Introduction
Infection with hepatitis C virus (HCV) leads to
chronic hepatitis in 60–80% of the patients, with the exception of
individuals infected with genotype 2 in Africa, who were found to
eliminate the virus more effectively (~50% of the cases). Chronic
hepatitis C (CH-C) leads to liver cirrhosis in at least 20% of
patients within 20 years after infection and to hepatocellular
carcinoma (HCC) in ~25% of HCV-infected patients (1,2).
Epidemiological studies showed that HCC develops in 3–6% patients
with HCV-related liver cirrhosis and in ~1% of HCV-positive
patients manifesting no signs of cirrhosis (3). In HCV-related carcinogenesis,
participation of viral proteins themselves used to be accentuated,
including at least three HCV proteins: capsid protein (core,
protein C) and two non-structural proteins, NS3 and NS5A (4,5). Such
products of HCV genome have a direct influence on the disturbance
of balance between proliferation and apoptosis of liver cells.
These processes are also regulated by liver-derived growth factors,
to which insulin-like growth factor (IGF)-1 and -2 belong (6,7). IGF-1
is a secretory protein consisting of a single polypeptide chain
with 70 amino acids, which manifests ~50% sequence identity to that
of insulin (8,9). In the postnatal period, liver remains
the main source of circulating IGF-1 and the protein is produced
mainly under effect of growth hormone (GH). Secretion of IGF-1 is
also affected by age, gender, genetic factors, nutrition, insulin
and disease conditions (9–11). IGF-1 produced in the liver exerts
mainly endocrine activity while IGF-1 synthesized by other tissues
acts in a para- and/or autocrine manner (8,9). Aside
from IGF-1, the system consists of the receptors IGF-1R, IGF-2R,
insulin receptor (IR), hybrid dimmers, and at least six high
affinity insulin-like growth factor binding proteins (IGFBPs)
(7). Most of the circulating IGF-1
is found in a ternary complex with IGF binding protein-3 (IGFBP-3)
and the glycoprotein acid-labile subunit (ALS) (11). Binding of IGFBP-3 to IGF-1 prevents
the ligand from interacting with the receptors and IGFBP-3 can
modulate, both in circulation and in extracellular environment, the
extent of IGF-1-dependent effects (12). IGF-1 acts primarily through the
binding and the activation of IGF-1R, and ligation of IGF-1R
initiates intracellular signalling cascades, involved in mitogenic,
cell-survival, anti-apoptotic and transforming activities (13). The IGFBPs may also induce
mitogenesis and cell migration (12). Serum concentration of IGF-1
(S-IGF-1) in childhood grows systematically, most rapidly before
and during pubescence, when it attains the highest levels, reaching
a plateau in early adulthood (10,14). A
systematic slow reduction in the IGF-1 level up to the 80th year of
age is noted (15). Discordant data
are available on a correlation between serum IGFBP-3 (S-IGFBP-3)
and age of patients (14,16–18).
Concentrations of IGF-1 and IGFBP-3 are stable during the day even
if GH, secreted in a pulsatory way, remains to be the principal
factor stimulating production and secretion of the two proteins.
Levels of IGF-1 are more sensitive to GH control than are the
levels of IGFBP-3 (19).
A decreased serum level of both IGF-1 and IGF-2 were
found to parallel progression of liver diseases, independently of
their etiology (20–23). In liver cirrhosis, lower IGF-1
levels were observed in comparison to control (24,25),
as well as its increased concentration following anti-viral therapy
(25). A continuous decline in the
serum concentration of IGF-1 and IGFBP-3 was observed during
progression of cirrhosis and the data correlated significantly with
the Child score index (21). Other
investigations documented decreased levels of IGF-1 and IGF-2 in
chronic liver diseases but the positive correlation with severity
of a disease was detected only for IGF-2 (26). The decreased S-IGF-1 level was more
pronounced in cases with virus-associated compared to
virus-negative HCCs (27). In CH-C,
lowered S-IGF-1 levels correlated with severity of liver
dysfunction (28) and staging
(25). Other investigations found
the reduction of S-IGF-1 level to precede by ~9 months the
development of HCV-associated HCC (29). There are also data on elevated
levels of S-IGF-1 and lowered levels of S-IGFBP-3 as compared to
the control in chronic viral hepatitis (HCV or HBV) (30). Lower levels of S-IGFBP-3 were
observed in liver steatosis (22),
CH-C and liver cirrhosis (18,23,28,31).
Serum levels of IGF-1 <30 ng/ml, IGF-2 <200 ng/ml and IGFBP-3
<6 ng/ml indicated a negative prognosis for patients with liver
cirrhosis (28). Other studies on
patients with already advanced HCC demonstrated markedly lower
concentrations of IGF-1 in HCC developed in the course of HCV
infection than those noted in hepatitis B virus (HBV)-related HCC
(32).
Few studies refer to the role of hepatic IGF-1 and
IGFBP-3 expression in chronic hepatitis as prognostic factors
related to development of HCC (33,34).
In the reports, descriptions of a lowered tissue expression of
IGF-1 (33,34) and of a tendency for an increased
production of IGF-2 prevail (33).
In human HCC samples, IGFBP-3 protein levels were either
undetectable or low compared with non-neoplastic liver tissue
examined by western blotting (35).
The role of the local expression of IGF-1 and IGFBP-3 in the
progression of chronic hepatitis to HCC remains unclear. The loss
of autocrine/paracrine IGFBP-3 loops is thought to potentially lead
to HCC tumor growth (35). Previous
results also suggest that IGFBP-3 has growth-inhibitory activity
which is independent of its IGF binding properties (36). Particularly little information is
available on the coexistence of HCV etiology and tissue expression
of IGF-1 and IGFBP-3 as prognostic factors of HCC development
(34). Following our earlier
studies (37), the aim of this
study was to evaluate the levels of S-IGF-1 and S-IGFBP-3 and
hepatic expression of both proteins in CH-C and HCC samples. The
ratio between IGF-1 and IGFBP-3 was also calculated.
Materials and methods
Patients and tissue material
Studies were performed on serum and liver biopsies
obtained from 37 adult patients (18 men and 19 women) aged 18–63
years (mean age 36±14 years) with CH-C (CH-C group) diagnosed at
the Department of Infectious Diseases, Poznan University of Medical
Sciences. The patients were referred to an anti-viral treatment and
had not previously been treated. The group was recruited in
2010–2012. Infections with other hepatotropic viruses (HBV, HCMV,
EBV) or other reasons of liver damage were excluded. Patients with
diabetes mellitus, kidney failure or any hormone disturbances were
not included in the group. HCV infection was confirmed by the
presence of anti-HCV antibodies (ELISA method, HCV version 3.0 AXYM
System; Abbott) and serum HCV RNA (AMPLICOR HCV™ test, version 2.0;
Roche, Mannheim, Germany). Fifteen healthy volunteers (blood
donors), age- and gender-matched with the cases, were used as a
serum control group (mean age 34±8 years). Plasma levels of IGF-1
were measured by ELISA method (IDS IGF-I ELISA kit;
Immunodiagnostic Systems Ltd., Boldon, UK). The quantitative
measurement of IGFBP-3 in serum was performed with immunoenzymetric
assay (DIAsource IGFBP-3-EASIA Kit; DIAsource Immunoassays S.A.,
Nivelles, Belgium). Results of both tests were expressed in ng/ml.
Liver biopsy and biochemical tests were performed in all cases as a
routine procedure prior to antiviral therapy. Based on USG tests
and α-fetoprotein (AFP) levels, neoplastic growth (HCC) was not
suspected in any patients. Written informed consent was obtained
from all patients prior to liver biopsy and approval for the study
was granted by the institution’s Ethics Committee. The archival
paraffin blocks (without serum samples) with HCC were obtained from
4 patients and tissue microarray panel (n=57) (Cybrdi, Inc.,
Rockville, MD, USA). Mean age of the HCC group was 51±13 years; 39
patients had histological grading of malignancy G2, 13 patients G3
and 9 patients G1. Only one patient from the HCC group was
HCV-positive, in the remaining patients (also in tissue microarray
panel) their serological status related to HCV infection remained
unknown.
The negative control tissue samples were obtained
from livers of serologically HCV-, HBV-, HCMV- and EBV-negative
organ donors and normal livers from tissue microarray panel
(Cybrdi, Inc.) (n=10) (mean age of the group was 50±16 years).
