Introduction
Hepatocellular carcinoma (HCC) is one of the most
common causes of cancer-related mortality and exhibits high
metastatic potential and is associated with poor patient prognosis.
More than 700,000 cases of HCC were diagnosed in 2008 (1–3).
Melanoma differentiation associated gene-7 (MDA-7)/interleukin-24
(IL-24) is a member of the IL-10 gene family, and several reports
have indicated that MDA-7/IL-24 overexpression causes tumor growth
suppression and tumor cell apoptosis in mesotheliomas,
osteosarcoma, melanoma, lung cancer, breast cancer, pancreatic
cancer, glioblastoma and prostate cancer (4–16),
suggesting that MDA-7/IL-24 may prove to be a potential strategy
for cancer therapy. However, the mechanisms through which MDA-7
expression exerts its anti-neoplastic activity, tumor-specificity
and efficacy across a spectrum of human cancer types have yet to be
fully elucidated. We, therefore, aimed to investigate the effect of
the ectopic production of MDA-7/IL-24 on the metastasis of HCC
HepG2 and BEL-7402 cells in vitro and attempted to identify
the underlying mechanisms involved in its suppression of
metastasis.
In the present study, we demonstrated for the first
time that MDA-7/IL-24 inhibits the adhesion and invasion of HCC
HepG2 and BEL-7402 cells by downregulating the expression of CD44,
ICAM-1, CyclinB, Twist, survivin, p-Akt and matrix
metalloproteinases (MMPs), and by upregulating the expression of
E-cadherin and p-ERK. Furthermore, we confirmed that MDA-7/IL-24
reduced the secretion of TGF-β and the transcriptional activity of
NF-κB and increased the transcriptional activity of AP-1 in HepG2
and BEL-7402 cells. Thus, MDA-7/IL-24 may provide multiple benefits
as an anticancer therapeutic strategy due to its inhibition of
tumor metastasis.
Materials and methods
Cell culture
Human HCC cell lines HepG2 and BEL-7402 were
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA), and the cell lines were cultured in Dulbecco's modified
Eagle's medium (DMEM) (10% FBS) under conditions of 37°C and 5%
CO2.
Plasmid construction
The mda-7/IL-24 coding sequences were amplified by
RT-PCR. The primers were: sense 1,
5′-AATAGGGCTAGCGCCACCATGAATTTTCAACAGAG GCT-3′ and sense 2,
5′-GAATTCGGTCTCCTCGAGGAGC TTGTAGAATTTCTGCA-3′ and were verified and
ligated into the pcDNA3.1 vector (Invitrogen).
Transfection of Mda-7/IL-24
The pcDNA3.1 empty vector plasmid and
pcDNA3.1-mda-7/IL-24 expression plasmid were transfected into HepG2
and BEL-7402 cells. A pool of transfectants was selected using G418
to build the mda-7/IL-24-overexpressing sublines (Ad.mda-7-1 and
Ad.mda-7-2) and the negative control empty vector subline
(Ad.vec).
Adhesion assay
Cells were cultured in Matrigel-coated 24-well
plates (Collaborative Biomedical), incubated, and washed with cold
PBS 6 h later. The adhesion assay was performed by MTS assay at 490
nm. The cell adhesion rate was calculated by the absorption of the
MDA-7/IL-24-overexpressing group or negative control group/the
absorption of the parent group.
Cell invasion assay
Cells were harvested, resuspended in serum-free
DMEM, and then transferred to hydrated Matrigel chambers (25,000
cells/well) of the Transwell system. The chambers were then
incubated in DMEM with 10% FBS in the bottom chambers before
examination overnight. The cells on the upper surface were scraped
and washed away, whereas the invaded cells in the lower surface
were fixed, stained and counted under a microscope, and the
relative number was calculated (magnification, ×20).
Cell cycle analysis
Cells were cultured, treated with serum-free DMEM
for 24 h for synchronization, and ixed in 70% ethanol at 4°C
overnight. Cells were then resuspended and incubated with 1 mg/ml
of RNase A and 0.5 mg/ml propidium iodide for 30 min in the dark.
Cell cycle analysis was analyzed by flow cytometry, and the
percentage of cells at each phase of the cell cycle was determined
using MultiCycle software.
ELISA assay
The cell culture supernatant was collected and added
to a microplate. The anti-TGF-β antibody was then added and
incubation was carried out at 37°C for 30 min. HRP was added and
incubation was carried out at 37°C for 30 min after washing with
wash buffer. TMB was added and incubated at room temperature for 20
min in the dark. The absorbance was read at 450 nm after the stop
solution was added, and the TGF-β content was calculated using a
standard curve.
Real-time quantitative PCR
Total RNA was isolated by TRIzol assay, and
real-time-PCR assays were performed by SYBR-Green incorporation.
The relative gene expression was determined by duct calculation
utilizing actin for normalization. GAPDH was used as a reference
gene. The following primers were used to amplify cDNA fragments:
human E-cadherin, (forward) 5′-CAGCATCACTGGCCAAGGAGC TGA-3′ and
(reverse) 5′-GACCACACTGATGACTCCTGT GT TCC-3′; human CD44, (forward)
5′-CGTGATGGCACCCGC TATGT-3′ and (reverse) 5′-CAGGGATTCTGTCTGTGCTG
TCG-3′; human ICAM-1, (forward) 5′-CTCCAATGTGCCA GGCTTG-3′ and
(reverse) 5′-CAGTGGGAAAGTGCCAT CCT-3′; human MMP-2, (forward)
5′-CTTCCAAGTCTGGAG CGATGT-3′ and (reverse) 5′-TACCGTCAAAGGGGTATC
CAT-3′; human MMP-9, (forward) 5′-GGGACGCAGACATC GTCATC-3′ and
(reverse) 5′-TCGTCATCGTCGAAATG GGC-3′; human CyclinB1, (forward)
5′-GGCCAAAATGC CTATGAAGA-3′ and (reverse) 5′-AGATGTTTCCATTGGGC
TTG-3′; human Twist, (forward) 5′-AGTCCGCAGTCTTAC GAGGA-3′ and
(reverse) 5′-GCAGAGGTGTGAGGATG GT-3′; human survivin, (forward)
5′-GCCCAGTGTTTCTT CTGCTT-3′ and (reverse) 5′-CCGGACGAATGCTTTTTA
TG-3′; human GAPDH (forward) 5′-TGTTGCCATCAATGAC CCCTT-3′ and
(reverse) 5′-CTCCACGACGTACTCAGCG-3.
