Introduction
Breast and ovarian cancer remain major health
concerns, and are the most common malignancies diagnosed in women
(1). Approximately 30% of
metastatic human breast and ovarian cancer are associated with the
overexpression or amplification of the HER2 receptor (2,3). HER2
is a 185-kDa transmembrane receptor with tyrosine kinase activity
and may dimerize with other epithelial growth factor receptor
(EGFR) family members, including EGF receptors HER1, HER3 and HER4.
Overexpression of HER2 has been frequently found in various types
of human cancers, such as breast, gastric, lung, ovarian, kidney
and bladder cancers. HER2 overexpression in cancer cells have been
proven to enhance cell proliferation, increase the tendency for
metastasis, shorten disease-free survival, induce clinical
drug-resistance, and lower overall survival rates (4,5).
HER2-mediated signaling has also been demonstrated
to be involved in the anti-apoptosis induced by certain
pro-apoptotic stimuli (4).
Moreover, previous studies have indicated that reducing the HER2
expression in cancer cells may attenuate anti-apoptotic signaling
and suppress HER2-mediated malignant phenotypes. Therefore, HER2 is
not only a potent oncogene, but also an excellent therapeutic
target for breast and ovarian cancers. Monoclonal antibodies were
the first anti-HER2 strategy to be used for clinical therapy
(6). Trastuzumab (Herceptin), a
recombinant humanized monoclonal antibody, is used to treat
metastatic breast cancer via directly counteracting the
extracellular domain of HER2. Although Herceptin is known as a
successful therapeutic antibody, only one-third of
HER2-overexpressing metastatic breast cancers respond to Herceptin
single-agent therapy, while almost two-thirds respond to combined
Taxol-Herceptin regimens. However, these responses are short-lived,
averaging less than 1 year (7), and
recurring resistance for Herceptin has been observed in the
majority of patients within 1 year (8,9).
Identification of the potential mechanisms of Herceptin-resistance
can be extremely helpful for the development of new compounds that
may overcome such resistance and demonstrate additive/synergistic
antitumor effects when administered in association with
Herceptin.
The 90-kDa heat shock protein 90 (Hsp90) is a
protein chaperone whose functions are to promote the maturation and
conformational stabilization of a subset of cellular proteins, and
it is crucial in signal transductions of cell proliferation and
survival (10). During client
processing, ATP binding to Hsp90 drives momentous conformational
change in the chaperone and ultimately leads to ATP hydrolysis
(11). HER2, a client protein of
Hsp90, is known to interact with Hsp90 to acquire proper protein
function; therefore, using inhibitors of Hsp90 to target HER2
through dissociation of HER2 from the chaperone leads to
degradation of HER2 by a proteasome-dependent manner (11,12).
The Hsp90 inhibitors such as geldanamycin and its less cytotoxic
analogue, 17-(allylamino)-17-demethoxygeldanamycin (17-AAG),
through binding to an ATP pocket in the NH2-terminal
domain of this protein, inhibits the Hsp90 chaperone function
(11,13).
Numerous structure-activity relationships and
biochemical assay data indicate that
1-benzyl-3-(5-hydroxymethyl-2-furyl)indazole (YC-1) displays high
potential as a new anticancer drug candidate (14,15).
In vivo xenograft studies have also revealed that YC-1
exhibits marked antitumor activity against various cancer cell
lines and prolongs the survival time of tumor-bearing mice, but
without evident toxic effects (16,17).
The anticancer effect of YC-1 appears to be a consequence of its
multiple actions, including anti-inflammation activity, suppression
of the hypoxia-inducible factor-1α (HIF-1α) expression, influence
on the differentiation of stem cells, and promotion of NK cell
differentiation by activating the p38-MAPK pathway (18–20).
In our previous study, we reported that YC-1
furopyrazole and thienopyrazole isosteric analogues exhibit greater
cytotoxicity against HL-60 cells than YC-1, and their
physiochemical properties and biological mechanisms appear to be at
variance to YC-1 (15,21). As part of our continuing search for
potential anticancer drug candidates among YC-1 analogues, we
further investigated the anticancer activity and biological
mechanisms of various YC-1 isosteric analogues in vitro and
in vivo. In this present study, 8 furopyrazole and
thienopyrazole compounds were chosen for evaluation of their effect
on HER2 expression. Among them, CLC604
(1-benzyl-3-(p-hydroxymethylphenyl)-5-methylfuro[3,2-c]pyrazol)
was more sensitive to HER2-overexpressing cancer cells. Thus, among
the tested YC-1 analogues, CLC604 was considered to be the most
promising compound for further study of its physiochemical
properties and biological mechanisms.
Combinative cancer therapy has become a common
approach for increasing curative rates and decreasing side effects
leading to the improvement in the quality of life of patients.
Consequently, we performed in vitro and in vivo
synergistic treatment on breast cancer cells with HER2
overexpression using a combination of CLC604 with a low-dose of
clinical drugs (Taxol, doxorubicin, and etoposide) in contrast with
single-agent treatment. We found that CLC604 suppressed the growth
of HER2-overexpressing breast tumors in MCF/Her18 tumor-bearing
mice and increased the potency of Taxol.
Materials and methods
Chemicals and reagents
YC-1, and furopyrazole and thienopyrazole analogues
of YC-1 were synthesized in our laboratory. Cell culture materials
were obtained from Invitrogen Life Technologies (Burlington,
Ontario, Canada). Antibodies and reagents were purchased from
commercial sources: antibodies against Akt and c-Raf were purchased
from Cell Signaling Technology, Inc. (Beverly, MA, USA). An
antibody against Hsp90 was from BD Transduction Laboratory and
antibodies against cyclin-dependent kinase 4 (CDK4) and HER2 (9G6)
were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). An
antibody against HER2 (Ab3) was obtained from Calbiochem Company
(San Diego, CA, USA). Anti-mouse and anti-rabbit antibodies
conjugated to horseradish peroxidase, a β-actin antibody, an
α-tubulin antibody, 3-(4,5-dimethylthiazol-2-yl)-2,
5-diphenyltetrazolium bromide (MTT), actinomycin D (AcD),
cycloheximide, 17-AAG, Z-Leu-Leu-Leu-al (MG132), G418, etoposide
(E1383), Taxol (T7402), and doxorubicin (D1515) were obtained from
Sigma Chemical Co. (St. Louis, MO, USA). Protein A/G-agarose was
from Upstate Company and SeaPlaque Agarose (low melting temperature
agarose) was purchased from Lonza (Rockland, ME, USA).
Cell lines and cell cultures
The human breast and ovarian cancer cell lines used
in this study were SKOV3, SKOV3.ip1, MDA-MB-453 and SKBr3, all of
which overexpress HER2. MCF-7, MDA-MB-231 and MDA-MB-435/neo cells
all express a basal level of HER2. The HBL-100 cell line, which is
derived from normal human breast tissue, was transformed by SV40
large T antigen and expresses a basal level of HER2. In addition,
we used MCF-7/HER18 and MDA-MB-435/HER2 cells; MCF-7 and MDA-MB-435
were stably transfected with pSV2/HER2 and had HER2 overexpression.
MDA-MB-453, MDA-MB-231 and MCF-7 cells were grown in Dulbecco’s
modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine
serum (FBS) and SKBr3 was cultured in MyCoy’s 5A medium (modified).
The other cells were cultured in DMEM/F12 supplemented with 10%
FBS. Cells were grown in a humidified incubator at 37°C under 5%
CO2 in air.
Preparation of cell lysates,
immunoblotting and immunoprecipitation
Cells were treated with various agents, as indicated
in the figure legends. After treatment, cells were washed with cold
phosphate-buffered saline (PBS) and lysed with a lysis buffer [1%
Triton X-100, 10% glycerol, 10 μg/ml leupeptin, 1 mmol/l sodium
orthovanadate, 1 mmol/l EGTA, 10 mmol/l NaF, 1 mmol/l sodium
pyrophosphate, 100 μmol/l β-glycerophosphate, 20 mmol/l Tris-HCl
(pH 7.9), 137 mmol/l NaCl, 5 mmol/l EDTA, and a protease inhibitor
(1:10,000)] for immunoblotting. For immunoprecipitation, cells were
lysed with RIPA-B buffer [1% Triton X-100, 150 mmol/l NaCl, 20
μmol/l NaHPO4 (pH 7.4), 0.1 mmol/l sodium orthovanadate
and 1 mol/l NaF, protease inhibitor (1:2,500)]. One milligram of
each sample was mixed with 1 μg antibody and 50 μl protein A
agarose at 4°C for 3 h. The immunoprecipitates were washed in
RIPA-B buffer without a protease inhibitor, eluted with the SDS
sample loading buffer, and processed for immunoblot analyses, as
described previously (22). For
preparation of Triton X-100-soluble and -insoluble fractions, cells
were lysed with a lysis buffer containing 1% Triton X-100, as
described above. After removal of Triton X-100-soluble cell lysate
supernatants by centrifugation, the pellets were washed once with
the lysis buffer, and a 1× SDS loading buffer (50 μl) was then
added to the pellets and heated at 95°C for 15 min to dissolve the
Triton X-100-insoluble proteins.
