Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common malignant tumor in the world (1). Clinically, the majority of HCC cases
are incompetently surgically resected and remain insensitive to
radiation and chemotherapy treatments (2,3). The
overall 5-year survival rate for patients with HCC worldwide is
approximately 10% (4). Thus, the
development of novel strategies against HCC is required.
Epidemiological studies have confirmed that the
chronic infection with hepatitis B virus (HBV) is strongly
associated with the development of HCC. HBV, belonging to the
hepadnavirus family, has a relaxed partially double-strand circular
DNA genome. This virus encodes four open reading frames with a
17-kDa non-structural multifunctional regulatory protein termed the
hepatitis B virus X protein (HBx) (5). Numerous studies have suggested that
HBx plays critical roles in regulating cell progression such as
cell cycle, proliferation, or apoptosis, which mostly depend on
impacting cellular signaling transduction. Meanwhile, HBx sequence
has been mapped with multi-epitopes which can elicit robust
specific cytolytic T lymphocyte (CTL) responses (6–8).
Studies has demonstrated that HBx expression in
hepatoma tissues is positive in 80% of HCC cases and the long-time
expression of HBx is considered to play a significant role in
cellular malignant transformation which contributes to the
development of HCC (2,3,9,10).
Thus, it had been applied as a target for immunotherapy when
HBx-specific CTL responses could be induced. It is well documented
that CTL epitope peptides from HBx could induce HBx-specific
CD8+ T cells and eradicate tumor in xenografted nude
mice (11). Ding et
al(8) also reported that
multi-epitope peptide-loaded virus-like particles as a vaccine
could elicit an immune response to protect against HBV-related HCC.
In addition, Wang et al(12)
reported that 2 oral HBx vaccines delivered by live attenuated
Salmonella could induce significantly specific antitumor
immunity. In our previous study, we demonstrated that HBx specific
immunity against HCC was elicited by adenovirus vaccine encoding
HBx (2).
Whole tumor cells are a source of tumor-associated
antigens (TAA) for vaccination purposes. Vaccination with
irradiated tumor cells has been studied in several animal models.
However, due to the weak immunogenicity of tumor antigen,
downregulated expression of MHC molecule and lacking costimulatory
molecules on tumor cells, the original tumor cell vaccine is often
unable to induce a strong immune response (9,13–15).
Thus, the development of novel methods to enhance antigen
presentation is urgently required. The genetic engineering of tumor
cells to express cytokines, co-stimulatory molecules and tumor
antigen has been used to improve the immunogenicity of tumor cell
vaccines. Moreover, a plurality of genetically modified tumor cell
vaccines has shown some promise in clinical trials (16–18).
In the present study, we developed an
adenoviral-mediated genetic engineering of hepatoma cells vaccine
to express HBx and to evaluate if the vaccine could elicit specific
immune responses against HCC. Our results demonstrated that the
irradiated HBx-modified hepatoma cell vaccines could elicit
significant specific antitumor immunity.
Materials and methods
Cell culture
The human embryonic kidney cell line 293A and the
murine HCC cell line Hepa1-6 were obtained from the American Type
Culture Collection (ATCC, Manassas, VA, USA). All cell lines were
maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing
10% fetal bovine serum (FBS) (both from Gibco-BRL, Carlsbad, CA,
USA), 2 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml
streptomycin. Stable Hepa1-6 cell line expressing HBx (Hepa1-6/HBx)
was constructed as previously described and cultured in complete
medium containing 200 μg/ml G418 (2). The cells mentioned above were
propagated at 37°C in humidified 5% CO2 conditions.
Construction and amplification of
recombinant adenovirus
The construction of recombinant adenovirus vectors
encoding HBx protein (AdHBx), including vectors expressing no
transgene (AdNull) was performed as previously described (19). Adenovirus vectors were amplified in
293A cells and viral titers were measured by a standard
plague-forming assay. All viruses were stored in aliquot at −80°C
until use.
Infection of Hepa1-6 cells with
adenovirus
Prior to adenovirus infection, the culture medium of
Hepa1-6 seeded on the culture plates or dishes was decanted and
washed with fresh medium without supplements. Viral dilution was
added to cells at a multiplicity of infection (MOI) of 20 in a
minimum culture volume and co-incubated with cells at 37°C for 2 h.
After a 2-h incubation, virus solution was replaced with complete
medium and cells were incubated for 24 h at 37°C. Then, the cells
were harvested for experiments in vivo or in
vitro.
Reverse transcription-polymerase chain
reaction (RT-PCR) assay
The total mRNA of Hepa1-6 cells after 24 h-infection
treatment was extracted by TRIzol Reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA) according to the protocol. The
expression of HBx was assayed using PrimeScript RT-PCR kit (Takara
Bio, Inc., Shiga, Japan) as previously described (20). Briefly, the RNA samples mentioned
above were reverse transcribed to generate cDNA using random
primers. Thereafter, the PCR was performed with primers for HBx
gene as follows: forward, 5′-ATGGCTGCTAGGCTGTGCTG-3′ and reverse,
5′-GGCAGAGGTGAAA AAGTTGC-3′ designed on the basis of Gene Bank. And
GAPDH was amplified as a control to validate accuracy and outcome
of the products of RT-PCR measured by 1% agarose gel
electrophoresis.
Vaccine preparation and immunization
The tumor cells infected with adenovirus for 24 h
were digested, washed 3 times with fresh serum-free medium,
irradiated with 50 Gy, and then adjusted to a concentration of
1×106 cells in 100 μl prior to administration. C57BL/6
female mice 6–8 weeks old were purchased from Beijing Weitong Lihua
Biological Technology Co., Ltd and maintained in pathogen-free and
isothermal conditions. All procedures were reviewed and approved by
the Animal Care and Use Committee of Sichuan University.
