Introduction
Prostate cancer is the most common non-cutaneous
malignancy in Western countries and a major fatal disease in men
(1). In particular, prostate
cancer-related mortality usually appears in men of advanced age
(2). In several different
therapeutic strategies for cancer cells, the key focus is the
inhibition of cellular proliferation or induction of apoptosis.
Previous studies have discovered a number of materials including
natural products (such as betulinic acid) and chemical compounds
(such as polyamine analogues and everolimus and docetaxel) to
prevent the proliferation of cancer cells, and the substances have
proven to be effective (3–7).
Recently, various methods including surgery,
radiation, chemotherapy and hormonal therapy have been used to
treat prostate cancer cells, but, among them, chemopreventive
methods are considered key in decreasing progression, mortality,
and invasive intervention (8). In
chemopreventive methods, administration of phytoestrogen,
antioxidant, and cyclooxygenase-2 (Cox-2) selective inhibitors are
represented in prostate cancer therapy (2). An alternative to prevent the growth of
prostate cancer cells, a selective combination of dietary
phytoestrogens (such as genistein, quercetin and biochanin A), was
reported to inhibit cell proliferation of androgen-responsive
prostate cancer cells (9).
Antioxidants, which protect cells from damage caused by oxidative
stress, are associated with pathological conditions including
inflammation that are a precursor in neoplastic transformation of
the prostate (8). Huang et
al(10) reported that
benzodithiazolium-based compound CX9051 is a selective inhibitor
for Cox-2 activity, which inhibits cell proliferation and induces
apoptosis in numerous human cancer cell types including prostate
cancer cells. In addition to these results, various strategies have
been extensively studied for prostate cancer therapy.
Previous studies have reported that various
monoamine oxidase (MAO) inhibitors including pargyline,
tranylcypromine, clorgyline and other derivatives are used for
cancer treatment in human cancer cells (11–16).
Tranylcypromine, clorgyline and pargyline effectively decreased
cell proliferation in various breast cancer cell lines such as
MDA-MB-231, MDA-MB-453, MCF-7 and T47D (11,16).
Also, in neuroblastoma cells, tranylcypromine showed similar
results with breast cancer cells (12). Cortez et al(13) reported that after human breast
cancer cells were injected into nude mice, regular treatment of
pargyline exhibited suppression of tumor growth. Based on these
previous studies (11–16), MAO inhibitors may have potential as
anticancer agents.
In order to verify the anticancer potential of
pargyline and tranylcypromine, we examined the effect of pargyline
and tranylcypromine on the cell viability of human prostate
carcinoma LNCaP-LN3 cells. After exposing LNCaP-LN3 cells to
pargyline or tranylcypromine, we investigated the cell
proliferation rate, the cell cycle distribution and the induction
of apoptosis in the cells. Furthermore, we analyzed the expression
of apoptosis-related genes by treatment of pargyline or
tranylcypromine in LNCaP-LN3 cells.
Materials and methods
Cell culture
Human prostate carcinoma cells (LNCaP-LN3; KCLB No.
80018) were obtained from the Korean Cell Line Bank (Seoul, Korea).
LNCaP-LN3 cells were grown in MEM, supplemented with 10% fetal
bovine serum, penicillin (100 U/ml)/streptomycin (100 μg/ml) (all
from Invitrogen Life Technologies, Carlsbad, CA, USA) at 37°C in a
5% CO2 atmosphere.
Cell proliferation assay
The proliferation of LNCaP-LN3 cells was evaluated
using a Premix WST-1 Cell Proliferation Assay System (Takara Bio,
Inc., Shinga, Japan). After exposing the cells to pargyline or
tranylcypromine (0.5, 1, 1.5 or 2 mM) for 24, 48, 72, 96 or 120 h,
the culture medium was removed and the cells were washed with
phosphate buffered saline (PBS). WST-1 reagent was then added, and
the cells were incubated for 4 h. The results of the WST-1 assay
were measured using a Model 680 microplate reader (Bio-Rad,
Hercules, CA, USA).
Cell cycle analysis
The cell cycle assay was performed as previously
described (17). The cells were
plated in 10-cm2 plates (Corning Inc., Corning, NY, USA)
and cultured in normal growth medium for 24 h before treatment with
pargyline or tranylcypromine (Sigma-Aldrich, St. Louis, MO, USA).
After treating with pargyline or tranylcypromine for 24 or 48 h,
the cells were harvested to analyze cell cycle using 0.25%
trypsin-EDTA (Invitrogen Life Technologies). The cells were washed
twice with PBS, and probed with BD CycleTest™ Plus DNA Reagent kit
(BD Biosciences, Franklin Lakes, NJ, USA) according to the
manufacturer’s instructions. Cell cycle distribution was analyzed
using FACSCalibur (BD Biosciences). The percentage of cells in
different cell cycle phases was calculated using ModFit LT 3.0
(Verity Software House, Topsham, ME, USA).
Real-time RT-PCR
After exposing LNCaP-LN3 cells to pargyline or
tranylcypromine, total RNA was isolated using RNAiso Plus (Takara
Bio, Inc.). Total RNA was reverse transcribed into cDNA using
PrimeScript™ Reverse Transcriptase (Takara Bio, Inc.). Real-time
PCR was performed using 7500 real-time PCR system (Applied
Biosystems, Foster City, CA, USA) and 2X SYBR-Green PCR Master Mix
(Takara Bio, Inc.). The sequences of the primers used in this study
were: BCL-2 forward, 5′-GGGGAGGATTGTGGCCTTC-3′ and reverse,
5′-CAGGGCGATGTTGTCCACC-3′; NOXA forward,
5′-ACCAAGCCGGATTTGCGATT-3′ and reverse,
5′-ACTTGCACTTGTTCCTCGTGG-3′; and β-actin forward,
5′-TGGAGAAAATCTGGCACCACACC-3′ and reverse,
5′-GATGGGCACAGTGTGGGTGACCC-3′. β-actin was used as an internal
standard. The gene expression levels were analyzed using the
2−ΔΔCT method (18).