Determination of cell viability by MTT
assay
The effects of YC-1, furopyrazole and thienopyrazole
analogues of YC-1, and 3 clinical drugs (doxorubicin, etoposide and
Taxol) on cell viability were examined by MTT assay. Cells were
treated with various doses of the drugs for the indicated times,
and the MTT dye was then added to each well. After a 4-h
incubation, the growth medium was removed and the formazan
crystals, generated by oxidation of the MTT dye by cell
mitochondria, were dissolved in 0.04 N HCl in isopropanol. The
absorbance was measured at 570 nm, and the cell survival ratio was
expressed as a percentage of the control viability (23).
Flow cytometric analysis
Cells were treated with various agents for the
indicated times, harvested by trypsinization, fixed with 70% (v/v)
ethanol at 4°C for 30 min, and washed twice with phosphate-buffered
saline (PBS). After centrifugation, the cells were incubated with
0.1 ml of a phosphate-citric acid buffer [0.2 M NaHPO4
and 0.1 M citric acid (pH 7.8)] for 30 min at room temperature. The
cells were then centrifuged and re-suspended in 0.5 ml propidium
iodide (PI) solution comprising Triton X-100 (0.1%, v/v), RNase
(100 mg/ml) and PI (80 mg/ml). The percentage of apoptosis was
analyzed with FACScan and CellQuest software (Becton-Dickinson,
Mountain View, CA, USA).
Soft agar colony formation assay
The effects of CLC604 on the soft agar colony
formation of various human breast cell lines were investigated.
Briefly, cells (1×104) were seeded in a 6-cm culture
dish containing 0.35% low-melting agarose over a 0.7% agarose layer
in the presence of varying concentrations of CLC604 or a control
vehicle and incubated for 3 weeks at 37°C. Colonies were stained
with p-iodonitrotetrazolium violet (1 mg/ml), and those colonies
larger than 100 μm were measured. The differences in the effects of
CLC604 between cell lines expressing a basal level of HER2 and
overexpressing HER2 were evaluated using ANOVA.
Cell transfection
Plasmid pSV2-erbB2, a constitutive expression
vector, carries 4.4-kb full-length human HER2 cDNA under the
control of the SV40 promoter/enhancer sequence. Cells
(6×105) were transfected with 5 μg of DNA mediated by 21
μl of a Lipofectin reagent. Experiments were performed after
transfection.
Immunofluorescence assay
MDA-MB-453 cells were placed on cover slides in
6-well plates. Experiments were performed 24 h after cell
attachment. Cells were fixed in PBS containing 4% formaldehyde for
10–15 min at room temperature. Cells were rinsed with PBS 2–3 times
followed by blocking with 5% BSA (Sigma-Aldrich, St. Louis, MO,
USA) for 30 min. Incubations were performed with primary antibodies
diluted in a blocking buffer at 4°C overnight, after which cover
slides were washed and incubated for 30 min with the isothiocyanate
(FITC)-conjugated secondary antibodies (Chemicon, Temecula, CA,
USA) diluted in blocking buffer. Cover slides were washed and
mounted. Fluorescence was visualized using a Nikon Optiphot-2
microscope.
In vitro Hsp90 assay
Hsp90 proteins expressed as His6-tagged fusions were
purified as described previously using TALON metal-affinity
chromatography, Q-Sepharose ion-exchange, and Superdex 75, 200, or
Sephacryl 400HR gel-filtration chromatography. Proteins were
concentrated in 20 mM Tris (pH 7.5) containing 0–25 mM NaCl, 1 mM
EDTA and 0.5 mM DTT. The Hsp90 ATPase assay was performed as
previously described (24).
In vivo studies
The animals used in this study were purchased from
the National Laboratory Animal Breeding and Research Center
(Taipei, Taiwan) following China Medical University Institutional
Animals Ethic Committee clearance (99–151-N). Female BALB/c SCID
mice (18–20 g; 6–8 weeks of age) were purchased from the National
Animal Center, Taipei, Taiwan, and maintained in pressurized
ventilated cages according to institutional regulations. Each SCID
mouse was subcutaneously inoculated in the right flank with
2×106 (group A) MCF-7 cells or (group B) MCF-7/Her18
cells in 0.5 ml PBS via a 24-gauge needle. Growth of MCF-7 tumors
was supplemented with 0.72 mg of 60-day release estrogen pellets
(Innovative Research of America, Sarasota, FL, USA) which were
implanted subcutaneously in the back of the animals 24 h prior to
cell inoculation. After the appearance of a 100-mm3
tumor nodule, tumor-bearing mice of group A and B were randomly
divided into 5 subgroups (n=6) for treatment with: vehicle; i.p.
injection of Taxol (5 mg/kg); Taxol (5 mg/kg) combined with CLC604
alone (50 mg/kg) or CLC604 (50 and 100 mg/kg, respectively) every 5
days each week for 4 consecutive weeks. The animals were weighed,
and the tumors were measured using calipers twice a week before,
during, and after drug treatment. The tumor volume was calculated
using the following formula: Volume = 1/2 (L × W2),
where L is the length and W is the width of the tumor. At the end
of the experiments, the animals were euthanized with carbon dioxide
followed by cervical dislocation.
Western blot analysis of expression of
HER2 protein in vivo
Protein extracts were prepared by homogenizing tumor
tissues obtained from the mice treated with vehicle, CLC604 alone,
Taxol alone, and a combination of CLC604 and Taxol using a lysis
buffer [20 mM Na2PO4 (pH 7.4), 150 mM NaCl,
1% Triton X-100, 1% aprotinin, 1 mM phenylmethylsulfonyl fluoride,
10 mg/ml leupeptin, 100 mM NaF and 2 mM
Na3VO4]. The protein content was determined
against a standardized control using the Bio-Rad protein assay kit
(Bio-Rad Laboratories, Hercules, CA, USA). A total of 50 μg of
protein was processed for immunoblotting analyses as described
previously (22).
Statistical analysis
All values are expressed as means ± SD. Each value
is the mean of at least 3 separate experiments for each group.
ANOVA was used for statistical comparison. Values are significantly
different from the control at p<0.05, p<0.01 and p<0.001
as indicated in the figure legends.
Results
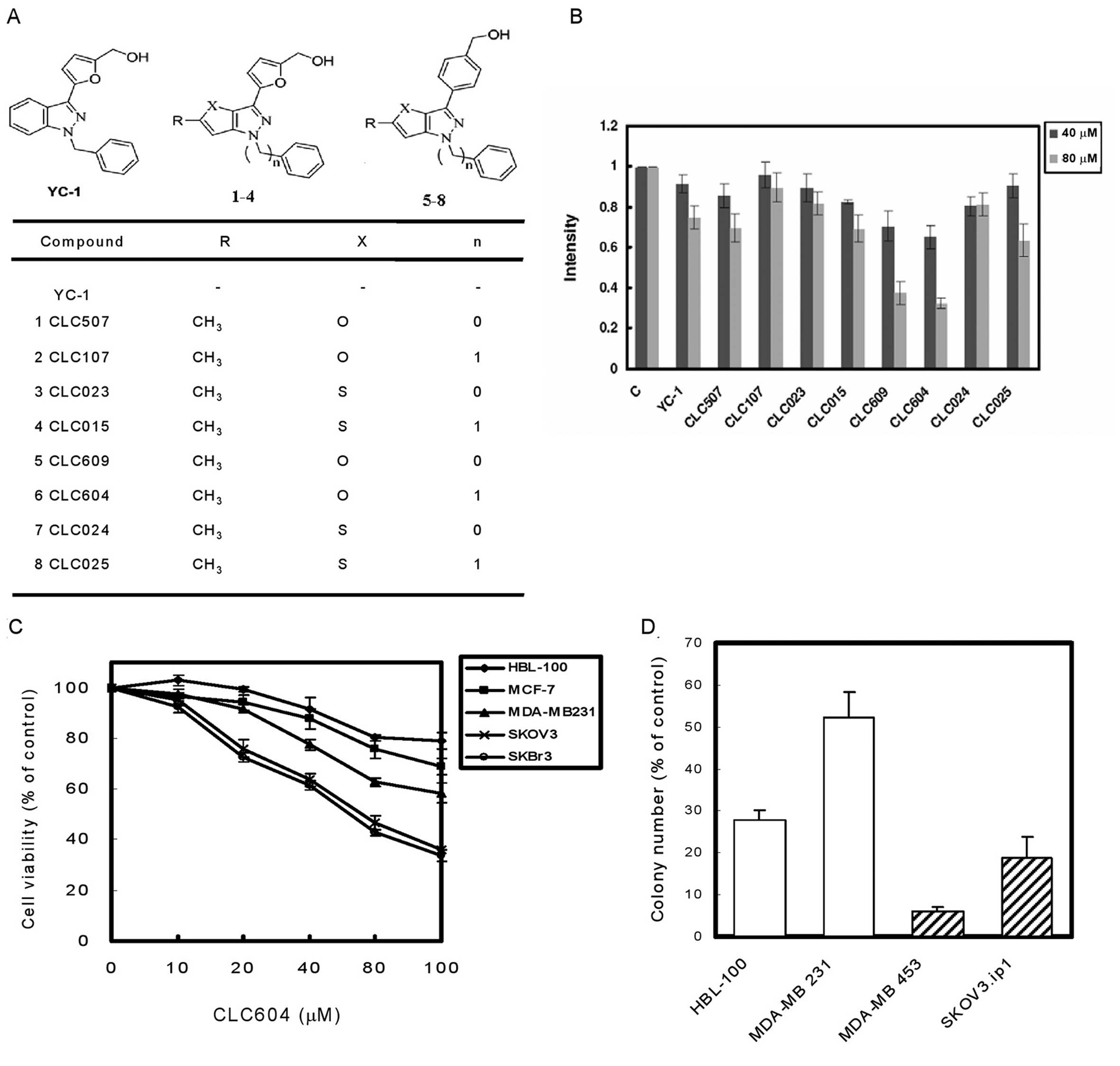
YC-1 and its furopyrazole and
thienopyrazole isosteric analogues promote degradation of HER2
To investigate the effects of YC-1 and its
furopyrazole and thienopyrazole isosteric analogues (Fig. 1A) on reducing the expression of HER2
protein, western blotting was performed to establish the HER2
protein level in HER2-overexpressing human breast cancer MDA-MB-453
cells. These results showed that the expression of HER2 in
MDA-MB-453 cancer cells was suppressed by YC-1 and its analogues in
a dose-dependent manner (Fig. 1B).
