Introduction
Colorectal cancer is the second most common cancer
in developed countries (1);
therefore, chemoprevention for colorectal cancer is indispensable.
Clinical and preclinical studies provide strong evidence that
non-steroidal anti-inflammatory drugs (NSAIDs) can prevent numerous
types of malignant tumors (2,3),
particularly colorectal cancer (4–7). Also
in Japan, double-blind randomized clinical trials have been
registered to elucidate the preventive effects of low-dose aspirin
on colorectal cancer and adenoma growth (8,9). Among
these studies, many reports on aspirin have been published;
however, a greater risk of bleeding complications remains with
aspirin (10), and its
chemopreventive potential might be limited. Thus, we focused on
ibuprofen, one of the most commonly used NSAIDs as a worldwide
over-the-counter (OTC) drug. The anti-inflammatory effect of
ibuprofen was found to exceed aspirin (11) and the side effects are fewer than
other NSAIDs.
In general, NSAIDs inhibit cyclooxygenase (COX)-1
and COX-2, which are key enzymes of prostaglandin biosynthesis.
Several independent molecular investigations have shown that COX-2
is overexpressed in 67–83% of human colon tumors (12,13).
The chemopreventive effects of NSAIDs have been reported due to
their ability to inhibit the production of proliferative and
inflammatory prostaglandins (PGs), particularly PGE2. Thus,
specific COX-2 inhibitors, developed as a means to reduce the
gastrointestinal toxicity associated with inhibiting COX-1, are
expected to be used for chemoprevention against colorectal cancer
(14). However, it has been
elucidated that treatment with specific COX-2 inhibitors is
associated with an increased incidence of adverse cardiovascular
events, even after short-term exposure (15,16).
Nevertheless, there is a similar reduction in the risk of human
colon cancer by selective and nonselective COX-2 inhibitors
(17,18). In contrast, ibuprofen inhibits both
COX-1 and COX-2 equally, and causes G1-phase arrest by decreasing
cyclin D1 expression and apoptosis by suppressing Bcl-2 and
survivin (19–21). However, there have been no clinical
studies on whether ibuprofen has a chemopreventive effect.
Moreover, we reported that many chemopreventive
agents enhance the apoptosis-inducing effects of tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL) (22–25).
TRAIL is a member of the TNF family and induces apoptosis in many
types of malignant tumor cells in vitro and in vivo,
but has little or no toxicity in normal cells (26,27).
Death receptor 5 (DR5; also called TRAIL-R2) and death receptor 4
(DR4) are receptors for TRAIL. A soluble recombinant human TRAIL is
undergoing phase I/II clinical trials for the treatment of solid
malignant tumors (28–30). Furthermore, endogenous TRAIL plays
an important role in the inhibition of carcinogenesis, since TRAIL
deficiency accelerates the growth of malignancies in mice (31). Activation of the TRAIL signaling
pathway is considered an attractive option for cancer treatment and
prevention.
In the present study, we found that ibuprofen
markedly stimulated the apoptosis-inducing efficacy of TRAIL
against human colon cancer HCT116 cells, but not against normal
human peripheral blood mononuclear cells, and ibuprofen may be a
safer chemopreventive candidate than aspirin.
Materials and methods
Reagents
Ibuprofen was purchased from Nacalai Tesque (Kyoto,
Japan) and dissolved in dimethyl sulfoxide (DMSO). Soluble
recombinant human TRAIL/Apo2L was obtained from PeproTech (London,
UK). The human recombinant DR5 (TRAIL-R2)/Fc chimera and caspase
inhibitor, zVAD-fmk, were purchased from R&D Systems
(Minneapolis, MN, USA).
Cell culture
Human colon cancer HCT116 cells were purchased from
the American Type Culture Collection (Manassas, VA, USA). Cells
were maintained in Dulbecco’s modified Eagle’s medium (DMEM)
supplemented with 10% fetal bovine serum (FBS), 4 mM glutamine, 100
U/ml penicillin and 100 μg/ml streptomycin. Normal peripheral blood
mononuclear cells (PBMCs) were isolated using Lymphoprep
(Axis-Shield, Oslo, Norway) and maintained in RPMI-1640 medium with
10% FBS and 2 mM glutamine. PBMCs were acquired from healthy
volunteers after obtaining informed consent. This study was
approved by the Kyoto Prefectural University of Medicine Research
Ethics Committee (permission no. C-919). All cells were incubated
at 37°C in a humidified atmosphere of 5% CO2.
