Introduction
Prostate cancer is generally the second leading
cause of cancer-related mortality in males of Western countries
(1,2). Androgen ablation therapy is an
effective treatment for hormone-dependent prostate cancer; however,
a subset of patients ultimately develops hormone refractory disease
(3–5). Therefore, it is necessary to identify
and characterize important regulators of aggressive prostate
cancer. Cellular heterogeneity is a common histopathological
feature in prostate cancer, and cancer cells undertake progressive
morphologic and behavioral changes during disease progression and
metastasis. Many aggressive prostate cancers recapitulate normal
developmental processes, such as epithelial-to-mesenchymal
transition (EMT), to enhance cell migration and invasion. The
conversion of an epithelial cell into a mesenchymal cell requires
alterations in morphology, cellular architecture, invasion and
migration (6–9).
The prostate cancer ARCaP cell is a well recognized
cell model for investigation of the molecular mechanisms of EMT in
prostate cancer (10). ARCaP cells
consist of two subtype cell lines, including ARCaPE and
ARCaPM. The parental ARCaPE cells were
isolated from the ascites fluid of a patient with bone metastasis
and display typical epithelial cell morphology and have only
limited tumorigenic potential. However, ARCaPE cells
have a high propensity for rapid and predictable bone and soft
tissue growth and metastases through orthotopic, intracardiac and
intraosseous injections in athymic mice, and can undergo EMT to
become ARCaPM cells, which exhibit mesenchymal cell
morphology and lose original epithelial cell markers but gain
various mesenchymal cell markers (11,12).
EGF signaling is proposed to participate in the
pathogenesis or maintenance of several types of human cancers of an
epithelial origin (13). In
prostate cancer cells, EGFR ligands are frequently elevated and
EGFR itself is commonly overexpressed (14). Furthermore, EGFR expression
increases during progression to a hormone-resistant stage (15–17).
Previous studies have demonstrated that epidermal growth factor
(EGF) signaling and Hedgehog (HH) signaling are both involved in
prostate cancer tumorigenesis and progression (18–23);
however, whether there is any ‘crosstalk’ between these two
important pathways requires clarification. In the present study, we
mainly explored the role of GLI-1, which is the transcription
factor of HH signaling, in EGF-regulated enhancement of the
invasiveness of ARCaPE prostate cancer cells in
vitro. We found that GLI-1 may function as one of the important
effectors, which is activated by EGF downstream signaling, to
promote the invasiveness of prostate cancer ARCaPE
cells. This finding indicates that EGF and HH signaling is
synergistically integrated in prostate cancer progression.
Materials and methods
Cell culture
The human prostate cancer ARCaPE and
ARCaPM cell lines were kindly provided as a gift by Dr
Leland Chung from the Cedars Sinai Medical Center, USA. The
ARCaPE and ARCaPM cells were maintained in
T-medium (Invitrogen, Carlsbad, CA, USA) and 5% fetal bovine serum
(FBS) at 37°C supplemented with 5% CO2 in a humidified
incubator. To study the effect of human recombinant EGF protein
(hrEGF) treatment, ARCaPE cells with 70% confluence were
cultured in non-serum T-medium overnight, then 100 ng/ml hrEGF was
added and treated for a consecutive 4 days. FBS and hrEGF, were
purchased from Sigma-Aldrich, Inc. (St. Louis, MO, USA).
Cell immunofluorescence staining
ARCaPE cells (5×104) were
added to poly-L-lysine-coated chamber slides and cultured for 24 h.
The cells were fixed in 4% paraformaldehyde, permeabilized with
0.25% Triton X-100 in PBS and blocked with 10% donkey serum, then
stained with goat polyclonal primary antibody against EGFR
(sc-31156, Santa Cruz Biotechnology, Dallas, TX, USA), and rabbit
polyclonal primary antibody against GLI-1 (ab92611, Abcam,
Cambridge, MA, USA). After rinsing, the primary antibodies were
respectively detected with Alexa Fluor® 488 donkey
anti-goat (A-11055, Invitrogen, Grand Island, NY, USA) or Alexa
Fluor® 488 goat anti-rabbit secondary antibodies
(Invitrogen), and the nuclei were labeled with DAPI (0.5 mg/ml) as
previously described (24,25). Chamber slides were mounted and
fluorescence images were visualized and captured with an Olympus
IX50 inverted fluorescence microscope (Olympus, Tokyo, Japan) and
processed using Adobe Photoshop 7.0 software.
Invasion assay
The invasive capability of prostate cancer cells was
determined by the Transwell assay. Before seeding the cells, 1 ml
of Matrigel (BD Biosciences, Shanghai, China) was dissolved in 4 ml
serum-free T-medium, and 60 μl diluted Matrigel was applied to the
upper chamber of 8-mm pore size polycarbonate membrane filters
(Corning Incorporation, Corning, NY, USA), and put into an
incubator overnight. ARCaPE and ARCaPM cells
were then harvested and seeded with serum-free T-medium into the
upper chamber (1×105 cells/well), and the bottom chamber
of the apparatus contained T-medium with 10% FBS. The hrEGF or/and
the GLI-1 inhibitor GANT61 (sc-202630, Santa Cruz Biotechnology,
Dallas, TX, USA) were added to both the upper and bottom chambers
for the ARCaPE cell treatment group at concentrations of
100 ng/ml and 10 μmol/l, respectively, and then incubated for 48 h
at 37°C. Following incubation, the cells that had invaded and
attached to the lower surface of the membrane were fixed with 4%
paraformaldehyde and stained with 1% toluidine blue. Cell numbers
were counted in five randomly chosen microscopic fields (x100) per
membrane using the IX50 inverted microscope (Olympus, Japan).
Western blot analysis
The expression levels of p-ERK, ERK and GLI-1 were
determined by western blot analysis according to previous studies
(24–27). Briefly, the cells were washed with
ice cold PBS (pH 7.4) and lysed in RIPA buffer (50 mM Tris, pH 7.4,
150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 1 mM EDTA
and 0.1% SDS) with the addition of mixed protease inhibitors.
Supernatants were collected after centrifuging at 14,000 rpm for 10
min at 4°C. Protein concentration was determined by the Bradford
method. Protein samples (20 μg) prepared in a final 1X sample
buffer (50 mM Tris/pH 6.8, 10% glycerol, 2% SDS, 0.1% bromophenol
blue and 0.5% 2-β-mercaptoethanol) were denatured for 5 min at
100°C and resolved on 10% SDS-polyacrylamide minigels.