Moreover, we found that CLC609 and CLC604 exhibited more potency in
suppressing the HER2 protein level than the other analogues.
Furthermore, we used the MTT growth assay to test the cytotoxicity
of CLC609 and CLC604. Among them, CLC604 was found to be less
cytotoxic to the immortalized noncancerous breast HBL-100 cell line
(data not shown). Thus, CLC604 was considered to be most promising
compound for further study. To assess the biological activity of
CLC604 in terms of cell proliferation, cells were treated with
CLC604 at different concentrations for 24 h. The growth inhibition
of the tested cell lines was in a dose-dependent manner, but to
various extents (Fig. 1C). For
example, CLC604 at 80 μM blocked >60% of growth in
HER2-overexpressing cancer cells (SKOV3 and SKBr3). However, the
inhibitory effect was much less effective in cells expressing a
basal level of HER2 (MDA-MB-231, MCF-7 and HBL-100) under the same
condition.
Effects of CLC604 on soft agar colony
formation
Examining the effects of CLC604 on
anchorage-independent growth is an important hallmark of the
transformation phenotype. We seeded a variety of human cells into
soft agar in the presence of a control vehicle or various
concentrations of CLC604 and monitored them for colony formation.
The colony-forming activity of HER2-overexpressing cancer cells
(MDA-MB-453 and SKOV3.ip1) was more significantly suppressed than
the colony formation in cells with a basal level of HER2
(MDA-MB-231 and HBL-100) at 80 μM CLC604 (Fig. 1D). The results suggest that CLC604
reduces the HER2-mediated transformation phenotype of cancer cell
lines. The above results indicate that CLC604 is safe and
preferentially inhibits the growth of HER2-overexpressing cancer
cell lines.
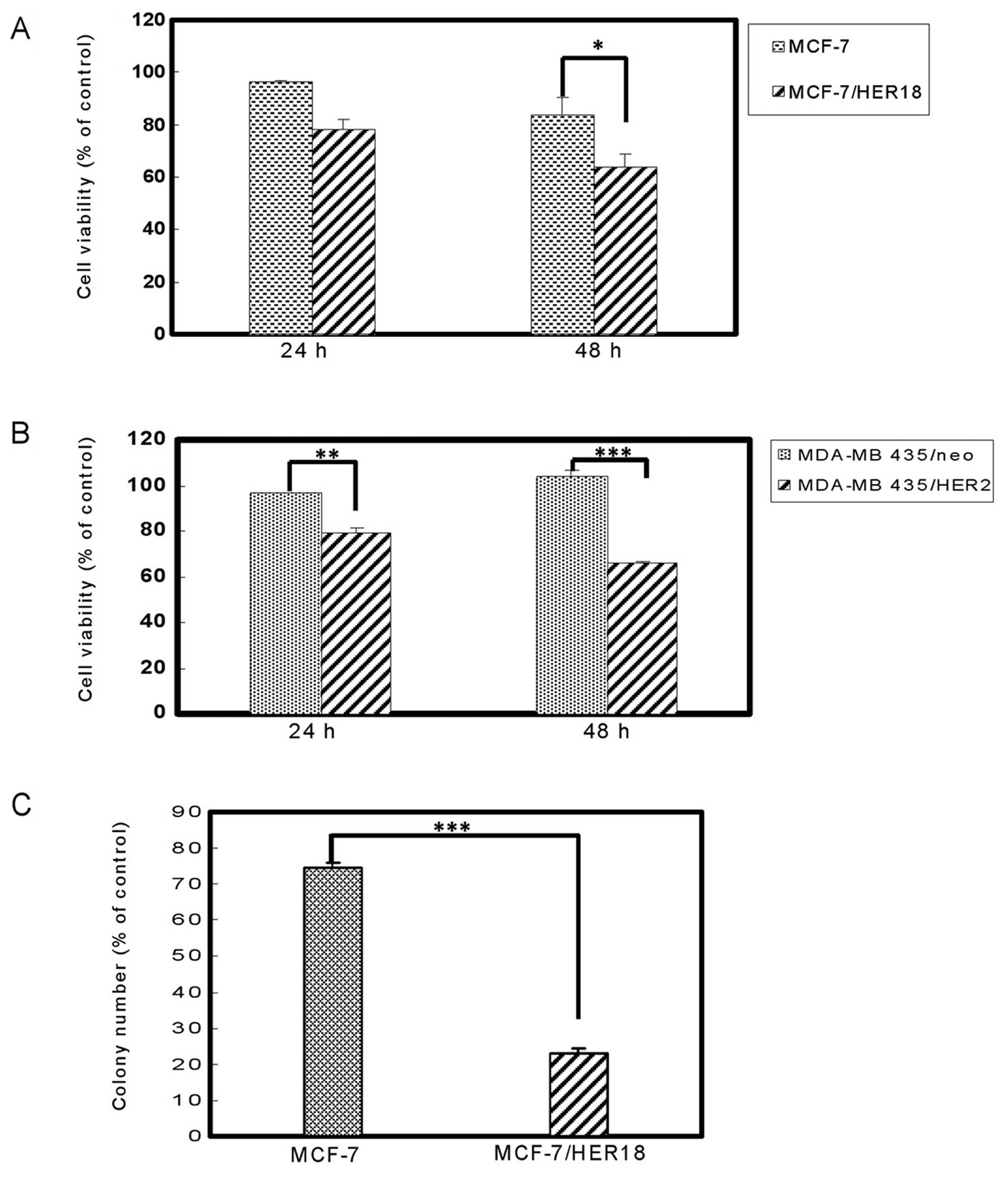
CLC604 preferentially inhibits the
proliferation of HER2-overexpressing cancer cells
To evaluate the effects of CLC604 on cell
proliferation, MCF-7 and MDA-MB-435 cells were stably transfected
with pSV2-erbB2 and then treated with 80 μM CLC604 for 24
and 48 h. The growth of these cell lines following CLC604 treatment
was inhibited in a time-dependent manner (Fig. 2A and B). Treatment with 80 μM CLC604
for 48 h inhibited over 40% of the growth in HER2-overexpressing
breast cancer cell lines which stably expressed HER2 (MCF-7/HER18
and MDA-MB-435/HER2). However, the growth inhibition by CLC604 was
marked lower in those parental cell lines expressing a basal level
of HER2 (MCF-7 and MDA-MB-435). Conversely, CLC604 significantly
inhibited the growth of HER2-overexpressing breast cancer cells,
which stably expressed HER2 as determined by cell
anchorage-independent growth assay (Fig. 2C). Overall, these results suggest
that CLC604 preferentially suppresses the growth of
HER2-overexpressing cancer cells.