Apoptosis analysis
Cells were plated at 1×106
cells/cm2 in 10-cm2 plates and grown for 24 h
before treatment with pargyline or tranylcypromine. After treating
with pargyline or tranylcypromine for 24 h, the cells were
harvested with 0.25% trypsin-EDTA and were washed twice with PBS.
The apoptosis analysis was performed using In Situ Cell
Death Detection kit, Fluorescein (Roche Diagnostics, Mannheim,
Germany), according to the manufacturer’s instructions, and
analyzed using a FACSCalibur (BD Biosciences).
Western blot analysis
Western blotting was performed as previously
described (19), with minor
modifications. After treating the cells with 0.5 mM pargyline or
tranylcyprominein for 24 h, extraction of total protein from the
cells was performed using RIPA buffer [50 mM Tris-HCl, pH 7.5; 150
mM NaCl; 1% (v/v) Nonidet P-40 (NP-40); 0.5% sodium deoxycholate;
0.1% SDS and protease inhibitors]. The protein was separated by
SDS-PAGE and transferred to polyvinylidene difluoride membranes
(Schleicher & Schuell BioScience Inc., Keene, NH, USA). The
membranes were incubated overnight at 4°C with a BCL-2 antibody,
cytochrome c antibody (both from Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA), caspase-3 antibody (Cell Signaling
Technology, Inc., Danvers, MA, USA) or β-actin antibody
(Sigma-Aldrich) followed by incubation with HRP-conjugated
anti-rabbit or anti-mouse IgG. After washing with TBS-T, the
proteins were visualized with ECLTM Western Blotting
Detection Reagents (GE Healthcare, Wauwatosa, WI, USA).
Statistical analyses
The data were analyzed using OriginPro 8 software
(OriginLab Corp., Northampton, MA, USA). Each value is expressed as
the means ± standard error of mean (SEM) from 3 independent
experiments. All statistical analyses were performed using SPSS
17.0 software (SPSS Inc., Chicago, IL, USA). P-values <0.05 were
considered to indicate statistically significant differences.
Results
Regulation of cell proliferation by
pargyline and tranylcypromine
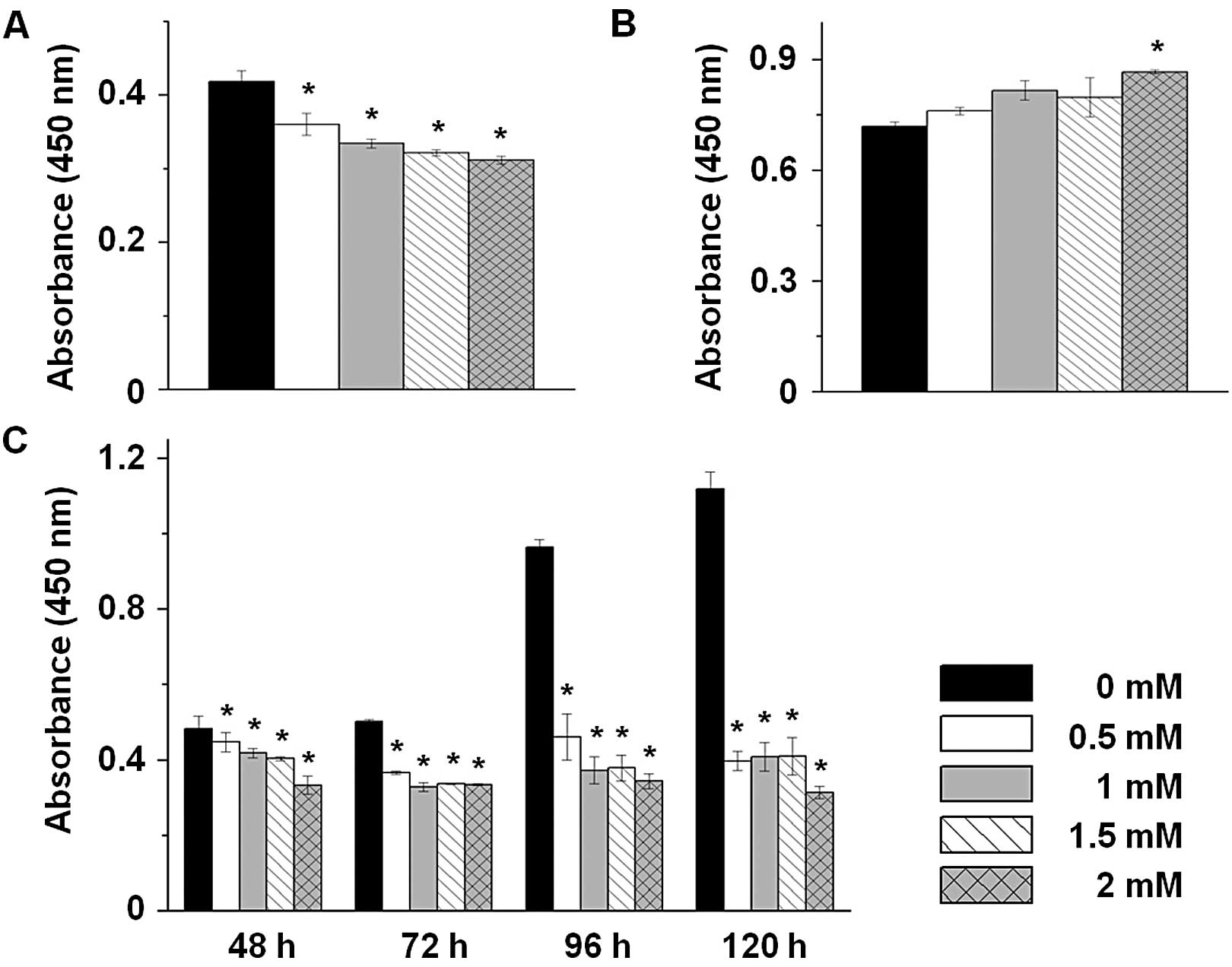
To investigate the cellular proliferation effect of