These normal controls were without morphological evidence of
pathology. Liver biopsy specimens and all tissue controls were
fixed in 10% buffered formalin, embedded in paraffin for purposes
of light microscopy and immunocytochemistry.
Histopathological lesions of CH-C patients were
analyzed following the classical H&E staining by two
pathologists employing a numerical scoring system for the grading
(G=0–3) and for the stage of fibrosis (S=0–4) according to the
METAVIR Cooperative Study Group (38). Liver steatosis was also
semiquantitatively appraised, scoring 0 when no fatty degeneration
was noted under a light microscope, and annotating grades 1 or 2
when, respectively, <30% of hepatocytes or 30–70% of hepatocytes
were affected (39).
Immunocytochemistry
For immunocytochemistry, 5 μm sections were cut and
mounted onto SuperFrost/Plus microscope slides. Monoclonal
anti-human IGF-1 and IGFBP-3 antibodies (in a dilution of 1:500 and
1:100, respectively; R&D Systems, Inc.) were employed. The
studies followed the classical (strapt)avidin-biotin-peroxidase
complex (ABC) technique (40), the
details of which were previously described (39). In the case of IGFBP-3,
microwave-oven pretreatment for antigen retrieval was used.
Internal negative control reactions were based on substituting
specific antibodies with normal sera of the respective species in
0.05 M Tris-HCl, pH 7.6, supplemented with 0.1% BSA and 15 mM
sodium azide.
Morphometric study using spatial
visualization method
Histological slides with IGF-1 and IGFBP-3 positive
immunocytochemical expression were examined under the optical
Olympus BH-2 Microscope, coupled to a digital camera. Colour
microscope images were recorded using ×40 objective (at least 10
fields in every slide), 2,560×1,920 pixels in size using LUCIA
Image 5.0 Computer Software, documenting them in jpg format on the
computer hard disc. The image analysis was conducted using a
technique based on spatial visualization of protein expression in
microscope images, elaborated and programmed in the A4D computer
software C++ language (41). Results obtained in the two software
for image analysis (LUCIA Image 5.0 and A4D) were exported to the
format of Microsoft Excel programme. Mean surface area of positive
immunocytochemical reaction for IGF-1 and IGFBP-3 per entire
surface area of liver parenchyma in every patient and every group
of patients was calculated, and expressed in percentage (%), as
previously described (37).
Statistical analysis
First, the parameters of descriptive statistics were
calculated (arithmetic mean, standard deviation, median value,
minimum and maximum values). For statistical analysis of
qualitative traits we employed the Mann-Whitney test for unlinked
samples. In cases of linked traits, Wilcoxon’s test was applied.
Correlations between data rows were determined with the Spearman’s
rank correlation index and Kruskal-Wallis test. The relationships
or differences were considered significant at the significance
level of P≤0.05. The statistical analysis took advantage of the
Statistica PL v. 8 software.
Results
Serum concentration of IGF-1 and IGFBP-3
in the CH-C group and control
S-IGF-1 level was significantly lower in CH-C
patients compared to controls. Notably, there was no significant
difference in S-IGFBP-3 level between the two examined groups. In
the CH-C group, a significantly lower ratio S-IGF-1/IGFBP-3 was
noted, as compared to the control group (Table I).
 | Table IMean serum level ± SD and range (in