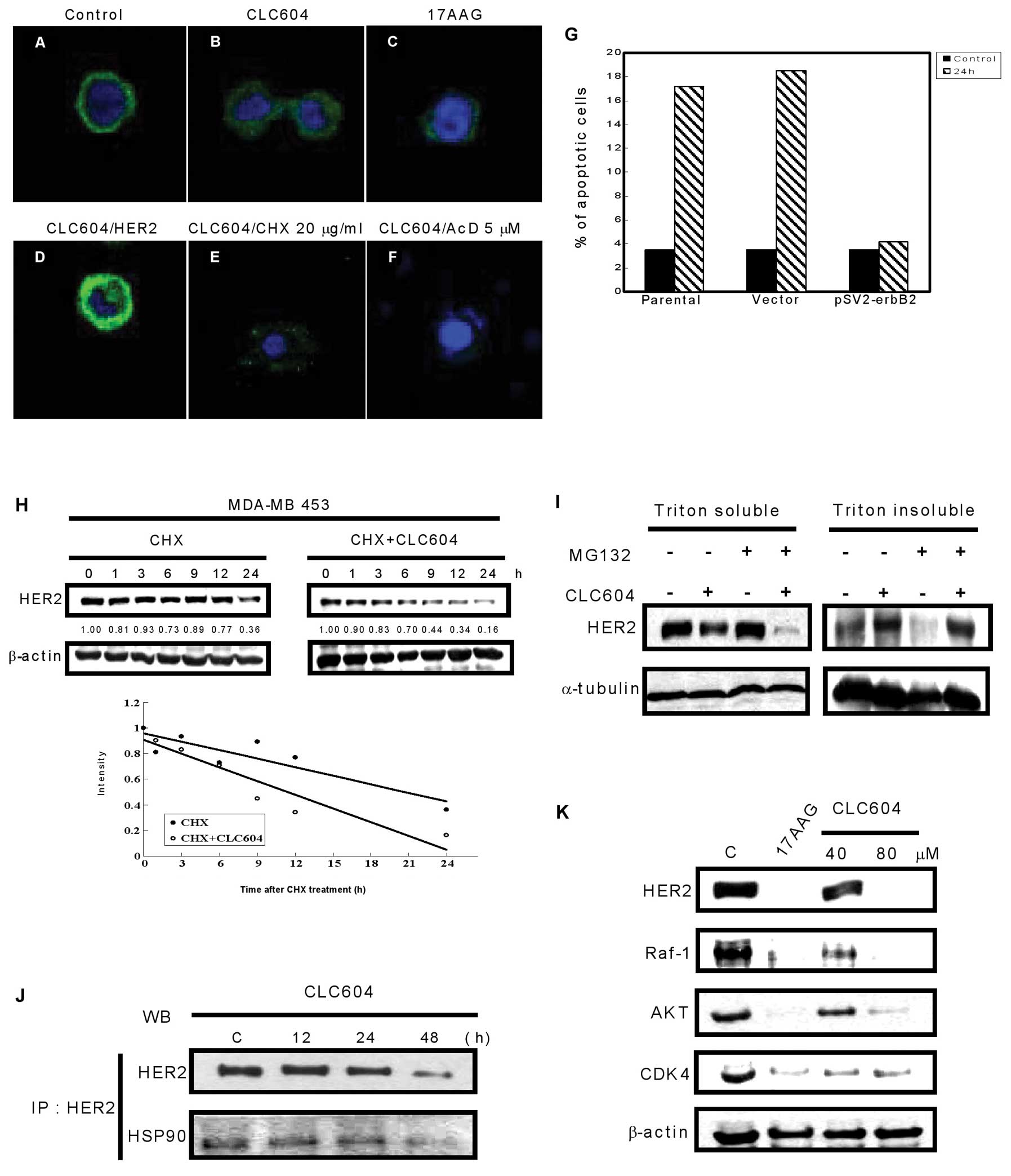
CLC604 alters the subcellular
distribution of HER2
To further confirm the inhibitory effect on HER2
expression by CLC604, an immunofluorescence study with the
anti-HER2 antibody (Ab-3) showed that the control cells had strong
immunofluorescence at the plasma membrane (Fig. 3A). However, after CLC604 treatment,
the immunofluorescence at the plasma membrane disappeared and was
replaced by diffused cytoplasmic punctate staining (Fig. 3B), which may be compatible with
localization in the endoplasmic reticulum or the Golgi apparatus.
Moreover, MDA-MB-453 cells were treated with 17-AAG (inhibitor of
Hsp90), and the immunofluorescence was also degraded (Fig. 3C), indicating that the stability of
HER2 on the plasma membrane may be associated with Hsp90.
HER2-mediated resistance to
CLC604-induced apoptosis
Cells transiently transfected with a human cDNA
encoding HER2 (pSV2-erbB2) recovered the immunofluorescence
at the membrane (Fig. 3D). This
phenomenon was not observed in cells transfected with a control
vector (data not shown). In addition, as shown in Fig. 3G, pSV2-erbB2-transfected
MDA-MB-453 cells demonstrated high resistance to CLC604-induced
apoptosis, whereas the untransfected cells progressively underwent
cell death. These results indicate that CLC604 reduced HER2 protein
levels and induced apoptosis in the HER2-overexpressing breast
cancer cells.
CLC604 inhibits HER2 expression by
decreasing HER2 stability
To delineate more effectively the mechanism of
CLC604-mediated HER2 downregulation, we tested the effect of CLC604
on the HER2 protein level compared with that on the mRNA level.
Combined with transcription inhibitor actinomycin D (AcD) or
translation inhibitor cycloheximide (CHX), we examined the effect
of CLC604 on the HER2 protein level in MDA-MB-453 breast cancer
cells. The HER2 protein level was detected by immunofluorescence
assay and observed by confocal microscopy. The addition of CHX
(Fig. 3E) or AcD (Fig. 3F) did not significantly alter the
effect of CLC604 on the immunofluorescence pattern, indicating that
CLC604 treatment did not alter HER2 mRNA levels or change the rate
of de novo synthesis of HER2. To determine whether HER2
degradation is accelerated by CLC604, MDA-MB-453 cells were treated
with CHX or with a combination of CHX and CLC604, and the relative
levels of HER2 protein in these cells were detected. As shown in
Fig. 3H, the degradation rate of
HER2 protein in MDA-MB-453 cells treated with CHX and CLC604 was
faster than that of the cells treated only with CHX. This indicates
that a posttranslational mechanism contributes to CLC604-inducd
HER2 depletion in HER2-overexpressing cancer cells. To investigate
further the role of proteolysis in CLC604-mediated HER2
downregulation, we conducted studies with the proteasome inhibitor
MG132. In the absence of MG132, CLC604 reduced the protein levels
of HER2 in both detergent (Triton X-100)-soluble and detergent
(Triton X-100)-insoluble cellular fractions. MG132 was found to
inhibit CLC604-mediated reduction in HER2 levels in the Triton
X-100-insoluble cellular fraction (Fig.
3I). These results suggest that proteosomal activity involves
CLC604-induced HER2 degradation.
Dissociation of HER2 from Hsp90 precedes
the depletion of HER2
HER2 must be bound to the Hsp90 molecular chaperone
complex, which is essential for HER2 stability and maturation
(25). Hsp90 is an ATP-binding
protein and has Mg2+-dependent ATPase activity. To
identify whether or not CLC604 disrupted the association of Hsp90
with HER2 resulting from the competition with ATP, an in
vitro Hsp90 ATPase activity assay was performed. Our results
showed that the efficacy of Hsp90 using ATP was inhibited by CLC604
treatment (IC50=14.32±0.46 μM). To study further the
mechanism of HER2 depletion by disassociating it with Hsp90,
MDA-MB-453 cells were treated with either the control vehicle or 80
μM CLC604 at a variety of periods, and the binding of HER2 with
Hsp90 was assessed. Equal amounts of fractionated proteins were
immunoprecipitated with 1 μg of an anti-HER2 monoclonal antibody,
and the immunoprecipitates were blotted with HER2 and Hsp90
antibodies. The binding of HER2 with Hsp90 had already
significantly decreased after CLC604 treatment (Fig. 3J). Moreover, Hsp90 inhibitor 17-AAG
and CLC604 (40 and 80 μM) were also found to decrease the levels of
client proteins of Hsp90 (HER2, Raf-1, AKT, CDK4) in MDA-MB-453
cells (Fig. 3K).