MAO inhibitors on prostate cancer cells, we performed a cell
proliferation assay in LNCaP-LN3 cells after exposing the cells to
pargyline or tranylcypromine treatment in a dose-dependent manner
(0, 0.5, 1, 1.5 and 2 mM) for 24 h. The cells exposed to pargyline
exhibited a decrease in cellular proliferation (Fig. 1A) that was dose-dependent. By
contrast, the cells exposed to tranylcypromine exhibited an
increase in cellular proliferation compared to the control cells
(Fig. 1B). To further investigate
the effect of pargyline in a time-dependent manner, we exposed the
cells to pargyline for 48, 72, 96 and 120 h. The proliferation in
the control cells increased continuously, while the proliferation
in the cells exposed to pargyline did not increase and, markedly,
the cells exposed to 2 mM pargyline for 120 h decreased 3-fold in
cellular proliferation compared to the control cells (Fig. 1C). Therefore, pargyline may inhibit
the proliferation of prostate cancer cells in a time- and
dose-dependent manner.
Regulation of cell cycle patterns by
pargyline and tranylcypromine
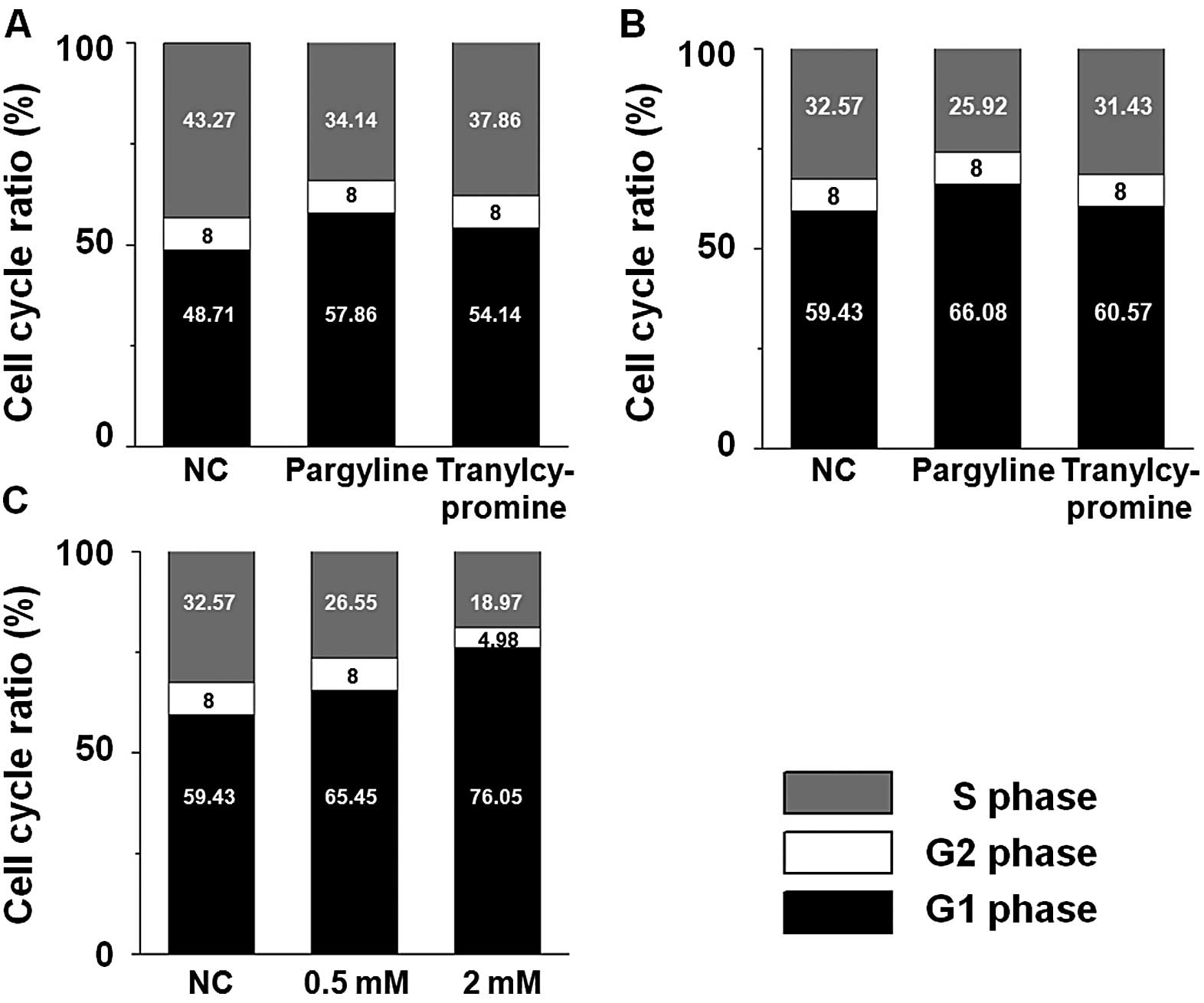
Based on these observations that pargyline and
tranylcypromine affect the cellular proliferation in prostate
cancer cells, we examined whether the proliferation changes in the
cells exposed to pargyline or tranylcypromine were induced by
alteration of the cell cycle pattern. The S phase ratio of the
cells exposed to pargyline for 24 and 48 h decreased, while their
G1 phase ratio increased compared to the control cells (Fig. 2A and B). In particular, the decrease
in the S phase or the increase in the G1 phase became more evident
with the progress of time. On the other hand, there was little
difference between the control and the tranylcypromine-exposed
cells at the alteration ratios of the S and the G1 phase. We
further analyzed whether pargyline affected the cell cycle pattern
in a dose-dependent manner. When the cells were exposed to 0.5 or 2
mM pargyline for 24 h, the decrease of S phase and the increase of
the G1 phase in the cells were dose-dependent compared to the
control cells (Fig. 2C).