Electrophoresis was initially carried out at 90 V through the
stacking gel and then at 120 V through the separation gel. After
electrophoresis, proteins were transferred to nitrocellulose
filters (Bio-Rad Laboratories, Shanghai, China). Filters were
subsequently blocked for 1.5 h at room temperature with blocking
solution (50 mM Tris/pH 7.4, 150 mM NaCl, 0.05% Tween-20 and 5%
skim milk), followed by incubation with rabbit anti-p-ERK,
anti-ERK, and anti-GLI1 polyclonal antibodies (Santa Cruz
Biotechnology) at 1:500 in blocking buffer and with mouse
anti-GAPDH monoclonal antibody (KangChen Bio-Tech Inc., Shanghai,
China) at 1:1,000 in blocking buffer for 1.5 h at room temperature.
After a 4 × 5-min rinse with TBST buffer (50 mM Tris/pH 7.4, 150 mM
NaCl and 0.05% Tween-20), blots were incubated with horseradish
peroxidase-conjugated anti-rabbit or anti-mouse IgG secondary
antibody (KangChen Bio-Tech) at 1:3,000 in blocking buffer for 1 h
at room temperature and rinsed 4 × 5 min with TBST. The
immunopositive bands were examined by an enhanced chemiluminescence
detection system (Thermo Fisher Scientific, Rockford, IL, USA), and
the signals were transferred and analyzed using the Odyssey
quantitative fluorescent imaging system (LI-COR Biosciences,
Lincoln, NE, USA). Protein equal loading was confirmed by GAPDH
expression.
Statistical analysis
All statistical analyses were performed using SPSS
11.5 software (SPSS Inc., Chicago, IL, USA). Quantitative data are
presented as means ± SEM, and the differences between two groups
were compared by the 2-tailed Student’s t-test. P<0.05 was
considered to indicate a statistically significant result.
Results
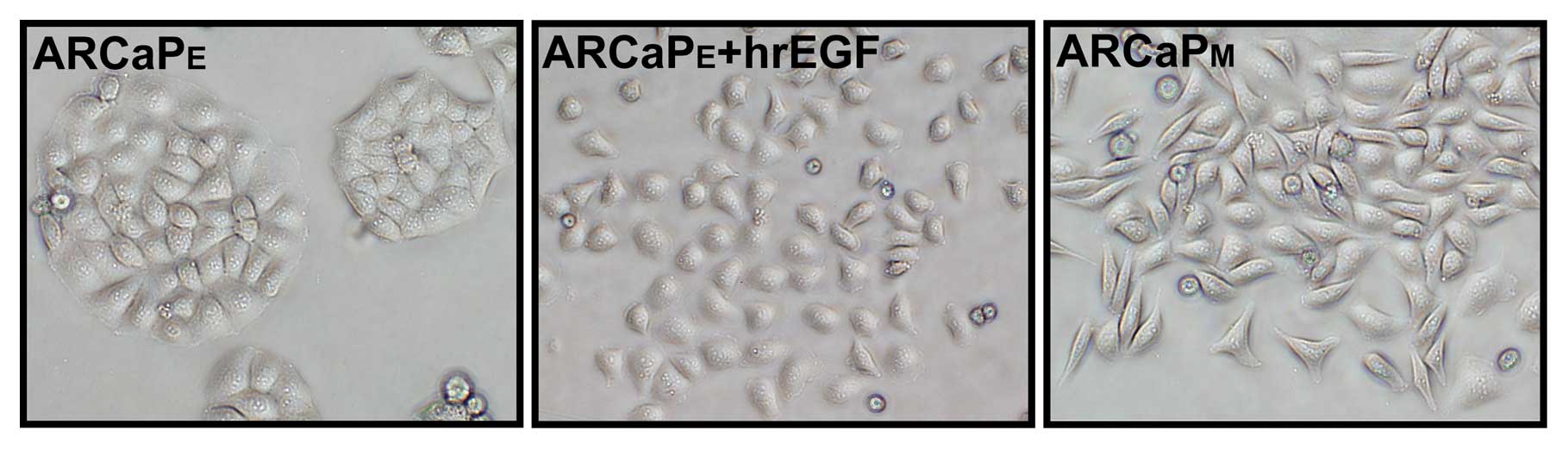
Morphology of the ARCaPE cells
is altered from an epithelial shape to a mesenchymal cell phenotype
following treatment with hrEGF
When grown in a two-dimensional culture,
ARCaPE cells exhibited a cobble-stone, epithelial-like
morphology and aggregated growth (Fig.
1), while the lineage-derived ARCaPM cells exhibited
a spindle-shaped mesenchymal morphology and scattered growth
(Fig. 1). The morphologic changes
observed in the ARCaPM cells resembled that of cells
undergoing EMT. However, after treatment with hrEGF (100 ng/ml) for
4 days, the morphology of the ARCaPE cells displayed
various characteristics of a mesenchymal cell-like phenotype with
dispersed growth (Fig. 1), which
largely resembled the morphology of the ARCaPM cells
(Fig. 1).