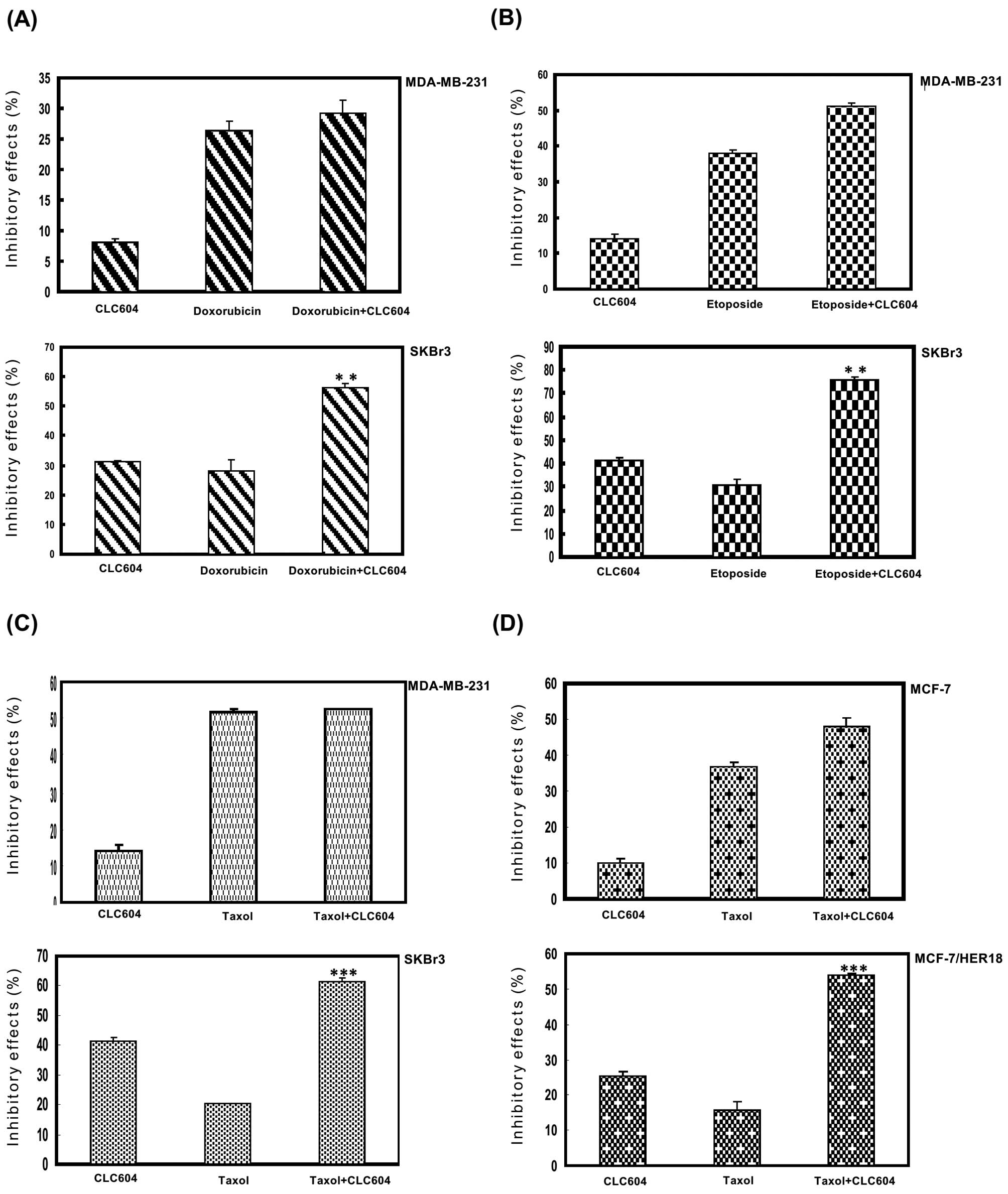
CLC604 enhances the sensitivity of
doxorubicin, etoposide, and Taxol on the growth of
HER2-overexpressing cancer cells
To investigate whether or not CLC604 sensitizes
HER2-overexpressing cancer cells to clinical drugs, we used an MTT
assay to investigate the effect of CLC604 treatment alone or in
combination with doxorubicin, etoposide or Taxol on the growth of
HER2-overexpressing cancer cells. To identify optimal conditions
for the combination treatment, we first examined the sensitivity of
the HER2-overexpressing cancer cells to doxorubicin, etoposide or
Taxol. As shown in Fig. 4, compared
with the cancer cell line that expressed low levels of HER2, the
HER2-overexpressing cancer cells demonstrated greater resistance to
doxorubicin (Fig. 4A), etoposide
(Fig. 4B) and Taxol (Fig. C). We then examined the combined
effects of CLC604 and doxorubicin, etoposide or Taxol on the growth
of MDA-MB-231 cells, which express low levels of HER2, and on the
growth of SKBr3 cells, which overexpress HER2. The combination of
CLC604 and doxorubicin (Fig. 4A),
etoposide (Fig. 4B) or Taxol
(Fig. 4C) synergistically inhibited
HER2-overexpressing SKBr3 cell growth. However, no significant
synergistic antiproliferative effect was noted in the MDA-MB-231
cells.
To investigate specifically the effects of CLC604 on
HER2-induced drug resistance, we required transformed cells whose
drug resistance phenotypes are induced solely by an HER2 oncogene.
To achieve this, we used the MCF-7/HER18 breast cancer cell line,
which stably expresses HER2. Next, we examined the effects of
CLC604 on the cell growth rate. As shown in Fig. 4D, the HER2-overexpressing cancer
(MCF-7/HER18) cells were much more resistant to Taxol than the
parental breast cancer (MCF-7) cells. Regarding the efficacy of
combinational treatment, the cytotoxicity of CLC604 when combined
with Taxol in HER2-overexpressing cancer cells was obviously
increased when compared to the cytotoxic effect following treatment
with CLC604 or Taxol alone. These results indicate that CLC604
enhances the cytotoxic effect of clinical drugs in
HER2-overexpressing cancer cells and reduces the HER2-induced drug
resistance of cancer cells.
CLC604 sensitizes HER2-overexpressing
tumors in SCID mice to Taxol
As mentioned above, CLC604 acts synergistically with
Taxol to inhibit the growth of HER2-overexpressing human breast
cancer cells in vitro (Fig.
4D); we therefore examined whether CLC604 sensitizes
HER2-overexpressing tumors in athymic SCID mice to Taxol. MCF-7 or
MCF-7/HER18 cells were injected s.c. into athymic BALB/c SCID mice.
When the solid tumors became palpable, mice were treated with
either control, CLC604 alone (50 and 100 mg/kg, respectively),
Taxol alone (5 mg/kg), or a combination of CLC604 (50 mg/kg) and
Taxol (5 mg/kg) given by i.p. injection every 5 days each week for
4 consecutive weeks. As shown in Fig.
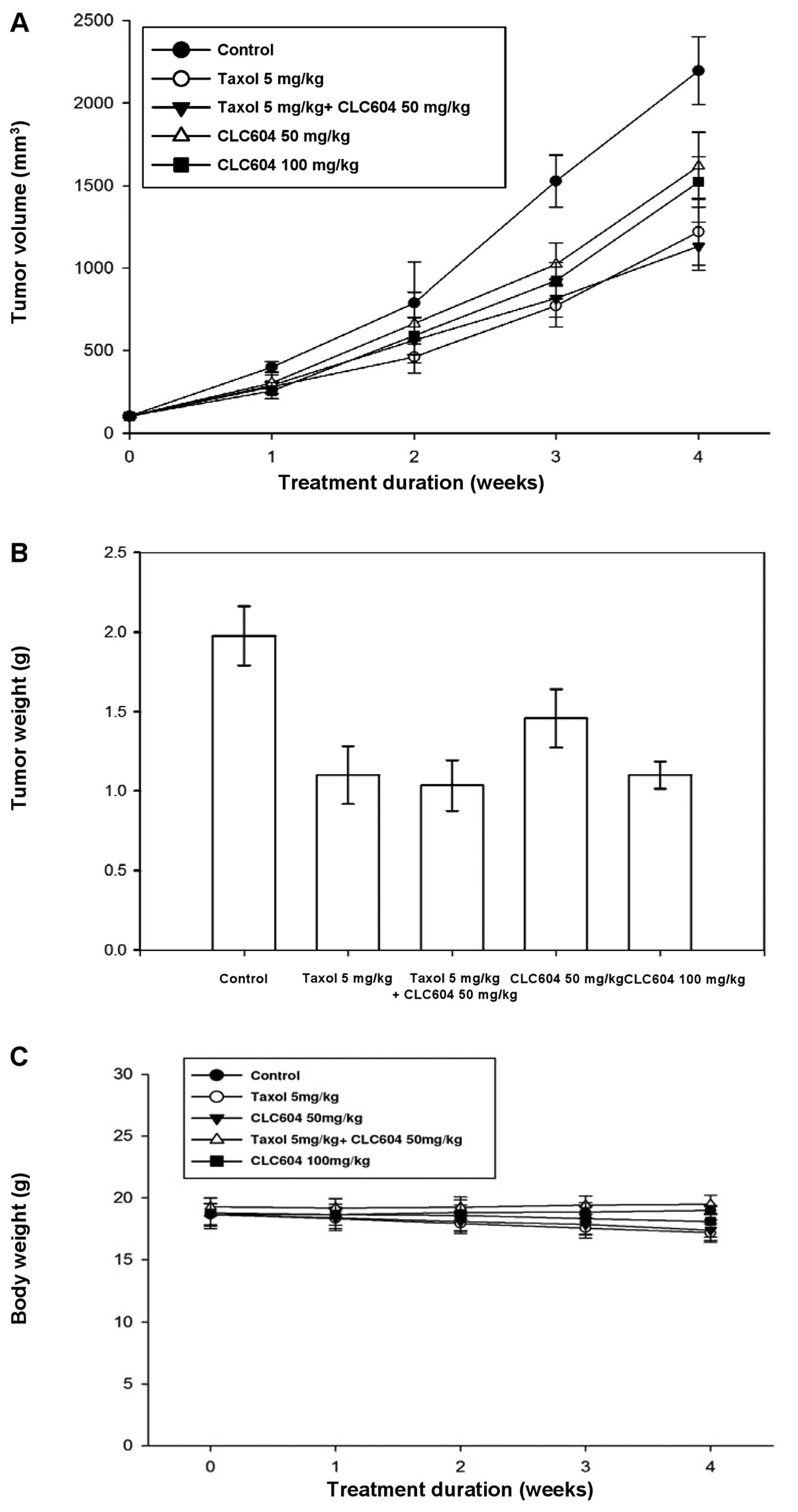
5, treatment of the MCF-7 tumor-bearing mice with Taxol alone
significantly inhibited tumor growth and tumor weight (Fig. 5A and B). Moreover, the inhibitory
effect on tumor growth was not enhanced by injection of CLC604
followed by Taxol (Fig. 5A and B).