brackets) of IGF-1, IGFBP-3 and IGF-1/IGFBP-3 ratio in the CH-C and
the control group. |
Table I
Mean serum level ± SD and range (in
brackets) of IGF-1, IGFBP-3 and IGF-1/IGFBP-3 ratio in the CH-C and
the control group.
| CH-C (n=37) | Control (n=15) | P-value |
|---|
| S-IGF-1
(ng/ml) | 66.18±38.99
(9.22–175.66) | 129.38±56.29
(70.62–289.72) | 0.0001 |
| S-IGFBP-3
(ng/ml) | 919.73±120.61
(727.86–1268.34) | 913.48±94.97
(759.26–1036.65) | 0.842 |
|
S-IGF-1/IGFBP-3 | 0.07±0.05
(0.01–0.19) | 0.14±0.06
(0.08–0.31) | 0.0001 |
Serum concentration of IGF-1 and IGFBP-3
vs. clinicopathological data in the CH-C group
In patients with CH-C, strong negative relationships
were demonstrated between concentration of S-IGF-1, on the one
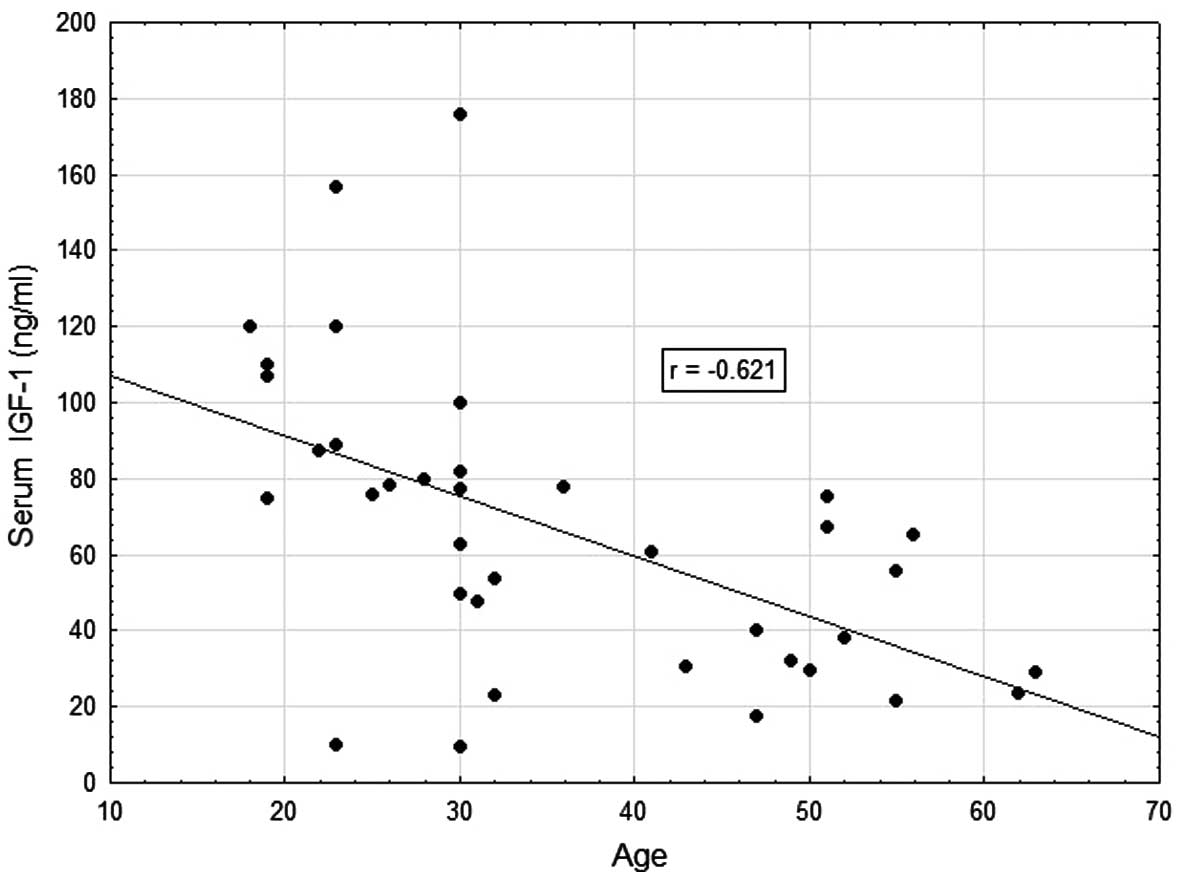
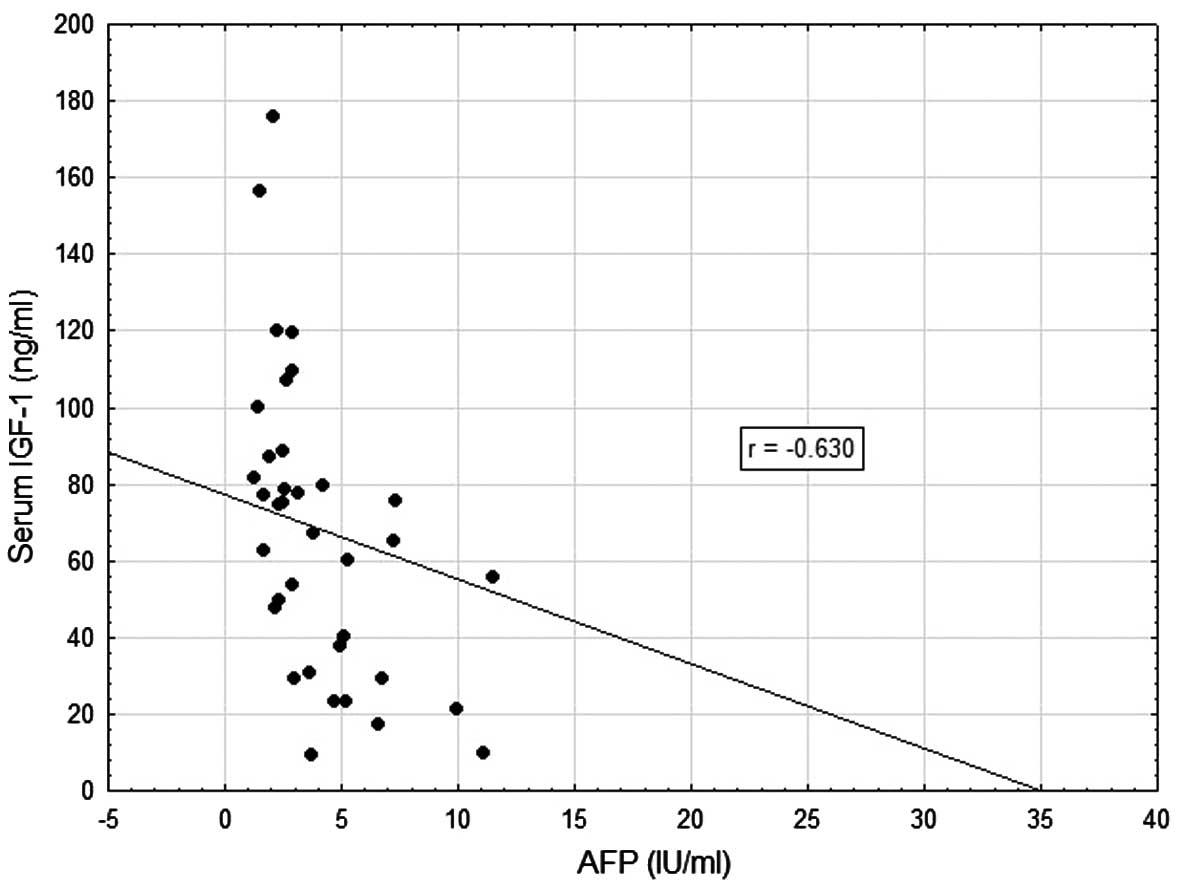
hand, and patient age (r=−0.621; P=0.001) and AFP concentration
(r=−0.630; P=0.001) on the other (Table II, Figs. 1 and 2). Very weak negative correlations were
documented between S-IGF-1, on the one hand, and BMI (r=−0.338;
P=0.04), ALT activity (r=−0.339; P=0.04) and concentration of HCV
RNA (r=−0.332; P=0.04) on the other (Table II). No significant correlations
were detected between S-IGF-1 level, liver steatosis and staging
(Table II) and other studied
biochemical parameters (AST, levels of total protein, albumins, γ
globulins, total bilirubin, glucose, and HOMA-IR) (data not shown).
Upon comparison of serum concentration of the two proteins (IGF-1
and IGFBP-3) using also Wilcoxon’s test for various intensities of
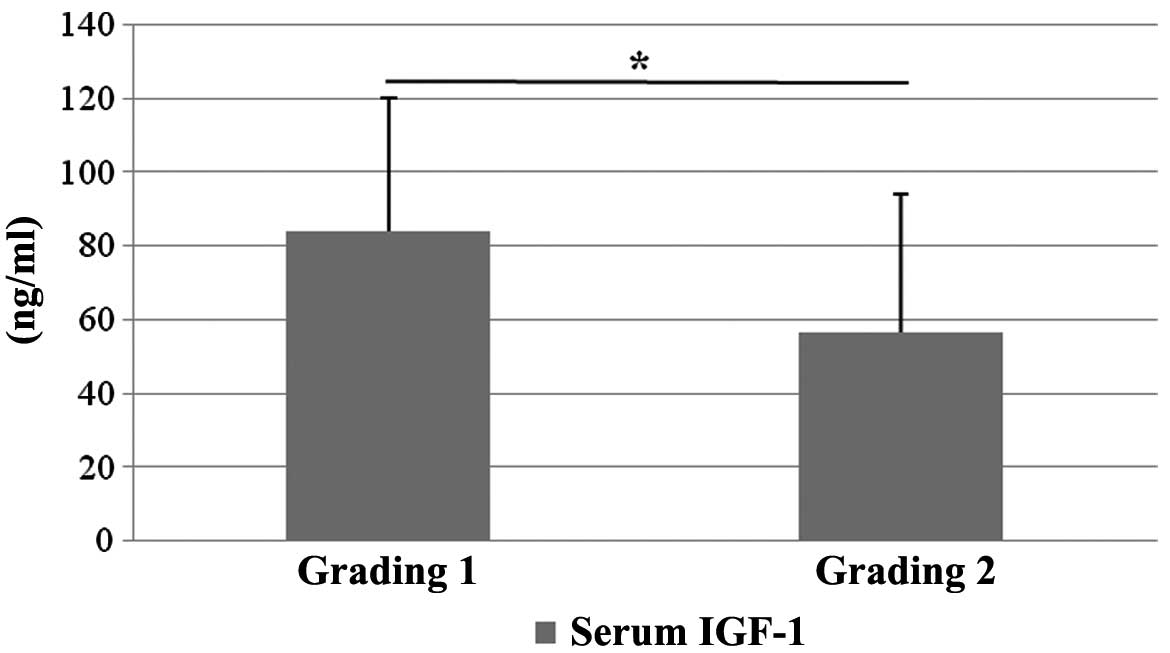
inflammation (grading), a significantly lower S-IGF-1 was detected
in patients with grading 2 (56.56±37.43) as compared to patients
with grading 1 (84.28±36.00; P=0.03) (Fig. 3). The group with grading 3 included
only 3 patients and, therefore, the respective data could not be
compared.
 | Table IISpearman’s correlation coefficients
between IGF-1, IGFBP-3 serum concentration (S) and hepatic
expression (H) vs. selected clinical data in the CH-C group. |
Table II
Spearman’s correlation coefficients
between IGF-1, IGFBP-3 serum concentration (S) and hepatic
expression (H) vs. selected clinical data in the CH-C group.
| Age (years) | BMI | Staginga | Steatosisa | ALT (U/l) | AFP (ng/ml) | HCV RNA
(IU/ml) |
|---|
| S-IGF-1
(ng/ml) | −0.621 | −0.338 | −0.311 | −0.296 | −0.339 | −0.630 | −0.332 |
| H-IGF-1 (% of
reaction) | 0.058 | 0.183 | −0.268 | −0.160 | 0.134 | −0.081 | −0.360 |
| S-IGFBP-3
(ng/ml) | 0.210 | 0.084 | 0.318 | 0.163 | −0.006 | 0.107 | −0.054 |
| H-IGFBP-3 (% of
reaction) | −0.173 | −0.433 | 0.062 | −0.200 | −0.056 | 0.012 | −0.092 |
| S-IGF-1/IGFBP-3
ratio | −0.600 | −0.316 | −0.348 | −0.306 | −0.301 | −0.626 | −0.307 |
| H-IGF-1/IGFBP-3
ratio | 0.313 | 0.479 | −0.158 | 0.242 | 0.099 | 0.019 | 0.001 |
Concentration of S-IGFBP-3 in the CH-C group failed
to manifest significant relationships with any clinical or
pathological variables in the patients (Table II). On the other hand, the
S-IGF-1/IGFBP-3 ratio demonstrated pronounced negative correlations
with: i) patient age (r=−0.600; P=0.001); ii) AFP concentration
(r=−0.626; P=0.001); and weak correlations between iii)
S-IGF-1/IGFBP-3 and liver staging (r=−0.348; P=0.03); and iv) a
tendency for negative correlation between S-IGF-1/IGFBP-3 and
steatosis (r=−0.305; P=0.06) (Table
II). A significantly lower S-IGF-1/IGFBP-3 ratio was detected
in patients with grading 2 (0.06±0.04) as compared to patients with
grading 1 (0.10±0.04; P=0.02) (data not shown).
