Introduction
Colorectal cancer (CRC) is the third most common
malignancy in males and the second most common in females
worldwide, with more than 1.2 million newly diagnosed cases and
608,700 deaths in 2008 (1). In
China, the incidence of CRC has been increasing annually in both
urban and rural areas since 1998, and it is estimated to rise
continuously in the next few years (2). In contrast, the incidence of CRC has
been reported to be stabilized in developed countries. Moreover,
the prognosis of CRC patients has improved over time (3,4), which
is at least partly attributed to the application of advanced
treatment modalities, such as individualized treatment. The key to
successful individualized treatment lies in the development of
reliable biomarkers, which can both guide treatment and help to
predict prognosis. However, most of the biomarkers evaluated in
previous studies lack the appropriate qualities. Therefore, efforts
in identifying suitable biomarkers are still needed.
The tumor immune microenvironment, a hallmark of the
tumor, plays an important role in the development as well as the
progression of CRC (5). Except for
local infiltrating immune cells, chemokines, such as CXCL5
(6) and CAECAM1 (7), have an effect on the growth and
metastasis of CRC. CCL21, known as 6Ckine/SLC, is a CC-type
chemokine with specificity to CCR7 receptors via a uniquely long
C-terminal tail containing 32 amino acids (8). Constitutive expression of CCL21
regulates cell recruitment in homeostasis, particularly in the
homing of dendritic cells (DCs) as well as subpopulations of T
cells (9). Previous studies have
shown that CCL21 plays different roles in different tumors, namely
presenting a metastasis-promoting effect on melanoma (10), non-small cell lung cancer (11) and gastric cancer (12), while functioning as a protecting
factor in prostate cancer (13).
However, the impact of CCL21 expression on the prognosis of CRC
patients has not been well defined. Thus, the aim of the present
study was to evaluate the prognostic value of CCL21 in a large
cohort of stage III/IV CRC patients.
In the present study, a cohort of stage III/IV CRC
patients with a long-term follow-up was enrolled. CCL21 density was
detected using immunohistochemical analysis (IHC) and the TMAJ
program. The cohort was divided into two groups based on the
optimal cut-point, which was generated by X-tile program. The
clinical prognostic significance of CCL21 in stage III/IV CRC
patients was subsequently analyzed.
Materials and methods
Patients and cohorts
To detect the expression dynamics of CCL21 in stage
III/IV CRC patients, 12 paired freshly frozen specimens from
primary CRC tissues and normal colorectal tissues were obtained
from the Tissue Bank of the Sixth Affiliated Hospital of Sun
Yat-Sen University (Guangzhou, China). All the samples were
confirmed histologically.
To detect the association between the expression of
CCL21 and the prognosis of patients, 143 CRC patients with TNM
stage III/IV disease, who underwent initial resection at the First
Affiliated Hospital of Sun Yat-Sen University (Guangzhou, China)
from 2000 to 2005, were enrolled for tissue microarray
construction. Patients who received preoperative chemotherapy
and/or radiotherapy were excluded from the study.
Clinicopathological variables collected from the database included
age, gender, body mass index (BMI), history of smoking and alcohol
consumption, family history of cancer, preoperative bowel
obstruction, preoperative serum CEA and CA199 levels, tumor
location, tumor diameter, histopathology, degree of
differentiation, depth of tumor invasion, nodal status and American
Joint Committee on Cancer (AJCC) stage (7th edition). This study
was approved by the Institutional Review Board (IRB) of Sun Yat-Sen
University (Guangzhou, China).
Patient follow-up was carried out according to an
original plan by two specialists. All CRC patients were evaluated
at the hospital, by telephone, or mail correspondence, quarterly
for the first year, semi-annually for the second year, and annually
thereafter. The primary endpoint was overall survival (OS), which
was defined as the time interval from surgery to death. The
secondary endpoint was disease-free survival (DFS) time, defined as
the time interval from surgery to the first event of either
recurrent disease or death.
Western blot analysis
Tissue protein extractions were prepared using T-PER
tissue protein extraction reagent (Pierce, Rockford, IL, USA) and a
protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA).
For each sample, 30 μg of protein was loaded and separated by 10%
SDS-polyacrylamide gel and then transferred to NC membranes
(Millipore Corp., Billerica, MA, USA). After blocking with 5%
non-fat dry milk in TBST, membranes were probed overnight at 4°C
with the CCL21 antibody (1:1,000; Abcam, Cambridge, UK) and the
β-actin antibody (Santa Cruz Europe). The membranes were then
washed with TBST 3 times and incubated with the secondary
antibodies at room temperature for 1 h (goat anti-rabbit IgG-HRP;
Santa Cruz Biotechnology). Fluorescence on the membrane was
generated using the ECL Plus kit after the membranes were washed 3
times with TBST. Protein densitometry was performed using Gel-Pro
Analyzer 4.0 software (Media Cybernetics, Inc., Silver Spring, MD,
USA), and CCL21 expression was normalized against β-actin.