However, the MCF-7/HER18 tumor-bearing mice were much more
resistant to Taxol than the MCF-7 tumor-bearing mice (Fig. 6A and B). Notably, the combination
treatment was significantly more effective than either of the
treatments alone in MCF-7/HER18 tumor-bearing mice (Fig. 6A and B). During evaluation of the
antitumor activity, no apparent changes in mouse body weight were
observed in either the treatment or the control group (Figs. 5C and 6C). These in vivo experimental
results unambiguously indicate that CLC604 significantly enhances
the antitumor efficacy of Taxol in HER-overexpressing tumors and
efficiently reduces the HER2-induced drug resistance.
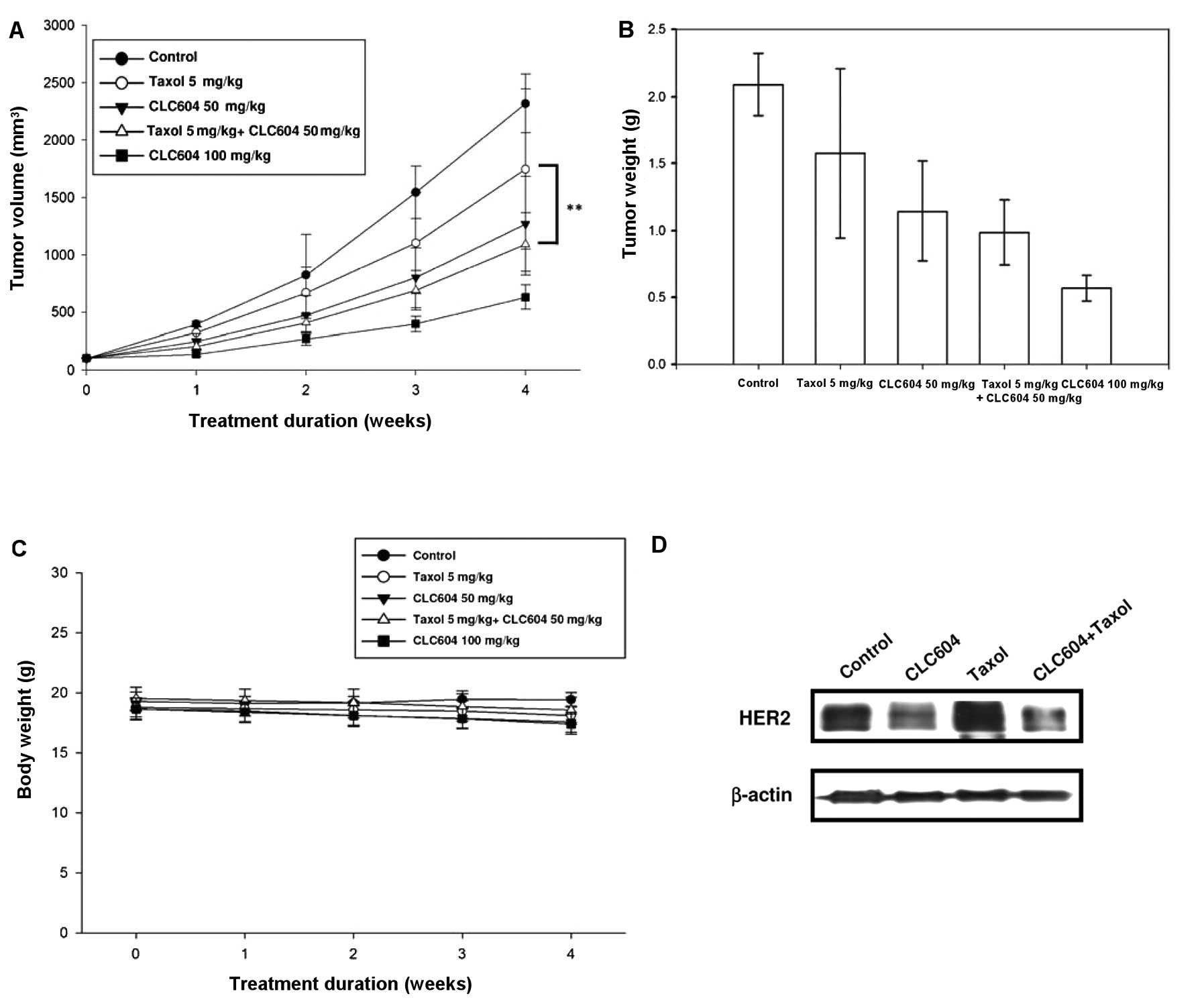 | Figure 6Effect of CLC604 alone or in
combination with Taxol in the HER2-overexpressing BALB/c SCID mouse
subcutaneous xenograft model. Female BALB/c SCID mice (n=6) were
subcutaneously inoculated with 2×106 MCF-7/HER18 cells.
When the solid tumors were palpable and tumor size was measured as
shown at day 0, the mice were given either a placebo, CLC604 (50
and 100 mg/kg, respectively), Taxol (5 mg/kg), or CLC604 (50 mg/kg)
plus Taxol (5 mg/kg) by i.p. injection every 5 days each week for 4
consecutive weeks. (A) Tumor volume (mm3), (B) tumor
weight (g), (C) body weight (g) are shown. Results are expressed as
means; bars, SD. (D) Western blot analysis of levels of HER2
protein in vivo. Protein extracts were prepared by
homogenizing tumor tissues obtained from the control,
CLC604-treated, Taxol-treated, and combined CLC604 and
Taxol-treated mice with lysis buffer. Western blotting was
conducted using an anti-HER2 antibody or an anti-β-actin antibody,
as described in ‘Materials and methods’. |
CLC604 suppresses the expression of HER2
in vivo
To investigate whether or not suppression of HER2
was associated with the therapeutic effects of CLC604 on tumors
in vivo, MCF-7/HER18 tumors from the mice in each group
(control, CLC604 alone (50 mg/kg), Taxol alone, or CLC604 plus
Taxol) were analyzed by western blot analysis. HER2 levels in the
CLC604-treated tumors were barely detectable, compared with levels
in the control tumors; however, HER2 levels in the Taxol-treated
tumors were not significantly altered (Fig. 6D). HER2 levels in the tumors treated
with CLC604 combined with Taxol were obviously markedly decreased
than levels in the tumors treated with CLC604 or Taxol alone. These
results indicate that CLC604 suppresses the growth of
HER2-overexpressing tumors in SCID mice by inhibiting the
expression of HER2 and reduces the HER2-induced drug resistance of
cancer cells.
Discussion
In the present study, we examined the relationship
between the chemical structures and the inhibitory activity of YC-1
and its furopyrazole and thienopyrazole isosteric analogues on the
expression of HER2 protein. We identified that 1 of the 8
derivatives, CLC604, was more effective than the other derivatives,
even more than YC-1 in suppressing the HER2 protein level. We
demonstrated that CLC604 preferentially inhibited the growth of
HER2-overexpressing cancer cells, but not the cell lines expressing
basal levels of HER2, and suppressed the transformation phenotype
induced by HER2 overexpression. Moreover, CLC604 was also found to
be more effective in inhibiting the proliferation of
HER2-overexpressing cancer cells, which were stably transfected
with pSV2-erbB2, when compared with breast cancer cells
expressing basal levels of HER2.
Resent studies have demonstrated that constitutive
phosphorylation of HER2 is associated with resistance to systemic
therapies and local radiation therapies. Activation of
HER2-containing heterodimers results in receptor
autophosphorylation on COOH-terminal tyrosine residues, which
become the docking sites for a number of signal transducers and
adaptor molecules that initiate a plethora of signaling programs
leading to cell proliferation, differentiation, migration,
adhesion, protection from apoptosis and transformation, among other
effects. Our present study showed that HER2 is essential for cell
survival. We treated HER2-overexpressing breast cancer MDA-MB-453
cells with 80 μM CLC604, and the cell survival rate was detected by
flow cytometry and compared with MDA-MB-453 cells transiently
transfected with pSV2-erbB2. These results demonstrated that
pSV2-erbB2-transfected MDA-MB-453 cells exhibited high
resistance to CLC604-induced apoptosis, whereas the untransfected
cells progressively underwent cell death. This is consistent with a
recent report that HER2-overexpressing cancer cells are dependent
on HER2 levels for survival and, thus, are more sensitive to
treatments that target HER2.