No significant correlations were detected between
IGF-1/IGFBP-3 index and the remaining clinical variables (BMI, ATL,
HCV-RNA) (Table II) or with levels
of total protein, albumins, γ globulins, total bilirubin, glucose
and HOMA-IR (data not shown).
Tissue expression of IGF-1 and
IGFBP-3
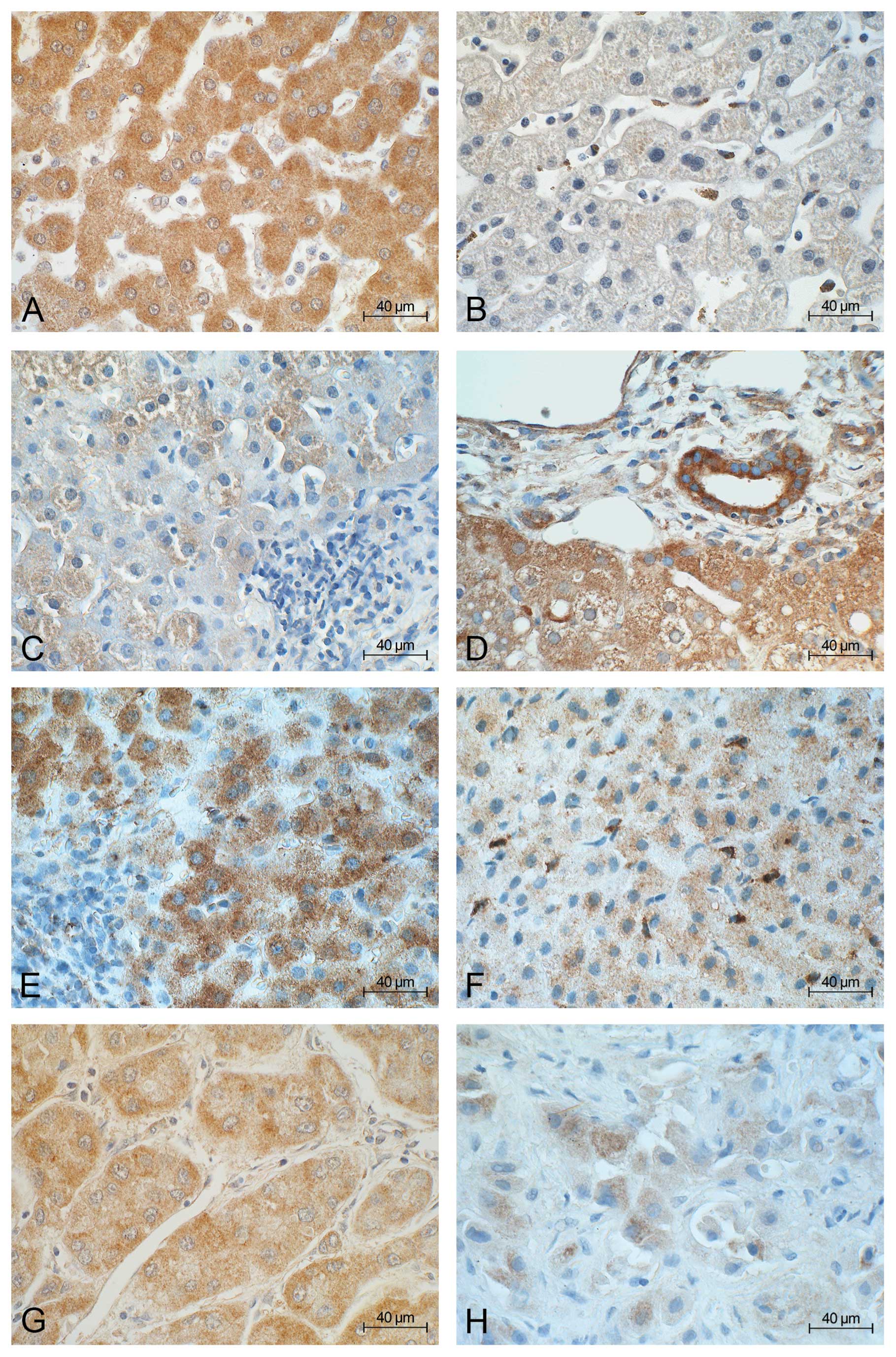
In control livers, expression of IGF-1 was detected
in the cytoplasm of almost all hepatocytes in a high power field
(Fig. 4A), and IGFBP-3 was noted
mainly in cells of liver sinusoids and, in lower amounts, in
hepatocytes as well (Fig. 4B). In
all HCV-infected patients, cytoplasmic expression of IGF-1
prevailed in hepatocytes (Fig. 4C)
and cholangiocytes (Fig. 4D).
IGFBP-3 was detected mainly in hepatocytes (Fig. 4E) but also in cells of liver
sinusoids (Fig. 4F). In HCC cells,
weak cytoplasmic expression of IGF-1 (Fig. 4G) and IGFBP-3 (Fig. 4H) were demonstrated. Mean hepatic
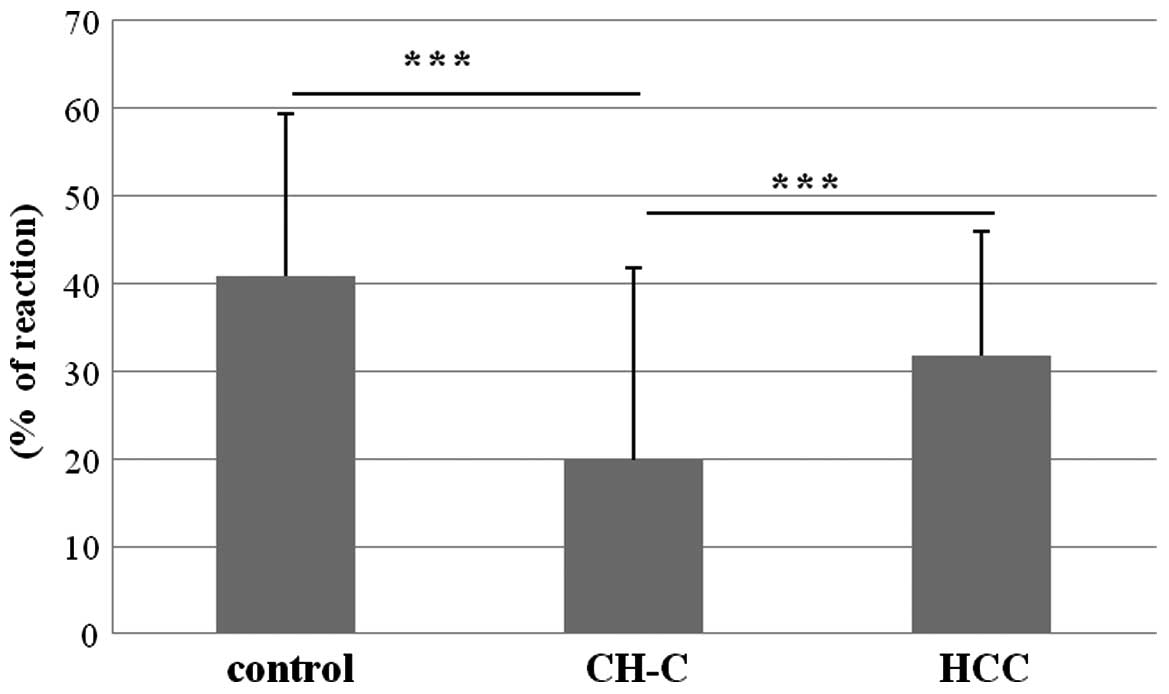
expression of IGF-1 (H-IGF-1) in patients with CH-C was
19.80±22.00% and it was significantly lower than that in the
control (40.81±18.53%) and HCC (31.80±14.25%) (Fig. 5). IGFBP-3 tissue (H-IGFBP-3)
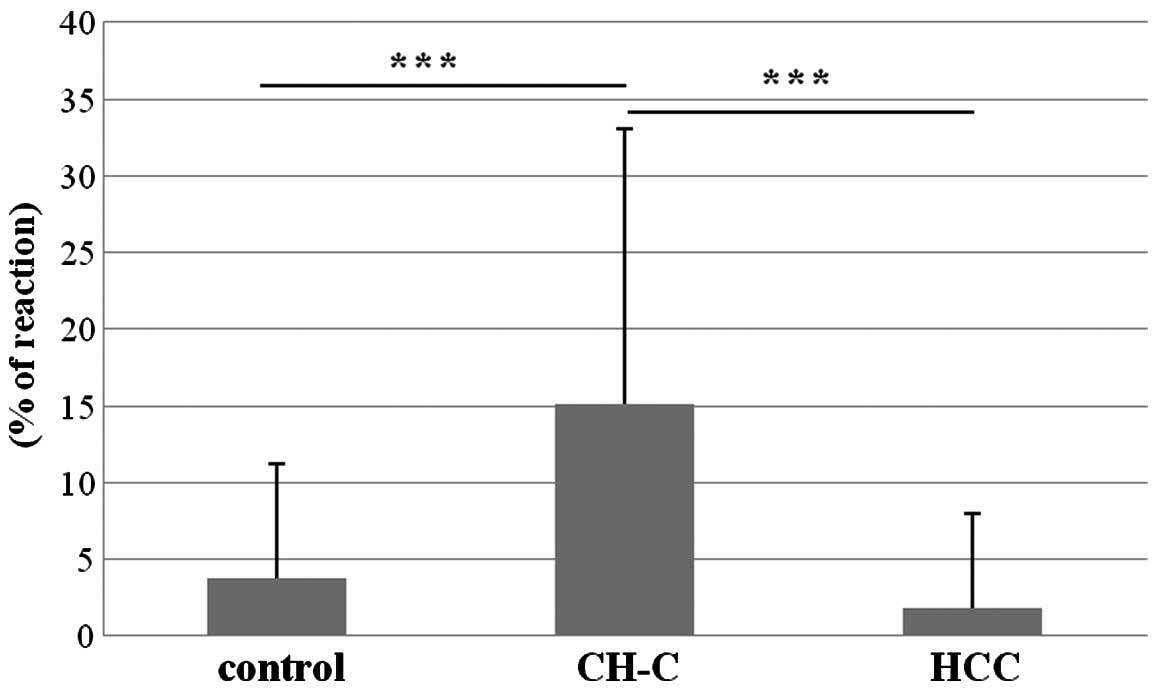
expression in the CH-C group amounted to 15.10±18.00% and it was
significantly higher than that in the control group (3.78±7.40%)
and HCC (1.78±6.16) (Fig. 6). The
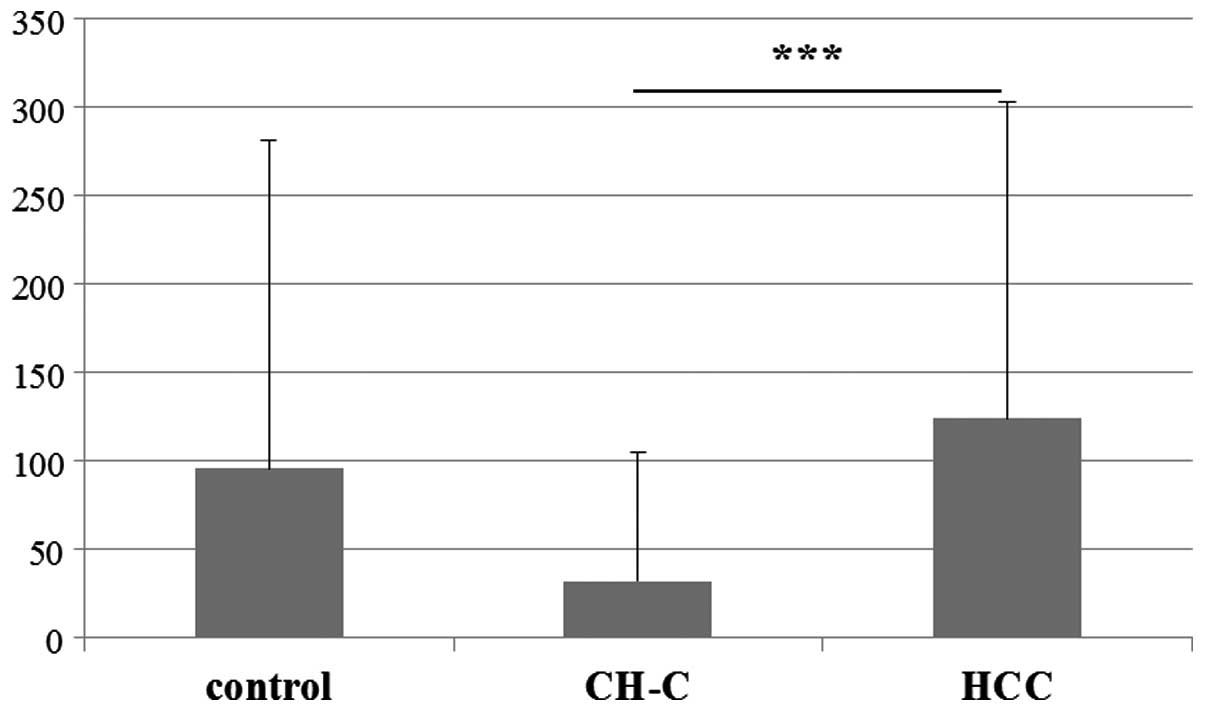
mean H-IGF-1/IGFBP-3 ratio for the CH-C group amounted to
32.20±73.00 and it was significantly lower than that in the HCC
group (124.20±179.24) (Fig. 7) but
it did not differ from that noted in the control group
(95.92±185.52).
