Introduction
The presence or absence of lymph node metastasis is
an important prognostic factor in breast cancer (1). However, the significance of lymph node
micro-involvement remains unclear (2,3). In
the UICC TNM classification (7th edition), micrometastases are
defined as metastases with a long diameter of 2 mm or less as
observed in section samples; those with diameters of 0.2 mm or less
are defined as isolated tumor cells (ITCs). While ITCs are not
thought to affect outcomes, micrometastases are considered to be a
prognostic factor (4).
In clinically node-negative breast cancer, the
presence or absence of lymph node metastasis is primarily
determined by sentinel node (SN) biopsy (5). When omitting axillary dissection based
on SN biopsy results, detection of micrometastases using a rapid
diagnostic method would be beneficial (4). However, the accuracy of conventional
intraoperative diagnosis remains low. Although the false-negative
rate could be reduced by preparing several 2-mm-thick sections, the
diagnostic potential of frozen sections is limited (6).
One of the current commonly used techniques for
intraoperative diagnosis of nodal metastasis is rapid
immunohistochemistry (IHC). Rapid IHC aims to improve the accuracy
of frozen-section diagnoses. However, this method has drawbacks
that make it difficult to use for the diagnosis of micrometastases
and ITCs (7). Qualitative diagnosis
of cells is poor given the lack of cytoplasmic staining, and
sections used for rapid IHC are prepared differently from those
used for hematoxylin and eosin (H&E) staining (8).
Therefore, we refined the rapid IHC technique using
nanocrystal beads labeled with an anti-cytokeratin antibody for
determining lymph node metastasis (9). This method uses a double-staining
procedure that combines rapid fluorescent immunostaining and
H&E staining on the same section. We refer to this method as
‘RDS’ (rapid double staining with H&E and immuno-nanocrystal
bead staining) (9). Using lymph
nodes extracted from breast cancer patients who had undergone SN
biopsies in our unit, we compared the false-negative rates of the
RDS and rapid H&E methods.
Materials and methods
Nanocrystal beads and antibody
We used quantum dots (QDs) Qdot®655
(Invitrogen, Carlsbad, CA, USA) as nanocrystal beads (9). The cytokeratin 8 antibody SC-8020
(Santa Cruz Biotechnology, Santa Cruz, CA, USA) was adopted as the
anti-cytokeratin antibody (8).
RDS procedure
QD labeling with anti-cytokeratin antibodies was
carried out according to the specified protocol (Fig. 1) (10). Stock solutions of antibody-labeled
QDs were stored at 4ºC. The procedure for RDS was as follows.
Frozen samples were first rapidly fixed for 30 sec in 100% acetone,
followed by rinsing with running water. After rinsing in
phosphate-buffered saline (PBS) for 1 min, a few drops of the
10-fold diluted QD solution were applied to the samples, which were
then incubated at 37ºC for 20 min. After rinsing with PBS for 1
min, samples were stained with hematoxylin for 10 sec. Samples were
then rinsed with running water for 30 sec, followed by staining
with eosin for 3 sec. Finally, the samples were fixed in alcohol
for 1 min and sealed with xylene (Table
I). The entire staining procedure can be completed within 30
min (8).
 | Table IProcedure for the hematoxylin and
eosin and immuno-nanocrystal bead staining. |
Table I
Procedure for the hematoxylin and
eosin and immuno-nanocrystal bead staining.
| Step | Procedure |
|---|
| 1 | Acetone solution for
1 min |
| 2 | Rinse with water for
30 sec |
| 3 | Rinse with PBS one
time |
| 4 | Staining with labeled
Qdot diluted 20 times for 20 min at 37ºC |
| 5 | Rinse with PBS
twice |
| 6 | Hematoxylin for 10
sec |
| 7 | Rinse with water for
30 sec |
| 8 | Eosin for 3 sec |
| 9 | Rinse with water for
30 sec |
| 10 | 99% alcohol for 1
min |
| 11 | Enclose in
xylene |
Observation of specimens
Fixed samples were examined under a fluorescence
microscope BX51-3 (Olympus, Tokyo, Japan) equipped with a U-MWU2
optical filter (Olympus) (11).
Both the bright-field views and the fluorescence views of the same
microscopic field are able to be observed with the BX51-3. The
observation method for the RDS slide was as follows. We first
looked for any fluorescent sites with the fluorescent view. If any
such site was found, we switched to bright-field observation and
examined whether it was a metastatic focus. Both bright-field and
fluorescent images are able to be observed merely by switching
filters.
Patients and lymph nodes
Samples used in the present study were from 372
lymph nodes from 100 patients who suffered from breast cancer.
Patients underwent partial mastectomy and SN biopsy without
neoadjuvant chemotherapy between October 2007 and March 2009. Our
institute adopts combination mapping with radioisotope (RI) and
blue dye to identify SN in breast cancer patients (12). For the RI tracer, we perform
subcutaneous injection around the tumor; for the dye, Patent Blue
is subcutaneously injected beneath the areola.