Tissue microarray construction and
immunohistochemical analysis
In the tissue microarray construction, two cores of
tissue were punched from the representative tumor areas and
deposited into a recipient block with the tissue array instrument,
MiniCore (Alphelys, Paris, France), and 5-μm sections were cut for
immunohistochemical staining. All slides were deparaffinized and
rehydrated through graded alcohols. Endogenous peroxidase was
blocked with 0.3% hydrogen peroxide for 10 min at room temperature
before the slides were exposed to the antigen retrieval system (10
mM sodium citrate, 0.05% Tween-20, pH 6.0 for 25 min). After
incubation with the primary rabbit multiclonal CCL21 antibody
(1:500; Abcam) overnight at 4°C, the sections were stained with
diaminobenzidine in an EnVision System (DakoCytomation, Glostrup,
Denmark). The slides were finally counterstained with hematoxylin
(Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing,
China).
Evaluation of immunohistochemical (IHC)
analysis
A digital image (2592×1944 pixels, 13.9
nanometers/pixel) for each tumor spot was captured (fold
magnification, ×200) with a Leica DMI4000B microscope (Leica
Microsystems, Wetzlar, Germany). The CCL21 expression index was
then estimated in the TMAJ project (Johns Hopkins TMA Core
Facility, Baltimore, MD, USA), calculated as the product of CCL21
staining intensity and the density. The intensity was provided by
the software automatically, and the density was the quotient
between the positive area and total area (Fig. 1C). Finally, the mean IHC index for
each patient was used for statistical analysis without knowledge of
the clinicopathological information. The median CCL21 expression
index was 7.2 (range, 0–187.7).
Selection of the cut-point value
Cut-point of the CCL21 expression index was
optimized with the X-tile program (version 3.6.1, Yale University
School of Medicine, New Haven, CT, USA) based on CRC patient
outcomes (14). In the X-tile
program, the cohort was divided randomly into a matched training
and validation sets, as a method for the optimal cut-point
selection. The cut-point value derived from the training set was
then parsed in the validation set, and statistical significance was
determined by using a standard log-rank method, while correcting
for the use of minimum P statistics by Miller-Siegmund P-value
correction (15).
Statistical analysis
The expression dynamics of CCL21 between CRC and
normal colorectal tissues was evaluated using the paired t-test.
Correlations between the CCL21 expression index and
clinicopathological characteristics were analyzed using the 2-tail
t-test (or Wilcoxon rank sum test as appropriate) for continuous
variables and the Chi-square test (or the Fisher’s exact test as
appropriate) for categorical variables. Patient survival was
depicted using Kaplan-Meier curves with a log-rank test. Both
univariate and multivariate analyses were performed to evaluate the
risk factors for both OS and DFS in the stage III/IV CRC patients.
Receiver operating characteristic (ROC) curve analysis was carried
out to assess the predictive value of the parameters, including a
combined parameter, which was defined as the combination of the
CCL21 expression index and TNM stage, ranging from 0 to 2. All
statistical analyses were performed using the SPSS software,
version 16 (SPSS, Inc., Chicago, IL, USA). P-value <0.05 was
considered to indicate a statistically significant result.
Results
Patient demographics and follow-up
A total of 143 patients with stage III/IV CRC met
the inclusion criteria and were enrolled for immunohistochemical
analysis. Seventy-eight patients (54.5%) were male and 65 (45.5%)
were female, with a mean age of 56.7±14.5 years. Based on the 7th
AJCC classification, there were 114 stage III patients and 29 stage
IV patients. Site of cancer was the rectum in 72 patients (50.3%)
and the colon in 71 patients (49.7%). After a mean follow-up of
49.5±30.6 months, 59 patients either died or had disease
recurrence.