Detection of apoptosis
DNA fragmentation was quantified based on the
percentage of hypodiploid DNA (sub-G1). After being washed with
phosphate-buffered saline (PBS), the collected cells were suspended
in a 0.1% Triton-X 100/PBS solution. They were then treated with
RNase A (Sigma, St. Louis, MO, USA) and the nuclei were stained
with propidium iodide (Sigma). The DNA content was measured using
FACSCalibur (Becton Dickinson, Franklin Lakes, NJ, USA). For each
experiment, 10,000 events were analyzed. Cell Quest software
(Becton Dickinson) was used to analyze the data.
Western blot analysis
The cell lysate was prepared as previously described
(32). The cell lysate was resolved
on a 5, 7.5, 10 or 12.5% SDS-polyacrylamide gel for
electrophoresis, and blotted onto polyvinylidene difluoride (PVDF)
membranes (Millipore, Bedford, MA, USA). Rabbit polyclonal
anti-DR4, anti-DR5 (Prosci Inc., Poway, CA, USA), anti-survivin
(R&D Systems), anti-Bcl-2 (Abcam, Cambridge, UK), anti-Bax,
anti-c-IAP1, anti-caspase-3, anti-Bid, anti-PARP (Santa Cruz
Biotechnology, Santa Cruz, CA, USA), and mouse monoclonal anti-XIAP
(R&D Systems), anti-caspase-8, anti-caspase-9 (MBL, Nagoya,
Japan), and anti-β-actin (Sigma) antibodies were used as the
primary antibodies. The blots were incubated with the appropriate
HRP-conjugated secondary antibody (GE Healthcare, Piscataway, NJ,
USA), and signals were detected with Chemilumi-One (Nacalai
Tesque).
Determination of TRAIL receptor
expression
Cells were harvested by short trypsinization, washed
once with ice-cold PBS containing 1% bovine serum albumin (BSA),
and resuspended in 100 μl PBS with 1% BSA. Then, PE-labeled mouse
anti-human DR4 or DR5 mAb (eBioscience, San Diego, CA, USA) was
added. To assess nonspecific staining, PE-labeled control IgG
isotypes (eBioscience) were applied. After a 30-min incubation on
ice, cells were washed and 2×104 cells were analyzed by
FACSCalibur.
RNA analysis
Total cellular RNA was extracted using Sepasol-RNA I
(Nacalai Tesque), according to the manufacturer’s instructions. For
quantitative real-time RT-PCR, total RNA (2 μg) was
reverse-transcribed to cDNA in a 20 μl reaction volume, using a
High Capacity cDNA reverse transcription kit (Applied Biosystems,
Foster City, CA, USA) according to the manufacturer’s instructions.
Quantitative real-time RT-PCR was carried out using an RT-PCR
system GeneAmp7300 (Applied Biosystems). Real-time quantitative
reverse transcription-PCR primer-probe sets for DR5 and GAPDH mRNA
were purchased from Applied Biosystems. The expression level of DR5
mRNA was normalized against the level of GAPDH mRNA in the same
sample.
Statistical analysis
Data are the means ± SD of three determinations.
Data were analyzed using the Student’s t-test, and differences were
considered significant at P<0.05.