Western blot analysis
The cells were lysed and analyzed using 12%
SDS-PAGE. The proteins were transferred to polyvinylidene
difluoride membranes and blocked at 4°C overnight with 5% milk and
incubated with the monoclonal antibodies against: MDA-7/IL-24
(1:800), ICAM-1 (1:800), MMP-2 (1:800), MMP-9 (1:500), CyclinB
(1:500), E-cadherin (1:800), CD44 (1:500), Twist (1:500), survivin
(1:500), p-ERK (1:800), ERK (1:800), p-Akt (1:800), Akt (1:800) and
mouse monoclonal β-actin (1:2,000). The results were visualization
using a chemiluminescence detection kit.
Reporter gene assay
Cells were transfected with pAP-1-luc or pNFκB-luc
and pRL-SV40 and plated in a 24-well plate at 1×105
cells/well at 37°C. The cells were then counted by luciferase assay
after 24 h.
Statistical analysis
Data are presented as the means ± standard deviation
(SD) and were evaluated with SPSS 11.0. Analysis of variance was
performed by one-way ANOVA, and differences were considered
significant at P<0.05.
Results
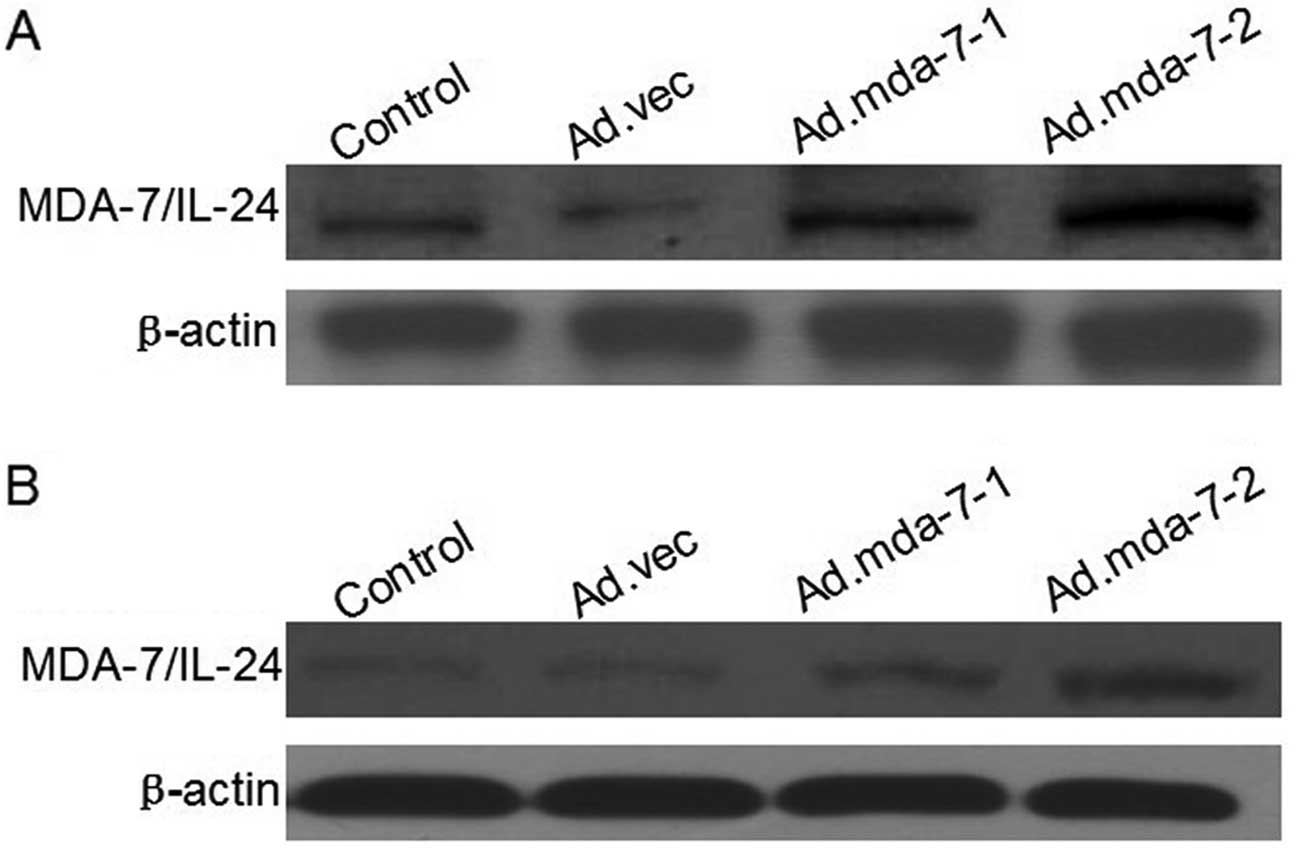
Expression of the MDA-7/IL-24 gene in the
establishment of stable HepG2 and BEL-7402 cells
The transfection efficiency of HepG2 and BEL-7402
cells was measured by western blotting. The protein expression in
the Ad.mda-7-1 and Ad.mda-7-2 groups was higher when compared with
the expression in the Ad.vec and parent groups (Fig. 1).