Cellular expression of IGF-1 and IGFBP-3
in HCC samples
Expression of both IGF-1 and IGFBP-3 was
demonstrated mainly in the cytoplasm of neoplastic cells (Fig. 4G and H). Intensity of two protein
expressions manifested extensive individual differences.
Quantitative analysis showed that expression of IGF-1 in studied
HCC samples (H-IGF-1) was reduced as compared to that in the
control (P=0.05) (Fig. 5). Hepatic
IGFBP-3 expression in HCC showed no significant differences as
compared to the control (P=0.088) (Fig.
6). Also, no significant differences were observed between the
hepatic IGF-1/IGFBP-3 ratio in this group of patients and in the
control (P=0.230) (Fig. 7). Results
of comparative tissue expression in HCC samples and in CH-C were
described above (Figs. 5–7). Although it was least pronounced in
grading 3 (mean of 24.64±14.14%), expression of IGF-1 in HCC
samples manifested no significant differences as compared to the
expression in grading 1 (33.36±16.78) or grading 2 (33.61±13.53)
(data not shown). Similar differences were related to H-IGFBP-3
expression, which amounted to the mean of 2.32±5.02% in grading 1,
2.19±7.29 in grading 2 and 0.17±0.29 in grading 3, none of which
were significantly different. The highest IGF-1/IGFBP-3 index was
noted in grading 2 (150.06±217.65), as compared to 91.60±117.07 in
grading 1 and 83.50±57.81 in grading 3. Statistical analysis
manifested no significant differences related to the degree of
histological malignancy. Also, no significant reciprocal
correlation was found between expressions of H-IGF-1 and H-IGFBP-3
in HCC samples (r=0.143; P>0.05) (data not shown).