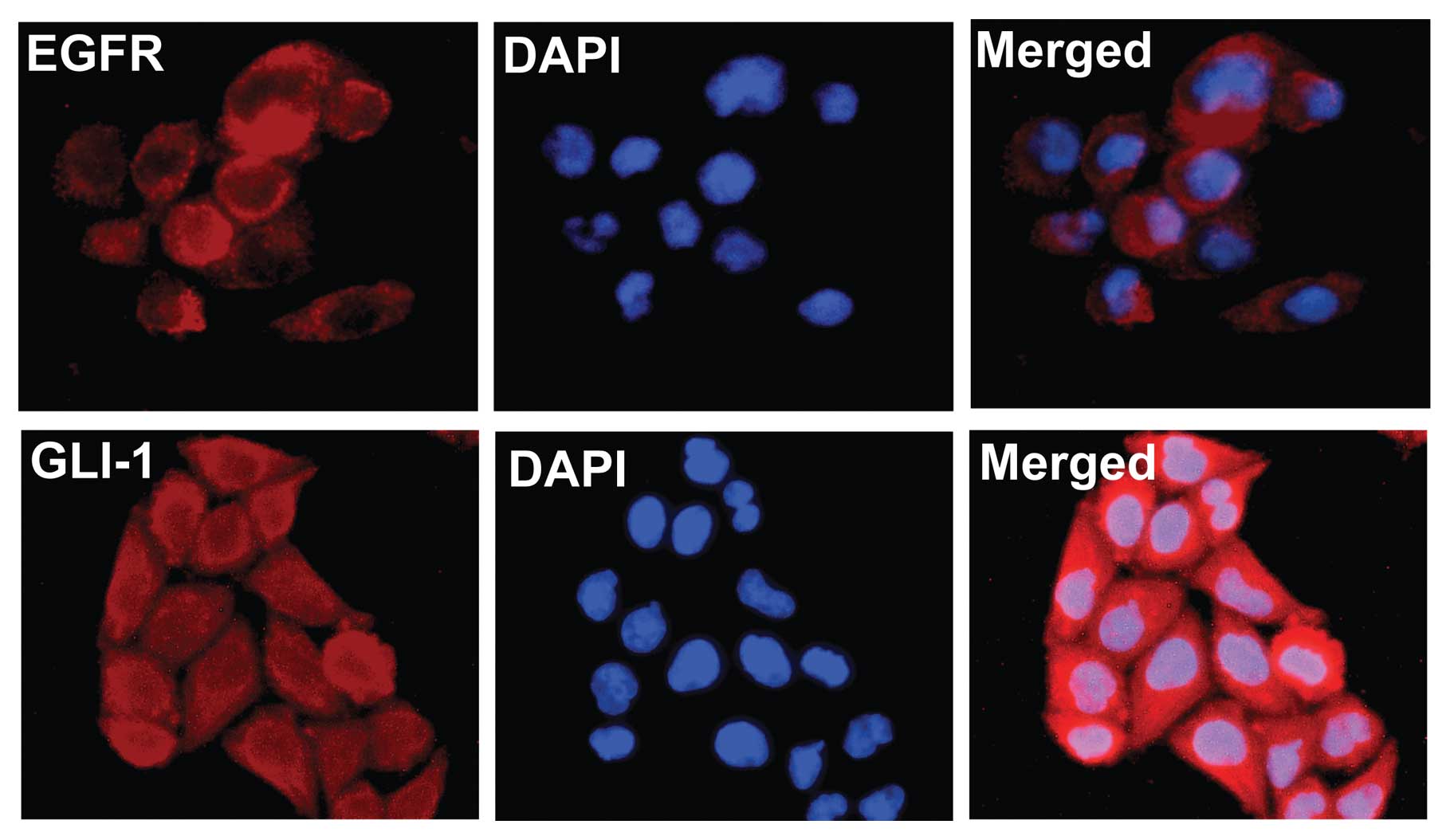
Expression of EGFR and GLI-1 in
ARCaPE cells
Using immunofluorescence detection assay, expression
of EGFR and GLI-1 was detected in ARCaPE prostate cancer
cells. Expression of EGFR was predominate in the cell membrane and
cytoplasm, while expression of GLI-1 was largely confined to the
cytoplasm and cell nucleus (Fig.
2).
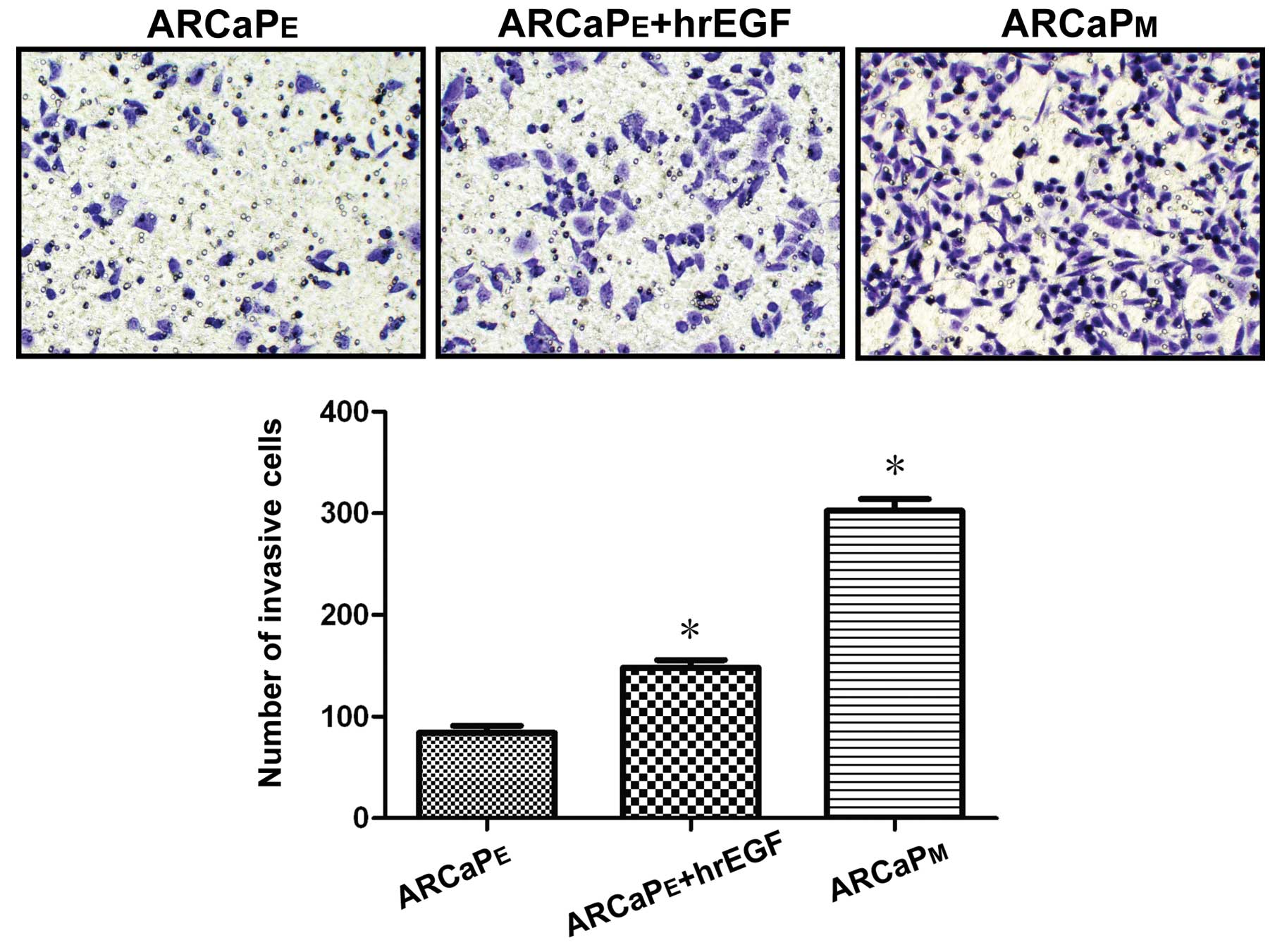
Treatment of EGF enhances the invasive
capability of ARCaPE cells
Using Transwell assay for 48 h, it was demonstrated
that the number of invasive cells noted in the parental
ARCaPE cells, hrEGF-treated ARCaPE cells, and
ARCaPM cells was 84±3, 148±5 and 302±18, respectively.