CCL21 expression dynamics and selection
of the optimal cut-point value
Regarding the expression dynamics, western blot
analysis revealed that CCL21 expression was comparable between the
tumor tissue and the normal colorectal specimens (P=0.619, Fig. 1A and B). CCL21 expression in all 143
CRC patients was evaluated through tissue microarrays, and the
index of CCL21 expression was calculated using the TMAJ project
(Fig. 1C). The CCL21 expression
index ranged from 0 to 187.7 (median 7.2). According to the X-tile
program, patients were divided into a low expression group and a
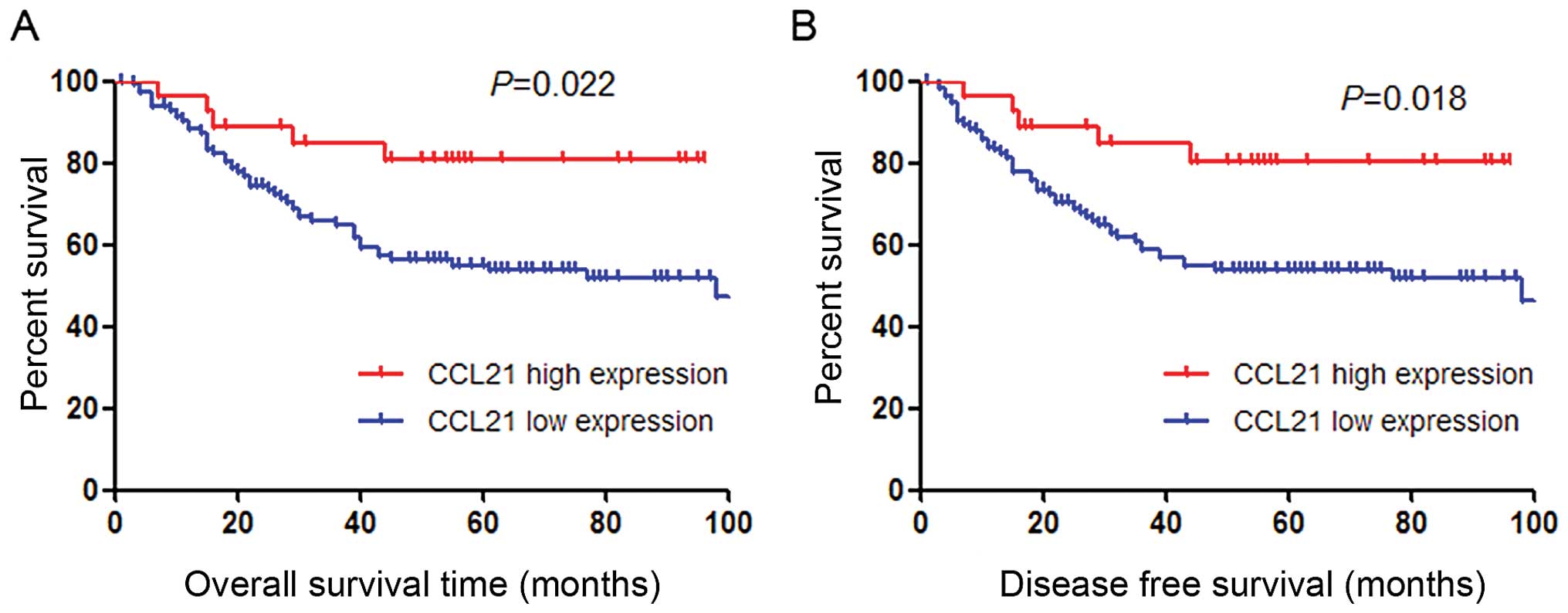
high expression group based on a cut-point of 56.1 (P=0.022,
Fig. 1D).
Association between CCL21 expression,
clinicopathological variables and patient survival
High expression of CCL21 was identified in 27
(18.9%) of 143 stage III/IV CRC patients (Table I). The tumor diameter was
significantly larger in patients with high CCL21 expression than in
those with low expression (P=0.007). Patients with high CCL21
expression were also found to have more mucinous or signet ring
cell carcinoma (P<0.001) and poor tumor differentiation
(P=0.001). However, no significant difference was identified
between patients with high and low expression in terms of the other
clinicopathological variables (P>0.05).
 | Table IClinicopathological characteristics of
the 143 stage III/IV CRC patients. |
Table I
Clinicopathological characteristics of
the 143 stage III/IV CRC patients.
| Characteristics | All cases | Low CCL21
expression | High CCL21
expression |
P-valuea |
|---|
| No. of patients | 143 | 116 | 27 | |
| Mean age (years) | 56.7±14.5 | 57.0±15.0 | 55.6±12.5 |
0.654b |
| Gender, n (%) | | | | 0.160 |
| Male | 78 | 60 (51.7) | 18 (66.7) | |
| Female | 65 | 56 (48.3) | 9 (33.3) | |
| BMI,
kg/m2 | 21.1±3.2 | 20.8±3.1 | 22.1±3.5 |
0.075b |
| Bowel obstruction, n
(%) | | | |
0.730c |
| Yes | 14 | 11 (9.6) | 3 (11.1) | |
| No | 128 | 104 (90.4) | 24 (88.9) | |
| CEA (ng/ml), n
(%) | | | | 0.659 |
| <5 | 74 | 59 (55.1) | 15 (60.0) | |
| ≥5 | 58 | 48 (44.9) | 10 (40.0) | |
| CA199 (ng/ml), n
(%) | | | | 0.331 |
| <37.5 | 89 | 74 (72.5) | 15 (62.5) | |
| ≥37.5 | 37 | 28 (27.5) | 9 (37.5) | |
| Tumor location, n
(%) | | | | 0.496 |
| Colon | 71 | 56 (48.3) | 15 (55.6) | |
| Rectum | 72 | 60 (51.7) | 12 (44.4) | |
| Tumor diameter,
cm | 5.1±2.2 | 4.9±2.1 | 6.1±2.5 |
0.007b |
| Histopathology, n
(%) | | | |
<0.001 |
|
Adenocarcinoma | 126 | 110 (94.8) | 16 (59.3) | |
| Mucinous carcinoma
or signet ring cell carcinoma | 17 | 6 (5.2) | 11 (40.7) | |
| Differentiation, n
(%) | | | | 0.001 |
| Well/moderate | 114 | 99 (85.3) | 15 (55.6) | |
| Poor | 29 | 17 (14.7) | 12 (44.4) | |
| Depth of tumor
invasion, n (%) | | | |
1.000c |
| T1 + T2 | 10 | 8 (6.9) | 2 (7.4) | |
| T3 + T4 | 133 | 108 (93.1) | 25 (92.6) | |
| Nodal status, n
(%) | | | |
0.675c |
| N0 | 9 | 7 (6.2) | 2 (7.7) | |
| N1 + N2 | 130 | 106 (93.8) | 24 (92.3) | |
| Distant metastasis,
n (%) | | | | 0.800 |
| M0 | 114 | 92 (79.3) | 22 (81.5) | |
| M1 | 29 | 24 (20.7) | 5 (18.5) | |
Both Kaplan-Meier analysis and univariate Cox
proportional hazard regression model were applied to investigate
the impact of CCL21 overexpression on stage III/IV CRC patient
survival. High expression of CCL21 was significantly correlated