Hepa1-6/HBx (2.5×106) tumor cells in 100 μl PBS were
injected s.c. in the posterior flank of mice. When the tumor
diameters were ~3 mm, mice were divided into 4 groups. The
irradiated Hepa1-6 cells infected with AdHBx described above were
vaccinated s.c. at a dose of 1×106 cells each mouse in
100 μl PBS (day 0). Other groups received inoculation of PBS,
irradiated Hepa1-6, or irradiated Hepa1-6 infected with AdNull in
the same volume as the control experiments. Vaccination was boosted
on day 14 and 21. The tumor size was measured using a sliding
caliper every 3 days and the volume was calculated by the equation:
0.52 × (length × width2).
Characterization of T-lymphocyte
subsets
Three mice of each group were sacrificed a week
after the last immunization. Spleens were aseptically removed and
single-cell suspensions were isolated as previously described
(2). Isolated cells
(105) were stained with fluorochrome-coupled antibodies
to CD4 and CD8 at 4°C for 30 min. The cell suspensions were
measured on FACSCalibur and the data were analyzed by cell quest
software (both from BD Biosciences, Franklin Lakes, NJ, USA).
Cell proliferation assay
To verify the T-cell proliferative ability of
immunized mice, lymphocytes were isolated from mice 1 week after
the last vaccination and labeled by 0.5 μM CFSE for 10 min at 37°C
in 5% CO2. Then an equal volume of fetal calf serum
(FCS) was added to stop the reaction. The cells were centrifuged at
400 × g for 5 min and washed twice with complete medium.
CFSE-labeled cells were resuspended in 10% RPMI medium, adjusted to
2×105 cells/ml and stimulated by irradiated Hepa1-6/HBx
at effector:target (E:T) ratios of 100:1. Cells were incubated in
24-well plates at 37°C for 5 days. The labeled dilution was
measured on FACSCalibur and the divisive generations were assayed
via FlowJo software version 7.6.1 (Tree Star, Inc., San Carlos, CA,
USA). Proliferation index was conveyed as the percentage of cells
that had divided and the average number of cell divisions.
CFSE-based cytotoxicity assay
The cytotoxicity assay was performed as previously
described with slight modifications (22,23).
Isolated splenocytes from mice were as effector cells. After 48–72
h of stimulation with irradiated Hepa1-6/HBx, effector
cells were washed twice and resuspended to a concentration of
107/ml. The Hepa1-6/HBx cells were used as target cells,
labeled with CFSE (2.5 μM) as described above and adjusted to a
concentration of 105/ml. Effector cells and target cells
were mixed in total volume of 200 μl at E:T ratios of 100, 50, 25,
12.5, 6.25 in round-bottom polystyrene tubes, centrifuged at 400 ×
g for 45–60 sec and incubated at 37°C in 5% CO2 for 4–6
h. The target cells of each sample were 104 cells in 200
μl volume, and only target cells in the tube served as a negative
control. Following incubation, each sample was supplemented with 20
μl PI (100 μg/ml) on the ice and the acquisition was performed by
flow cytometry within 1 h. CFSE+/PI+ cells
were considered as dead target cells. Percentage of specific lysis
was then expressed as: % specific lysis = [dead targets in the
sample (%) − spontaneously dead targets (%)/100 − spontaneously
dead targets (%)]× 100.
Enzyme-linked immunospot assay
Specific IFN-γ-secreting T cells were measured using
the Mouse IFN-γ ELISPOT kit (U-CyTech Biosciences, The Netherlands)
according to directions of the manufacturer. The 96-well ELISPOT
plates were coated with antibodies at 4°C overnight before
splenocytes were harvested. Plates were washed 3 times with PBS and
blocked at 37°C for 1 h. Splenocytes (3×105) co-cultured
with irradiated Hepa1-6/HBx at 100:1 responder: stimulator ratios
were added to wells in triplicate and incubated for 48 h at 37°C.
Subsequently, cells were decanted and plates were washed 6 times
with wash buffer (PBS containing 0.05% Tween-20). Diluted
biotinylated detection antibodies were added to each well and
plates were incubated for 1 h at 37°C. Then, antibodies dilution
was removed by 6X wash using wash buffer. GABA (φ-labeled
anti-biotin antibodies) solution was added to wells. After 1 h of
incubation at 37°C, plates were washed 6 times. Thereafter 35 μl
AEC substrate solution was applied to every well and then plates
were incubated for 10–20 min in the dark at room temperature. When
distinct spots were developed, the reaction was stopped by the
addition of demineralized water. Plates were dried at room
temperature and spots were counted using a dissecting microscope.
IFN-γ-producing cells were calculated by the numbers of
spot-forming cells per 106 splenocytes.
Electron microscopy
The cellular ultrastructural analysis by electron
microscopy was performed as previously described. Hepa1-6 cells
were infected with AdHBx or AdNull and irradiated with X-rays.
Infected cells were prepared without X-rays as controls. Cells were
harvested and fixed with 3% glutaraldehyde in 0.1 M cacodylate
buffer overnight. Then, cells were treated in 1% OsO4,
dehydrated in ethanol and embedded in Epon. Fixed cells were made
into 0.1 mm sections, stained and then viewed with the electron
microscope (Hitachi, H-7650, Japan).
Statistical analyses
Statistical significance of experimental groups was
analyzed using Student’s t-test and performed on GraphPad InStat
version 3.06. The data were means ± SEM and P-values <0.05 were
considered to indicate statistically significant differences,
presented in figure captions.