Regulation of apoptosis-related genes by
pargyline and tranylcypromine
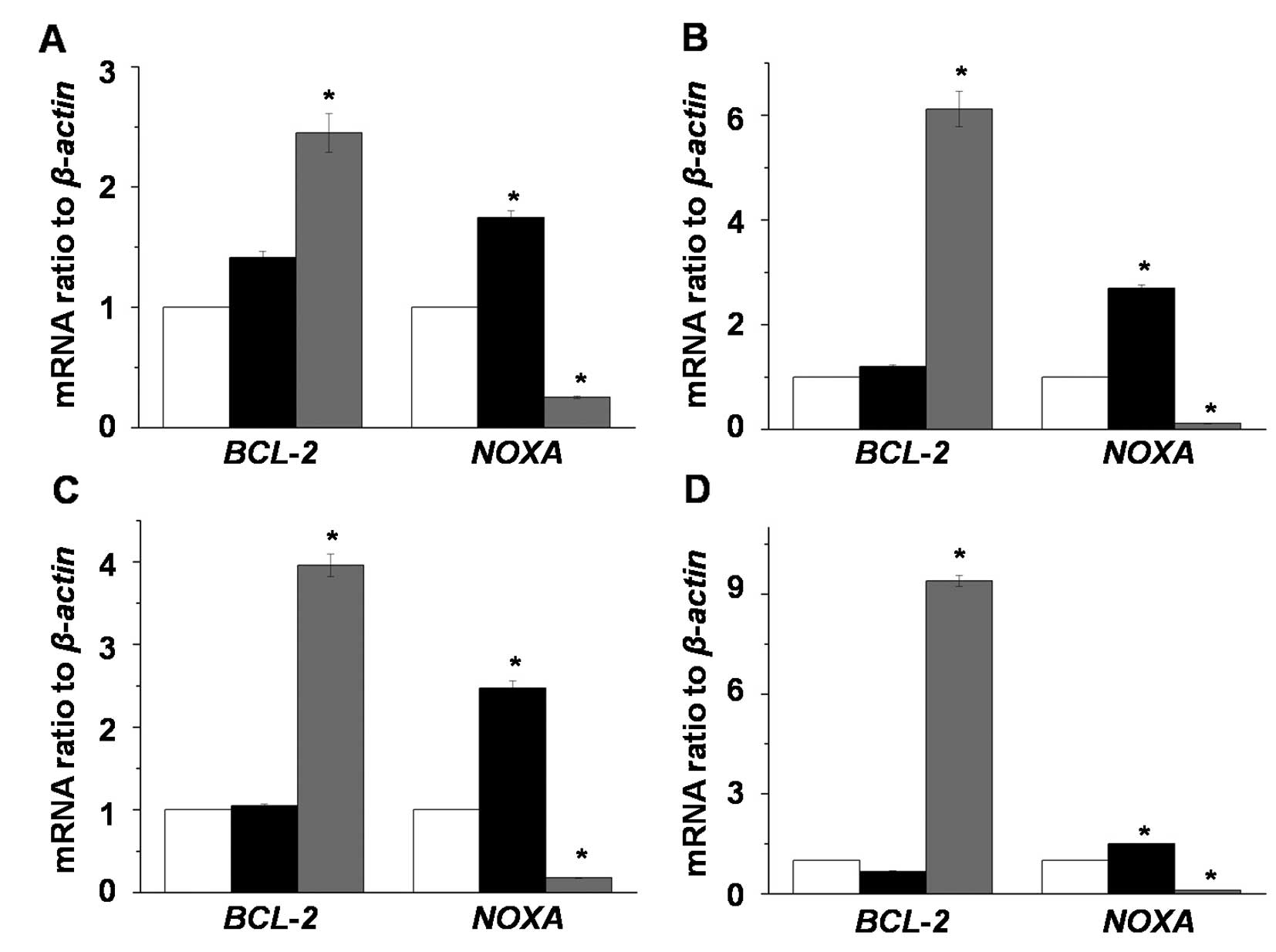
To investigate the induction of apoptosis by MAO
inhibitors in LNCaP-LN3 cells, we analyzed the expression changes
of apoptosis-related genes (BCL-2 and NOXA) after
exposing LNCaP-LN3 cells to 2 mM pargyline or tranylcypromine in a
time-dependent manner (Fig. 3). The
expression level of BCL-2 mRNA did not change in the
pargyline-treated cells, while its expression levels in the
tranylcypromine-treated cells were significantly increased compared
to the control and pargyline-treated cells. On the other hand, the
expression level of NOXA mRNA in the pargyline-treated cells
significantly increased, while its expression in
tranylcypromine-treated cells decreased.