Results
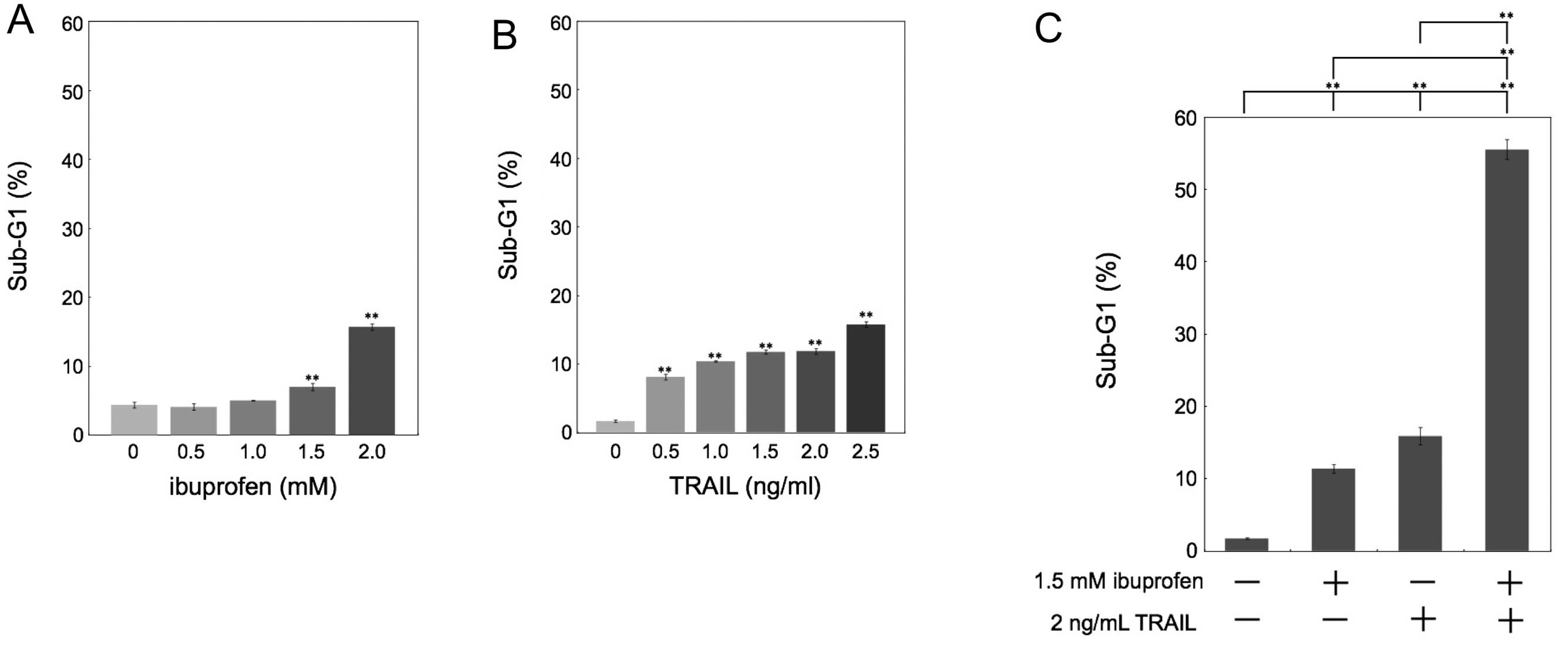
Combined treatment with ibuprofen and
TRAIL induces synergistic apoptosis in HCT116 cells
It has been reported that NSAIDs have
antiproliferative effects on human colon cancer. In the present
study, we investigated the effect of an NSAID, ibuprofen, and/or
exogenous recombinant human TRAIL on apoptosis in human colon
cancer HCT116 cells. We quantified apoptotic cells by measuring the
sub-G1 population. Whereas treatment with ibuprofen or TRAIL only
weakly induced apoptosis (Fig. 1A and
B), the combination of ibuprofen and TRAIL synergistically
induced apoptosis (Fig. 1C).