with better overall survival (log-rank, P=0.022) (Fig. 2A and Table II). Additional clinicopathological
variables, including preoperative serum CEA level (P=0.001),
preoperative serum CA199 level (P=0.003), nodal status (P=0.006)
and distant metastasis (P<0.001), were also found to be
significant prognostic factors for patient overall survival
(Table II). Similar results were
also identified for patient disease-free survival (Fig. 2B and Table II). Of note, after being stratified
by the tumor location, CCL21 expression was still found to have an
impact on patient survival (OS, P=0.025; DFS, P=0.020).
 | Table IIUnivariate analyses of CCL21
expression and clinicopathological variables in the 143 stage
III/IV CRC patients. |
Table II
Univariate analyses of CCL21
expression and clinicopathological variables in the 143 stage
III/IV CRC patients.
| Overall
survival | Disease-free
survival |
|---|
|
|
|
|---|
|
Characteristics | HR (95% CI) | P-valuea | HR (95% CI) | P-valuea |
|---|
| Age (years) | | 0.257 | | 0.298 |
| <56.7b | 1.0 | | 1.0 | |
| ≥56.7 | 1.355
(0.801–2.294) | | 1.322
(0.781–2.237) | |
| Gender | | 0.765 | | 0.740 |
| Female | 1.0 | | 1.0 | |
| Male | 0.925
(0.554–1.543) | | 0.917
(0.549–1.530) | |
| BMI,
kg/m2 | | 0.458 | | 0.417 |
| <21.1b | 1.0 | | 1.0 | |
| ≥21.1 | 0.820
(0.487–1.383) | | 0.805
(0.478–1.358) | |
| Bowel
obstruction | | 0.515 | | 0.596 |
| No | 1.0 | | 1.0 | |
| Yes | 1.284
(0.605–2.724) | | 1.225
(0.578–2.597) | |
| CEA (ng/ml) | | 0.001 | | 0.001 |
| <5 | 1.0 | | 1.0 | |
| ≥5 | 2.576
(1.454–4.567) | | 2.720
(1.524–4.854) | |
| CA199 (ng/ml) | | 0.003 | | 0.004 |
| <37.5 | 1.0 | | 1.0 | |
| ≥37.5 | 2.461
(1.371–4.417) | | 2.387
(1.330–4.285) | |
| Tumor location | | 0.240 | | 0.271 |
| Colon | 1.0 | | 1.0 | |
| Rectum | 1.362
(0.813–2.283) | | 1.336
(0.798–2.237) | |
| Tumor diameter
(cm) | | 0.635 | | 0.689 |
| <5b | 1.0 | | 1.0 | |
| ≥5 | 0.884
(0.530–1.473) | | 0.901
(0.540–1.502) | |
| Histopathology | | 0.910 | | 0.985 |
|
Adenocarcinoma | 1.0 | | 1.0 | |
| Mucous
carcinoma | 0.952
(0.408–2.222) | | 0.992
(0.425–2.314) | |
|
Differentiation | | 0.071 | | 0.058 |
| Well +
moderate | 1.0 | | 1.0 | |
| Poor | 1726
(0.955–3.117) | | 1.771
(0.981–3.199) | |
| Depth of
invasion | | 0.564 | | 0.589 |
| T1 + T2 | 1.0 | | 1.0 | |
| T3 + T4 | 1.408
(0.440–4.507) | | 1.378
(0.430–4.413) | |
| Nodal status | | 0.006 | | 0.013 |
| N0 | 1.0 | | 1.0 | |
| N1 + N2 | 0.329
(0.148–0.730) | | 0.366
(0.165–0.812) | |
| Distant
metastasis | |
<0.001 | |
<0.001 |
| M0 | 1.0 | | 1.0 | |
| M1 | 6.563
(3.839–11.218) | | 6.214
(3.621–10.662) | |
| CCL21
expression | | 0.029 | | 0.025 |
| Low | 1.0 | | 1.0 | |
| High | 0.360
(0.144–0.901) | | 0.349
(0.139–0.875) | |
CCL21 expression as a significant
prognostic factor in the multivariate analysis
As expected, high expression of CCL21 remained a
significant independent prognostic factor for favorable overall
survival in the multivariate analysis after adjusting for
preoperative serum CEA level, preoperative serum CA199 level, nodal
status and distant metastasis [hazard ratio (HR), 10.204; 95%
confidence interval (CI), 2.358–45.455; P=0.002; Table III]. As expected, analysis of
disease-free survival showed similar results (HR, 9.524; 95% CI,
2.232–40.000; P=0.002; Table
III).