Induction of apoptosis by pargyline and
tranylcypromine
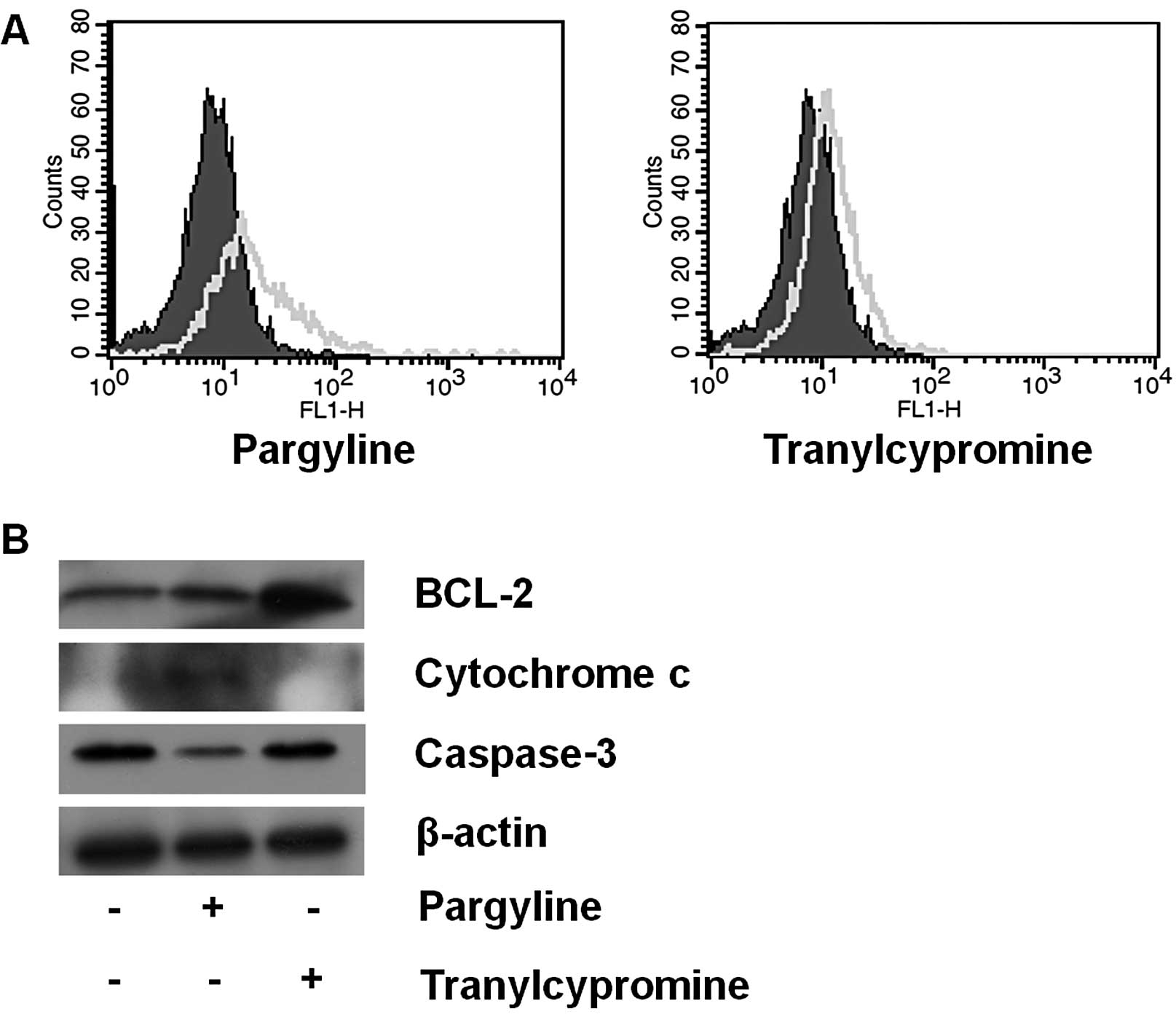
Based on the results described above, we observed
the apoptotic signals after exposing the cells to pargyline or
tranylcypromine for 24 h. The pargyline-treated cells showed more
apoptotic induction, whereas the tranylcypromine-treated cells
showed a similar pattern compared to the control cells (Fig. 4A). In addition to the increase in
the apoptotic cells after pargyline treatment, we further examined
the expression level of the apoptosis regulatory proteins (BCL-2,
cytochrome c and caspase-3) after pargyline or
tranylcypromine treatment for 48 h (Fig. 4B). Pargyline treatment induced an
increase of cytochrome c and a decrease of caspase-3 in the
cells, but did not lead to a change of BCL-2 expression. On the
other hand, tranylcypromine promoted a significant increase of
BCL-2, showing no change of cytochrome c and caspase-3
expressions. Therefore, pargyline may induce cell death in prostate
cancer cells.
Discussion
Prostate cancer is the most common malignancy in
Western countries, particularly in >50-year-old men (1,2).
Prostate cancer-related mortality was reported as the second most
common among all types of cancer (20). Treatments such as chemopreventive
methods have been systematically studied for prostate cancer
therapy (8). Additionally, various
chemical reagents are being used to prevent cancer growth due to
the importance of inhibition of proliferation in cancer cells.
However, there are currently no effective target materials to
prevent the growth of the cancer cells. For this reason, we
analyzed the possibility of using pargyline and tranylcypromine as
candidates for the treatment of prostate cancer.