Compared to the number of invasive cells noted in the parental
ARCaPE cells, there was a significant difference in the
invasive cell numbers noted in both the hrEGR-treated
ARCaPE and ARCaPM cells (Fig. 3, P<0.05). This result indicates
that EGF treatment increased the invasive capability of
ARCaPE cells.
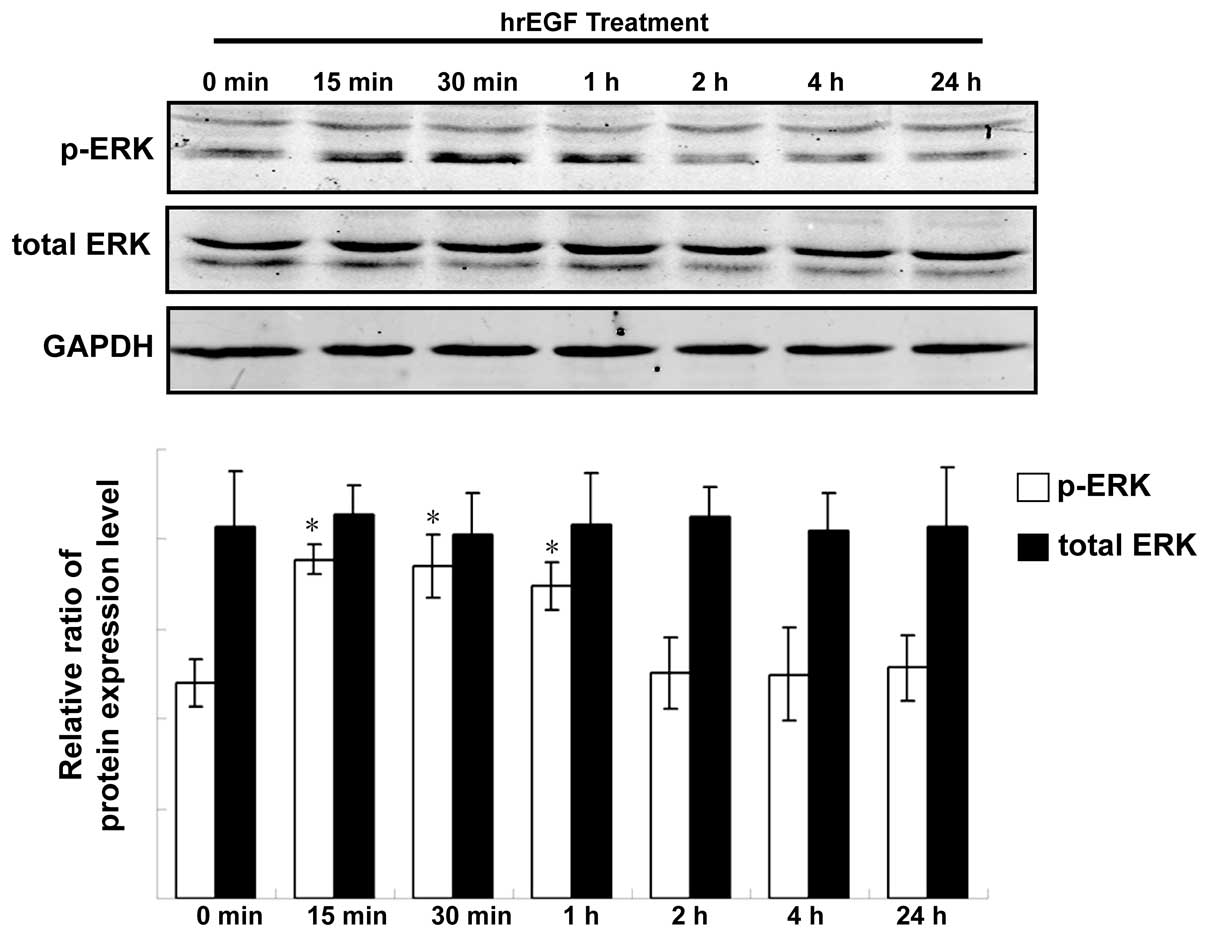
EGF activates ERK signaling in
ARCaPE cells
Following treatment with hrEGF, the expression of
total ERK and phosphorylated ERK (p-ERK) in ARCaPE cells
was detected at a different time-point by western blot assay. The
results showed that the expression level of p-ERK was initially
upregulated after 15 min of treatment with hrEGF. The level
continued to increase after 30 min and 1 h, but decreased to its
baseline level after a 2-h treatment (Fig. 4, P<0.05). However, the expression
level of total ERK in ARCaPE cells was relatively
unchanged following treatment of hrEGF (Fig. 4).