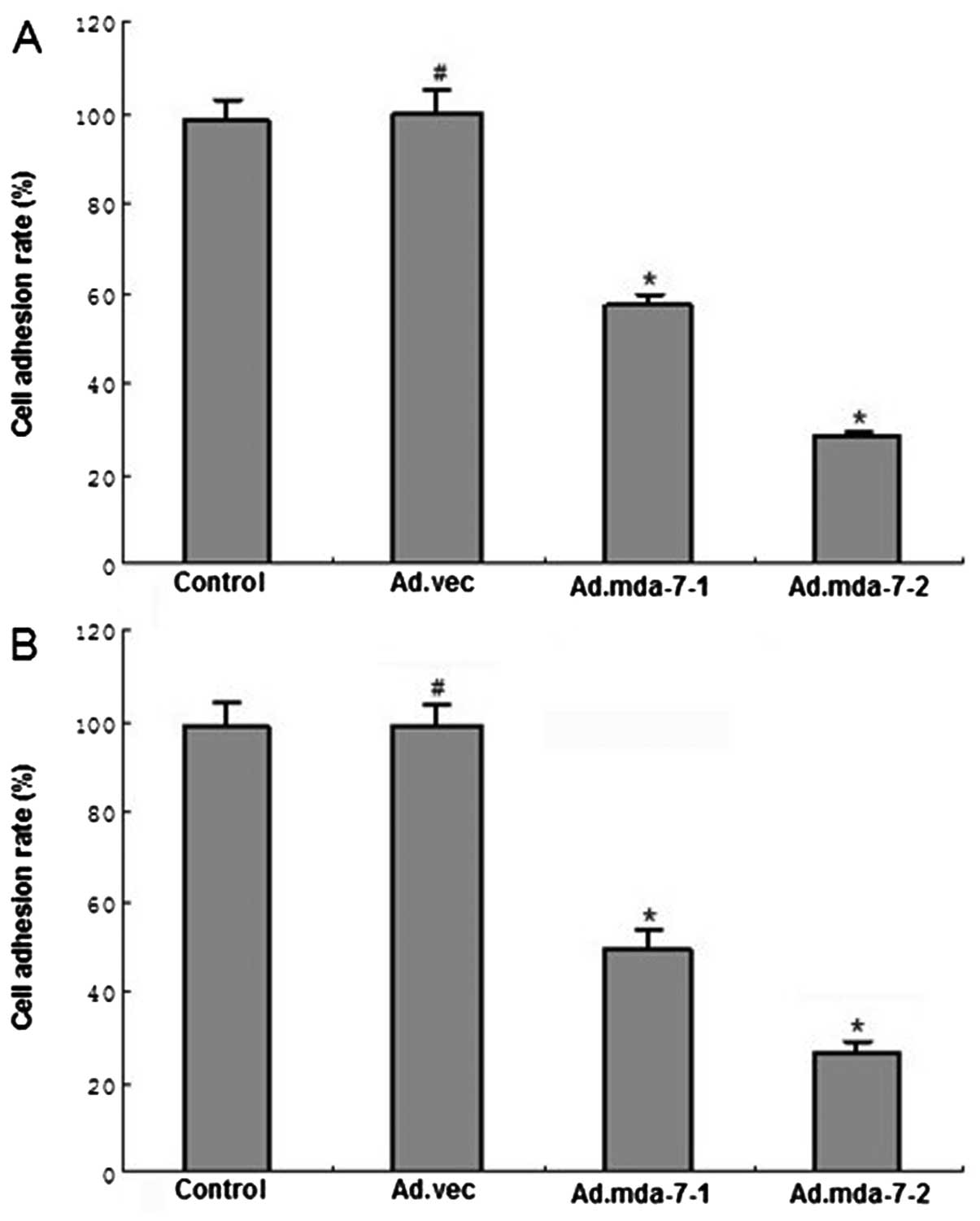
MDA-7/IL-24 inhibits tumor cell adhesion
and invasive potential in HepG2 and BEL-7402 cells
We assessed the ability of MDA-7/IL-24 to inhibit
cell adhesion in HepG2 and BEL-7402 cell lines. HCC cells
overexpressing MDA-7/IL-24 were significantly less able to adhere
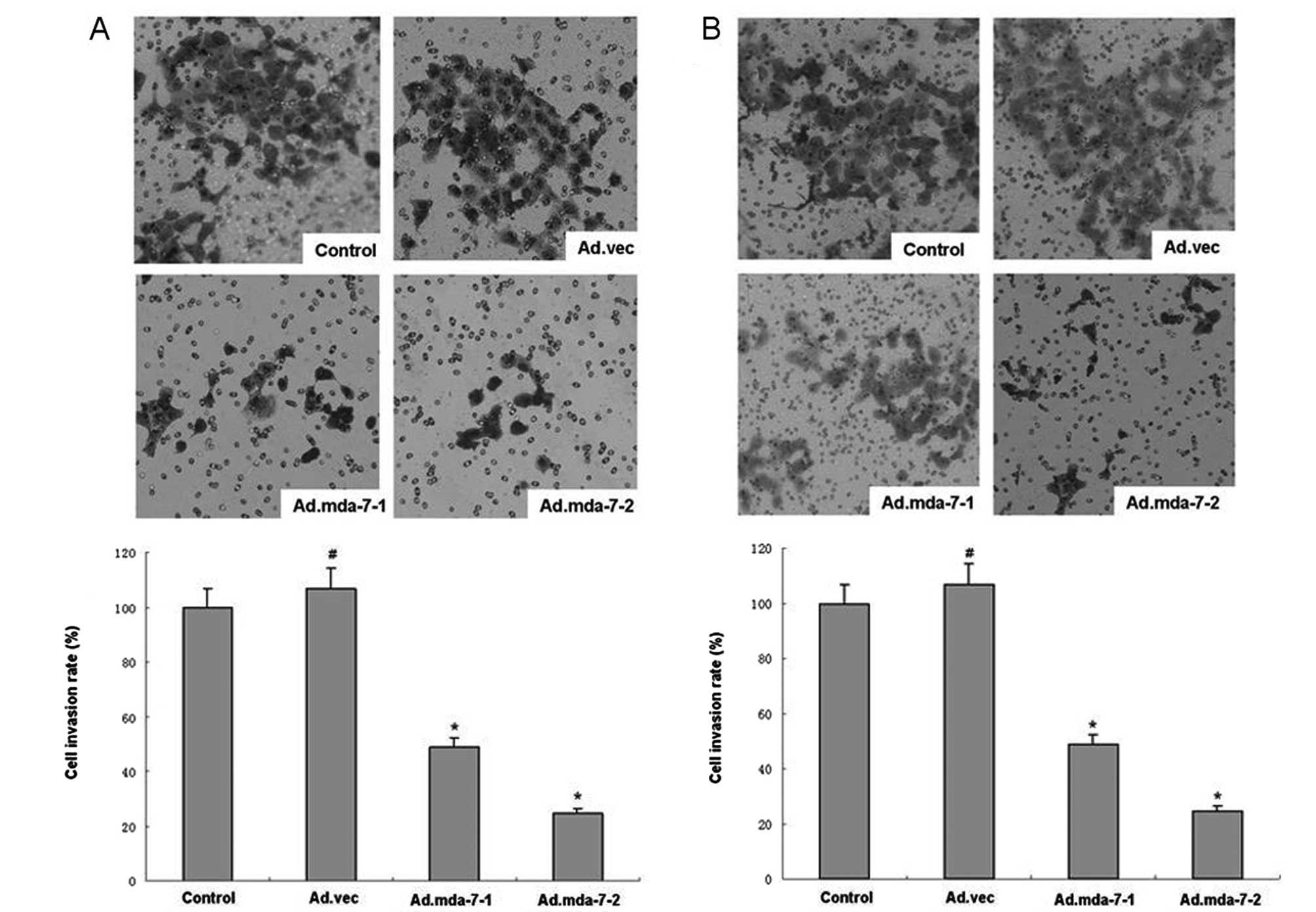
when compared to the empty vector or control groups (Fig. 3). Furthermore, HepG2 and BEL-7402
cells overexpressing MDA-7/IL-24 were much less invasive than that
of the empty vector or control groups (Fig. 2), indicating that MDA-7/IL-24