Results
Characterization of tumor cell
vaccines
To prepare tumor cell vaccines engineered to express
HBx, we infected Hepa1-6 cells with recombinant adenoviral vectors
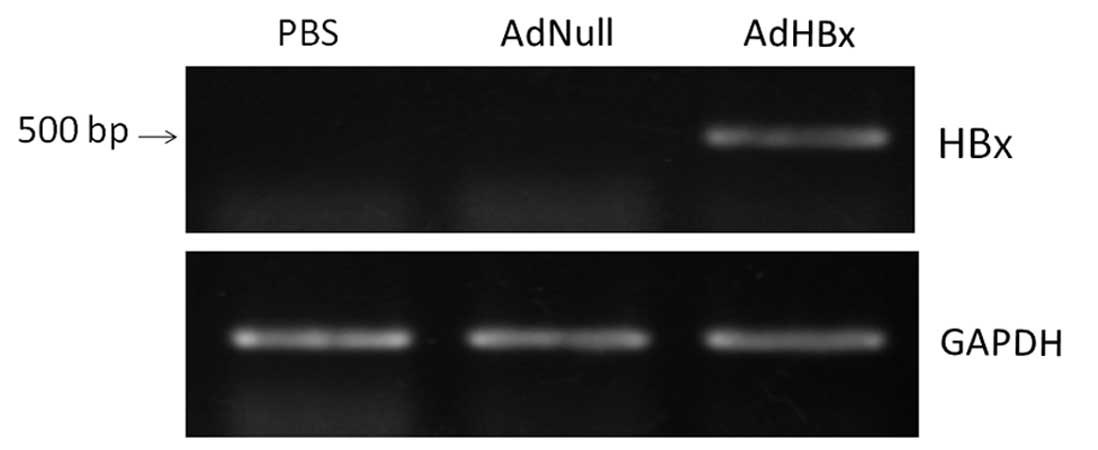
encoding HBx at an MOI of 20. We detected the expression of HBx in
tumor cell vaccines by the means of RT-PCR. As shown in Fig. 1, only RNA extracts from Hepa1-6
cells infected with Ad-HBx, but not RNA extracts from cells
infected with AdNull or uninfected, amplified a single distinct
band.
Induction of therapeutic antitumor
immunity
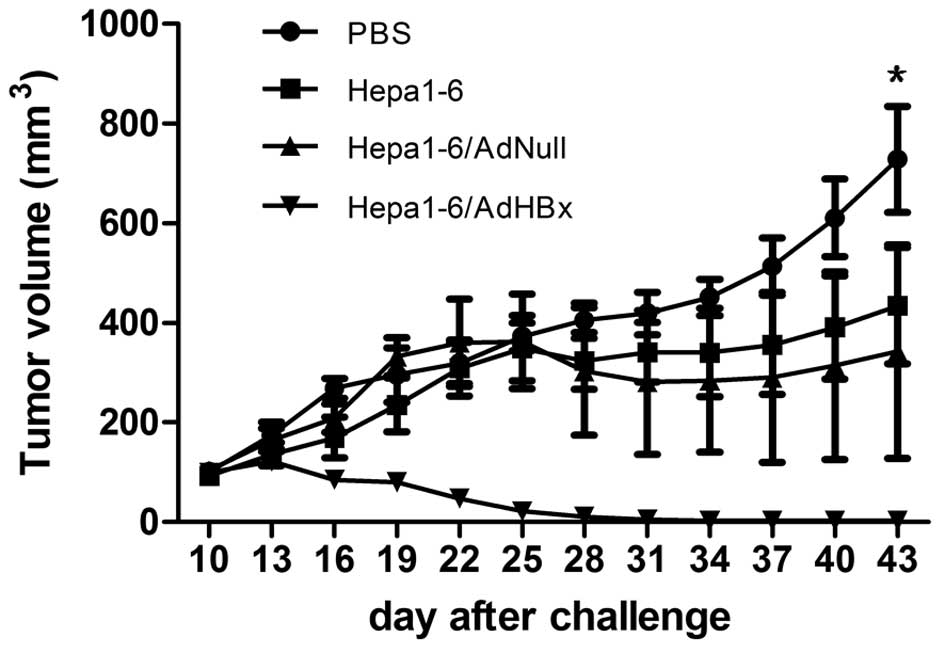
To investigate the therapeutic effect of this
cellular vaccine in vivo, mice were challenged with
2.5×106 Hepa-6/HBx cells. After 7 days, the first
vaccination was administered (day 0), and then vaccination was
boosted on day 14 and 21. We observed a significant delay in the
growth of tumors in AdHBx-infected Hapa1-6 immunized mice with a
statistically significant reduction (P<0.001) (Fig. 2). Thirty-four days after challenge,
only 1 mouse bore a tumor, which was 18.1 mm3 in size,
while the tumors of the other 5 mice were completely eradicated. By
contrast, the tumor regression was not observed in the mice
administered PBS, Hepa1-6 or Hepa1-6/AdNull. The results confirmed
that the Hepa1-6/AdHBx tumor vaccine induced significant specific
antitumor immunity to inhibit tumor growth and even eliminate
tumors.
