullin RJ, et al:
Assessment of epidermal growth factor receptor (EGFR, ErbB1) and
HER2 (ErbB2) protein expression levels and response to lapatinib
(Tykerb, GW572016) in an expanded panel of human normal and tumour
cell lines. Cell Prolif. 40:580–594. 2007. View Article : Google Scholar
|
|
20
|
Xia W, Mullin RJ, Keith BR, et al:
Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor
blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT
pathways. Oncogene. 21:6255–6263. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mimura K, Kono K, Maruyama T, et al:
Lapatinib inhibits receptor phosphorylation and cell growth and
enhances antibody-dependent cellular cytotoxicity of EGFR- and
HER2-overexpressing esophageal cancer cell lines. Int J Cancer.
129:2408–2416. 2011. View Article : Google Scholar
|
|
22
|
Kim HP, Yoon YK, Kim JW, et al: Lapatinib,
a dual EGFR and HER2 tyrosine kinase inhibitor, downregulates
thymidylate synthase by inhibiting the nuclear translocation of
EGFR and HER2. PLoS One. 4:e59332009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Blackwell KL, Burstein HJ, Storniolo AM,
et al: Randomized study of Lapatinib alone or in combination with
trastuzumab in women with ErbB2-positive, trastuzumab-refractory
metastatic breast cancer. J Clin Oncol. 28:1124–1130. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cameron D, Casey M, Press M, et al: A
phase III randomized comparison of lapatinib plus capecitabine
versus capecitabine alone in women with advanced breast cancer that
has progressed on trastuzumab: updated efficacy and biomarker
analyses. Breast Cancer Res Treat. 112:533–543. 2008. View Article : Google Scholar
|
|
25
|
Chung KY, Shia J, Kemeny NE, et al:
Cetuximab shows activity in colorectal cancer patients with tumors
that do not express the epidermal growth factor receptor by
immunohistochemistry. J Clin Oncol. 23:1803–1810. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Geyer CE, Forster J, Lindquist D, et al:
Lapatinib plus capecitabine for HER2-positive advanced breast
cancer. N Engl J Med. 355:2733–2743. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wainberg ZA, Anghel A, Desai AJ, et al:
Lapatinib, a dual EGFR and HER2 kinase inhibitor, selectively
inhibits HER2-amplified human gastric cancer cells and is
synergistic with trastuzumab in vitro and in vivo. Clin Cancer Res.
16:1509–1519. 2010. View Article : Google Scholar
|
|
28
|
Zhang D, Pal A, Bornmann WG, et al:
Activity of lapatinib is independent of EGFR expression level in
HER2-overexpressing breast cancer cells. Mol Cancer Ther.
7:1846–1850. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Konecny GE, Pegram MD, Venkatesan N, et
al: Activity of the dual kinase inhibitor lapatinib (GW572016)
against HER-2-overexpressing and trastuzumab-treated breast cancer
cells. Cancer Res. 66:1630–1639. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guo XF, Zhu XF, Zhong GS, Deng BG, Gao ZT
and Wang H: Lapatinib, a dual inhibitor of EGFR and HER2, has
synergistic effects with 5-fluorouracil on esophageal carcinoma.
Oncol Rep. 27:1639–1645. 2012.PubMed/NCBI
|
|
31
|
Nolan GP: What’s wrong with drug screening
today. Nat Chem Biol. 3:187–191. 2007.
|
|
32
|
Kamb A: What’s wrong with our cancer
models? Nat Rev Drug Discov. 4:161–165. 2005.
|
|
33
|
Masters JR: HeLa cells 50 years on: the
good, the bad and the ugly. Nat Rev Cancer. 2:315–319.
2002.PubMed/NCBI
|
|
34
|
Scaltriti M, Verma C, Guzman M, et al:
Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization
and accumulation of HER2 and potentiates trastuzumab-dependent cell
cytotoxicity. Oncogene. 28:803–814. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dragovich T, McCoy S, Fenoglio-Preiser CM,
et al: Phase II trial of erlotinib in gastroesophageal junction and
gastric adenocarcinomas: SWOG 0127. J Clin Oncol. 24:4922–4927.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ferry DR, Anderson M, Beddard K, et al: A
phase II study of gefitinib monotherapy in advanced esophageal
adenocarcinoma: evidence of gene expression, cellular, and clinical
response. Clin Cancer Res. 13:5869–5875. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Janmaat ML, Gallegos-Ruiz MI, Rodriguez
JA, et al: Predictive factors for outcome in a phase II study of
gefitinib in second-line treatment of advanced esophageal cancer
patients. J Clin Oncol. 24:1612–1619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hecht J, Urba S, Koehler M, et al:
Lapatinib monotherapy in recurrent upper gastrointestinal
malignancy: phase II efficacy and biomarker analyses. Proc GI
Cancer Symposium. 2008.
|