 | Table IIICox multivariate analyses of
prognostic factors on patient survival. |
Table III
Cox multivariate analyses of
prognostic factors on patient survival.
|
Characteristics | Hazard ratio | 95% CI | P-valuea |
|---|
| Overall
survival |
| CEA, ng/ml (≥5 vs.
<5) | 1.783 | 0.917–3.467 | 0.088 |
| CA199, ng/ml
(≥37.5 vs. <37.5) | 1.793 | 0.934–3.440 | 0.079 |
| Nodal status
(N1+N2 vs. N0) | 0.964 | 0.338–2.747 | 0.945 |
| Distant metastasis
(M0 vs. M1) | 11.196 | 5.156–24.313 | <0.001 |
| CCL21 density (low
vs. high) | 10.204 | 2.358–45.455 | 0.002 |
| Disease-free
survival |
| CEA, ng/ml (≥5 vs.
<5) | 1.952 | 1.003–3.797 | 0.049 |
| CA199, ng/ml
(≥37.5 vs. <37.5) | 1.977 | 1.034–3.781 | 0.039 |
| Nodal status
(N1+N2 vs. N0) | 1.396 | 0.494–3.939 | 0.529 |
| Distant metastasis
(M0 vs. M1) | 11.574 | 5.300–25.275 | <0.001 |
| CCL21 density (low
vs. high) | 9.524 | 2.232–40.000 | 0.002 |
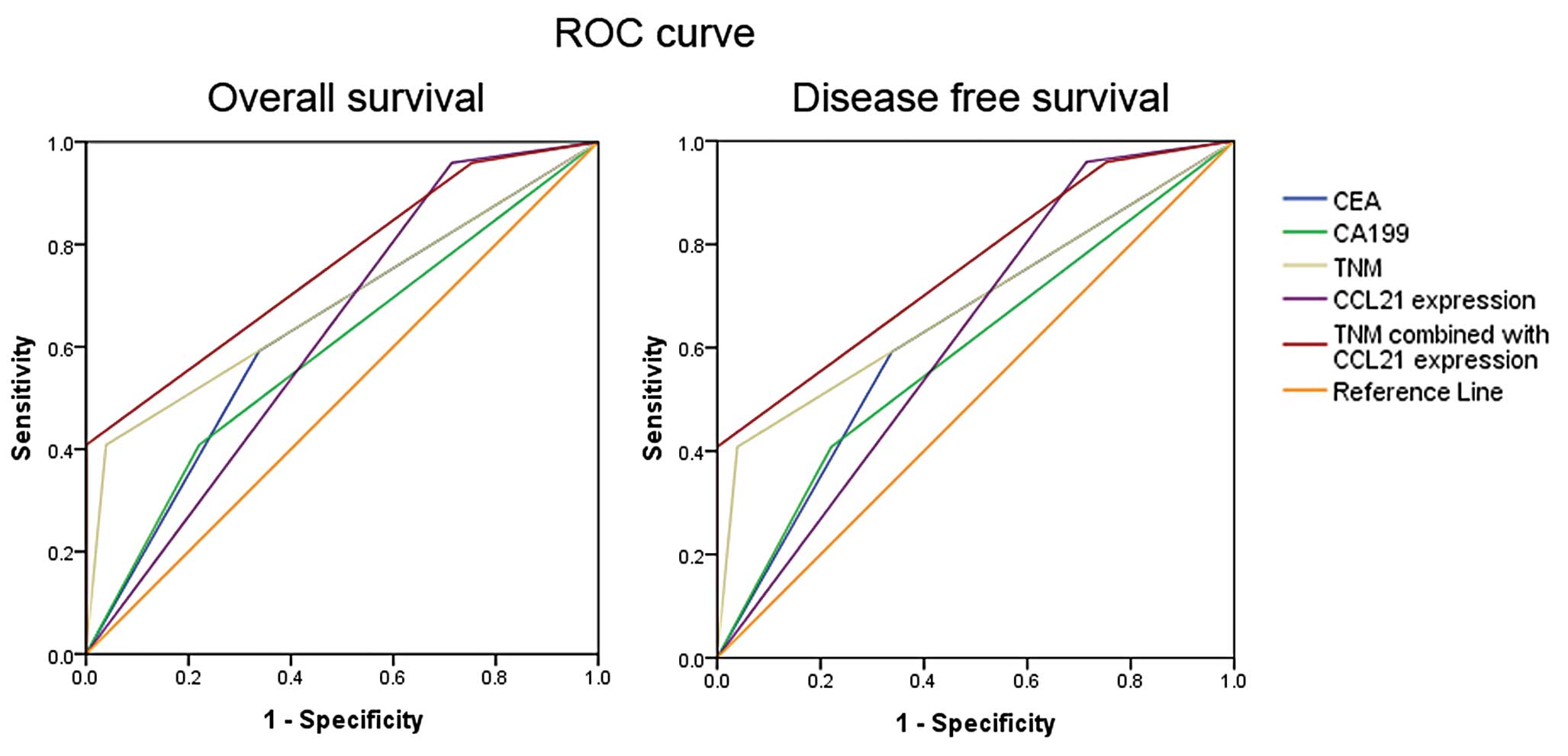
ROC curve analysis was also applied to further
assess the prognostic value of CCL21 expression, which showed an
encouraging role of CCL21 expression in predicting CRC patient
overall survival and disease-free survival [area under the curve
(AUC)=0.622 and 0.622; Fig. 3].
More importantly, the AUC value was increased after incorporating
CCL21 expression into TNM stage (AUC=0.757 and 0.757, Fig. 3).
Discussion
Despite the improved diagnostic techniques and
advanced treatments, CRC remains a major public health issue. Due
to the limitations of the current AJCC staging system (16), numerous studies in the literature
have evaluated the prognostic roles of biomarkers for CRC patients
(17,18). In vitro, CCL21 was found to
be able to promote the invasive ability of colorectal cancer cells
by inducing MMP-9 expression (19).
However, CCL21 was also shown to enhance the infiltration of immune
cells into the tumor region, inhibiting the growth of tumors by
generating an antitumor cellular immune response (20,21).
Although the prognostic value of CCL21 has been studied in several
types of cancers, including gastric (12) and pancreatic cancer (20), its role in CRC is not well defined.
Mumtaz et al(22) found that
the expression of CCL21 was decreased in rectal cancer, but the
prognostic value of CCL21 was not further evaluated due to the lack
of appropriate follow-up.
In the present study, western blot analysis of 12
paired freshly frozen specimens from primary stage III/IV CRC and