In previous studies, YC-1 has been identified as a
novel class of NO-dependent stimulators of soluble gualylate
cyclase (sGC) that exhibit therapeutic potential for the treatment
of a range of vascular diseases, including hypertension,
thrombosis, erectile dysfunction, and postangioplasty restenosis
(14,26,27).
Currently, YC-1 has been proven to suppress proliferation of HA22T
cells through G0-G1 arrest via inhibition of
CDK2 and CDK4 by upregulation of p21CIP1/WAP1 and
p27KIP1(28).
Furthermore, YC-1 was observed to suppress NF-κB activity via
inhibition of the phosphorylation and degradation of I-κBα and to
induce apoptosis in prostate cancer PC-3 cells (17). Moreover, YC-1 was recognized to
inhibit angiogenesis via repression of VEGF by downregulating HIF-1
resulting in reducing cancer cell proliferation (16,29–31).
In the present study, our results showed that YC-1 repressed the
protein level of HER2. Interestingly, we found that its isosteric
analogue, CLC604, was more effective than YC-1 and its known
derivatives in repressing the protein level of HER2. Moreover,
CLC604 preferentially inhibited the growth of HER2-overexpressing
cancer cells.
Hsp90 is required for refolding unfolded proteins
and for cellular survival under environmental stress, and plays a
key role in transducing proliferative and anti-apoptotic signals
particularly in tumor cells; but in normal tissues, Hsp90 exists in
a free, uncomplexed, or latent state (32). Consequently, inhibition of Hsp90 has
emerged as a possible strategy for the treatment of advanced
cancers (10). Research found that
the benzoquinone ansamycins such as geldanamycin and other Hsp90
inhibitors, such as 17-AAG, enhanced the intracellular degradation
of HER2 which involved targeting of the Hsp90 (13). Hsp90 forms complexes with HER2 and
other client proteins. Recently, the mechanistic basis of Hsp90
client proteins sensitive to 17-AAG has been demonstrated. The data
of client protein half-life has showed that HER2 < mutant EGFR
< Raf-1 < Akt < mutant BRAF < wild-type EGFR or HER2 is
more sensitive to 17-AAG than other client proteins (33). Once 17-AAG blocks ATP binding to
Hsp90, the chaperone complex associated with the client protein is
biased toward a degradative fate, resulting in polyubiquitylation
and subsequent destruction of the client. The mature HER2 requires
Hsp90 association with its kinase domain to maintain the
conformation necessary to heterodimerize with other
ligand-activated HER proteins.
Our present study found that the HER2 protein level
decreased more rapidly in cells treated with CHX plus CLC604 than
in cells treated with CHX alone. This result suggests that a
post-translational mechanism contributes to CLC604-induced HER2
instability and depletion in HER2-overexpressing cancer cells. We
then attempted to identify whether or not CLC604 disrupts the
association of Hsp90 with HER2 resulting from competition with ATP.
Our in vitro Hsp90 ATPase activity assay showed that the
efficacy of Hsp90 was inhibited by CLC604 treatment. CLC604
dissociated the complex between HER2 and Hsp90, and such
dissociation precedes the depletion of mature HER2 at the plasma
membrane. The depletion of mature membrane HER2 and the concomitant
accumulation of HER2 in the cytoplasmic organelles are compatible
with the notion that the complex of HER2 with Hsp90 is necessary
for its maturation and subsequent transport to the plasma membrane.
We thus hypothesized that CLC604 may also disrupt the association
of HER2 and the chaperone complex through competition with ATP, and
this may explain why CLC604 can deplete HER2 protein. Aside from
HER2, other Hsp90 client proteins, such as Akt, c-Raf and CDK4,
were also reduced by CLC604.
Recently, research has suggested that
HER2-overexpressing cancer cells develop drug resistance and
relapse capacity following treatment with clinical drugs (34). This supports the notion that HER2
overexpression is associated with chemoresistance. Moreover, data
from clinical trials in breast cancer also suggest an association
between HER2 overexpression and resistance to chemotherapy
(35–38). Their results indicate that
node-negative breast cancer patients whose tumors contains HER2
overexpression have a less favorable prognosis due to a lack of
response to adjuvant cyclophosphamide, methotrexate, and
5-fluorouracil-based chemotherapy. A study of HER2 overexpression
in epithelial ovarian cancer also demonstrated that patients whose
tumors had the HER2 alteration were more likely to have a failed
response to chemotherapy with cyclophosphamide and carboplatin
(39). These reports support the
notion that HER2 overexpression is associated with
chemoresistance.
In the present study, we demonstrated that CLC604
was able to sensitize SKBr3 and MCF-7/HER18 breast cancer cells
which overexpress HER2 to the anticancer drugs doxorubicin,
etoposide and Taxol in vitro, but did not have the same
effect on the MDA-MB-231 and MCF-7 cell lines, which express basal
levels of HER2. These results suggest that HER2 is required for
cell growth and promotes doxorubicin, etoposide and Taxol
resistance; and tumor suppression by CLC604 alone and the
synergistic effect of CLC604 plus Taxol on tumor growth in mice may
be due to decreased HER2. In the mice with HER2-overexpressing
MCF-7/HER18 tumors, CLC604 significantly reduced tumor volume and
weight. Western blot analysis indicated that the HER2 level was
significantly reduced in the tumors by CLC604 treatment when
compared with the control treatment. These results indicate that
CLC604 functions as an Hsp90 inhibitor and causes
HER2-overexpressing cancer cells to become sensitized to Taxol
in vivo. Our data corroborated a previous report that higher
efficiency was obtained when combining treatment with 17-AAG, an
Hsp90 inhibitor, causing cancer cells to become sensitive to Taxol
specifically and other clinical drugs when treating
HER2-overexpressing cancer (40).
Taken together, the central and novel findings in
the present study are that i) CLC604 is more effective than YC-1
and its known derivatives; ii) CLC604 decreases the expression
level of HER2 in HER2-overexpressing cancer cells in vitro;
iii) CLC604 significantly suppresses the growth of
HER2-overexpressing cancer cells and transformed breast cancer
cells in soft agarose; iv) CLC604 decreases the protein half-life
of HER2 by proteasome activity; and v) CLC604 may act as an Hsp90
inhibitor and cause HER2-overexpressing cancer cells to become
sensitive to Taxol, doxorubicin and etoposide. The above findings
may help improve the efficacy of preventive or therapeutic
compounds against HER2-overexpressing cancer cells.
Acknowledgements
The investigation was supported by a research grants
from the National Science Council (NSC 101-2325-B-039-005) to S.C.
Kuo. Thanks are also due for the support (in part) by a grant from
the Department of Health (Taiwan), China Medical University
Hospital Cancer Research Center of Excellence (DOH100-TD-C-111-005)
and a grant from the China Medical University (CMU100-TS-10). We
thank Professor Mien-Chie Hung (The University of Texas M.D.
Anderson Cancer Center, Houston, TX, USA) for generously providing
cancer cell lines MDA-MB 453/neo, MDA-MB 435/HER2 and
MCF-7/HER18.
Abbreviations:
|
17-AAG
|
17-(allylamino)-17-demethoxygeldanamycin
|
|
CHX
|
cycloheximide
|
|
CLC604
|
(1-benzyl-3-(p-hydroxymethylphenyl)-5-methylfuro[3,2-c]pyrazol)
|
|
EGFR
|
epithelial growth factor receptor
|
|
HIF-1α
|
hypoxia-inducible factor-1α
|
|
Hsp90
|
90-kDa heat shock protein
|
|
MG132
|
Z-Leu-Leu-Leu-al
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,
5-diphenyltetrazolium bromide
|
|
PBS
|
phosphate-buffered saline
|
|
YC-1
|
1-benzyl-3-(5-hydroxymethyl-2-furyl)indazole
|
References
|
1
|
Stearns V, Schneider B, Henry NL, Hayes DF
and Flockhart DA: Breast cancer treatment and ovarian failure: risk
factors and emerging genetic determinants. Nat Rev Cancer.
6:886–893. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chiang CT, Way TD and Lin JK: Sensitizing
HER2-overexpressing cancer cells to luteolin-induced apoptosis
through suppressing p21WAF1/CIP1 expression with
rapamycin. Mol Cancer Ther. 6:2127–2138. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Slamon DJ, Clark GM, Wong SG, Levin WJ,
Ullrich A and McGuire WL: Human breast cancer: correlation of
relapse and survival with amplification of the HER-2/neu oncogene.