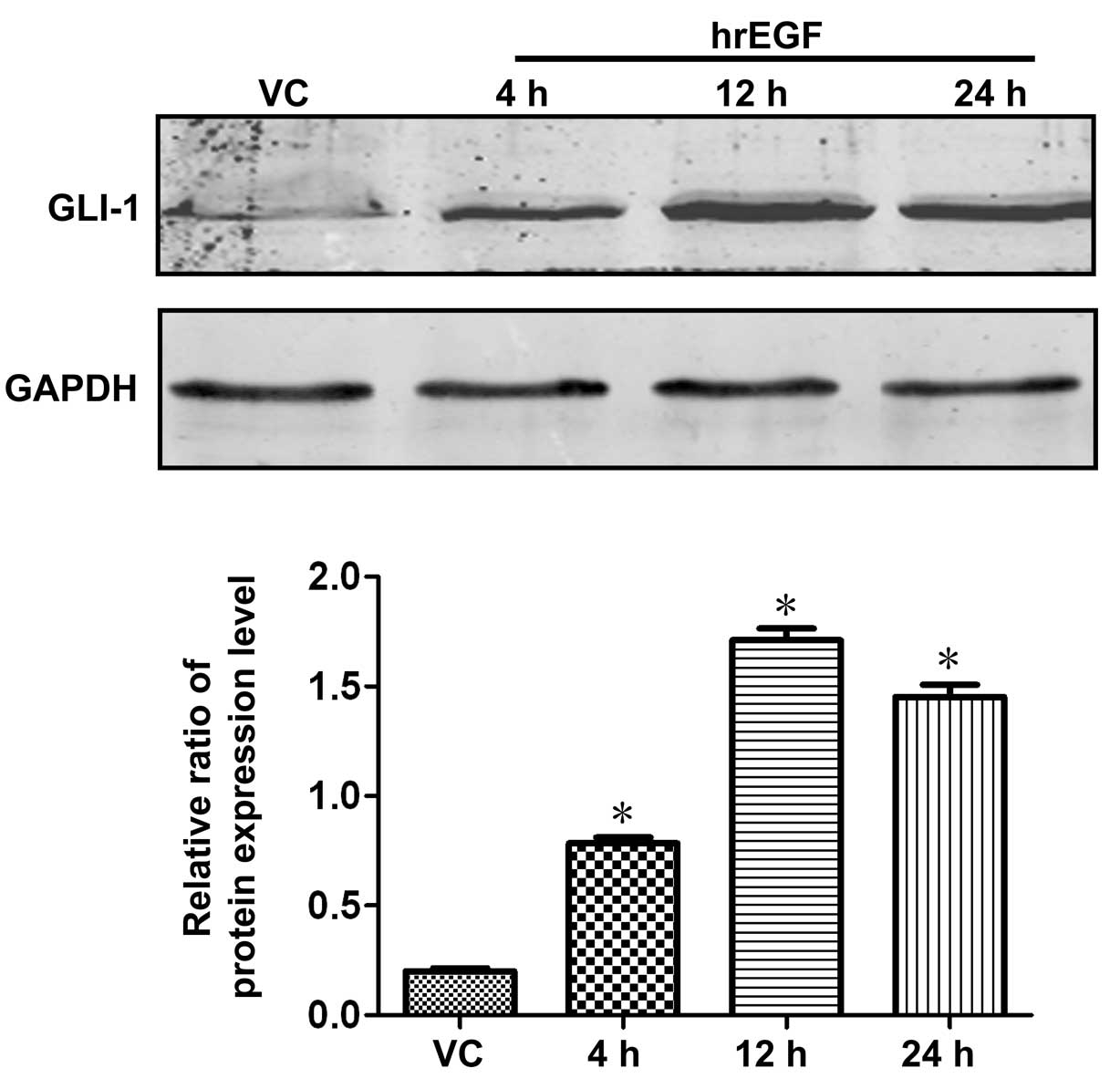
Expression of GLI-1 is upregulated
following treatment with EGF in ARCaPE cells
The expression of GLI-1 in ARCaPE cells
was timely detected by western blot analysis and the cell lysates
were harvested at 4, 12 and 24 h time-points after a 30-min
treatment with hrEGF (100 ng/ml). Compared with the negative
control cells (parental ARCaPE cells treated with DMSO),
the expression level of GLI-1 in ARCaPE cells treated
with hrEGF was upregulated at a 4-h time-point following treatment
with EGF, and its expression was gradually increased to a peak
level at the time-point of 12 h and reached a relatively higher
level at 24 h when compared with its baseline level (Fig. 5; P<0.05).
GLI-1-specific inhibitor GNT61 reverses
the enhanced invasive efficacy induced by EGF in ARCaPE
cells
Using Transwell assay for 48 h, it was demonstrated
that GLI-1 inhibitor GANT61 dramatically reversed the enhanced
invasive effect induced by EGF in ARCaPE cells. The
number of invasive ARCaPE cells treated with DMSO,
GANT61, hrEGF and hrEGF+GANT61 was 85±8, 25±2, 177±10 and 41±4,
respectively. There was a statistically significant increase in the
invasive cell number noted in the hrEGF-treated ARCaPE
cells when compared to the DMSO-treated control (Fig. 6, P<0.05), and a statistically
significant decrease in the invasive cell number of
hrEGF+GANT61-treated ARCaPE cells (Fig. 6, P<0.05) was noted when compared
to the invasive cell number of hrEGF-treated cells.
Discussion
GLI-1 is the key transcriptional regulator of HH
signaling, which is very essential both for normal prostate
development and prostate cancer progression (28–37).
In mammals, the general schema of this signaling activation
involves the binding of Hedgehog ligands to its receptor, Patched
(PTC), which is released as an inhibitory function of the protein
Smoothened (SMO), a co-receptor for HH signaling, and allows
activation of downstream GLI-1 transcriptional factors to activate
target genes (38–40). Previous studies have demonstrated
that sonic Hedgehog (SHH) signaling pathway activity is common in
localized PCa and that it may promote tumor cell proliferation by a
combination of autocrine and paracrine signaling, and its activity
is dramatically increased in advanced metastatic PCa (32–37).
Specifically, Karhadkar et al(35) reported that HH pathway activity is
required for regeneration of prostate epithelium, propagation of
prostate cancer in xenografts and expression of the stem cell
renewal factors in cancer cells, and additionally it was also found
that forced HH pathway activity could produce malignant
transformation of primitive prostate epithelial progenitor cells,
suggesting that prostate cancer might be initiated by trapping of a
normal stem cell in a HH-dependent state of continuous renewal.
Clinically, Shaw et al(36)
reported that the HH pathway was activated in patients with
androgen-independent prostate cancer (AIPC), and PTC-positive
circulating tumor cells could be identified in patients with
metastatic AIPC.
Based on the systematic and detailed studies
concerning GLI-1 in cancer biology, a growing body of evidence
suggests that activation of GLI-1 is not controlled exclusively by
HH signaling itself but also by other pathways frequently activated
in human malignancies (41–43). GLI-1 activity can be modulated by
PI3K/AKT, MEK/ERK, protein kinase C, and transforming growth
factor-β/SMAD, which affect stability, subcellular localization, or
expression of GLI proteins in various types of cancers (41,44,45).
However, the precise role of GLI-1 and its relationship with other
signaling cascades in manipulating prostate cancer progression are
largely unknown.