effectively inhibits tumor cell adhesion and invasive potential in
HepG2 and BEL-7402 cells.
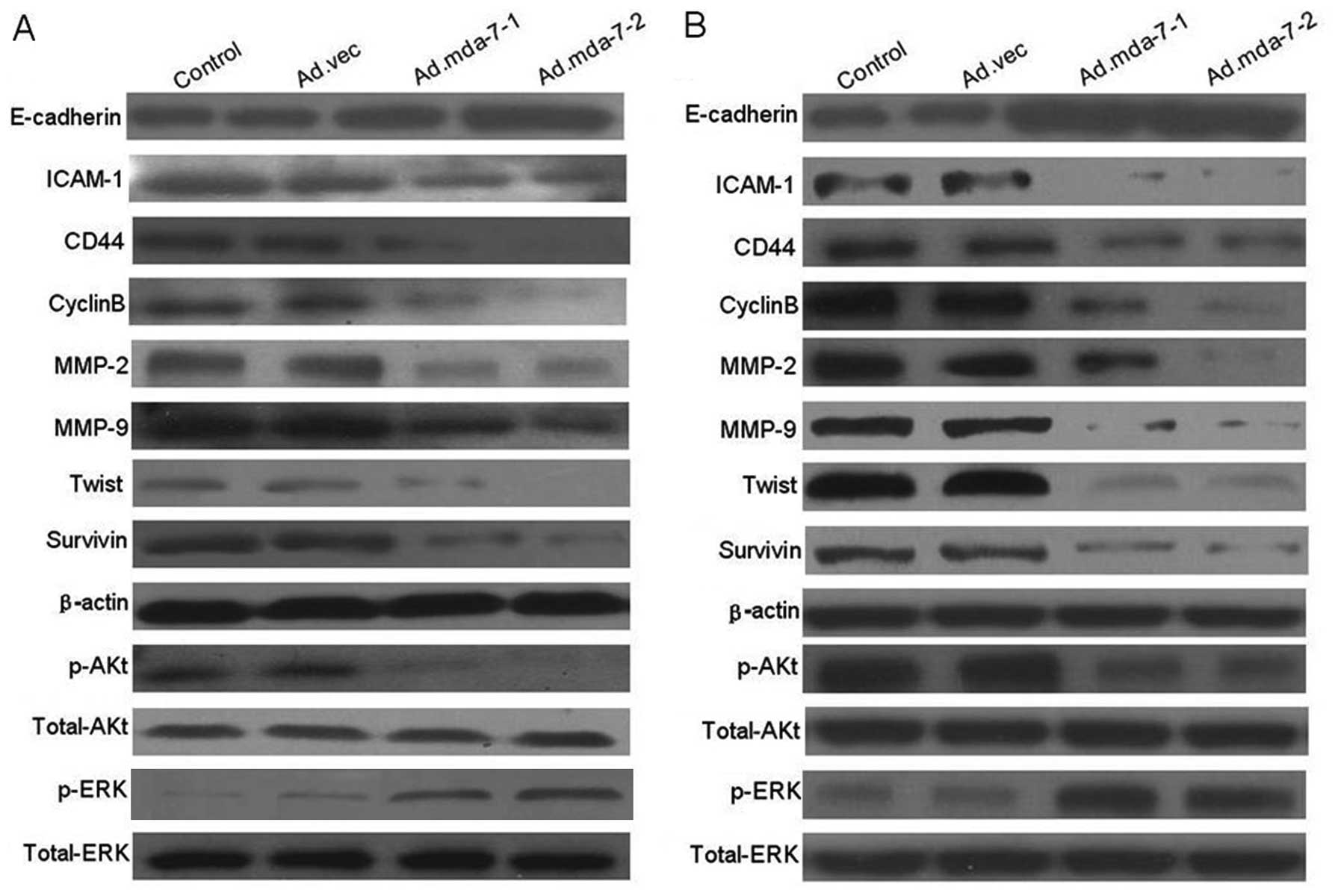
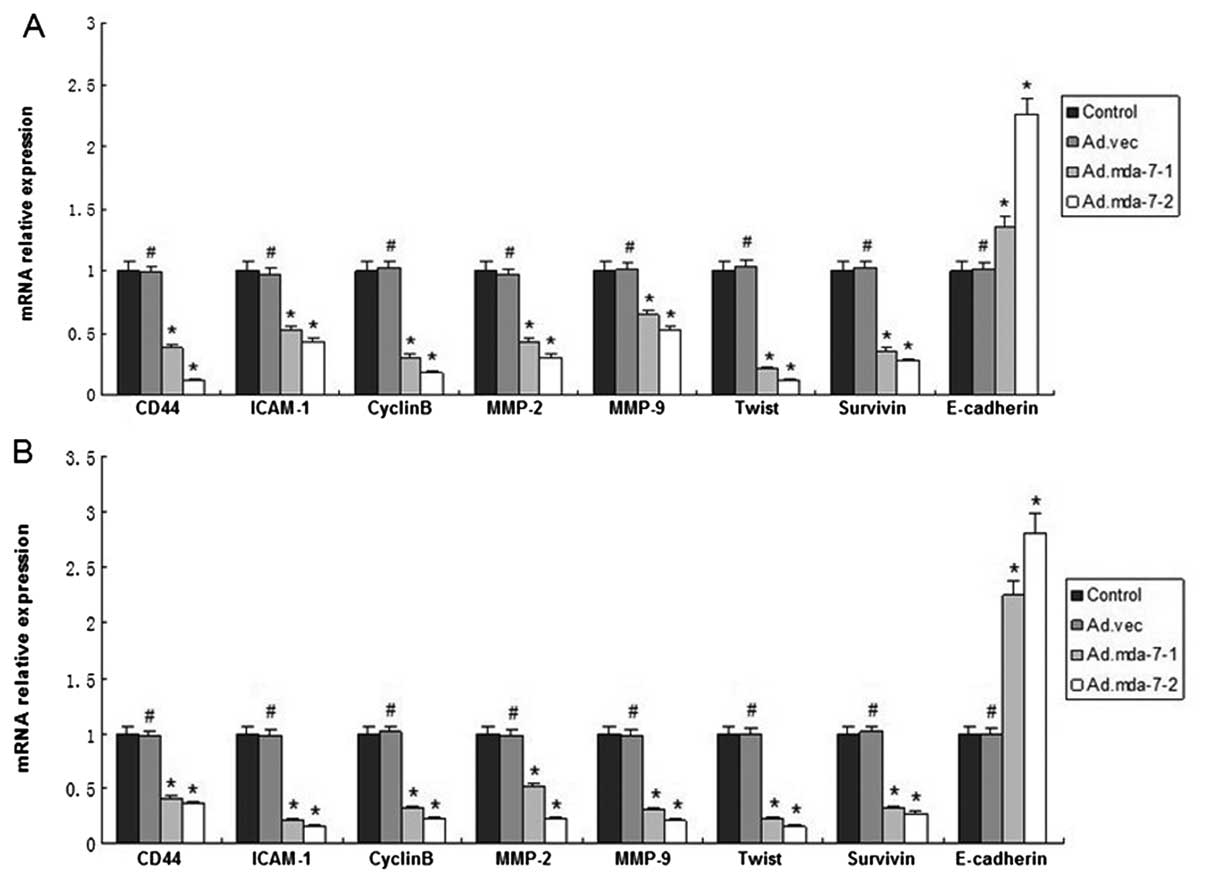
MDA-7/IL-24 regulates expression of
metastasis-related genes in HepG2 and BEL-7402 cells
We next examined the regulatory effect of
MDA-7/IL-24 on tumor metastasis-related genes which influence tumor
adhesion and invasion by western blot analysis and RT-PCR assay.
The results showed that the expression levels of CD44, ICAM-1,
MMP-2/−9, CyclinB, Twist, survivin and p-Akt in
MDA-7/IL-24-overexpressing cells were significantly decreased when
compare to these levels in the empty vector or control groups at
the transcription and translation levels. Moreover, the expression
of E-cadherin and p-ERK was significantly increased (Figs. 4 and 5). The results revealed that MDA-7/IL-24
inhibits expression of tumor metastasis-related genes in HepG2 and
BEL-7402 cells.
MDA-7/IL-24 inhibits the TGF-β production
in tumor cells
We used ELISA assay to detect the expression of
TGF-β. Results showed that production of TGF-β was decreased in the
HepG2 and BEL-7402 cells overexpressing MDA-7/IL-24, when compared
with the production in the empty vector negative control or parent
groups (Fig. 6).