For SNs dissected during surgery, multiple 2-mm
slices were cut parallel to the central slice containing the hilus.
Two sets of sections for pathological diagnosis during surgery, one
for conventional rapid H&E staining and the other for RDS, were
prepared in the pathology department of our hospital. We compared
the false-negative rates of the rapid H&E and RDS methods using
the final pathology results from permanent preparations as a
reference. The final pathology results were categorized as
metastasis (pN1), micrometastasis [pN1(mi)], or isolated tumor
cells [pN0(i+)], according to the UICC classification (7th
edition). In our pathology department, the diagnostic procedure for
final pathology results involves diagnosing metastases using
H&E-stained, formalin-fixed permanent preparations; for lymph
nodes diagnosed as n0, including ITCs, cytokeratin immunostaining
is then carried out to confirm negative diagnoses of metastasis
(12).
Ethical guidelines of the study
All the patients were fully informed in regards to
the purpose and content of the study and provided informed consent
for participation in the study, according to the Helsinki
Declaration.
Results
Detection of metastasis by RDS
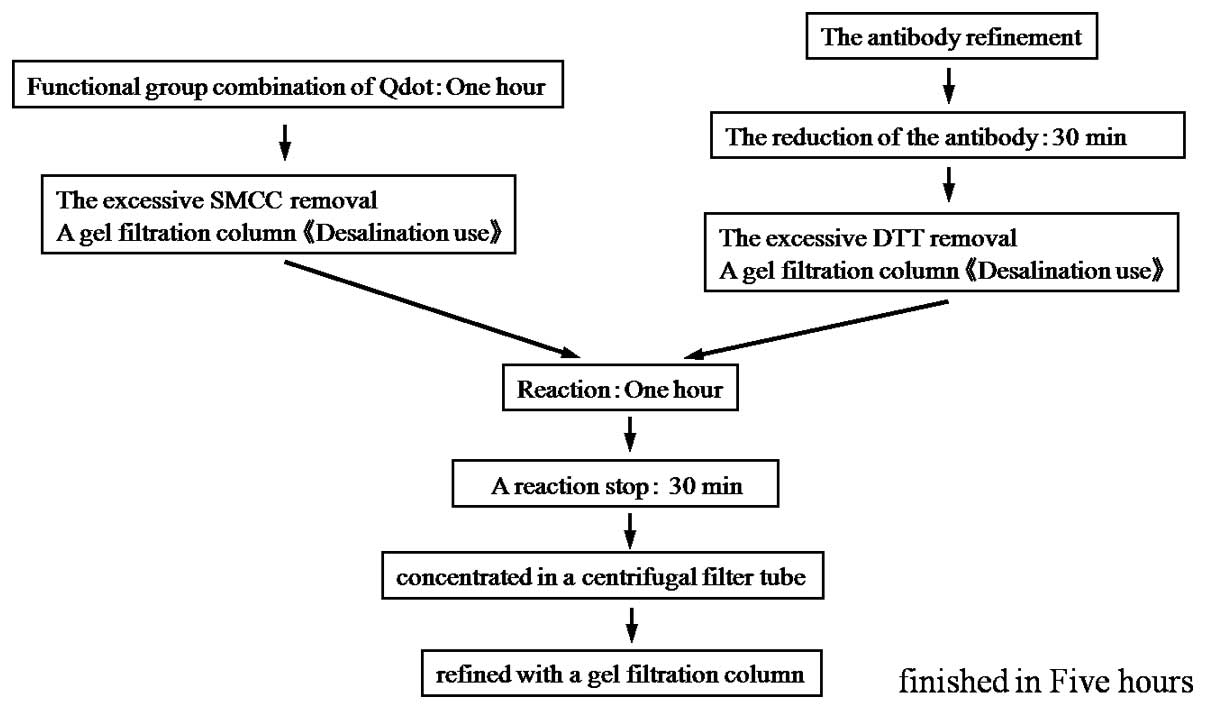
Fig. 2 shows the
images of metastatic lymph nodes stained by RDS. In the
bright-field observation, metastatic foci of lymph nodes were
observed with H&E staining. For observation of fluorescence,
the cytoplasm of cancer cells was clearly stained orange by the
QDs, while normal lymphocytes appeared green due to eosin staining.
This provides a clear contrast that facilitates the diagnosis of
metastasis.