EGF signaling is well known for its multifaceted
functions in development and tissue homeostasis. Binding of EGF to
EGFR modulates cellular function by activating EGFR through
autophosphorylation, which results in a downstream cascade that
leads to increased cellular proliferation (46). EGF signaling results in activation
of phosphoinositol-3 kinase. The latter activates the Akt family of
kinases and signal transducer and activator of transcription
(STAT), resulting in downstream events that regulate cellular
proliferation, survival and migration (47). The EGF family has been extensively
investigated for their roles in promoting tumorigenesis and
metastasis in a variety of cancer types, including prostate cancer
(18,48,49).
Furthermore, its receptor, EGFR, is overexpressed in prostate
cancers, and the EGF signaling pathway is involved in prostate
cancer hormone resistance development (15–17).
Previous studies have demonstrated that EGFR is
highly expressed in high grade prostatic intraepithelial neoplasia
and in neoplastic cells. Both the ligand and its signaling receptor
partner are frequently upregulated in advanced stages of prostate
cancer, and are correlated with a high Gleason score and tumor
progression from an androgen-dependent to an androgen-independent
state (50,51). Targeting EGFR with monoclonal
antibodies or with tyrosine kinase inhibitors suppresses the growth
and invasion of androgen-dependent and independent prostate cancer
cells in vitro(52).
Although EGFR was demonstrated to play a key role in prostate
cancer invasion and metastasis, the precise mechanism of its
downstream signaling with other essential molecular pathways in
prostate cancer progression is still unclear.
In the present study, we used the exogenous hrEGF to
treat prostate cancer ARCaPE cells in vitro,
which dramatically increased the cell invasive capability.
Additionally, we found that the expression of GLI-1 protein in
ARCaPE cells was upregulated after treatment with EGF
when compared with the DMSO control, and p-ERK was also activated
upon EGF treatment, although the total expression level of ERK was
largely unchanged. Base on these findings, we hypothesized that the
signaling pathway, mediated via p-ERK ‘crosstalk’ with GLI-1, may
play an important role in the elevated invasiveness of
ARCaPE cells. Thus, we further blocked the function of
GLI-1 using the specific inhibitor, GANT61. As expected, GANT61
dramatically reversed the enhanced invasive capacity of the
EGF-treated ARCaPE cells. Thus, taken together, the role
of EGF in ARCaPE cell invasiveness may partially depend
on the induction of HH signaling transcriptional factor GLI-1 to
achieve its function.
This novel finding is significant not only because
it is consistent with published reports showing their specific
roles in prostate cancer aggressiveness and metastasis, but it may
also indicate the possible ‘crosstalk’ between these key molecules
in prostate cancer progression. Actually, several similar studies
have also implicated the EGFR pathway in the modulation of HH/GLI
activity. For instance, EGF and Sonic HH cooperate to stimulate
neural stem cell proliferation and invasive growth of keratinocytes
(53–56), and recently Schnidar et
al(57) reported that
synergistic integration of GLI activator function and EGFR
signaling is a critical step in oncogenic transformation and
provides a molecular basis for therapeutic opportunities relying on
combined inhibition of the HH/GLI and EGFR/MEK/ERK/JUN pathway in
human basal cell carcinoma. However, few previous studies have
shown the direct upregulation of HH transcriptional factor GLI-1
protein by EFG induction in prostate cancer cells as documented in
our present study. Although the mechanisms by which EGF induces
GLI-1 expression requires further investigation, our data clearly
suggest that activation of GLI-1 by p-ERK leads to increased PCa
cell invasion, and inhibition of GLI-1 may reverse this enhanced
invasiveness induced by exogenous EGF in human ARCaPE
prostate cancer cells.
In conclusion, our preliminary in vitro study
showed that EGF signaling increases ARCaPE human
prostate cell invasive capability via upregulation of p-ERK and the
HH signaling transcriptional factor GLI-1. Additionally, this
enhanced cell invasive capacity was reversed by a GLI-1-specific
inhibitor in vitro. According to these results, we
hypothesize that the EGF and HH pathway may have possible
‘crosstalk’ with their downstream effectors through unknown
molecular mechanisms which synergistically contribute to the
enhanced invasiveness of prostate cancer cells. Targeting this
molecular ‘crosstalk’ may be a possible therapeutic strategy which
warrants future exploration and drug design concerning anti-EGF
signaling for the treatment of patients with advanced prostate
cancer.
Acknowledgements
Financial support from the National Natural Science
Foundation of China (NSFC: 30901501 to Guodong Zhu) is
acknowledged, and we thank the technical support provided by Ms.
Lin Chen.
References
|
1
|
Heidenreich A, Bellmunt J, Bolla M, Joniau
S, Mason M, Matveev V, Mottet N, Schmid HP, van der Kwast T, Wiegel
T and Zattoni F; European Association of Urology. EAU guidelines on
prostate cancer. Part 1: Screening, diagnosis, and treatment of
clinically localised disease. Eur Urol. 59:61–71. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bosetti C, Bertuccio P, Chatenoud L, Negri
E, La Vecchia C and Levi F: Trends in mortality from urologic
cancers in Europe, 1970–2008. Eur Urol. l60:1–15. 2011.
|
|
3
|
Mottet N, Bellmunt J, Bolla M, Joniau S,
Mason M, Matveev V, Schmid HP, Van der Kwast T, Wiegel T, Zattoni F
and Heidenreich A: EAU guidelines on prostate cancer. Part II:
Treatment of advanced, relapsing, and castration-resistant prostate
cancer. Eur Urol. 59:572–583. 2011. View Article : Google Scholar
|
|
4
|
Hammerer P and Madersbacher S: Landmarks
in hormonal therapy for prostate cancer. BJU Int. 110(Suppl 1):
23–29. 2012. View Article : Google Scholar
|
|
5
|
Wolff JM and Mason M: Drivers for change
in the management of prostate cancer - guidelines and new treatment
techniques. BJU Int. 109(Suppl 6): 33–41. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gao D, Vahdat LT, Wong S, Chang JC and
Mittal V: Microenvironmental regulation of epithelial-mesenchymal
transitions in cancer. Cancer Res. 72:4883–4889. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lim J and Thiery JP:
Epithelial-mesenchymal transitions: insights from development.