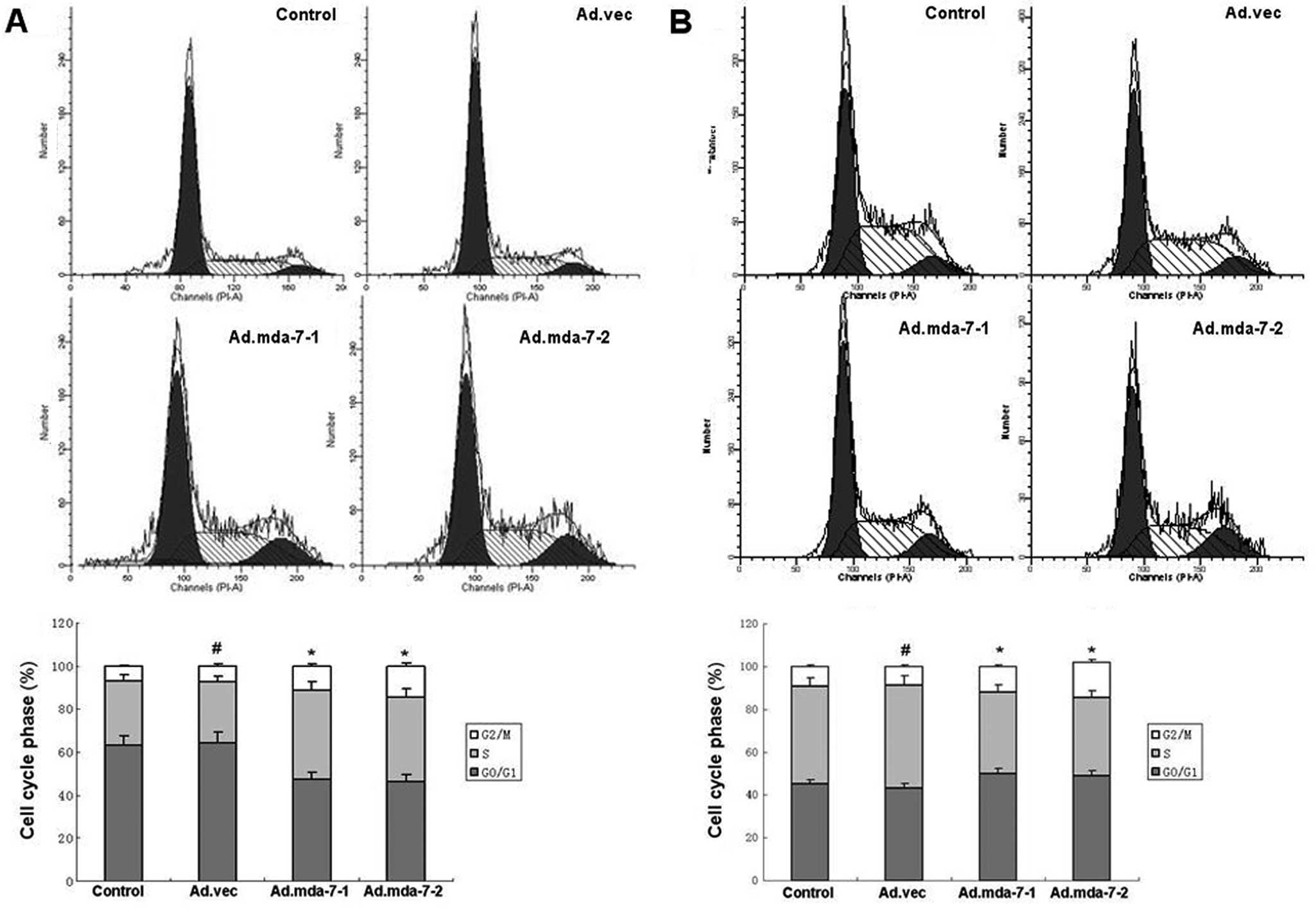
MDA-7/IL-24 induces accumulation of tumor
cells in the G2/M phase of the cell cycle
As shown in Fig. 7,
MDA-7/IL-24 induced the accumulation of HepG2 and BEL-7402 cells in
the G2/M phase. We found that the percentages of HepG2 and BEL-7402
cells in the G2/M phase were 11.26 and 14.62% in the
MDA-7/IL-24-overexpressing groups, 7.25% in the empty vector group
and 6.74% in the control group (P<0.01), while the percentages
of HepG2 and BEL-7402 cells in the S phase were 38.75 and 41.25% in
the MDA-7/IL-24-overexpressing groups, 30.06% in the empty vector
group and 28.13% in the control group (P<0.01). The results
revealed that MDA-7/IL-24 induced an accumulation of HepG2 and
BEL-7402 cells in the G2/M phase.
MDA-7/IL-24 downregulates the
transcriptional activation of AP-1 and NF-κB in the HepG2 and
BEL-7402 cells
We examined the effect of MDA-7/IL-24 on the
transcriptional activation of AP-1 and NF-κB in HepG2 and BEL-7402
cells by luciferase reporter assay. We found that MDA-7/IL-24
downregulated the transcriptional activation of NF-κB and
upregulated the transcriptional activation of AP-1 in HepG2 and
BEL-7402 cells (Fig. 8).
Discussion
MDA-7/IL-24 is a cytokine-like protein of the IL-10
cytokine family and has been reported to decrease survival in
adjacent tumor cells while sparing normal cells (17–20).