In recent years, MAO inhibitors such as pargyline,
tranylcypromine and clorgyline (11,12,14–16,21)
began to be tested for cancer cell treatment. In the present study,
we found that pargyline efficiently inhibited the proliferation of
prostate cancer cells. In a study reported by Flamand et al
(14), clorgyline suppressed the enzyme activity of MAO A as
well as the cellular proliferation in prostate cancer cells. A
previous study observed that pargyline, tranylcypromine and
clorgyline significantly inhibited the growth of neuroblastoma
cells in a concentration-dependent manner (15). In addition, the MAO inhibitors are
known to suppress the growth of breast cancer cell lines such as
MDA-MB-231, MDA-MB-453, MCF-7 and T47D (11,16,21).
Therefore, it may be that pargyline inhibits the proliferation of
various cancer cells including prostate and breast cancer cells. On
the other hand, Benelkebir et al(22) reported that tranylcypromine
analogues suppressed cell growth more effectively than
tranylcypromine in prostate cancer cells. In the present study,
tranylcypromine did not inhibit the cell proliferation in prostate
cancer cells. Therefore, it is believed that tranylcypromine
differentially affects cell proliferation according to cancer type
whereas pargyline inhibits cellular proliferation in cancer cells
regardless of cancer type.
It was reported that cell cycle arrest at the G1
phase negatively affects cell proliferation (23). Our data showed a decrease in the S
phase proportion and an increase in the G1 phase proportion by
pargyline treatment in prostate cancer cells. In particular,
pargyline led to a decrease in the S phase proportion and an
increase in the G1 phase proportion in a dose-dependent manner. A
previous study reported that pargyline induced cell cycle arrest by
the decrease of cyclin B1 protein in human cervical adenocarcinoma
HeLa cells (24). In the present
study, tranylcypromine, unlike pargyline, did not show a
significant regulation in the proportion of S phase compared to the
control. Contrary to our result, Gatta and Mantovani (25) observed that tranylcypromine
treatment (2 μM) in human colon carcinoma HCT116 cells efficiently
suppressed progression to the S phase. Based on the previous report
and our results, tranylcypromine may show a different effect
depending on the concentration of tranylcypromine and type of
cancer cells. Therefore, these results suggest that pargyline is
more powerful to induce cell cycle arrest in prostate cancer cells
than tranylcypromine.
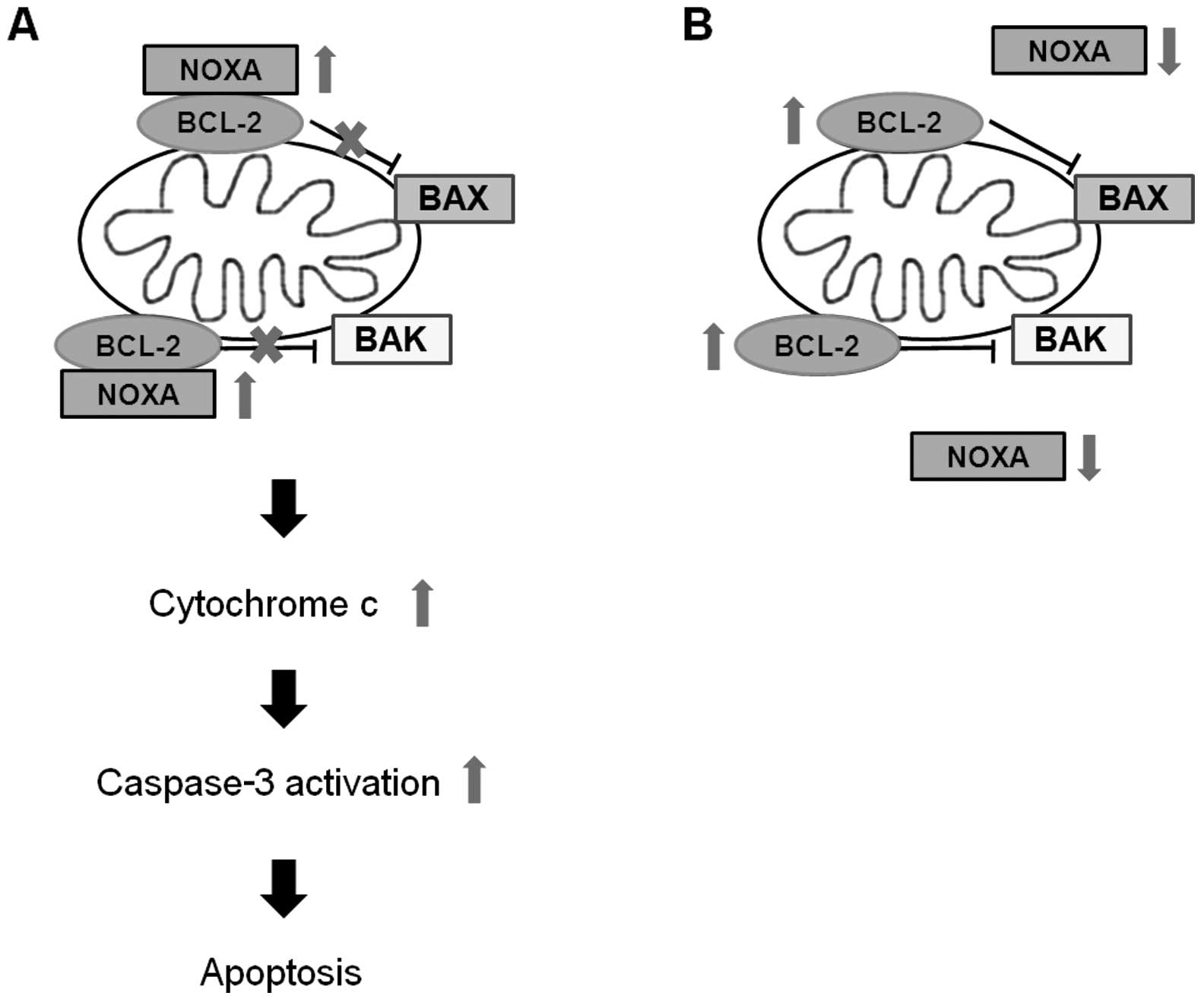
We observed that the expression of apoptosis
related-genes such as BCL-2 and NOXA was regulated by
pargyline or tranylcypromine. BCL-2 is an anti-apoptotic protein
that regulates apoptosis by inhibition of the release of
pro-apoptotic factors such as cytochrome c(26–28).