T-cell subsets following vaccination
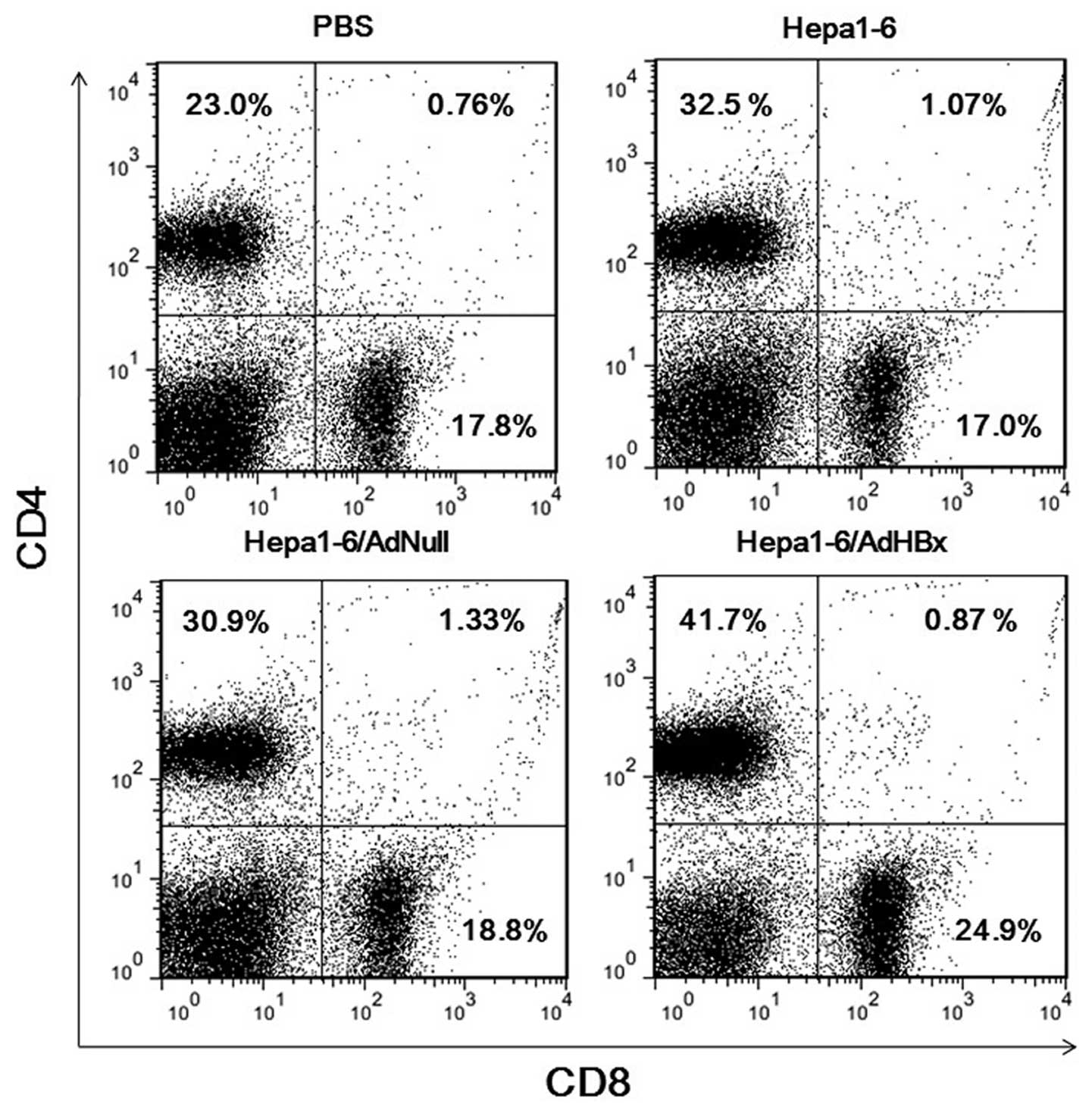
Next, we characterized which type of T cells
participated in the antitumor immunity. After 3 vaccinations,
splenocytes were harvested and flow cytometry with 2-color staining
for CD4 and CD8 was performed to characterize T-cell subsets. The
component of CD4+ and CD8+ T cells of
Hepa1-6/AdHBx treatment were 41.7 and 24.7% which were higher than
those of the PBS, Hepa1-6, and Hepa1-6/AdNull groups (Fig. 3). The results demonstrated that
CD4+ and CD8+ T cells both participated in
the therapeutic immunological responses in mice vaccinated with
Hepa1-6/AdHBx vaccines; i.e., MHC class I-and II-restricted
immunity responses are involved in the antitumor therapy.
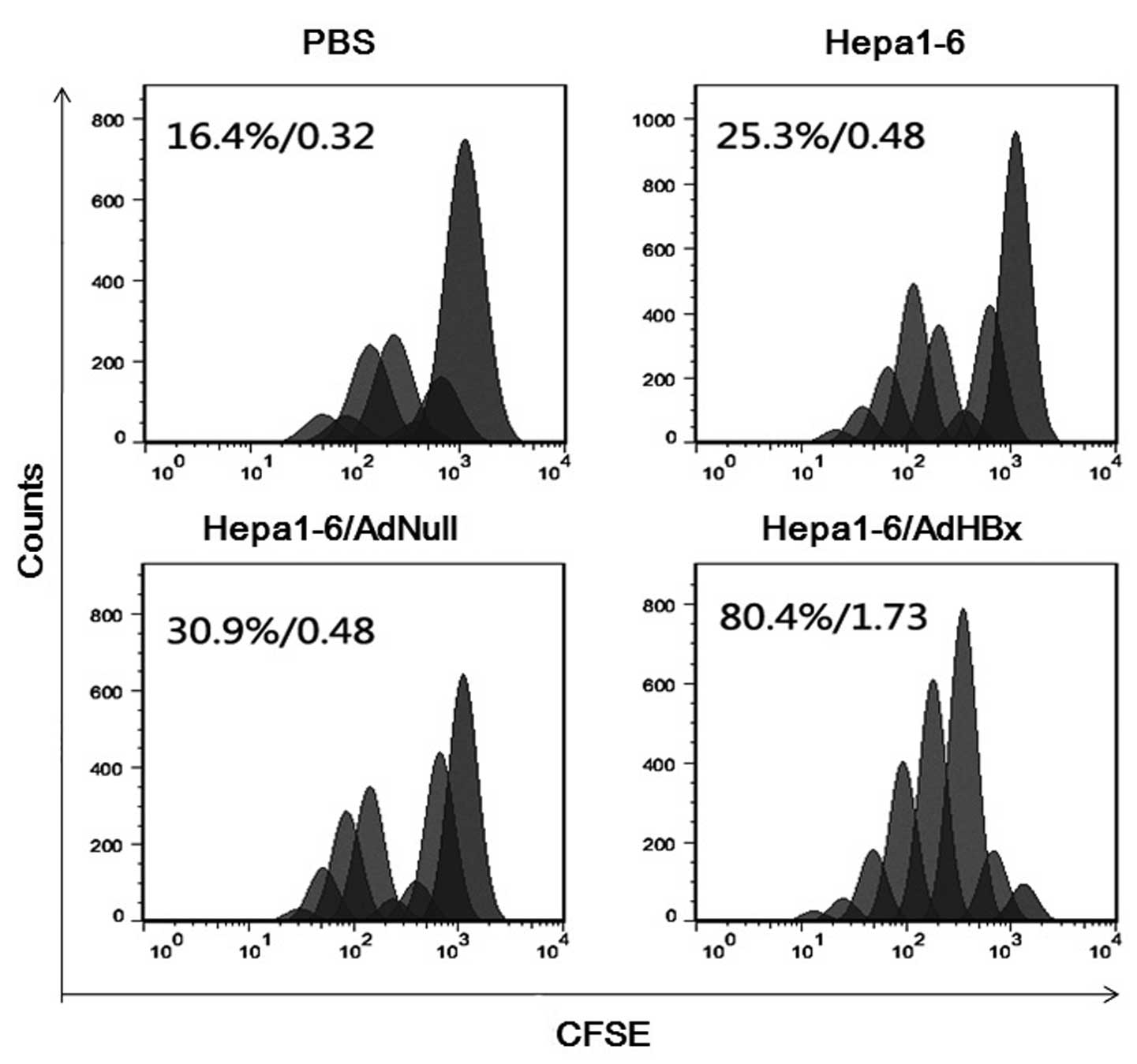
Proliferative ability of lymphocytes
The lymphocyte proliferation assay was performed to
evaluate the memory response in vaccinated mice. Spleen lymphocytes