normal colorectal tissues was performed to examine the expression
dynamics of CCL21. In contrast with a previous study (22), CCL21 expression was not found to be
significantly different from that in normal colorectal tissues in
our study. This might be explained by the nature of the
heterogeneity of CRCs, which results in varied expression of CCL21
in different CRC patient tumors. To avoid a predetermined arbitrary
cut-point, we constructed X-tile plots for assessment of scores
which divide CCL21 expression into two populations, in which we
corrected for the use of minimum P statistics by Miller-Siegmund
P-value correction (15). In the
present study, our results revealed that CCL21 expression in CRC
was closely associated with tumor diameter, tumor histology and
tumor differentiation. In this perspective, CCL21 expression may
increase as tumor progress. Furthermore, survival analysis revealed
that stage III/IV CRC patients with high expression of CCL21 had a
prolonged survival, even after being stratified by the tumor
location. In the multivariate analyses, CCL21 expression was proven
to be a prognostic factor independent of certain well-established
clinicopathologcial parameters. CCL21 expression may constitute a
useful biomarker in addition to the AJCC TNM staging system for
these patients, and identify patients whose tumors are more likely
to progress and recur and who are good candidates to receive more
aggressive treatment.
Although the underlying mechanisms of the predictive
value of CCL21 for stage III/IV CRC patients were not further
explored in the present study, there were several lines of evidence
to support our findings. The tumor microenvironment consists of
cancer cells, immune inflammatory cells, endothelial cells as well
as other elements (5). CCL21 may
inhibit tumor progression through inducing the function of immune
inflammatory cells and endothelial cells. Previous research
demonstrated that the antitumor activity of CCL21 was mediated by
enhancing the infiltration of mature dendritic cells (DCs) and
CD8+ T cells to the tumor (23–25).
Further data also suggest that modification of the tumor immune
microenvironment may lead to an effective tumor-specific response.
Inside, DCs are highly effective at secretion of IL-12 and
interferon-γ, and are thus expected to be beneficial for efficient
local antitumor responses, as well as T, NK and NKT cells (26,27).