Development. 139:3471–3486. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nieto MA and Cano A: The
epithelial-mesenchymal transition under control: global programs to
regulate epithelial plasticity. Semin Cancer Biol. 22:361–368.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Acloque H, Adams MS, Fishwick K,
Bronner-Fraser M and Nieto MA: Epithelial-mesenchymal transitions:
the importance of changing cell state in development and disease. J
Clin Invest. 119:1438–1449. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhau HE, Odero-Marah V, Lue HW, Nomura T,
Wang R, Chu G, Liu ZR, Zhou BP, Huang WC and Chung LW: Epithelial
to mesenchymal transition (EMT) in human prostate cancer: lessons
learned from ARCaP model. Clin Exp Metastasis. 25:601–610. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhau HY, Chang SM, Chen BQ, Wang Y, Zhang
H, Kao C, Sang QA, Pathak SJ and Chung LW: Androgen-repressed
phenotype in human prostate cancer. Proc Natl Acad Sci USA.
93:15152–15157. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhau HE, Li CL and Chung LW: Establishment
of human prostate carcinoma skeletal metastasis models. Cancer.
88(12 Suppl): 2995–3001. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seshacharyulu P, Ponnusamy MP, Haridas D,
Jain M, Ganti AK and Batra SK: Targeting the EGFR signaling pathway
in cancer therapy. Expert Opin Ther Targets. 16:15–31. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gregg J and Fraizer G: Transcriptional
regulation of EGR1 by EGF and the ERK signaling pathway in prostate
cancer cells. Genes Cancer. 2:900–909. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Teixeira AL, Ribeiro R, Cardoso D, Pinto
D, Lobo F, Fraga A, Pina F, Calais-da-Silva F and Medeiros R:
Genetic polymorphism in EGF is associated with prostate cancer
aggressiveness and progression-free interval in androgen
blockade-treated patients. Clin Cancer Res. 14:3367–3371. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cathomas R, Rothermundt C, Klingbiel D,
Bubendorf L, Jaggi R, Betticher DC, Brauchli P, Cotting D, Droege
C, Winterhalder R, Siciliano D, Berthold DR, Pless M, Schiess R,
von Moos R and Gillessen S; Swiss Group for Clinical Cancer
Research SAKK. Efficacy of cetuximab in metastatic
castration-resistant prostate cancer might depend on EGFR and PTEN
expression: results from a phase II trial (SAKK 08/07). Clin Cancer
Res. 18:6049–6057. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Humez S, Monet M, Legrand G, Lepage G,
Delcourt P and Prevarskaya N: Epidermal growth factor-induced
neuroendocrine differentiation and apoptotic resistance of
androgen-independent human prostate cancer cells. Endocr Relat
Cancer. 13:181–195. 2006. View Article : Google Scholar
|
|
18
|
Traish AM and Morgentaler A: Epidermal
growth factor receptor expression escapes androgen regulation in
prostate cancer: a potential molecular switch for tumour growth. Br
J Cancer. 101:1949–1956. 2009. View Article : Google Scholar
|
|
19
|
Gan Y, Shi C, Inge L, Hibner M, Balducci J
and Huang Y: Differential roles of ERK and Akt pathways in
regulation of EGFR-mediated signaling and motility in prostate
cancer cells. Oncogene. 29:4947–4958. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen M, Carkner R and Buttyan R: The
Hedgehog/Gli signaling paradigm in prostate cancer. Expert Rev
Endocrinol Metab. 6:453–467. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim TJ, Lee JY, Hwang TK, Kang CS and Choi
YJ: Hedgehog signaling protein expression and its association with
prognostic parameters in prostate cancer: a retrospective study
from the view point of new 2010 anatomic stage/prognostic groups. J
Surg Oncol. 104:472–479. 2011. View Article : Google Scholar
|
|
22
|
Efstathiou E, Karlou M, Wen S, Hoang A,
Pettaway CA, Pisters LL, Maity S, Troncoso P and Logothetis CJ:
Integrated Hedgehog signaling is induced following castration in
human and murine prostate cancers. Prostate. 73:153–161. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sheng T, Li C, Zhang X, Chi S, He N, Chen
K, McCormick F, Gatalica Z and Xie J: Activation of the Hedgehog
pathway in advanced prostate cancer. Mol Cancer. 3:292004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu K, Fan J, Zhang L, Ning Z, Zeng J, Zhou
J, Li L, Chen Y, Zhang T, Wang X, Hsieh JT and He D: PI3K/Akt to
GSK3β/β-catenin signaling cascade coordinates cell colonization for
bladder cancer bone metastasis through regulating ZEB1
transcription. Cell Signal. 24:2273–2282. 2012.
|
|
25
|
Xue Y, Li L, Zhang D, Wu K, Chen Y, Zeng
J, Wang X and He D: Twisted epithelial-to-mesenchymal transition
promotes progression of surviving bladder cancer T24 cells with
hTERT-dysfunction. PLoS One. 6:e277482011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu G, Zhau HE, He H, Zhang L, Shehata B,
Wang X, Cerwinka WH, Elmore J and He D: Sonic and desert Hedgehog
signaling in human fetal prostate development. Prostate.
67:674–684. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li L, He D, He H, Wang X, Zhang L, Luo Y
and Nan X: Overexpression of PML induced apoptosis in bladder
cancer cell by caspase dependent pathway. Cancer Lett. 236:259–268.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Podlasek CA, Barnett DH, Clemens JQ, Bak
PM and Bushman W: Prostate development requires Sonic hedgehog
expressed by the urogenital sinus epithelium. Dev Biol. 209:28–39.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Berman DM, Desai N, Wang X, Karhadkar SS,
Reynon M, Abate-Shen C, Beachy PA and Shen MM: Roles for Hedgehog
signaling in androgen production and prostate ductal morphogenesis.