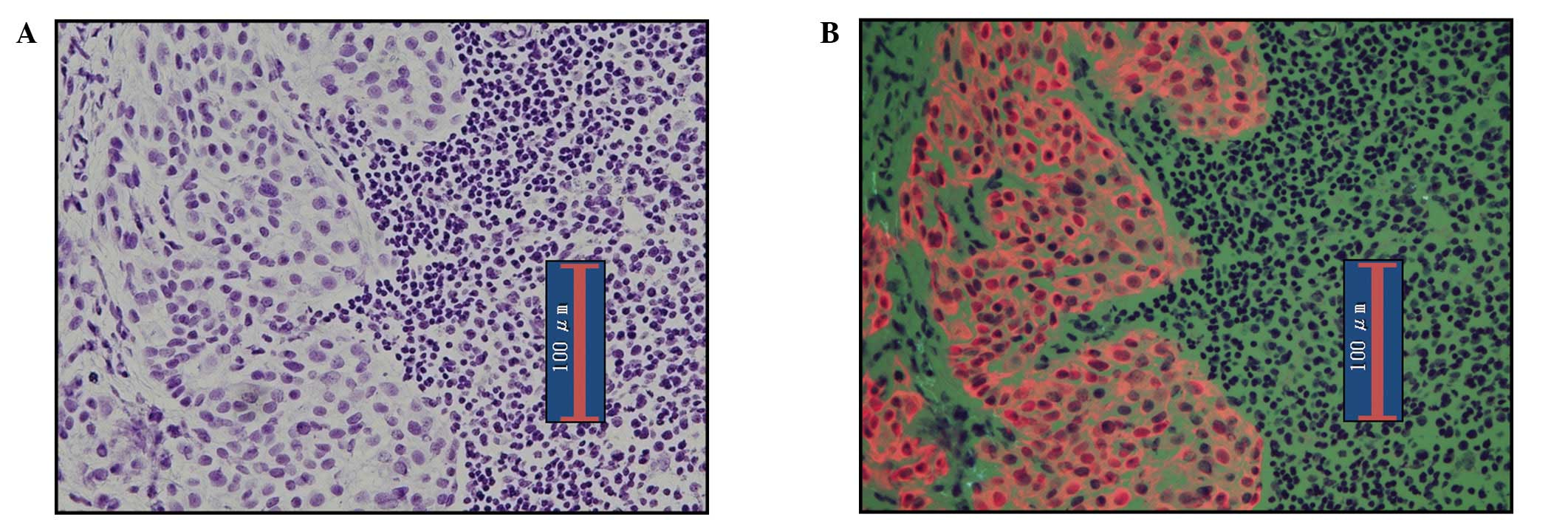
RDS images of micrometastasis in lymph nodes are
shown in Fig. 3. As in pN1(mi)
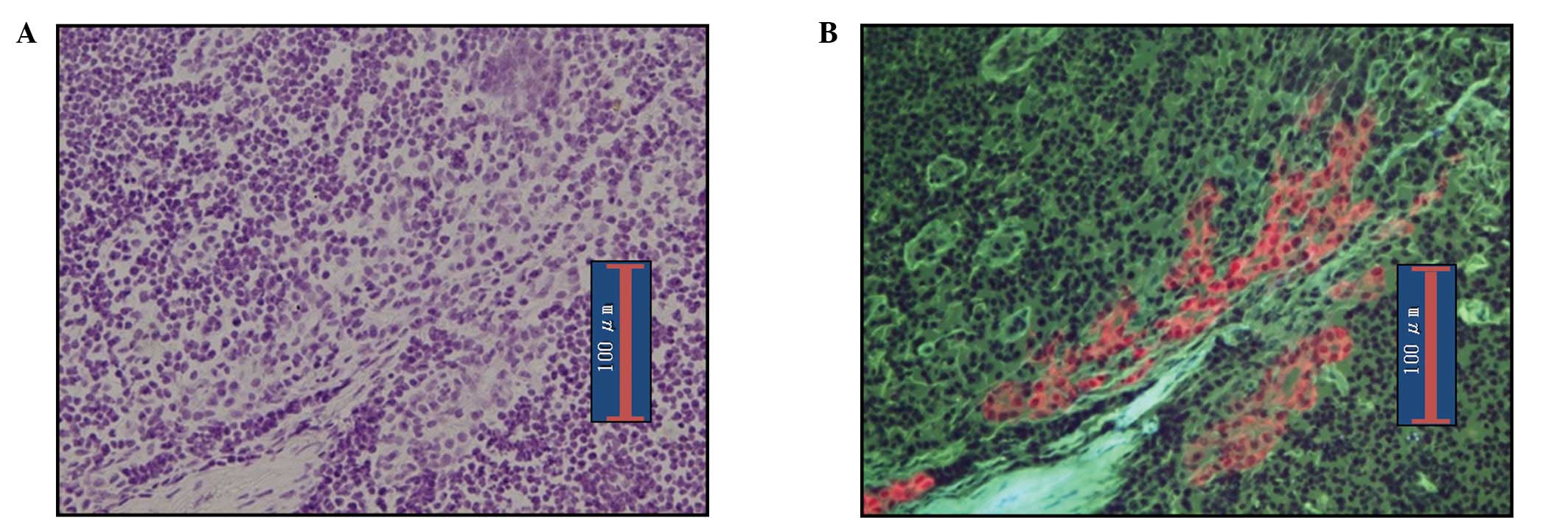
nodes, making a diagnosis of metastasis is easy. Fig. 4 shows ITC images detected by RDS.