isolated were labeled by CFSE and incubated with irradiated
Hepa1-6/HBx as a stimulator for 5 days. The assay was performed by
flow cytometry and analyzed with FlowJo. As shown in Fig. 4, the percentage of cells that
divided at least once and the average number of cell divisions were
80.4% and 1.73 respectively for lymphocytes from mice administered
AdHBx-infected Hepa1-6 vaccines, which were higher than those from
control mice. The results demonstrated that immunization with
Hepa1-6/AdHBx vaccine was effective at inducing memory immune
responses in animals.
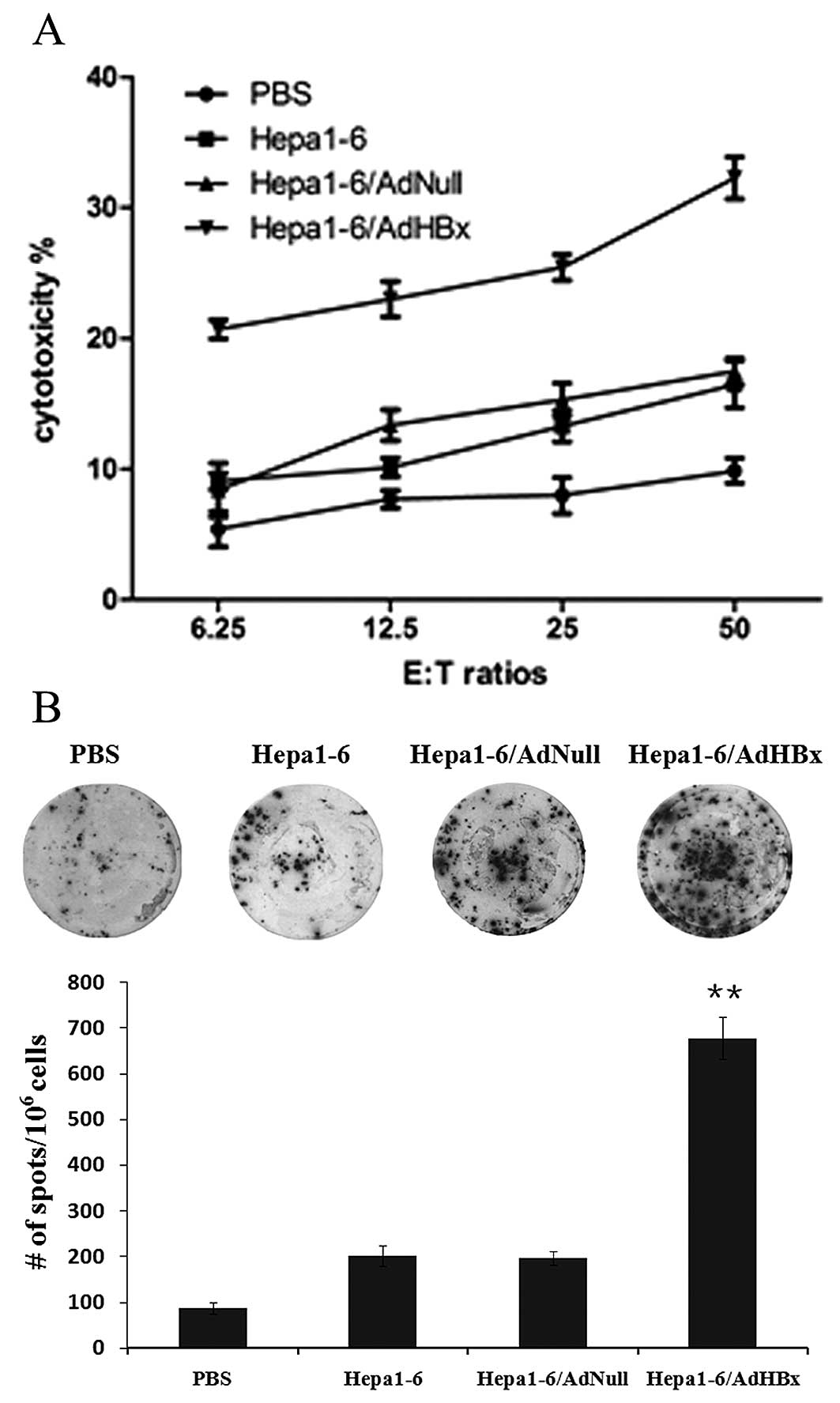
Activation of HBx-specific CTLs
To determine whether vaccination of irradiated
Hepa1-6 infected with AdHBx elicit HBx-specific CTL responses,
spleen lymphocytes were isolated and stimulated with irradiated
Hepa1-6/HBx cells for 72 h. Then, the reconstituted lymphocytes
were co-cultured with CFSE-labeled Hepa1-6 stably expressing HBx as
target cells for 4 h. As shown in Fig.