Dev Biol. 267:387–398. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Freestone SH, Marker P, Grace OC,
Tomlinson DC, Cunha GR, Harnden P and Thomson AA: Sonic Hedgehog
regulates prostatic growth and epithelial differentiation. Dev
Biol. 264:352–362. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pu Y, Huang L and Prins GS: Sonic
Hedgehog-patched Gli signaling in the developing rat prostate
gland: lobe-specific suppression by neonatal estrogens reduces
ductal growth and branching. Dev Biol. 273:257–275. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sanchez P, Hernández AM, Stecca B, Kahler
AJ, DeGueme AM, Barrett A, Beyna M, Datta MW, Datta S and Ruiz i
Altaba A: Inhibition of prostate cancer proliferation by
interference with SONIC HEDGEHOG-GLI1 signaling. Proc Natl Acad Sci
USA. 101:12561–12566. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stecca B, Mas C and Ruiz i Altaba A:
Interference with HH-GLI signaling inhibits prostate cancer. Trends
Mol Med. 11:199–203. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Azoulay S, Terry S, Chimingqi M, Sirab N,
Faucon H, Gil Diez de Medina S, Moutereau S, Maillé P, Soyeux P,
Abbou C, Salomon L, Vacherot F, de La Taille A, Loric S and Allory
Y: Comparative expression of Hedgehog ligands at different stages
of prostate carcinoma progression. J Pathol. 216:460–470. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Karhadkar SS, Bova GS, Abdallah N, Dhara
S, Gardner D, Maitra A, Isaacs JT, Berman DM and Beachy PA:
Hedgehog signalling in prostate regeneration, neoplasia and
metastasis. Nature. 431:707–712. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shaw G, Price AM, Ktori E, Bisson I,
Purkis PE, McFaul S, Oliver RT and Prowse DM: Hedgehog signalling
in androgen independent prostate cancer. Eur Urol. 54:1333–1343.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen G, Goto Y, Sakamoto R, Tanaka K,
Matsubara E, Nakamura M, Zheng H, Lu J, Takayanagi R and Nomura M:
GLI1, a crucial mediator of sonic Hedgehog signaling in prostate
cancer, functions as a negative modulator for androgen receptor.
Biochem Biophys Res Commun. 404:809–815. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ingham PW, Nakano Y and Seger C:
Mechanisms and functions of Hedgehog signalling across the metazoa.
Nat Rev Genet. 12:393–406. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang L, Xie G, Fan Q and Xie J: Activation
of the Hedgehog-signaling pathway in human cancer and the clinical
implications. Oncogene. 29:469–481. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Varjosalo M and Taipale J: Hedgehog:
functions and mechanisms. Genes Dev. 22:2454–2472. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lauth M and Toftgård R: Non-canonical
activation of GLI transcription factors: implications for targeted
anti-cancer therapy. Cell Cycle. 6:2458–2463. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jenkins D: Hedgehog signalling: emerging
evidence for non-canonical pathways. Cell Signal. 21:1023–1034.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Stecca B and Ruiz i Altaba A:
Context-dependent regulation of the GLI code in cancer by Hedgehog
and non-Hedgehog signals. J Mol Cell Biol. 2:84–95. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ruiz i Altaba A, Mas C and Stecca B: The
Gli code: an information nexus regulating cell fate, stemness and
cancer. Trends Cell Biol. 17:438–447. 2007.PubMed/NCBI
|
|
45
|
Riobo NA, Lu K and Emerson CP Jr: Hedgehog
signal transduction: signal integration and cross talk in
development and cancer. Cell Cycle. 5:1612–1615. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Migliaccio A, Castoria G, Di Domenico M,
Ciociola A, Lombardi M, De Falco A, Nanayakkara M, Bottero D, De
Stasio R, Varricchio L and Auricchio F: Crosstalk between EGFR and
extranuclear steroid receptors. Ann NY Acad Sci. 1089:194–200.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Brand TM, Iida M, Li C and Wheeler DL: The
nuclear epidermal growth factor receptor signaling network and its
role in cancer. Discov Med. 12:419–432. 2011.PubMed/NCBI
|
|
48
|
Dhomen NS, Mariadason J, Tebbutt N and
Scott AM: Therapeutic targeting of the epidermal growth factor
receptor in human cancer. Crit Rev Oncog. 17:31–50. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Peraldo-Neia C, Migliardi G, Mello-Grand
M, Montemurro F, Segir R, Pignochino Y, Cavalloni G, Torchio B,
Mosso L, Chiorino G and Aglietta M: Epidermal growth factor
receptor (EGFR) mutation analysis, gene expression profiling and
EGFR protein expression in primary prostate cancer. BMC Cancer.
11:312011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Schlomm T, Kirstein P, Iwers L, Daniel B,
Steuber T, Walz J, Chun FH, Haese A, Kollermann J, Graefen M,
Huland H, Sauter G, Simon R and Erbersdobler A: Clinical
significance of epidermal growth factor receptor protein
overexpression and gene copy number gains in prostate cancer. Clin
Cancer Res. 13:6579–6584. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Shariat SF, Bensalah K, Karam JA,
Roehrborn CG, Gallina A, Lotan Y, Slawin KM and Karakiewicz PI:
Preoperative plasma HER2 and epidermal growth factor receptor for
staging and prognostication in patients with clinically localized
prostate cancer. Clin Cancer Res. 13:5377–5384. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Festuccia C, Gravina GL, Angelucci A,
Millimaggi D, Muzi P, Vicentini C and Bologna M: Additive antitumor
effects of the epidermal growth factor receptor tyrosine kinase
inhibitor, gefitinib (Iressa), and the nonsteroidal antiandrogen,
bicalutamide (Casodex), in prostate cancer cells in vitro. Int J
Cancer. 115:630–640. 2005. View Article : Google Scholar
|
|
53
|
Palma V, Lim DA, Dahmane N, Sánchez P,
Brionne TC, Herzberg CD, Gitton Y, Carleton A, Alvarez-Buylla A and
Ruiz i Altaba A: Sonic Hedgehog controls stem cell behavior in the
postnatal and adult brain. Development. 132:335–344. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Palma V and Ruiz i Altaba A: Hedgehog-GLI
signaling regulates the behavior of cells with stem cell properties
in the developing neocortex. Development. 131:337–345. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bigelow RL, Jen EY, Delehedde M, Chari NS
and McDonnell TJ: Sonic Hedgehog induces epidermal growth factor
dependent matrix infiltration in HaCaT keratinocytes. J Invest
Dermatol. 124:457–465. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kasper M, Schnidar H, Neill GW, Hanneder
M, Klingler S, Blaas L, Schmid C, Hauser-Kronberger C, Regl G,
Philpott MP and Aberger F: Selective modulation of Hedgehog/GLI
target gene expression by epidermal growth factor signaling in
human keratinocytes. Mol Cell Biol. 26:6283–6298. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Schnidar H, Eberl M, Klingler S,
Mangelberger D, Kasper M, Hauser-Kronberger C, Regl G, Kroismayr R,
Moriggl R, Sibilia M and Aberger F: Epidermal growth factor
receptor signaling synergizes with Hedgehog/GLI in oncogenic
transformation via activation of the MEK/ERK/JUN pathway. Cancer
Res. 69:1284–1292. 2009. View Article : Google Scholar : PubMed/NCBI
|