The diagnosis of ITCs are known to be somewhat difficult by
bright-field observation; however, in our series ITCs were readily
diagnosable with observation of epifluorescence given the clear
contrast between cancerous and normal cells.
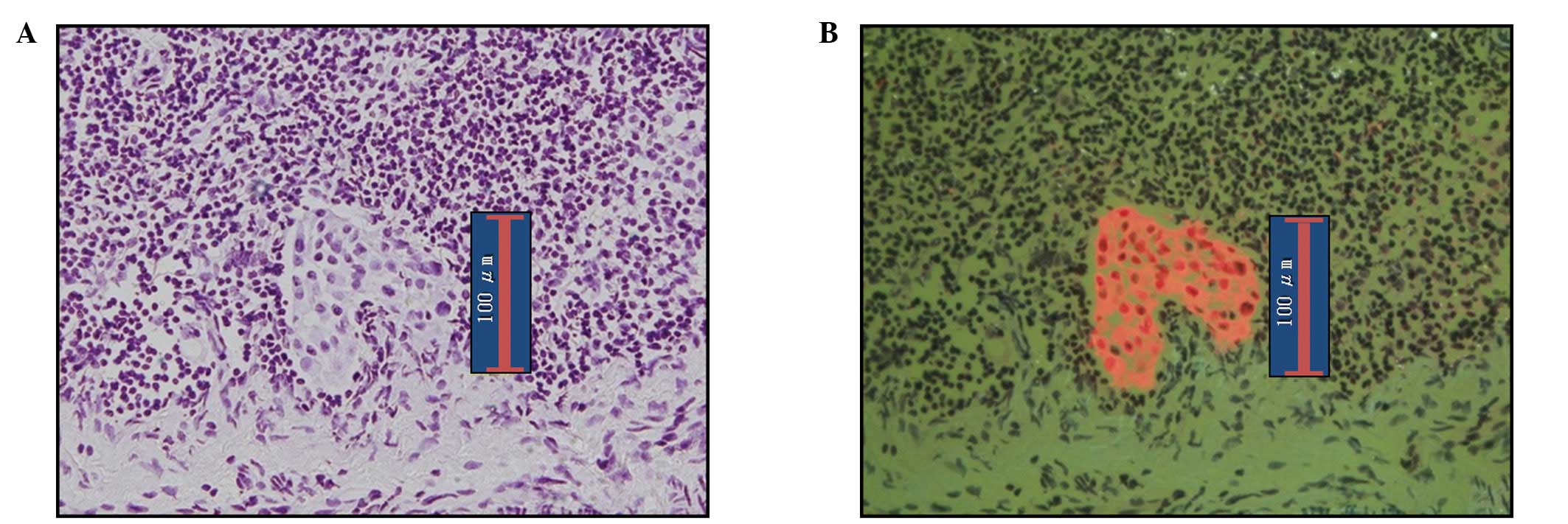
Fig. 5 shows plasma
cells in lymph nodes. Since anti-cytokeratin antibodies react with
plasma cells, they sometimes appear orange under epifluorescence
microscopy, as do cancer cells. However, H&E staining
facilitates determination of the absence of cancer cells when
viewed under bright-field observation.