Gender differences in IGF-1 and IGFBP-3
serum levels and liver expression in the CH-C group
Compared to male patients, a significantly higher
concentration of S-IGF-1 was detected in women with CH-C but no
gender-related differences were detected in concentrations of
S-IGFBP-3. Furthermore, no gender-related differences were revealed
in tissue expressions of IGF-1 and IGFBP3. A higher S-IGF-1/IGFBP-3
index was found in women, as compared to men, in the patient group,
with no gender-related differences in values of IGF-1/IGFBP-3
tissue ratio (Table III).
 | Table IIIGender differences in serum levels
and hepatic expression of IGF-1, IGFBP-3 and IGF-1/IGFBP-3 ratio in
the CH-C group. |
Table III
Gender differences in serum levels
and hepatic expression of IGF-1, IGFBP-3 and IGF-1/IGFBP-3 ratio in
the CH-C group.
| Gender | n | Mean | SD | Min | Max | P-value |
|---|
| S-IGF-1
(ng/ml) | Female | 19 | 80.85 | 42.04 | 23.35 | 175.66 | 0.022 |
| Male | 18 | 50.70 | 29.21 | 9.22 | 99.95 | |
| S-IGFBP-3
(ng/ml) | Female | 19 | 919.10 | 134.61 | 726.86 | 1268.34 | 0.916 |
| Male | 18 | 920.35 | 107.78 | 754.03 | 1188.43 | |
| H-IGF-1 (% of
reaction) | Female | 17 | 17.43 | 23.90 | 0.00 | 77.23 | 0.140 |
| Male | 17 | 22.18 | 19.47 | 3.35 | 57.48 | |
| H-IGFBP-3 (% of
reaction) | Female | 18 | 18.88 | 18.15 | 0.02 | 54.18 | 0.135 |
| Male | 16 | 10.85 | 16.34 | 0.00 | 53.05 | |
| S-IGF-1/IGFBP-3
ratio | Female | 19 | 0.09 | 0.05 | 0.02 | 0.19 | 0.029 |
| Male | 18 | 0.01 | 0.03 | 0.01 | 0.12 | |
| H-IGF-1/IGFBP-3
ratio | Female | 17 | 26.23 | 76.99 | 0.00 | 313.00 | 0.075 |
| Male | 15 | 38.90 | 71.06 | 0.06 | 264.33 | |
Reciprocal correlation between serum
concentration and tissue expression of IGF-1 and IGFBP-3 in the
CH-C group
No significant relationships were found between
concentrations of S-IGF-1 and S-IGFBP-3 (r=−0.172; P>0.05). No
significant correlations were found between concentration of
S-IGF-1 and hepatic expression of the protein (r=0.251; P>0.005)
(data not shown). No significant relationships were detected
between levels of S-IGF-1 and H-IGFBP-3 expression (r=0.244;
P>0.05). Also, no correlation was detected between expressions
of H-IGF-1 and H-IGFBP-3 (r=0.042; P>0.05) (data not shown).
Tissue expression of IGF-1 and IGFBP-3
vs. clinical data in the CH-C group
Liver IGF-1 expression in the CH-C group
demonstrated only a weak negative correlation with serum load of
HCV RNA (r=−0.360; P=0.04). Expression of H-IGFBP-3 manifested a
weak negative correlation with patient BMI (r=−0.433; P=0.01)
(Table II). No significant
correlations were detected between H-IGF-1 and H-IGFBP-3
expressions, on the one hand, and ALT, AFP, staging and steatosis
(Table II) and other biochemical
parameters (AST, levels of total protein, albumins, γ globulins,
total bilirubin, glucose, and HOMA-IR) on the other (data not
shown). Liver IGF-1/IGFBP-3 ratio was directly related to patient
BMI (r=0.479; P=0.006) (Table II).
The remaining correlations of the index with clinicopathological
data proved to be insignificant (data not shown).
In the CH-C group, the hepatic IGF-1/IGFBP-3
(32.20±73.00) ratio was significantly higher than the serum
IGF-1/IGFBP-3 ratio (0.07±0.05) (P=0.0001) (data not shown).
Discussion
In the stabilization of circulating IGF-1 levels,
the factors involved include age, body nutrition, genetic factors,
pregnancy dependent factors and disease conditions. The effect of
gender remains insufficiently defined (11). In liver diseases, serum level of
IGF-1 is thought to provide a useful marker of the organ function,
independently of etiology and patient age (20,24,42).
In the CH-C group, our investigations confirmed the negative
correlation between concentration of S-IGF-1 and patient age
described by other authors (43,44).
Moreover, in the CH-C group, we demonstrated that mean S-IGF-1
concentration is higher in women as compared to men. In women and
men with normal liver function, a similar situation was described
by Yu et al(15). Other
authors demonstrated no differences in S-IGF-1 values between men
and women (17,45). Reports are also available which
suggest slightly lower levels in females as compared to males
(11). In our patients with CH-C,
serum concentration of IGFBP-3 showed no differences related to age
and gender although higher concentrations of S-IGFBP-3 were
detected in women as compared to those in men (17). The serum ratio of IGF-1/IGFBP-3 was
also higher in the women compared to the men with CH-C in our study
and value of the parameter was shown to depend on patient age. This
is partially consistent with previous data on healthy adults in
whom S-IGFBP3 and ratio of S-IGF-1/IGFBP-3 were age-dependent
(14). The relationship between
S-IGF-1 concentration and nutritional status is well recognized; in
persons with BMI <21 and BMI >29 decreased levels of IGF-1
are noted (11,46). This has been corroborated by our
studies on CH-C patients although, in this case, the negative
correlation between S-IGF-1 concentration and BMI was very weak. On
the other hand, concentration of S-IGFBP-3 manifested no
correlation with BMI in our patients with CH-C, which is also
consistent with previous literature (47).
In our studies, examination of serum IGF-1
concentration confirmed previous reports which observed lower
concentrations in CH-C or in HCV-related liver cirrhosis, as
compared to the control (25,26,29,31,32,48).
Patients with chronic HCV infection manifested GH insufficiency and
persistent GH resistance of hepatocytes, which may also promote a
decrease in liver production of IGF-1 (48). In patients with HCV-related
cirrhosis, it was even demonstrated that the reduction in IGF-1
level preceded the diagnosis of HCC by 9.3±3.1 months (29). In the present study, we demonstrated
significantly lower concentrations of S-IGF-1 in HCV-infected
patients with a more intense inflammatory activity (grading 2), as
compared to patients with less advanced lesions (grading 1). On the
other hand, our study is not in agreement with Lorenzo-Zúñiga et
al, who showed in HCV infection that mean IGF-1 values in serum
were lower in patients with advanced fibrosis compared to the other
patients (25). Absence of such a
relationship as related to staging and liver steatosis in our
findings most probably reflects the fact that in most of our
patients liver steatosis and advancement of fibrosis (staging) were
insignificant. On the other hand, a highly pronounced negative
correlation was found between concentrations of IGF-1 and AFP in
serum of patients with CH-C. This indicates that the two markers of
a potential prognostic significance coexist.
In our CH-C group, tissue expression of IGF-1 failed
to correlate with concentration of S-IGF-1. However, studies on
tissue level have confirmed a significantly lower local expression
of IGF-1 as compared to the control. Morali et al(33) also observed a significant reduction
in hepatic production of IGF-1 in chronic hepatitis and cirrhosis,
although the etiology of respective liver lesions in the studies
was unknown. In another study on 34 HCV-infected patients, no
significant differences were detected in expression of IGF-1 mRNA
as compared to the control (34).
As previously described, this study also confirmed a lower than
that in the control hepatic expression of IGF-1 in HCC samples
(36).
Current investigations have demonstrated no
differences in concentration of S-IGFBP-3 in CH-C and in the
control. No significant relationships have been detected between
serum levels of IGFBP-3 and clinical data. Other studies on the
significance of this component of IGF axis in chronic hepatitis
were focused mainly on alcohol- or HBV-related liver injuries
(28,47). Although the lowered concentration of
IGFBP-3 was also documented in patients with CH-C, as compared to
the control (30,31), no correlation was detected with the
clinicopathological data (grading, staging, transaminase levels)
(30) or negative correlations were
demonstrated with age and AST activity, and positive correlations
with serum albumin and prothrombin concentration (31).
In the present study, mean value of serum
IGF-1/IGFBP-3 index was significantly lower in CH-C patients
compared to control individuals. Introduction of the parameter
permitted us to additionally manifest the relatively more or less
pronounced negative correlations of the parameter with several
clinical data (age, grading, AFP concentration, staging). In our
patients, we were not able to confirm the apparently evident
coexistence of lowered S-IGF-1 and serum IGF-1/IGFBP-3 values and
the highest IGFBP-3 levels with liver steatosis, reported earlier
by other authors in their studies on isolated fatty degeneration of
the liver (22) or non-alcoholic
fatty liver disease and portal fibrosis (49).