The present study adds to the literature findings regarding the
metastatic inhibitory function of MDA-7/IL-24 in solid human
malignancies, with particular reference to HCC. In the present
study, the results revealed that MDA-7/IL-24 inhibited the
potential of adhesion and invasion of human HCC HepG2 and BEL-7402
cells, and the invasion and adhesion inhibition ability of the
cells was enhanced along with the increased expression of
MDA-7/IL-24. Several reports have demonstrated that MDA-7/IL-24
overexpression results in growth inhibition and apoptosis in many
types of cancer. MDA-7/IL-24 is considered as an ideal gene for
tumor gene therapy due to its low deleterious effects on normal
cells (21). We transfected the
MDA-7/IL-24 expression plasmid into HepG2 and BEL-7402 cell lines,
and western blot assay showed that MDA-7/IL-24 was overexpressed in
cells transfected with Ad.mda-7-1 and Ad.mda-7-2, while the
parental and Ad.vec groups did not show protein overexpression.
MDA-7/IL-24 induces tumor-suppressive activity
through multiple mechanisms. In order to explore its mechanisms, we
investigated the protein and mRNA expression of E-cadherin, CD44,
ICAM-1, CyclinB, Twist, survivin, p-ERK and p-Akt in tumor cells.
The results indicated that MDA-7/IL-24 overexpression decreased the
expression of CD44, ICAM-1, CyclinB, Twist, survivin and p-Akt,
while the expression of E-cadherin and p-ERK was upregulated.
E-cadherin, CD44 and ICAM-1 are known as significant
adhesion molecules, and are necessary for cell adhesion, migration,
proliferation, apoptosis and cell signal transmission (22). Overexpression of MDA-7/IL-24 induced
the downregulation of CD44/ICAM-1 and the upregulation of
E-cadherin, indicating that one mechanism involved in the
suppression of tumor cell adhesion and invasion by MDA-7/IL-24 is
through the regulation of the expression of adhesion molecules.
CyclinB is a key protein in cell cycle regulation by
regulating cytoskeletal dynamics. Recent research suggests that
CyclinB may regulate tumor growth, invasion and metastasis, and
upregulation of CyclinB expression increases tumor metastasis
potential. MDA-7/IL-24 induced the accumulation of HepG2 and
BEL-7402 cells in the G2/M phase of the cell cycle. Twist is a
transcription factor which relies on the basic DNA-binding region
and the helix-loop-helix structure that allows monomers to form
functional dimers that can identify and bind to the E-box DNA
motif. Twist has been considered to inhibit E-cadherin expression,
promote tumor cell adhesion and invasion. Survivin promotes cell
adhesion and invasion by cell cycle-related kinases and regulation
of the cell division process. Our results suggest that MDA-7/IL-24
suppresses tumor adhesion and invasion by controlling the
expression of E-cadherin, CD44, ICAM-1, CyclinB, Twist and survivin
and that together they are important to human HCC metastasis.
The activity of proteolysis enzymes is attributed to
degradation of the tumor cell extracellular matrix (ECM). Among the
proteases implicated in tumor cell dissemination are the MMPs
(23), which are required by cells
for tissue remodeling. However, the production of MMPs has been
observed in many invasive tumor cell lines and during tumor growth
(24). In fact, MMP-2/−9 levels
appear to be prognostically significant during tumor progression in
many tumor types (11,25–27),
and the extent of MMP overproduction correlates with prognosis.
Therefore, we investigated and found that MDA-7/IL-24
overexpression decreased the expression of MMP-2/−9 at the protein
and mRNA levels. Additionally, MDA-7/IL-24 influenced TGF-β
activity in tumor cells. ELISA assay was used to investigate the
secretion of TGF-β in HCC cancer cells, and we found that
MDA-7/IL-24 induced the downregulation of TGF-β.
NF-κB is a transcription factor which upregulates
many types of metastasis-related genes, and research has
demonstrated that NF-κB regulates tumor metastasis potential. AP-1
is composed of the c-Jun and c-fos and also controls numerous genes
contributing to the process of tumor metastasis. We hypothesized
the regulatory role of MDA-7/IL-24 in the control of the NF-κB and
AP-1 transcription activation and demonstrated that MDA-7/IL-24
suppresses tumor metastasis potential by regulating their
transcription activation.
Additionally, we explored the contribution of
MDA-7/IL-24 on tumor metastasis signaling molecules. Numerous
reports have shown that Akt and ERK are involved in the processes
of adhesion and invasion in many types of tumors, and also control
expression of transcription factors. In the present study, we
examined the effect of MDA-7/IL-24 on the Akt and ERK pathway and
demonstrated that MDA-7/IL-24 regulates Akt and ERK phosphorylation
(28,29). Therefore, we presumed that the
modulation of the Akt and ERK signaling pathway contributing to the
AP-1 and NF-κB transcriptional activation regulation is the basis
for the suppression of tumor metastasis by MDA-7/IL-24.
In conclusion, we demonstrated for the first time
that MDA-7/IL-24 inhibits the metastatic potential of human HepG2
and BEL-7402 cells in vitro. Thus, MDA-7/IL-24 may provide
an effective therapeutic strategy for HCC and may reduce tumor
metastasis.
References
|
1
|
Ferlay J, Shin HR, Bray F, et al:
Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int
J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar
|
|
3
|
Tung-Ping Poon R, Fan ST and Wong J: Risk
factors, prevention, and management of postoperative recurrence
after resection of hepatocellular carcinoma. Ann Surg. 232:10–24.
2000.PubMed/NCBI
|
|
4
|
Fickenscher H, Hör S, Küpers H, et al: The
interleukin-10 family of cytokines. Trends Immunol. 23:89–96. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiang H, Su ZZ, Lin JJ, et al: The
melanoma differentiation associated gene mda-7 suppresses cancer
cell growth. Proc Natl Acad Sci USA. 93:9160–9165. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang H, Lin JJ, Su ZZ, et al: Subtraction
hybridization identifies a novel melanoma differentiation
associated gene, mda-7, modulated during human melanoma
differentiation, growth and progression. Oncogene. 11:2477–2486.