Diagnosis of metastasis by examining SN
in breast cancer
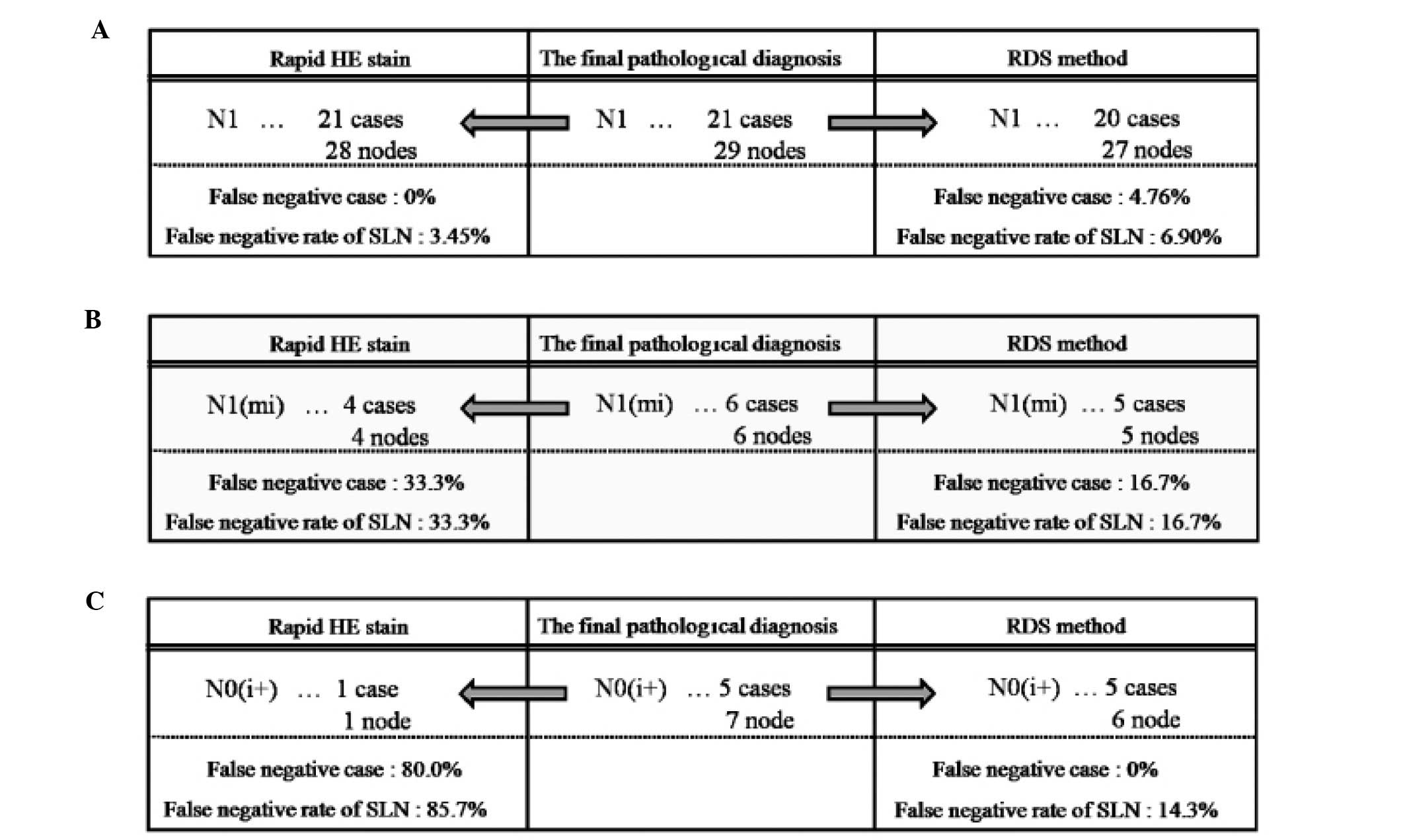
Of the 100 patients, 21 (21.0%) were diagnosed as
pN1 in the final pathological examination. Of the 372 lymph nodes,
metastasis was identified in 29 (7.8%). While all 21 cases were
diagnosed as having metastatic disease by rapid H&E staining,
20 cases were diagnosed as having metastatic disease by RDS. Thus,
the false-negative rate was 0% for rapid H&E staining and 4.8%
for RDS. The reason for the false-negative case by RDS was lack of
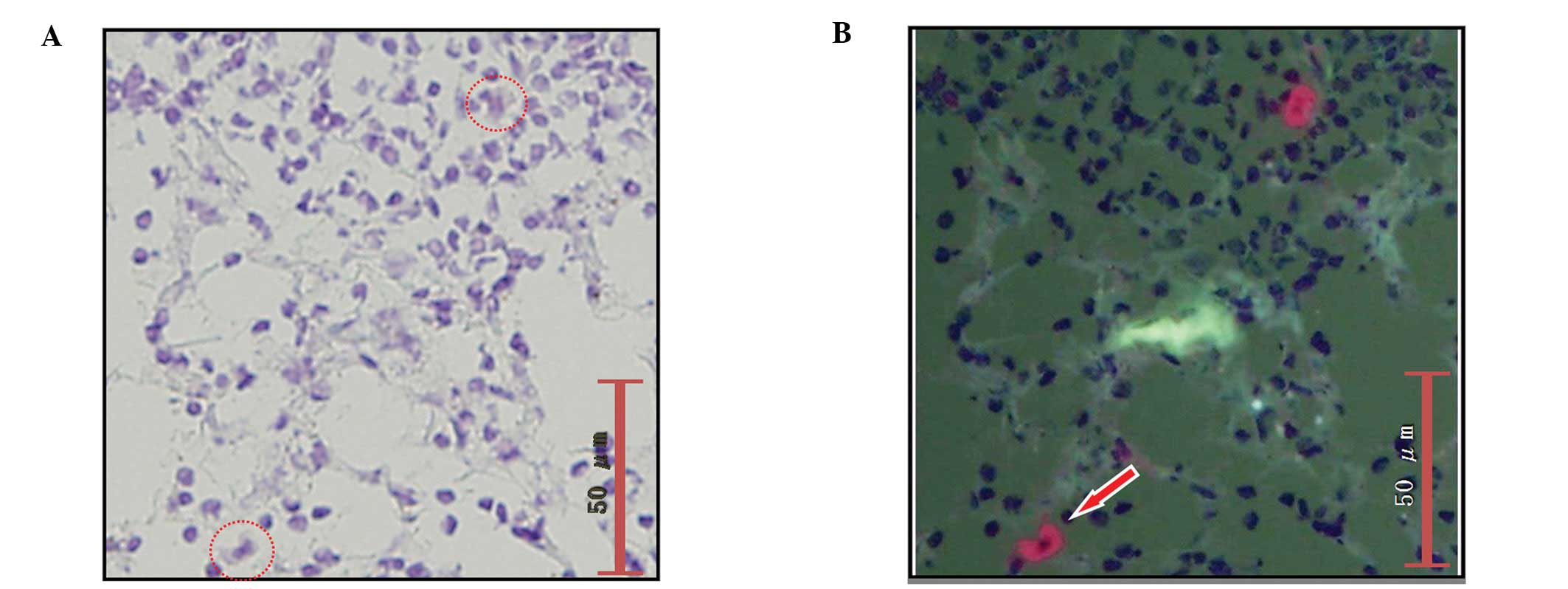
a metastatic focus in the RDS frozen section; the metastatic focus
was likely lost from the section in the slicing step (Fig. 6A).