In the present study, quantitative evaluation of
tissue IGFBP-3 expression in HCV-infected livers demonstrated a
notably significant overexpression of the protein in CH-C patients
as compared to the control and HCC patients. Expression of IGFBP-3
protein in livers of patients with CH-C has been demonstrated
mainly in hepatocytes while in a normal liver, expression of
IGFBP-3 has been demonstrated mainly in Kupffer cells and in portal
venous and sinusoidal endothelium, in line with observations of
other authors (50). Previous
studies in hepatocytes of a normal or cirrhotic human liver
observed gene expression of three IGF-1 binding proteins (IGFBP-1,
-2 and -3). However, parallel estimations of the three protein
concentrations in serum of the patients detected lowered
concentrations of IGFBP-3 in cirrhotic liver, as compared to the
control (51). The increased local
expression of IGFBP-3 in the studied patients with CH-C and the
higher hepatic IGF-1/IGFBP-3 index, in comparison to serum
IGF-1/IGFBP-3 ratio may suggest a disturbed release of IGFBP-3 from
HCV-infected cells. Since no significant correlations have been
detected between H-IGFBP-3 and clinical data (except for the weak
negative correlation with BMI), the role of local IGFBP-3
overexpression in patients with CH-C requires further studies. In
HCC samples, we demonstrated a high value of tissue IGF-1/IGFBP-3
index, a significantly higher one than that detected in the CH-C
group. By contrast, Mattera et al detected an augmented
serum IGF-1/IGFBP-3 index in patients with HCC, as compared to
patients with liver cirrhosis (52). No reciprocal relationships were
found between serum concentration and hepatic expression of either
IGF-1 or IGFBP-3 in our CH-C group.
In conclusion, we noted lowered serum level of IGF-1
in CH-C as compared to controls. The lowered concentration of
S-IGF-1 was linked to an increased inflammatory activity (grading).
Tissue expression of IGF-1 was also lower in CH-C, and that of
IGFBP-3 protein was increased as compared to healthy liver. In HCC
samples, a decreased liver expression of IGF-1 was observed as
compared to the control and a higher hepatic ratio of
IGF-1/IGFBP-3, but only as compared to the CH-C group. Considering
the serum concentration of both studied proteins, concentrations of
S-IGF-1 in particular may be accepted as a suitable marker of liver
injury in CH-C. Moreover, the studies suggest that inflammatory
lesions induced by chronic HCV infection result in a lowered
hepatic production of IGF-1, with the resulting decrease in serum
IGF-1 concentration. A lowered concentration of S-IGF-1 and H-IGF-1
coexists with other exponents of liver injury in HCV infection.
Apart from IGF-1, evaluation of serum IGF-1/IGFBP-3 ratio provides
another index allowing to evaluate hepatic function. An increased
hepatic expression of IGFBP-3 with no parallel increase in
concentration of IGFBP-3 in sera of patients with CH-C may point to
a blocked release of IGFBP-3 to circulation as well as to a
potential autocrine/paracrine role of the protein in HCV infection.
The results indicate a disturbed functioning of IGF axis in HCV
infection. Apart from IGF-1 alone and AFP, determination of serum
IGF-1/IGFBP-3 ratio may serve as an additional non-invasive marker
of a progressive liver injury.
Acknowledgements
The present study was supported by a grant from the
Ministry of Education and Science, Warsaw, Poland (no.
NN401009437).
References
|
1
|
Fattovich G, Stroffolini T, Zagni I and
Donato F: Hepatocellular carcinoma in cirrhosis: incidence and risk
factors. Gastroenterology. 127:S35–S50. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rehermann B and Nascimbeni M: Immunology
of hepatitis B virus and hepatitis C virus infection. Nat Rev
Immunol. 5:215–229. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Colombo M: Hepatitis C virus and
hepatocellular carcinoma. Semin Liver Dis. 19:263–269. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Anzola M: Hepatocellular carcinoma: role
of hepatitis B and hepatitis C viruses proteins in
hepatocarcinogenesis. J Viral Hepat. 11:383–393. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kasprzak A and Adamek A: Role of hepatitis
C virus proteins (C, NS3, NS5A) in hepatic oncogenesis. Hepatol
Res. 38:1–26. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alexia C, Fallot G, Lasfer M,
Schweizer-Groyer G and Groyer A: An evaluation of the role of
insulin-like growth factors (IGF) and of type-I IGF receptor
signalling in hepatocarcinogenesis and in the resistance of
hepatocellular cells against drug-induced apoptosis. Biochem
Pharmacol. 86:1003–1015. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kasprzak A and Adamek A: The insulin-like
growth factor (IGF) signaling axis and hepatitis C virus-associated
carcinogenesis (Review). Int J Oncol. 41:1919–1931. 2012.PubMed/NCBI
|
|
8
|
Daughday WH and Rotweien P: Insulin-like
growth factors I and II: peptide, messenger ribonucleic acid, and
gene structures, serum and tissue concentrations. Endocr Rev.
10:68–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zarilli R, Bruni CB and Riccio A: Multiple
levels of control of insulin-like growth factor gene expression.
Mol Cell Endocrinol. 101:R1–R14. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Juul A, Bang P, Hertel NT, et al: Serum
insulin-like growth factor-I in 1030 healthy children, adolescents,
and adults: relation to age, sex, stage of puberty, testicular
size, and body mass index. J Clin Endocrinol Metab. 78:744–752.
1994.PubMed/NCBI
|
|
11
|
Brabant G and Wallaschofski H: Normal
levels of serum IGF-I: determinants and validity of current
reference ranges. Pituitary. 10:129–133. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kostecka Y and Blahovec J: Insulin-like
growth factor binding proteins and their functions (minireview).
Endocr Regul. 33:90–94. 1999.PubMed/NCBI
|
|
13
|
Le Roith D, Bondy C, Yakar S, Liu JL and
Butler A: The somatomedin hypothesis: 2001. Endocr Rev. 22:53–74.
2001.
|
|
14
|
Elmlinger MW, Kühnel W, Weber MM and Ranke
MB: Reference ranges for two automated chemiluminescent assays for
serum insulin-like growth factor I (IGF-I) and IGF-binding protein
3 (IGFBP-3). Clin Chem Lab Med. 42:654–664. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu H, Mistry J, Nicar MJ, Khosravi MJ,
Diamandis A, van Doorn J and Juul A: Insulin-like growth factors
(IGF-I, free IGF-I, and IGF-II) and insulin-like growth factor
binding proteins (IGFBP-2, IGFBP-3, IGFBP-6, and ALS) in blood
circulation. J Clin Lab Anal. 13:166–172. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Juul A, Dalgaard P, Blum WF, et al: Serum
levels of insulin-like growth factor (IGF)-binding protein-3
(IGFBP-3) in healthy infants, children, and adolescents: the
relation to IGF-I, IGF-II, IGFBP-1, IGFBP-2, age, sex, body mass
index, and pubertal maturation. J Clin Endocrinol Metab.
80:2534–2542. 1995.PubMed/NCBI
|
|
17
|
Mattsson A, Svensson D, Schuett B,
Osterziel KJ and Ranke MB: Multidimensional reference region for
IGF-I, IGFBP-2 and IGFBP-3 concentrations in serum healthy adults.
Growth Horm IGF Res. 18:506–516. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Raslan HM, Elhosary Y, Ezzat WM, Rasheed
EA and Rasheed MA: The potential role of insulin-like growth factor
1, insulin-like growth factor binding protein 3 and bone mineral
density in patients with chronic hepatitis C virus in Cairo, Egypt.
Trans R Soc Trop Med Hyg. 104:429–432. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Blum WF, Albertsson-Wikland K, Rosberg S
and Ranke MB: Serum levels of insulin-like growth factor I (IGF-I)
and IGF binding protein 3 reflect spontaneous growth hormone
secretion. J Clin Endocrinol Metab. 76:1610–1616. 1993.PubMed/NCBI
|
|
20
|
Wu JC, Daughaday WH, Lee SD, et al:
Radioimmunoassay of serum IGF-I and IGF-II in patients with chronic
liver diseases and hepatocellular carcinoma with or without
hypoglycemia. J Lab Clin Med. 112:589–594. 1988.PubMed/NCBI
|
|
21
|
Kratzsch J, Blum WF, Schenker E and Keller
E: Regulation of growth hormone (GH), insulin-like growth factor
(IGF) I, IGF binding proteins -1, -2, -3 and GH binding protein
during progression of liver cirrhosis. Exp Clin Endocrinol
Diabetes. 103:285–291. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Völzke H, Nauck M, Rettig R, Dörr M,
Higham C, Brabant G and Wallaschofski H: Association between
hepatic steatosis and serum IGF1 and IGFBP-3 levels in a
population-based sample. Eur J Endocrinol. 161:705–713.