NOXA, BH3-only protein, is a pro-apoptotic member of the BCL-2
protein family, and interacts with MCL1, BCL-2 like 1 and BCL-2
(29). In the present study, the
expression levels of mRNA and protein of BCL-2 were
increased only by tranylcypromine treatment, while the mRNA
expression of NOXA exhibited an increase by pargyline but a
decrease by tranylcypromine. Previous studies reported that NOXA
BH3 domain interacts with BCL-2 BH3 binding groove, and it
indirectly promotes mitochondrial dysfunction through BAX and BAK
by inhibiting anti-apoptotic protein BCL-2 (29–32).
BCL-2 is known to prevent the release of cytochrome c from
mitochondria, which activates caspase-9, followed by caspase-3
cleavage in the intrinsic pathway of apoptotic events (33,34).
We identified that pargyline led to an increase of cytochrome
c and a decrease of caspase-3 in prostate cancer cells.
Therefore, pargyline may promote apoptosis through regulation of
BCL-2 and NOXA expression, while tranylcypromine does not affect
apoptosis in our condition. As an alternative, we confirmed that
pargyline effectively induced apoptosis compared with
tranylcypromine treatment in prostate cancer cells using an in
situ cell death detection technique. Similar to our results,
Malorni et al(35) reported
that apoptosis in human melanoma M14 cells exposed to 1 mM
pargyline increased. In addition, it has been reported that the
periodic administration of pargyline into nude mouse models
significantly induced apoptosis of tumors formed by breast cancer
MCF-7 cells (13). On the other
hand, tranylcypromine treatment, in combination with HDAC
inhibitors, induced synergistic apoptotic cell death in
glioblastoma multiforme cells (36). Collectively, the differential
expression of BCL-2 and NOXA may cause the opposite effects of
pargyline and tranylcypromine to cellular proliferation in prostate
cancer cells (Fig. 5).
In summary, we observed the mechanisms associated
with cell proliferation and apoptosis in prostate cancer cells
after exposing the cells to the MAO inhibitors, pargyline and
tranylcypromine. Pargyline not only induced growth arrest in the
cells but also showed a more significant increase of the G1 phase
and a decrease of the S phase compared with tranylcypromine.
Furthermore, when pargyline was used to treat prostate cancer
cells, the expression of pro-apoptotic member NOXA increased
significantly, but the expression of anti-apoptotic protein BCL-2
decreased compared with tranylcypromine treatment. The increased
expression of cytochrome c by pargyline treatment induces a
decrease in the expression of caspase-3 under pargyline-exposed
conditions. In addition, the treatment of pargyline effectively
induces apoptosis more than the treatment of tranylcypromine. Based
on our results and previous studies, it is believed that pargyline
has greater pharmaceutical potential as an anticancer drug than
tranylcypromine for human prostate cancer.
Acknowledgements
The authors thank Professor Adam Turner for
linguistic review of the manuscript. This work was supported by the
National Research Foundation of Korea (NRF) grant funded by the
Korea government (MSIP) (No. 2011-0030768 to Y.G.C.) and the Basic
Science Research Program through the National Research Foundation
of Korea (NRF) funded by the Ministry of Education, Science and
Technology (No. 2010-0023808 to M.R.C.).
References
|
1
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: the impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dunn MW and Kazer MW: Prostate cancer
overview. Semin Oncol Nurs. 27:241–250. 2011. View Article : Google Scholar
|
|
3
|
McCloskey DE, Casero RA Jr, Woster PM and
Davidson NE: Induction of programmed cell death in human breast
cancer cells by an unsymmetrically alkylated polyamine analogue.
Cancer Res. 55:3233–3236. 1995.PubMed/NCBI
|
|
4
|
Huang Y, Hager ER, Phillips DL, et al: A
novel polyamine analog inhibits growth and induces apoptosis in
human breast cancer cells. Clin Cancer Res. 9:2769–2777.
2003.PubMed/NCBI
|
|
5
|
Zhang L and Webster TJ:
Poly-lactic-glycolic-acid surface nanotopographies selectively
decrease breast adenocarcinoma cell functions. Nanotechnology.
23:1551012012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mertens-Talcott SU, Noratto GD, Li X,
Angel-Morales G, Bertoldi MC and Safe S: Betulinic acid decreases
ER-negative breast cancer cell growth in vitro and in vivo: role of
Sp transcription factors and microRNA-27a:ZBTB10. Mol Carcinog. Mar
7–2012.(Epub ahead of print). View
Article : Google Scholar
|
|
7
|
Zhang X, Zhang S, Liu Y, et al: Effects of
the combination of RAD001 and docetaxel on breast cancer stem
cells. Eur J Cancer. 48:1581–1592. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thapa D and Ghosh R: Antioxidants for
prostate cancer chemoprevention: challenges and opportunities.