Of the 100 patients, 6 (6.0%) were diagnosed as
pN1(mi) in the final pathological examination. Of the 372 lymph
nodes, metastasis was identified in 6 (1.61%). We were able to
detect metastatic foci in 4 of these 6 cases with rapid H&E
staining; metastatic foci were detected in 5 of the cases with RDS.
Thus, the false-negative rate was 33.3% for rapid H&E staining
and 16.7% for RDS. With regard to the one case in which metastasis
was not detected by RDS, the specimen was ultimately determined to
be from a case with ITCs rather than micrometastasis (Fig. 6B).
Of the 100 patients, 5 (5.0%) were diagnosed as
pN0(i+) in the final pathological examination. Of the 372 lymph
nodes, 7 (1.88%) had ITCs. While rapid H&E staining detected
ITCs in only 1 of the 5 cases, RDS detected ITCs in all 5 cases.
Thus, the false-negative rate was 80.0% for rapid H&E staining
and 0% for RDS. RDS failed to detect ITCs in 1 lymph node, probably
due to a lack of cancer cells in the frozen section (Fig. 6C).
Discussion
In the present study, we demonstrated that RDS was
superior to conventional rapid H&E staining for the diagnosis
of ITCs. This method, therefore, represents a promising improvement
in the accuracy of frozen section diagnosis of sentinel-node
biopsy.
Needless to say, in order to achieve more accurate
diagnoses of metastases, it will be necessary to improve the
intraoperative diagnostic effectiveness of the procedure (13,14).
In the present study, the accuracy of diagnosis of
intraoperative rapid H&E was very high (91%). To improve the
accuracy of intraoperative diagnosis of lymph node metastases, we
cut lymph nodes into 2-mm slices and prepared multiple slices
(6). However, for breast cancer,
the accuracy of intraoperative frozen section diagnosis of
metastatic lymph node lesions (metastatic lesions >0.2 mm)
varies among studies (1). For
example, Ali et al(1)
reported the accuracy to be 76%, while Tanis et al(15) found it to be 74%. To increase the
accuracy of detection of lymph node metastases, multiple
intraoperative immunohistochemical evaluation methods using
anti-cytokeratin antibodies have been developed (16,17).
Many of these staining procedures, however, are complicated,
time-consuming and therefore impractical.
In order to observe the immunostaining results more
clearly, we used a fluorescence immunostaining method (9). Traditionally, fluorescence
immunostaining has been available only as a two-step procedure in
which a luminescence reagent is added after antibody molecules are
bound to cells. Such a technique has multiple drawbacks: the
procedure is time-consuming and the period of light emission is
short (8).
To overcome these drawbacks, we developed RDS, which
has the following characteristics: a convenient one-step procedure
for immunostaining, very clear staining results, rapid H&E and
immunofluorescence staining of the same slice of specimen, easy
staining procedures, no requirement for special equipment except
for a fluorescence microscope and low cost.
Using the RDS method, the limitations of the
fluorescence immunostaining method were overcome by using
nanocrystal beads, which allow for clearer visualization and a
shorter procedure time. We used QDs as nanocrystal beads. The
energy acceptor modules consisted of CdSe/ZnS semiconductor
nanocrystals coated with a polymer shell containing 5–7 biotin
molecules per dot on their surfaces (18). The beads also have stable, bright
optical characteristics; furthermore, through a maleimide-thiol
binding reaction, they can be conjugated with any antibody
(18). Various QDs are available,
each with a different emission spectrum (19). Herein, we used QD655, which produces
maximal emission at 655 nm. The color of QD655 fluorescence is red,
clearly contrasting with normal lymphocytes, which appear green
upon eosin staining. Based on the procedure reported in a study by
Ishii et al(3), we
conjugated these QDs to anti-cytokeratin 8/18 antibodies
(SC-8020).