5A, the lymphocytes from mice administered AdHBx-infected
Hepa-6 vaccines were able to lyse specific Hepa1-6 cells stably
expressing HBx (target cells) in an E:T ratio-dependent manner with
statistical significance at E:T ratios ≥6.25 as compared with
controls (P<0.01). These findings suggested that irradiated HBx
gene modified tumor cells could induce a specific CTL response to
recognize and lyse HBx-positive Hepa1-6 cells.
Production of IFN-γ by lymphocytes
The frequency of IFN-γ secreting cells in
splenocytes reflected the number of CTL and cellular immune
response. To further evaluate HBx-specific CTL response, the
IFN-γ-producing T cells relative to the number spleen cells were
detected using IFN-γ ELISPOT assay. In these studies, mice were
immunized with Hepa1-6 infected with AdHBx, AdNull or uninfected
and PBS respectively. Splenocytes were harvested and stimulated
with irradiated Hepa1-6/HBx cells and the spot-forming cells were
measured after 48 h. As shown in Fig.
5B, the number of spot-forming cells produced by 106
splenocytes from the mice treated with Hepa1-6/AdHBx were ~8-fold
compared with PBS treatment, and 4-fold compared with groups
vaccinated with Hepa1-6 alone or Hapa1-6/AdNull. The results
further indicated that vaccination with Hepa1-6/AdHBx could elicit
specific CTL response.
HBx sensitizes cells to
irradiation-induced autophagy
To explore the impact of HBx and X-ray irradiation
on Hepa1-6 cells, we observed the cellular ultrastructure of tumor
cells using transmission electron microscopy. Hepa1-6 cells were
infected with AdHBx or AdNull and irradiated by X-rays. Cells were
treated and analyzed by electron microscopy. The results indicated
that autophagy was induced in the AdHBx-infected cells treated with
X-ray irradiation. Numerous autophagosomes or autolysosomes are
presented in Fig. 6D-F. However, no
or few autophagic vacuoles were observed in the other groups
(Fig. 6A-C).
Discussion
HCC is one of the most aggressive tumors worldwide.
In China, the number of people diagnosed with advanced HCC
increases annually. However, at present, HCC is often incompetently
surgically resected and insensitive to radiation and chemotherapy
treatments and the postsurgical recurrence and metastasis present
further challenges to the treatments of HCC. A new efficacious
therapy is required. In recent years, immunotherapy has emerged as
a promising mode of treatment for cancer. HBx is closely associated
with hepatic carcinogenesis (6,23–26).
HBx expression is present in ~80% of the HCC cases including both
carcinomas and pericancerous liver tissues. The multiple epitopes
of HBx can elicit robust specific CTL responses and induce regress
HBx-positive tumor (6–8).
In our previous study, we developed an adenoviral
vaccine against HBx oncoproteins to prevent growth of
HBV-associated HCC (2).
Replication-deficient adenoviral vectors are widely utilized for
transient transgene delivery due to their high transfection
efficiency and low toxicity (27,28);
they have transitioned from tools for gene replacement therapy to
vaccine delivery vehicles and are attractive vaccine vectors as
they can induce both innate and adaptive immune responses in
mammalian hosts (29). However, one
major obstacle to the use of adenoviral vectors is the high
prevalence of virus-neutralizing antibodies (VNAs) in humans.
Pre-existing VNAs to the vaccine carrier prevent the vector from
transducing target cells, which reduces the amount of vaccine
antigen production and attenuates the adaptive immune responses
(30).
In order to overcome the shortcoming of adenoviral
vaccines, in the present study we infected hepatoma cells with
adenovirus expressing HBx and developed irradiated tumor cells
engineered to express HBx as a therapeutic vaccine against HCC. The
irradiated tumor cells engineered to express HBx could
significantly induce antitumor immune responses in vivo.
While genetically unmodified tumor cells were also effective to a
certain degree, the antitumor immunity was too weak to inhibit
tumor growth significantly. The unmodified tumor cell vaccine could
not induce strong and specific antitumor immunity, this was
contributed to the poor immunogenicity of the tumor antigen,
downregulated expression of MHC molecule and deficient
co-stimulatory molecules on tumor cells (9, 13–15).
In this study, tumor cells genetically engineered to express HBx
may elicit significantly HBx-specific antitumor immunity besides
faintly whole-cell antigen-mediated antitumor immune responses. The
novel immunotherapy strategy may represent an advancement in the
development of treatments for HCC.