1995.
|
|
7
|
Gopalkrishnan RV, Sauane M and Fisher PB:
Cytokine and tumor cell apoptosis inducing activity of mda-7/IL-24.
Int Immunopharmacol. 4:635–647. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lebedeva IV, Sauane M, Gopalkrishnan RV,
et al: mda-7/IL-24: exploiting cancer's Achilles' heel. Mol Ther.
11:4–18. 2005. View Article : Google Scholar
|
|
9
|
Allen M, Pratscher B, Roka F, et al: Loss
of novel mda-7 splice variant (mda-7s) expression is associated
with metastatic melanoma. J Invest Dermatol. 123:583–588. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sarkar D, Su ZZ, Lebedeva IV, et al: mda-7
(IL-24) mediates selective apoptosis in human melanoma cells by
inducing the coordinated overexpression of the GADD family of genes
by means of p38 MAPK. Proc Natl Acad Sci USA. 99:10054–10059. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ramesh R, Ito I, Gopalan B, Saito Y, et
al: Ectopic production of MDA-7/IL-24 inhibits invasion and
migration of human lung cancer cells. Mol Ther. 9:510–518. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McKenzie T, Liu Y, Fanale M, et al:
Combination therapy of Ad-mda7 and trastuzumab increases cell death
in Her-2/neu-overexpressing breast cancer cells. Surgery.
136:437–442. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lebedeva IV, Su ZZ, Sarkar D, et al:
Induction of reactive oxygen species renders mutant and wild-type
K-ras pancreatic carcinoma cells susceptible to Ad. mda-7-induced
apoptosis. Oncogene. 24:585–596. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Su ZZ, Lebedeva IV, Sarkar D, et al:
Melanoma differentiation associated gene-7, mda-7/IL-24,
selectively induces growth suppression, apoptosis and
radiosensitization in malignant gliomas in a p53-independent
manner. Oncogene. 22:1164–1180. 2003. View Article : Google Scholar
|
|
15
|
Yacoub A, Mitchell C, Hong Y, et al: MDA-7
regulates cell growth and radiosensitivity in vitro of primary
(non-established) human glioma cells. Cancer Biol Ther. 3:739–751.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lebedeva IV, Su ZZ, Sarkar D, et al:
Melanoma differentiation associated gene-7, mda-7/interleukin-24,
induces apoptosis in prostate cancer cells by promoting
mitochondrial dysfunction and inducing reactive oxygen species.
Cancer Res. 63:8138–8144. 2003.
|
|
17
|
Su Z, Lebedeva IV, Gopalkrishnan RV, et
al: A combinatorial approach for selectively inducing programmed
cell death in human pancreatic cancer cells. Proc Natl Acad Sci
USA. 98:10332–10337. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lebedeva IV, Su ZZ, Chang Y, et al: The
cancer growth suppressing gene mda-7 induces apoptosis selectively
in human melanoma cells. Oncogene. 21:708–718. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mhashilkar AM, Schrock RD, Hindi M, et al:
Melanoma differentiation associated gene-7 (mda-7): a novel
anti-tumor gene for cancer gene therapy. Mol Med. 7:271–282.
2001.PubMed/NCBI
|
|
20
|
Tian H, Wang J, Zhang B, et al:
MDA-7/IL-24 induces Bcl-2 denitrosylation and ubiquitin-degradation
involved in cancer cell apoptosis. PLoS One. 7:e372002012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kawabe S, Nishikawa T, Munshi A, et al:
Adenovirus-mediated mda-7 gene expression radiosensitizes non-small
cell lung cancer cells via TP53-independent mechanisms. Mol Ther.
6:637–644. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fan H, Suzuki T, Ogata M, et al:
Expression of PCNA, ICAM-1, and vimentin in lens epithelial cells
of cataract patients with and without type 2 diabetes. Tokai J Exp
Clin Med. 37:51–56. 2012.PubMed/NCBI
|
|
23
|
Matrisian LM: The matrix-degrading
metalloproteinases. Bioessays. 14:455–463. 1992. View Article : Google Scholar
|
|
24
|
Shapiro SD: Matrix metalloproteinase
degradation of extracellular matrix: biological consequences. Curr
Opin Cell Biol. 10:602–608. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakopoulou L, Tsirmpa I, Alexandrou P, et
al: MMP-2 protein in invasive breast cancer and the impact of
MMP-2/TIMP-2 phenotype on overall survival. Breast Cancer Res
Treat. 77:145–155. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao XL, Sun T, Che N, et al: Promotion of
hepatocellular carcinoma metastasis through matrix
metalloproteinase activation by epithelial-mesenchymal transition
regulator Twist1. J Cell Mol Med. 15:691–700. 2011. View Article : Google Scholar
|
|
27
|
Sun T, Zhao N, Zhao XL, et al: Expression
and functional significance of Twist1 in hepatocellular carcinoma:
its role in vasculogenic mimicry. Hepatology. 51:545–556. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kang MH, Oh SC, Lee HJ, et al: Metastatic
function of BMP-2 in gastric cancer cells: The role of PI3K/AKT,
MAPK, the NF-κB pathway, and MMP-9 expression. Exp Cell Res.
317:1746–1762. 2011.PubMed/NCBI
|
|
29
|
Lu JT, Zhao WD, He W and Wei W: Hedgehog
signaling pathway mediates invasion and metastasis of
hepatocellular carcinoma via ERK pathway. Acta Pharmacol Sin.
33:691–700. 2012. View Article : Google Scholar : PubMed/NCBI
|