Science. 235:177–182. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu D and Hung MC: Overexpression of ErbB2
in cancer and ErbB2-targeting strategies. Oncogene. 19:6115–6121.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meric-Bernstam F and Hung MC: Advances in
targeting human epidermal growth factor receptor-2 signaling for
cancer therapy. Clin Cancer Res. 12:6326–6330. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Esteva FJ, Yu D, Hung MC and Hortobagyi
GN: Molecular predictors of response to trastuzumab and lapatinib
in breast cancer. Nat Rev Clin Oncol. 7:98–107. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vogel CL, Cobleigh MA, Tripathy D, et al:
Efficacy and safety of trastuzumab as a single agent in first-line
treatment of HER2-overexpressing metastatic breast cancer. J Clin
Oncol. 20:719–726. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cardoso F, Piccart MJ, Durbecq V and Di
Leo A: Resistance to trastuzumab: a necessary evil or a temporary
challenge? Clin Breast Cancer. 3:247–257. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lan KH, Lu CH and Yu D: Mechanisms of
trastuzumab resistance and their clinical implications. Ann NY Acad
Sci. 1059:70–75. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Trepel J, Mollapour M, Giaccone G and
Neckers L: Targeting the dynamic HSP90 complex in cancer. Nat Rev
Cancer. 10:537–549. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu W, Mimnaugh E, Rosser MF, Nicchitta C,
Marcu M, Yarden Y and Neckers L: Sensitivity of mature Erbb2 to
geldanamycin is conferred by its kinase domain and is mediated by
the chaperone protein Hsp90. J Biol Chem. 276:3702–3708. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Richter K, Reinstein J and Buchner J: A
Grp on the Hsp90 mechanism. Mol Cell. 28:177–179. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Solit DB, Zheng FF, Drobnjak M, et al:
17-Allylamino-17-demethoxygeldanamycin induces the degradation of
androgen receptor and HER-2/neu and inhibits the growth of prostate
cancer xenografts. Clin Cancer Res. 8:986–993. 2002.PubMed/NCBI
|
|
14
|
Ko FN, Wu CC, Kuo SC, Lee FY and Teng CM:
YC-1, a novel activator of platelet guanylate cyclase. Blood.
84:4226–4233. 1994.PubMed/NCBI
|
|
15
|
Chou LC, Huang LJ, Yang JS, Lee FY, Teng
CM and Kuo SC: Synthesis of furopyrazol analogs of
1-benzyl-3-(5-hydroxymethyl-2-furyl)indazole (YC-1) as novel
anti-leukemia agents. Bioorg Med Chem. 15:1732–1740. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pan SL, Guh JH, Peng CY, et al: YC-1
[3-(5′-hydroxymethyl-2′-furyl)-1-benzyl indazole] inhibits
endothelial cell functions induced by angiogenic factors in vitro
and angiogenesis in vivo models. J Pharmacol Exp Ther. 314:35–42.
2005.
|
|
17
|
Huang YT, Pan SL, Guh JH, Chang YL, Lee
FY, Kuo SC and Teng CM: YC-1 suppresses constitutive nuclear
factor-κB activation and induces apoptosis in human prostate cancer
cells. Mol Cancer Ther. 4:1628–1635. 2005.PubMed/NCBI
|
|
18
|
Chun YS, Yeo EJ, Choi E, Teng CM, Bae JM,
Kim MS and Park JW: Inhibitory effect of YC-1 on the hypoxic
induction of erythropoietin and vascular endothelial growth factor
in Hep3B cells. Biochem Pharmacol. 61:947–954. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mujoo K, Sharin VG, Bryan NS, et al: Role
of nitric oxide signaling components in differentiation of
embryonic stem cells into myocardial cells. Proc Natl Acad Sci USA.
105:18924–18929. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yun S, Lee SH, Kang YH, et al: YC-1
enhances natural killer cell differentiation from hematopoietic
stem cells. Int Immunopharmacol. 10:481–486. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chou LC, Huang LJ, Hsu MH, et al:
Synthesis of
1-benzyl-3-(5-hydroxymethyl-2-furyl)selenolo[3,2-c]pyrazole
derivatives as new anticancer agents. Eur J Med Chem. 45:1395–1402.
2010.PubMed/NCBI
|
|
22
|
Way TD, Lee JC, Kuo DH, et al: Inhibition
of epidermal growth factor receptor signaling by Saussurea
involucrata, a rare traditional Chinese medicinal herb, in
human hormone-resistant prostate cancer PC-3 cells. J Agric Food
Chem. 58:3356–3365. 2010.PubMed/NCBI
|
|
23
|
Lin VC, Chou CH, Lin YC, et al: Osthole
suppresses fatty acid synthase expression in HER2-overexpressing
breast cancer cells through modulating Akt/mTOR pathway. J Agric
Food Chem. 58:4786–4793. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Panaretou B, Prodromou C, Roe SM, O’Brien
R, Ladbury JE, Piper PW and Pearl LH: ATP binding and hydrolysis
are essential to the function of the Hsp90 molecular chaperone in
vivo. EMBO J. 17:4829–4836. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oude Munnink TH, Korte MA, Nagengast WB,
et al: 89 Zr-trastuzumab PET visualises HER2 downregulation by the
HSP90 inhibitor NVP-AUY922 in a human tumour xenograft. Eur J
Cancer. 46:678–684. 2010.PubMed/NCBI
|
|
26
|
Evgenov OV, Pacher P, Schmidt PM, Haskó G,
Schmidt HH and Stasch JP: NO-independent stimulators and activators
of soluble guanylate cyclase: discovery and therapeutic potential.
Nat Rev Drug Discov. 5:755–768. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu XM, Peyton KJ, Mendelev NN, Wang H,
Tulis DA and Durante W: YC-1 stimulates the expression of gaseous
monoxide-generating enzymes in vascular smooth muscle cells. Mol
Pharmacol. 75:208–217. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang SW, Pan SL, Guh JH, et al: YC-1
[3-(5′-Hydroxymethyl-2′-furyl)-1-benzyl indazole] exhibits a novel
antiproliferative effect and arrests the cell cycle in G0-G1 in
human hepatocellular carcinoma cells. J Pharmacol Exp Ther.
312:917–925. 2005.
|
|
29
|
Yeo EJ, Chun YS, Cho YS, Kim J, Lee JC,
Kim MS and Park JW: YC-1: a potential anticancer drug targeting
hypoxia-inducible factor 1. J Natl Cancer Inst. 95:516–525. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yeh WL, Lu DY, Lin CJ, Liou HC and Fu WM:
Inhibition of hypoxia-induced increase of blood-brain barrier
permeability by YC-1 through the antagonism of HIF-1α accumulation
and VEGF expression. Mol Pharmacol. 72:440–449. 2007.PubMed/NCBI
|
|
31
|
Zhao Q, Du J, Gu H, Teng X, Zhang Q, Qin H
and Liu N: Effects of YC-1 on hypoxia-inducible factor 1-driven
transcription activity, cell proliferative vitality, and apoptosis
in hypoxic human pancreatic cancer cells. Pancreas. 34:242–247.
2007. View Article : Google Scholar
|
|
32
|
Kamal A, Thao L, Sensintaffar J, Zhang L,
Boehm MF, Fritz LC and Burrows FJ: A high-affinity conformation of
Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature.
425:407–410. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sawai A, Chandarlapaty S, Greulich H, et
al: Inhibition of Hsp90 down-regulates mutant epidermal growth
factor receptor (EGFR) expression and sensitizes EGFR mutant tumors
to paclitaxel. Cancer Res. 68:589–596. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Morrow PK, Zambrana F and Esteva FJ:
Recent advances in systemic therapy: advances in systemic therapy
for HER2-positive metastatic breast cancer. Breast Cancer Res.
11:2072009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Allred DC, Clark GM, Tandon AK, et al:
HER-2/neu in node-negative breast cancer: prognostic significance
of overexpression influenced by the presence of in situ
carcinoma. J Clin Oncol. 10:599–605. 1992.PubMed/NCBI
|
|
36
|
Harris JR, Lippman ME, Veronesi U and
Willett W: Breast cancer (1). N Engl J Med. 327:319–328. 1992.
View Article : Google Scholar
|
|
37
|
Harris JR, Lippman ME, Veronesi U and
Willett W: Breast cancer (2). N Engl J Med. 327:390–398. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Harris JR, Lippman ME, Veronesi U and
Willett W: Breast cancer (3). N Engl J Med. 327:473–480. 1992.
View Article : Google Scholar
|
|
39
|
Felip E, Del Campo JM, Rubio D, Vidal MT,
Colomer R and Bermejo B: Overexpression of c-erbB-2 in epithelial
ovarian cancer. Prognostic value and relationship with response to
chemotherapy. Cancer. 75:2147–2152. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ramalingam SS, Egorin MJ, Ramanathan RK,
et al: A phase I study of 17-allylamino-17-demethoxygeldanamycin
combined with Taxol in patients with advanced solid malignancies.
Clin Cancer Res. 14:3456–3461. 2008. View Article : Google Scholar : PubMed/NCBI
|