It has been well demonstrated that CD8+ T
cells participate in antitumor immune response induced by various
HBx-based vaccines (7,8). Antigen-specific CD8+ CTLs
play an important role in antitumor immune responses (31). In the present study, we also found
that the frequency of CD8+ T cells and the expression of
T cytotoxic (Tc) 1-type cytokine (IFN-γ) markedly increased in mice
immunized with irradiated HBx modified tumor cell vaccine compared
with the other groups. Moreover, the vaccine could induce a
specific CTL response to recognize and lyse HBx-positive hepatoma
cells. However, aside from the frequency of CD8+ T
cells, that of CD4+ T cells more markedly increased in
mice immunized with irradiated HBx modified tumor cell vaccine in
our present study. Accumulating evidence indicates CD4+
T cells play important roles in inducing MHC class I-restricted
tumor-specific immunity and in the delivery of help for priming of
tumor-specific CTLs (32). Our
study demonstrated that both CD8+ and CD4+ T
lymphocytes participated in antitumor immune responses induced by
the irradiated HBx-modified tumor cell vaccine.
A previous study found that HBx sensitized cells to
starvation-induced autophagy via upregulation of beclin 1
expression (33). Another separate
report indicated HBV could also induce autophagy in cell cultures,
mouse liver and an infected patient via HBx (34). In this study, a considerable number
of autophagosomes or autolysosomes was observed in irradiated
HBx-modified tumor cells compared with control groups. Autophagy
plays a physiological process that is important for maintaining
intracellular homeostasis by promoting the transit of cytoplasmic
materials, such as proteins, organelles and pathogens, for
degradation within acidic organelles (35). In addition to maintaining cellular
homeostasis, increasing studies have also revealed that autophagy
plays an important role in innate and adaptive immunity. Autophagy
participated in enhancing extracellular antigens for MHC class II
presentation, regulating intracellular antigen processing for MHC
class I presentation, and packaging antigens for optimal
cross-presentation (36). Thus,
autophagy may contribute to enhancing CD8+ and
CD4+ T lymphocyte-mediated antitumor immune responses
which were induced by the irradiated HBx-modified tumor cell
vaccine. The mechanism by which autophagy contributes to enhancing
antitumor immune responses induced by the novel vaccine requires
further study.
In conclusion, our results demonstrated that the
irradiated HBx-modified tumor cell vaccine is a potent and
promising therapeutic agent against HBx-positive HCC by inducing
autophagy-enhanced CD8+ and CD4+ T
lymphocyte-mediated antitumor immune responses. The present
findings have implications for the development of clinical
immunotherapy against HBV-associated HCC.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81101728), the National
Science and Technology Major Projects of New Drugs (grant no.
2012ZX09103301-036), the National Science and Technology Major
Project for Infectious Diseases Control (grant no.
2012ZX10002014-004) and the Research Fund for the Doctoral Program
of Higher Education of China (grant no. 20110181120086).
References
|
1
|
Du Y, Kong G, You X, et al: Elevation of
highly up-regulated in liver cancer (HULC) by hepatitis B virus X
protein promotes hepatoma cell proliferation via down-regulating
p18. J Biol Chem. 287:26302–26311. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li Y, Cheng P, Wen Y, et al: T lymphocyte
responses against hepatitis B virus-related hepatocellular
carcinoma induced by adenovirus vaccine encoding HBx. Int J Mol
Med. 26:869–876. 2010.PubMed/NCBI
|
|
3
|
Dan Q, Sanchez R, Delgado C, et al:
Non-immunogenic murine hepatocellular carcinoma Hepa1-6 cells
expressing the membrane form of macrophage colony stimulating
factor are rejected in vivo and lead to CD8+ T-cell immunity
against the parental tumor. Mol Ther. 4:427–437. 2001.PubMed/NCBI
|
|
4
|
Altekruse SF, McGlynn KA and Reichman ME:
Hepatocellular carcinoma incidence, mortality, and survival trends
in the United States from 1975 to 2005. J Clin Oncol. 27:1485–1491.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rehermann B and Nascimbeni M: Immunology
of hepatitis B virus and hepatitis C virus infection. Nat Rev
Immunol. 5:215–229. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hsieh JL, Wu CL, Lee CH and Shiau AL:
Hepatitis B virus X protein sensitizes hepatocellular carcinoma
cells to cytolysis induced by E1B-deleted adenovirus through the
disruption of p53 function. Clin Cancer Res. 9:338–345. 2003.
|
|
7
|
Malmassari S, Lone YC, Zhang M, Transy C
and Michel ML: In vivo hierarchy of immunodominant and subdominant
HLA-A*0201-restricted T-cell epitopes of HBx antigen of hepatitis B
virus. Microbes Infect. 7:626–634. 2005.
|
|
8
|
Ding FX, Wang F, Lu YM, et al:
Multiepitope peptide-loaded virus-like particles as a vaccine
against hepatitis B virus-related hepatocellular carcinoma.
Hepatology. 49:1492–1502. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rosenthal FM, Zier KS and Gansbacher B:
Human tumor vaccines and genetic engineering of tumors with
cytokine and histocompatibility genes to enhance immunogenicity.
Curr Opin Oncol. 6:611–615. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yannelli JR and Wroblewski JM: On the road
to a tumor cell vaccine: 20 years of cellular immunotherapy.
Vaccine. 23:97–113. 2004.PubMed/NCBI
|
|
11
|
Chun E, Lee J, Cheong HS and Lee KY: Tumor
eradication by hepatitis B virus X antigen-specific CD8+
T cells in xenografted nude mice. J Immunol. 170:1183–1190. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang YJ, Hou Y, Huang H, Liu GR, White AP
and Liu SL: Two oral HBx vaccines delivered by live attenuated
Salmonella: both eliciting effective anti-tumor immunity.