CCL21 is not only important in recruiting DCs and T cells. It has
been classified as a CC chemokine, which binds to the CCR7
receptor, but it also has been shown to bind to the CXC chemokine
receptor CXCR3 (28). Therefore,
the angiostatic activity of CCL21 by binding to the CXCR3 receptor
may also be important in its antitumor capability. CXCR3 is
expressed on human microvascular endothelial cells under special
conditions, and engagement of CXCR3 by CCL21 may inhibit
endothelial cell proliferation in vitro(29). Furthermore, T cells expressing CXCR3
were found to be actively recruited into the invasive margin of CRC
and induce the Th1-shifted cellular immune responses (30).
The prognostic value of CCL21 in cancer patients
appears to be dependent on tumor type. Although a recent study
showed that overexpression of CCL21 was a negative prognostic
factor for gastric cancer (12), it
was shown to be a favorable prognostic factor for pancreatic and
breast cancer (20). Wu et
al(31) investigated the effect
of exogenous CCL21 expression in breast cancer MCF-7 cells on human
monocyte-derived DCs. Overexpression of CCL21 improved the
immunogenicity of tumor cells and the function of DCs in migration,
antigen uptake and presentation, giving rise to increased Th 1 type
cytokine, transformation of perforin-forming CD8+ T
cells and final T cell-associated clearance of tumor cells. Due to
the immune regulatory and angiostatic effect, overexpression of
CCL21 inhibits the progression of CRCs and can be used as a
favorable prognostic factor. Moreover, expression of CCL21 may
enhance the predictive capability of TNM staging. When combined
with CCL21 expression, TNM staging showed a more prominent
predictive capability. The possible reason is that the combination
of clinicopathological and molecular parameters may be a better
representation of tumor characteristics and prognosis.
To the best of our knowledge, the present study is
the first to evaluate the prognostic significance of CCL21
expression in stage III/IV CRC patients in a large cohort. Our
study revealed that high expression of CCL21 was an independent
predictor of more favorable survival, and CCL21 analysis may be
used as an additional parameter to TNM staging in predicting
patient survival outcomes and in guiding treatment. One limitation
of our study was the small cohort of paired tissues for analysis of
CCL21 expression dynamics.
In conclusion, in the present study of a large
cohort of stage III/IV CRC patients, we provide evidence that high
expression of CCL21 is a strong and independent predictor of
prolonged survival. Examination of CCL21 expression by IHC analysis
can be used as an additional and effective way to identify patients
at high risk for tumor progression, thus optimizing individualized
treatment for stage III/IV CRC patients.
Acknowledgements
This study was partially sponsored by the National
Natural Science Foundation of China (nos. 91029702 and 81072046),
the Medical Scientific Research Foundation of Guangdong Province,
China (no. WSTJJ20101107445221197902271012) and the Young Teachers
Training Program Foundation by Sun Yat-Sen University, China (no.
10ykpy01).
References
|
1
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Dai Z, Zheng RS, Zou XN, et al: Analysis
and prediction of colorectal cancer incidence trend in China.
Zhonghua Yu Fang Yi Xue Za Zhi. 46:598–603. 2012.(In Chinese).
|
|
3
|
Center MM, Jemal A, Smith RA, et al:
Worldwide variations in colorectal cancer. CA Cancer J Clin.
59:366–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Edwards BK, Ward E, Kohler BA, et al:
Annual report to the nation on the status of cancer, 1975–2006,
featuring colorectal cancer trends and impact of interventions
(risk factors, screening, and treatment) to reduce future rates.
Cancer. 116:544–573. 2010.
|
|
5
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kawamura M, Toiyama Y, Tanaka K, et al:
CXCL5, a promoter of cell proliferation, migration and invasion, is
a novel serum prognostic marker in patients with colorectal cancer.
Eur J Cancer. 48:2244–2251. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arabzadeh A, Chan C, Nouvion AL, et al:
Host-related carcinoembryonic antigen cell adhesion molecule 1
promotes metastasis of colorectal cancer. Oncogene. 32:849–860.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoshida R, Nagira M, Kitaura M, et al:
Secondary lymphoid-tissue chemokine is a functional ligand for the
CC chemokine receptor CCR7. J Biol Chem. 273:7118–7122. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rot A and von Andrian UH: Chemokines in
innate and adaptive host defense: basic chemokinese grammar for
immune cells. Annu Rev Immunol. 22:891–928. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takeuchi H, Fujimoto A, Tanaka M, et al:
CCL21 chemokine regulates chemokine receptor CCR7 bearing malignant
melanoma cells. Clin Cancer Res. 10:2351–2358. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koizumi K, Kozawa Y, Ohashi Y, et al:
CCL21 promotes the migration and adhesion of highly lymph node
metastatic human non-small cell lung cancer Lu-99 in vitro.