Biochem Pharmacol. 83:1319–1330. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kumar R, Verma V, Jain A, Jain RK,
Maikhuri JP and Gupta G: Synergistic chemoprotective mechanisms of
dietary phytoestrogens in a select combination against prostate
cancer. J Nutr Biochem. 22:723–731. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang CH, Guh JH, Chen GS, Lu PH and Chern
JW: Anticancer activity of a cyclooxygenase inhibitor, CX9051, in
human prostate cancer cells: the roles of NF-κB and crosstalk
between the extrinsic and intrinsic apoptotic pathways. Naunyn
Schmiedebergs Arch Pharmacol. 382:159–169. 2010.PubMed/NCBI
|
|
11
|
Lim S, Janzer A, Becker A, et al:
Lysine-specific demethylase 1 (LSD1) is highly expressed in
ER-negative breast cancers and a biomarker predicting aggressive
biology. Carcinogenesis. 31:512–520. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schmidt DM and McCafferty DG:
trans-2-Phenylcyclopropylamine is a mechanism-based inactivator of
the histone demethylase LSD1. Biochemistry. 46:4408–4416. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cortez V, Mann M, Tekmal S, et al:
Targeting the PELP1-KDM1 axis as a potential therapeutic strategy
for breast cancer. Breast Cancer Res. 14:R1082012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Flamand V, Zhao H and Peehl DM: Targeting
monoamine oxidase A in advanced prostate cancer. J Cancer Res Clin
Oncol. 136:1761–1771. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schulte JH, Lim S, Schramm A, et al:
Lysine-specific demethylase 1 is strongly expressed in poorly
differentiated neuroblastoma: implications for therapy. Cancer Res.
69:2065–2071. 2009. View Article : Google Scholar
|
|
16
|
Lee HT, Jung KH, Kim SK, Choi MR and Chai
YG: Effects of pargyline on cellular proliferation in human breast
cancer cells. Mol Cell Toxicol. 8:393–399. 2012. View Article : Google Scholar
|
|
17
|
Lee HT, Kim SK, Choi MR, Park JH, Jung KH
and Chai YG: Effects of the activated mitogen-activated protein
kinase pathway via the c-ros receptor tyrosine kinase on the
T47D breast cancer cell line following alcohol exposure. Oncol Rep.
29:868–874. 2013.PubMed/NCBI
|
|
18
|
Baik SY, Jung KH, Choi MR, et al:
Fluoxetine-induced up-regulation of 14-3-3zeta and tryptophan
hydroxylase levels in RBL-2H3 cells. Neurosci Lett. 374:53–57.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi MR, Oh DH, Kim SH, et al: Fluoxetine
up-regulates Bcl-xL expression in rat C6 glioma cells. Psychiatry
Investig. 8:161–168. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
21
|
Pollock JA, Larrea MD, Jasper JS,
McDonnell DP and McCafferty DG: Lysine-specific histone demethylase
1 inhibitors control breast cancer proliferation in ERα-dependent
and -independent manners. ACS Chem Biol. 7:1221–1231.
2012.PubMed/NCBI
|
|
22
|
Benelkebir H, Hodgkinson C, Duriez PJ, et
al: Enantioselective synthesis of tranylcypromine analogues as
lysine demethylase (LSD1) inhibitors. Bioorg Med Chem.
19:3709–3716. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim SJ, Min HY, Lee EJ, et al: Growth
inhibition and cell cycle arrest in the G0/G1 by schizandrin, a
dibenzocyclooctadiene lignan isolated from Schisandra
chinensis, on T47D human breast cancer cells. Phytother Res.
24:193–197. 2010.PubMed/NCBI
|
|
24
|
Chuang JY, Chang WC and Hung JJ: Hydrogen
peroxide induces Sp1 methylation and thereby suppresses cyclin B1
via recruitment of Suv39H1 and HDAC1 in cancer cells. Free Radic
Biol Med. 51:2309–2318. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gatta R and Mantovani R: NF-Y substitutes
H2A-H2B on active cell-cycle promoters: recruitment of CoREST-KDM1
and fine-tuning of H3 methylations. Nucleic Acids Res.
36:6592–6607. 2008. View Article : Google Scholar
|
|
26
|
Martinou JC and Youle RJ: Mitochondria in
apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev
Cell. 21:92–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rosser CJ, Gaar M and Porvasnik S:
Molecular fingerprinting of radiation resistant tumors: can we
apprehend and rehabilitate the suspects? BMC Cancer. 9:2252009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lucken-Ardjomande S and Martinou JC:
Regulation of Bcl-2 proteins and of the permeability of the outer
mitochondrial membrane. CR Biol. 328:616–631. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oda E, Ohki R, Murasawa H, et al: Noxa, a
BH3-only member of the Bcl-2 family and candidate mediator of
p53-induced apoptosis. Science. 288:1053–1058. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Smith AJ, Dai H, Correia C, et al:
Noxa/Bcl-2 protein interactions contribute to bortezomib resistance
in human lymphoid cells. J Biol Chem. 286:17682–17692. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ploner C, Kofler R and Villunger A: Noxa:
at the tip of the balance between life and death. Oncogene.
27(Suppl 1): S84–S92. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shibue T, Takeda K, Oda E, et al: Integral
role of Noxa in p53-mediated apoptotic response. Genes Dev.
17:2233–2238. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Adams JM and Cory S: The Bcl-2 protein
family: arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Elmore S: Apoptosis: a review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Malorni W, Giammarioli AM, Matarrese P, et
al: Protection against apoptosis by monoamine oxidase A inhibitors.
FEBS Lett. 426:155–159. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Singh MM, Manton CA, Bhat KP, et al:
Inhibition of LSD1 sensitizes glioblastoma cells to histone
deacetylase inhibitors. Neuro Oncol. 13:894–903. 2011. View Article : Google Scholar : PubMed/NCBI
|