As frozen-section diagnosis at our hospital is
highly effective and accurate overall, the advantage of RDS in this
study was limited to the diagnosis of ITCs (4). Nevertheless, we believe that RDS has
sufficient value for clinical use. In many institutions, if the
diagnosis of ITCs can be established by intraoperative rapid
diagnostic methods applied to lymph nodes, axillary lymph node
dissection is not routinely performed (1). Galimberit et al(20) reported, however, that 15–19% of
non-sentinel nodes were found to be metastatic in cases where ITCs
were present in SNs. In a study by de Boer et al(4), the presence or absence of
micrometastases and ITCs was also shown to be associated with
survival in patients who did not receive postoperative adjuvant
chemotherapy. Additionally, a case in which RDS failed to find
micrometastasis was diagnosed as having ITCs. We speculate that
this false-negative case was attributable to issues related to
preparation of the sections; a micrometastasis is very small and a
section does not always represent the maximal cutting size.
Therefore, the diagnosis should be made intraoperatively in any
case, although whether lymph node dissection is needed for patients
with ITCs is a separate issue that needs to be addressed (21,22).
For rapid diagnosis of lymph node metastasis,
one-step nucleic acid amplification (OSNA) assay was recently
developed (23). With this method,
the expression level of cytokeratin 19 (CK19) mRNA is assessed with
specific primers, and amplification and detection of CK19 mRNA can
be achieved in ~30 min. Tamaki et al(24) reported the sensitivity and
specificity of the OSNA assay for detection of metastases to be 95
and 97.1%, respectively.
Some metastatic lymph nodes of breast cancer
patients, however, are CK19-negative. The OSNA assay may provide
false-negative results in such cases (24). Parikh et al(25) reported lack of CK19 expression in
20.5% of 158 breast carcinomas in a tissue microarray. Moreover,
they found a statistically significant association between lack of
CK19 expression and the triple-negative (TN) phenotype (30% of TN
breast cancers were CK19-negative). Since the RDS method also
detects the expression level of cytokeratin 8/18 (CK8/18),
CK8/18-negative metastatic lymph nodes would display no
fluorescence signals. While no such cases were observed in this
study, our RDS method would avoid such false-negative results by
switching filters for bright-field observation, allowing
morphological detection of cancerous structures by H&E staining
of the same section.
Moreover, in the OSNA assay, sample lymph nodes must
be homogenized in lysis buffer. Thus, generally, when performing a
rapid intraoperative diagnosis, half of the lymph node sample is
used for the OSNA assay and the other half for imprint cytology
(24). For the samples used for
OSNA, morphological information regarding lymph node metastases is
completely lost during the lysis step and consequently cannot be
used later to confirm the diagnosis. Unlike the OSNA assay, the RDS
method allows morphological examination for the detection of
metastases, because the same frozen sections are used for
fluorescence immunostaining and H&E staining.
RDS would also be useful for SN biopsy in
gastrointestinal cancer cases. Since these patients cannot be
re-operated, the presence or absence of SN micrometastases must be
diagnosed intraoperatively.
As long as tumor cells are present in the sections,
the RDS method facilitates the diagnosis of ITCs even when
involvement is minimal. Our RDS method is clinically useful for
examination of the SN biopsy of breast cancers and can contribute
to the intraoperative diagnosis of lymph node metastases and
standardization of cancer treatments.
Acknowledgements
The authors are grateful to Dr Koichi Miwa of
Kanazawa University for making the present study possible.
References
|
1
|
Ali R, Hanly AM, Naughton P, et al:
Intraoperative frozen section assessment of sentinel lymph nodes in
the operative management of women with symptomatic breast cancer.
World J Surg Oncol. 6:692008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Giobuin SM, Kavanagh DO, Myers E, et al:
The significance of immunohistochemistry positivity in sentinel
nodes which are negative on haematoxylin and eosin in breast
cancer. Eur J Surg Oncol. 35:1257–1260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gobardhan PD, Elias SG, Madsen EV, et al:
Prognostic value of micrometastases in sentinel lymph nodes of
patients with breast carcinoma: a cohort study. Ann Oncol.