Oncol Rep. 17:1511–1516. 2007.PubMed/NCBI
|
|
12
|
Hwang TL, Lee LY, Wang CC, et al: CCL7 and
CCL21 overexpression in gastric cancer is associated with lymph
node metastasis and poor prognosis. World J Gastroenterol.
18:1249–1256. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yousefieh N, Hahto SM, Stephens AL, et al:
Regulated expression of CCL21 in the prostate tumor
microenvironment inhibits tumor growth and metastasis in an
orthotopic model of prostate cancer. Cancer Microenviron. 2:59–67.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Camp RL, Dolled-Filhart M and Rimm DL:
X-tile: a new bio-informatics tool for biomarker assessment and
outcome-based cut-point optimization. Clin Cancer Res.
10:7252–7259. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Raeside DE: Monte Carlo principles and
applications. Phys Med Biol. 21:181–197. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
O’Connell JB, Maggard MA and Ko CY: Colon
cancer survival rates with the new American Joint Committee on
Cancer sixth edition staging. J Natl Cancer Inst. 96:1420–1425.
2004.
|
|
17
|
Huang Y, Li W, Chu D, et al:
Overexpression of matrix metalloproteinase-21 is associated with
poor overall survival of patients with colorectal cancer. J
Gastrointest Surg. 15:1188–1194. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin KY, Tai C, Hsu JC, et al:
Overexpression of nuclear protein kinase CK2 alpha catalytic
subunit (CK2alpha) as a poor prognosticator in human colorectal
cancer. PLoS One. 6:e171932011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun RH, Wang GB, Li J, et al: Role of
CCL21/CCR7 in invasion of colorectal carcinoma cell line SW480. Ai
Zheng. 28:708–713. 2009.(In Chinese).
|
|
20
|
Turnquist HR, Lin X, Ashour AE, et al:
CCL21 induces extensive intratumoral immune cell infiltration and
specific anti-tumor cellular immunity. Int J Oncol. 30:631–639.
2007.PubMed/NCBI
|
|
21
|
Sharma S, Stolina M, Luo J, et al:
Secondary lymphoid tissue chemokine mediates T cell-dependent
antitumor responses in vivo. J Immunol. 164:4558–4563. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mumtaz M, Wagsater D, Lofgren S, et al:
Decreased expression of the chemokine CCL21 in human colorectal
adenocarcinomas. Oncol Rep. 21:153–158. 2009.PubMed/NCBI
|
|
23
|
Kirk CJ, Hartigan-O’Connor D, Nickoloff
BJ, et al: T cell-dependent antitumor immunity mediated by
secondary lymphoid tissue chemokine: augmentation of dendritic
cell-based immunotherapy. Cancer Res. 61:2062–2070. 2001.PubMed/NCBI
|
|
24
|
Nomura T, Hasegawa H, Kohno M, et al:
Enhancement of anti-tumor immunity by tumor cells transfected with
the secondary lymphoid tissue chemokine EBI-1-ligand chemokine and
stromal cell-derived factor-1alpha chemokine genes. Int J Cancer.
91:597–606. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sharma S, Stolina M, Zhu L, et al:
Secondary lymphoid organ chemokine reduces pulmonary tumor burden
in spontaneous murine bronchoalveolar cell carcinoma. Cancer Res.
61:6406–6412. 2001.PubMed/NCBI
|
|
26
|
Maldonado-Lopez R, Maliszewski C, Urbain
J, et al: Cytokines regulate the capacity of CD8α+ and
CD8α− dendritic cells to prime Th1/Th2 cells in vivo. J
Immunol. 167:4345–4350. 2001.
|
|
27
|
Tatsumi T, Kierstead LS, Ranieri E, et al:
Disease-associated bias in T helper type 1 (Th1)/Th2 CD4(+) T cell
responses against MAGE-6 in HLA-DRB10401(+) patients with renal
cell carcinoma or melanoma. J Exp Med. 196:619–628. 2002.PubMed/NCBI
|
|
28
|
Soto H, Wang W, Strieter RM, et al: The CC
chemokine 6Ckine binds the CXC chemokine receptor CXCR3. Proc Natl
Acad Sci USA. 95:8205–8210. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Romagnani P, Annunziato F, Lasagni L, et
al: Cell cycle-dependent expression of CXC chemokine receptor 3 by
endothelial cells mediates angiostatic activity. J Clin Invest.
107:53–63. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Musha H, Ohtani H, Mizoi T, et al:
Selective infiltration of CCR5+CXCR3+ T
lymphocytes in human colorectal carcinoma. Int J Cancer.
116:949–956. 2005.PubMed/NCBI
|
|
31
|
Wu S, Xing W, Peng J, et al: Tumor
transfected with CCL21 enhanced reactivity and apoptosis resistance
of human monocyte-derived dendritic cells. Immunobiology.
213:417–426. 2008. View Article : Google Scholar : PubMed/NCBI
|