2009.PubMed/NCBI
|
|
23
|
Rehem RN and El-Shikh WM: Serum IGF-1,
IGF-2 and IGFBP-3 as a parameters in the assessment of liver
dysfunction in patients with hepatic cirrhosis and in the diagnosis
of hepatocellular carcinoma. Hepatogastroenterology. 58:949–954.
2011.PubMed/NCBI
|
|
24
|
Vyzantiadis T, Theodoridou S, Giouleme O,
Harsoulis P, Evgenidis N and Vyzantiadis A: Serum concentrations of
insulin-like growth factor-I (IGF-I) in patients with liver
cirrhosis. Hepatogastroenterology. 50:814–816. 2003.PubMed/NCBI
|
|
25
|
Lorenzo-Zúñiga V, Bartolí R, Masnou H,
Montoliu S, Morillas RM and Planas R: Serum concentration of
insulin-like growth factor-I (igf-I) as a marker of liver fibrosis
in patients with chronic hepatitis C. Dig Dis Sci. 52:3245–3250.
2007.PubMed/NCBI
|
|
26
|
Nikolić JA, Todorović V, Bozić M, et al:
Serum insulin-like growth factor (IGF)-II is more closely
associated with liver disfunction than is IGF-I in patients with
cirrhosis. Clin Chim Acta. 294:169–177. 2000.PubMed/NCBI
|
|
27
|
Stuver SO, Kuper H, Tzonou A, Lagiou P,
Spanos E, Hsieh CC, Mantzoros C and Trichopoulos D: Insulin-like
growth factor 1 in hepatocellular carcinoma and metastatic liver
cancer in men. Int J Cancer. 87:118–121. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu YL, Ye J, Zhang S, Zhong J and Xi RP:
Clinical significance of serum IGF-I, IGF-II and IGFBP-3 in liver
cirrhosis. World J Gastroenterol. 10:2740–2743. 2004.PubMed/NCBI
|
|
29
|
Mazziotti G, Sorvillo F, Morisco F, et al:
Serum insulin-like growth factor I evaluation as a useful tool for
predicting the risk of developing hepatocellular carcinoma in
patients with hepatitis C virus-related cirrhosis: a prospective
study. Cancer. 95:2539–2545. 2002. View Article : Google Scholar
|
|
30
|
Okan A, Cömlekçi A, Akpinar H, Okan I,
Yeşil S, Tankurt E and Simşek I: Serum concentration of
insulin-like growth factor-I and insulin-like growth factor binding
protein-3 in patients with chronic hepatitis. Scand J
Gastroenterol. 35:1212–1215. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Raslan H, Ezzat W, Ahmed M and Rasheed E:
Insulin growth-factor-1 and insulin-like growth factor binding
protein-3 in Egyptian patients with chronic hepatitis C. Arch Med
Sci. 3:46–51. 2007.
|
|
32
|
Su WW, Lee KT, Yeh YT, Soon MS, Wang CL,
Yu ML and Wang SN: Association of circulating insulin-like growth
factor 1 with hepatocellular carcinoma: one cross-sectional
correlation study. J Clin Lab Anal. 24:195–200. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Morali G, Shitrit AB, Eran M, Freier S,
Reinus C and Braverman D: Hepatic production of insulin-like growth
factors in normal and diseased liver. Hepatogastroenterology.
52:1511–1515. 2005.PubMed/NCBI
|
|
34
|
Stefano JT, Correa-Giannella ML, Ribeiro
CMF, Alves VAF, Massarollo PCB, Machado MCC and Giannella-Neto D:
Increased hepatic expression of insulin-like growth factor-I
receptor in chronic hepatitis C. World J Gastroenterol.
28:3821–3828. 2006.PubMed/NCBI
|
|
35
|
Huynh H, Chow PKH, Ooi LLP and Soo KC: A
possible role for insulin-like growth factor-binding protein-3
autocrine/paracrine loops in controlling hepatocellular carcinoma
cell proliferation. Cell Growth Differ. 13:115–122. 2002.
|
|
36
|
Clemmons DR: Insulin-like growth factor
binding proteins and their role in controlling IGF actions.
Cytokine Growth Factor Rev. 8:45–62. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kasprzak A, Adamek A, Przybyszewska W, et
al: Expression of IGF-1 and viral proteins (C, NS3, NS5A) in the
livers of patients with chronic HCV infection. Adv Clin Exp Med.
20:263–273. 2011.
|
|
38
|
Bedossa P and Poynard T: An algorithm for
the grading of activity in chronic hepatitis C. The METAVIR
Cooperative Study Group. Hepatology. 24:289–293. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kasprzak A, Adamek A, Biczysko W, et al:
Intracellular expression of the proliferative marker Ki-67 and
viral proteins (NS3, NS5A and C) in chronic, long lasting hepatitis
C virus (HCV) infection. Folia Histochem Cytobiol. 45:357–366.
2007.PubMed/NCBI
|
|
40
|
Hsu SM, Raine L and Fanger H: Use of
avidin-biotin-peroxidase complex (ABC) in immunoperoxidase
techniques: a comparison between ABC and unlabeled antibody (PAP)
procedures. J Histochem Cytochem. 29:577–580. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kaczmarek E and Strzelczyk R: From two to
three-dimensional visualisation of structures in light and confocal
microscopy - applications for biomedical studies. Current Issues on
Multidisciplinary Microscopy Research and Education. Mendez-Vilas A
and Labajos-Broncano L: (FORMATEX microscopy book series no II).
Formatex Research Centre; Badajoz: pp. 289–295. 2005
|
|
42
|
Mahdy KA, Ahmed HH, Mannaa F and
Abdel-Shaheed A: Clinical benefits of biochemical markers of bone
turnover in Egyptian children with chronic liver diseases. World J
Gastroenterol. 13:785–790. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Aimaretti G, Boschetti M, Corneli G, et
al: Normal age-dependent values of serum insulin growth factor-I:
results from a healthy Italian population. J Endocrinol Invest.
31:445–449. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Andreassen M, Nielsen K, Raymond I,
Kristensen LØ and Faber J: Characteristics and reference ranges of
insulin-like growth factor-I measured with a commercially available
immunoassay in 724 healthy adult Caucasians. Scand J Clin Lab
Invest. 69:880–885. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rosario PW: Normal values of serum IGF-1
in adults: results from a Brazilian population. Arq Bras Endocrinol
Metabol. 54:477–481. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Holmes MD, Pollak MN and Hankinson SE:
Lifestyle correlates of plasma insulin-like growth factor I and
insulin-like growth factor binding protein 3 concentrations. Cancer
Epidemiol Biomarkers Prev. 11:862–867. 2002.
|
|
47
|
Colakošlu O, Taşkiran B, Colakoğlu G,
Kizildağ S, Ari Ozkan F and Unsal B: Serum insulin like growth
factor-1 (IGF-1) and insulin growth factor binding protein-3
(IGFBP-3) levels in liver cirrhosis. Turk J Gastroenterol.
18:245–249. 2007.
|
|
48
|
Plöckinger U, Krüger D, Bergk A, Weich V,
Wiedenmann B and Berg T: Hepatitis-C patients have reduced growth
hormone (GH) secretion which improves during long-term therapy with
pegylated interferon-alpha. Am J Gastroenterol. 102:2724–2731.
2007.PubMed/NCBI
|
|
49
|
Ichikawa T, Nakao K, Hamasaki K, et al:
Role of growth hormone, insulin-like growth factor 1 and
insulin-like growth factor-binding protein 3 in development on
non-alcoholic fatty liver disease. Hepatol Int. 1:287–294. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Arany E, Afford S, Strain AJ, Winwood PJ,
Arthur MJ and Hill DJ: Differential cellular synthesis of
insulin-like growth factor binding protein-1 (IGFBP-1) and IGFBP-3
within human liver. J Clin Endocrinol Metab. 79:1871–1876.
1994.PubMed/NCBI
|
|
51
|
Ross RJM, Chew SL, D’Souza Li L, et al:
Expression of IGF-I and IGF-binding protein genes in cirrhotic
liver. J Endocrinol. 149:209–216. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mattera D, Capuano G, Colao A, Pivonello
R, Manguso F, Puzziello A and D’Agostino L: Increased IGF-I:IGFBP-3
ratio in patients with hepatocellular carcinoma. Clin Endocrinol
(Oxf). 59:699–706. 2003. View Article : Google Scholar : PubMed/NCBI
|