20:41–48. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Boer M, van Deurzen CH, van Dijck JA,
et al: Micrometastases or isolated tumor cells and the outcome of
breast cancer. N Engl J Med. 361:653–663. 2009.
|
|
5
|
Mikhitarian K, Martin RH, Mitas M, et al:
Molecular analysis improves sensitivity of breast sentinel lymph
node biopsy: results of a multi-institutional prospective cohort
study. Surgery. 138:474–481. 2005. View Article : Google Scholar
|
|
6
|
Fritzsche FR, Reineke T, Morawietz L, et
al: Pathological processing techniques and final diagnosis of
breast cancer sentinel lymph nodes. Ann Surg Oncol. 17:2892–2898.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsujimoto M, Nakabayashi K, Yoshidome K,
et al: One-step nucleic acid amplification for intraoperative
detection of lymph node metastasis in breast cancer patients. Clin
Cancer Res. 13:4807–4816. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ishii K, Kinami S, Funaki K, et al:
Detection of sentinel and non-sentinel lymph node micrometastases
by complete serial sectioning and immunohistochemical analysis for
gastric cancer. J Exp Clin Cancer Res. 27:72008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chattopadhyay PK, Price DA, Harper TF, et
al: Quantum dot semiconductor nanocrystals for immunophenotyping by
polychromatic flow cytometry. Nat Med. 12:972–977. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chung I, Witkoskie JB, Cao J and Bawendi
MG: Description of the fluorescence intensity time trace of
collections of CdSe nanocrystal quantum dots based on single
quantum dot fluorescence blinking statistics. Phys Rev E Stat
Nonlin Soft Matter Phys. 73:0111062006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schmidt H, Brown EB, Schwaller B and
Eilers J: Diffusional mobility of parvalbumin in spiny dendrites of
cerebellar Purkinje neurons quantified by fluorescence recovery
after photobleaching. Biophys J. 84:2599–2608. 2003. View Article : Google Scholar
|
|
12
|
Noguchi M, Inokuchi M and Zen Y:
Complement of peritumoral and subareolar injection in breast cancer
sentinel lymph node biopsy. J Surg Oncol. 100:100–105. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Turner RR, Ollila DW, Stern S and Giuliano
AE: Optimal histopathologic examination of the sentinel lymph node
for breast carcinoma staging. Am J Surg Pathol. 23:263–267. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Viale G, Dell'Orto P, Biasi MO, et al:
Comparative evaluation of an extensive histopathologic examination
and a real-time reverse-transcription-polymerase chain reaction
assay for mammaglobin and cytokeratin 19 on axillary sentinel lymph
nodes of breast carcinoma patients. Ann Surg. 247:136–142. 2008.
View Article : Google Scholar
|
|
15
|
Tanis PJ, Boom RP, Koops HS, et al: Frozen
section investigation of the sentinel node in malignant melanoma
and breast cancer. Ann Surg Oncol. 8:222–226. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hughes SJ, Xi L, Raja S, et al: A rapid,
fully automated, molecular-based assay accurately analyzes sentinel
lymph nodes for the presence of metastatic breast cancer. Ann Surg.
243:389–398. 2006. View Article : Google Scholar
|
|
17
|
Carmon M, Olsha O, Rivkin L, Spira RM and
Golomb E: Intraoperative palpation for clinically suspicious
axillary sentinel lymph nodes reduces the false-negative rate of
sentinel lymph node biopsy in breast cancer. Breast J. 12:199–201.
2006. View Article : Google Scholar
|
|
18
|
Lifshitz E, Brumer M, Kigel A, et al:
Air-stable PbSe/PbS and PbSe/PbSexS1-x core-shell nanocrystal
quantum dots and their applications. J Phys Chem B.
110:25356–25365. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Charbonnière LJ, Hildebrandt N, Ziessel RF
and Löhmannsröben HG: Lanthanides to quantum dots resonance energy
transfer in time-resolved fluoro-immunoassays and luminescence
microscopy. J Am Chem Soc. 128:12800–12809. 2006.PubMed/NCBI
|
|
20
|
Galimberti V: Axillary sentinel lymph
node: how low can you go? Breast. 18(Suppl 1): S122009. View Article : Google Scholar
|
|
21
|
Nissan A, Jager D, Roystacher M, et al:
Multimarker RT-PCR assay for the detection of minimal residual
disease in sentinel lymph nodes of breast cancer patients. Br J
Cancer. 94:681–685. 2006.PubMed/NCBI
|
|
22
|
Bostick PJ, Huynh KT, Sarantou T, et al:
Detection of metastases in sentinel lymph nodes of breast cancer
patients by multiple-marker RT-PCR. Int J Cancer. 79:645–651. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schem C, Maass N, Bauerschlag DO, et al:
One-step nucleic acid amplification - a molecular method for the
detection of lymph node metastases in breast cancer patients;
results of the German study group. Virchows Arch. 454:203–210.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tamaki Y, Akiyama F, Iwase T, et al:
Molecular detection of lymph node metastases in breast cancer
patients: results of a multicenter trial using the one-step nucleic
acid amplification assay. Clin Cancer Res. 15:2879–2884. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Parikh RR, Yang Q, Higgins SA and Haffty
BG: Outcomes in young women with breast cancer of triple-negative
phenotype: the prognostic significance of CK19 expression. Int J
Radiat Oncol Biol Phys. 70:35–42. 2008. View Article : Google Scholar : PubMed/NCBI
|