Cancer Lett. 263:67–76. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bailly M, Bertrand S and Doré JF:
Increased spontaneous mutation rates and prevalence of karyotype
abnormalities in highly metastatic human melanoma cell lines.
Melanoma Res. 3:51–61. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hicklin DJ, Marincola FM and Ferrone S:
HLA class I antigen downregulation in human cancers: T-cell
immunotherapy revives an old story. Mol Med Today. 5:178–186. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li MO, Wan YY, Sanjabi S, Robertson AK and
Flavell RA: Transforming growth factor-β regulation of immune
responses. Annu Rev Immunol. 24:99–146. 2006.
|
|
16
|
Nelson WG, Simons JW, Mikhak B, et al:
Cancer cells engineered to secrete granulocyte-macrophage
colony-stimulating factor using ex vivo gene transfer as vaccines
for the treatment of genitourinary malignancies. Cancer Chemother
Pharmacol. 46(Suppl): S67–S72. 2000. View Article : Google Scholar
|
|
17
|
Salgia R, Lynch T, Skarin A, et al:
Vaccination with irradiated autologous tumor cells engineered to
secrete granulocyte-macrophage colony-stimulating factor augments
antitumor immunity in some patients with metastatic non-small-cell
lung carcinoma. J Clin Oncol. 21:624–630. 2003. View Article : Google Scholar
|
|
18
|
Lutz E, Yeo CJ, Lillemoe KD, et al: A
lethally irradiated allogeneic granulocyte-macrophage colony
stimulating factor-secreting tumor vaccine for pancreatic
adenocarcinoma. A Phase II trial of safety, efficacy, and immune
activation. Ann Surg. 253:328–335. 2011. View Article : Google Scholar
|
|
19
|
Cheng P, Li Y, Yang L, et al: Hepatitis B
virus X protein (HBx) induces G2/M arrest and apoptosis through
sustained activation of cyclin B1-CDK1 kinase. Oncol Rep.
22:1101–1107. 2009.PubMed/NCBI
|
|
20
|
Kong G, Zhang J, Zhang S, Shan C, Ye L and
Zhang X: Hepatitis B virus X protein promotes hepatoma cell
proliferation via upregulation of MEKK2. Acta Pharmacol Sin.
32:1173–1180. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Godoy-Ramirez K, Mäkitalo B, Thorstensson
R, Sandström E, Biberfeld G and Gaines H: A novel assay for
assessment of HIV-specific cytotoxicity by multiparameter flow
cytometry. Cytometry A. 68:71–80. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hao S, Li P, Zhao J, Hu Y and Hou Y:
17β-estradiol suppresses cytotoxicity and proliferative capacity of
murine splenic NK1. 1+ cells. Cell Mol Immunol.
5:357–364. 2008.
|
|
23
|
Lee YI, Kang-Park S, Do SI and Lee YI: The
hepatitis B virus-X protein activates a phosphatidylinositol
3-kinase-dependent survival signaling cascade. J Biol Chem.
276:16969–16977. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shan C, Xu F, Zhang S, et al: Hepatitis B
virus X protein promotes liver cell proliferation via a positive
cascade loop involving arachidonic acid metabolism and p-ERK1/2.
Cell Res. 20:563–575. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ou DP, Tao YM, Tang FQ and Yang LY: The
hepatitis B virus X protein promotes hepatocellular carcinoma
metastasis by upregulation of matrix metalloproteinases. Int J
Cancer. 120:1208–1214. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gearhart TL and Bouchard MJ: The hepatitis
B virus X protein modulates hepatocyte proliferation pathways to
stimulate viral replication. J Virol. 84:2675–2686. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Neering SJ, Hardy SF, Minamoto D, Spratt
SK and Jordan CT: Transduction of primitive human hematopoietic
cells with recombinant adenovirus vectors. Blood. 88:1147–1155.
1996.PubMed/NCBI
|
|
28
|
Nilsson M, Ljungberg J, Richter J, et al:
Development of an adenoviral vector system with adenovirus serotype
35 tropism; efficient transient gene transfer into primary
malignant hematopoietic cells. J Gene Med. 6:631–641. 2004.
View Article : Google Scholar
|
|
29
|
Tatsis N and Ertl HC: Adenoviruses as
vaccine vectors. Mol Ther. 10:616–629. 2004. View Article : Google Scholar
|
|
30
|
Pichla-Gollon SL, Lin SW, Hensley SE, et
al: Effect of preexisting immunity on an adenovirus vaccine vector:
in vitro neutralization assays fail to predict inhibition by
antiviral antibody in vivo. J Virol. 83:5567–5573. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vatakis DN, Koya RC, Nixon CC, et al:
Antitumor activity from antigen-specific CD8 T cells generated in
vivo from genetically engineered human hematopoietic stem cells.
Proc Natl Acad Sci USA. 108:E1408–E1416. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Martorelli D, Muraro E, Merlo A, Turrini
R, Rosato A and Dolcetti R: Role of CD4+ cytotoxic T
lymphocytes in the control of viral diseases and cancer. Int Rev
Immunol. 29:371–402. 2010.
|
|
33
|
Tang H, Da L, Mao Y, et al: Hepatitis B
virus X protein sensitizes cells to starvation-induced autophagy
via up-regulation of beclin 1 expression. Hepatology. 49:60–71.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sir D, Tian Y, Chen WL, Ann DK, Yen TS and
Ou JH: The early autophagic pathway is activated by hepatitis B
virus and required for viral DNA replication. Proc Natl Acad Sci
USA. 107:4383–4388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Crotzer VL and Blum JS: Autophagy and
adaptive immunity. Immunology. 131:9–17. 2010.
|
|
36
|
Münz C: Antigen processing via autophagy -
not only for MHC class II presentation anymore? Curr Opin Immunol.
22:89–93. 2010.
|