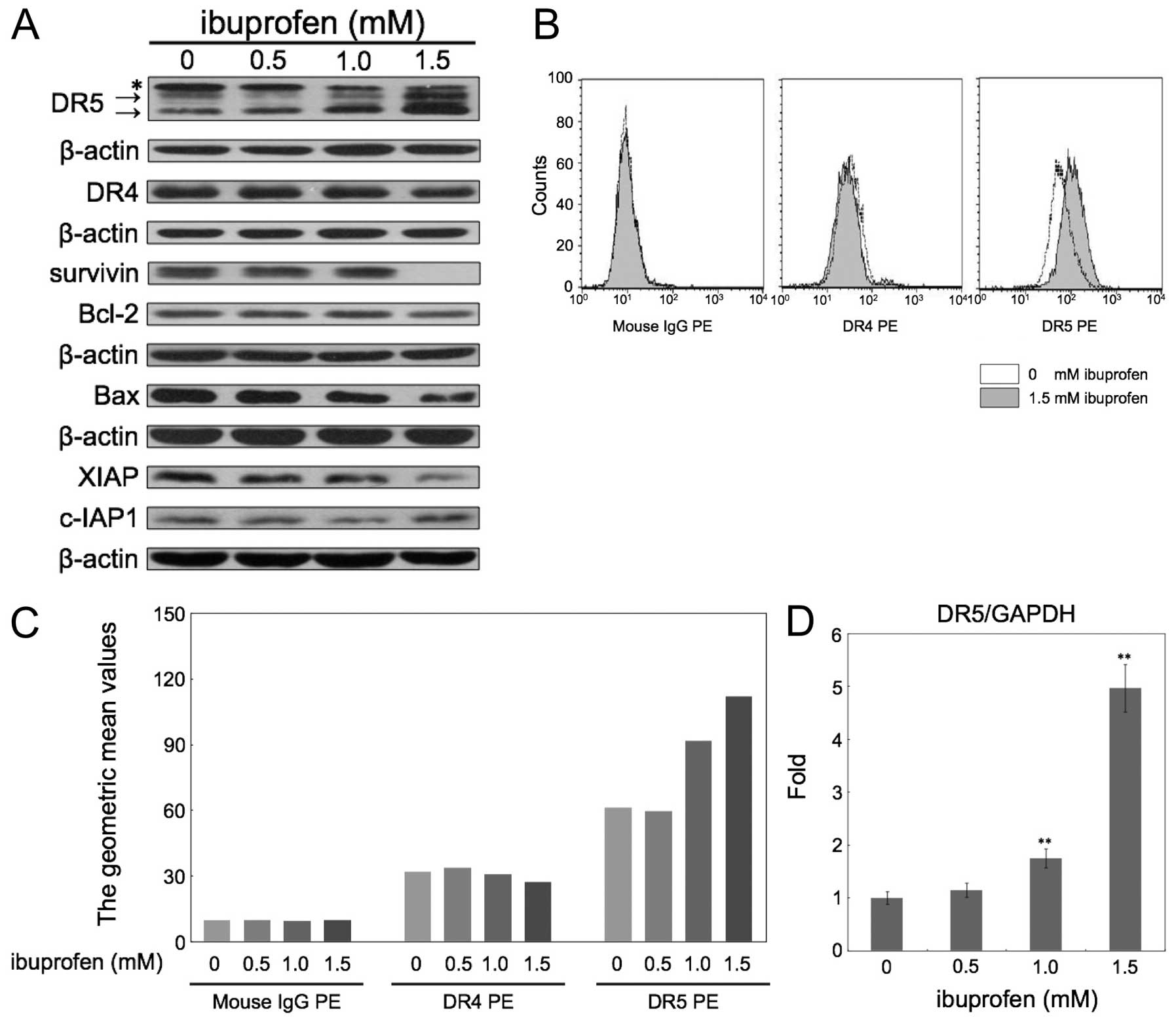
Ibuprofen induces DR5 expression in
HCT116 cells
To investigate the underlying mechanisms by which
ibuprofen enhances TRAIL-induced apoptosis, we examined the
alterations in protein expression by treating HCT116 cells with
ibuprofen for 24 h. First, we carried out western blotting for
TRAIL receptors, DR4 and DR5, and several anti- and pro-apoptotic
proteins (Fig. 2A). Ibuprofen
significantly upregulated the expression of DR5, but not DR4.
Ibuprofen also decreased the expression of survivin and XIAP. In
contrast, Bcl-2, c-IAP1 and Bax were not significantly changed.
Next we examined the expression levels of DR4 and DR5 in the
membrane fraction measured by flow cytometry (Fig. 2B and C). Ibuprofen at 1.5 mM did not
change the expression of DR4, but apparently increased DR5
expression. Furthermore, we analyzed the DR5 mRNA level after
treatment with ibuprofen at the indicated concentrations for 24 h
by quantitative real-time RT-PCR (Fig.
2D). Ibuprofen at 1.5 mM increased DR5 mRNA expression
~5-fold.
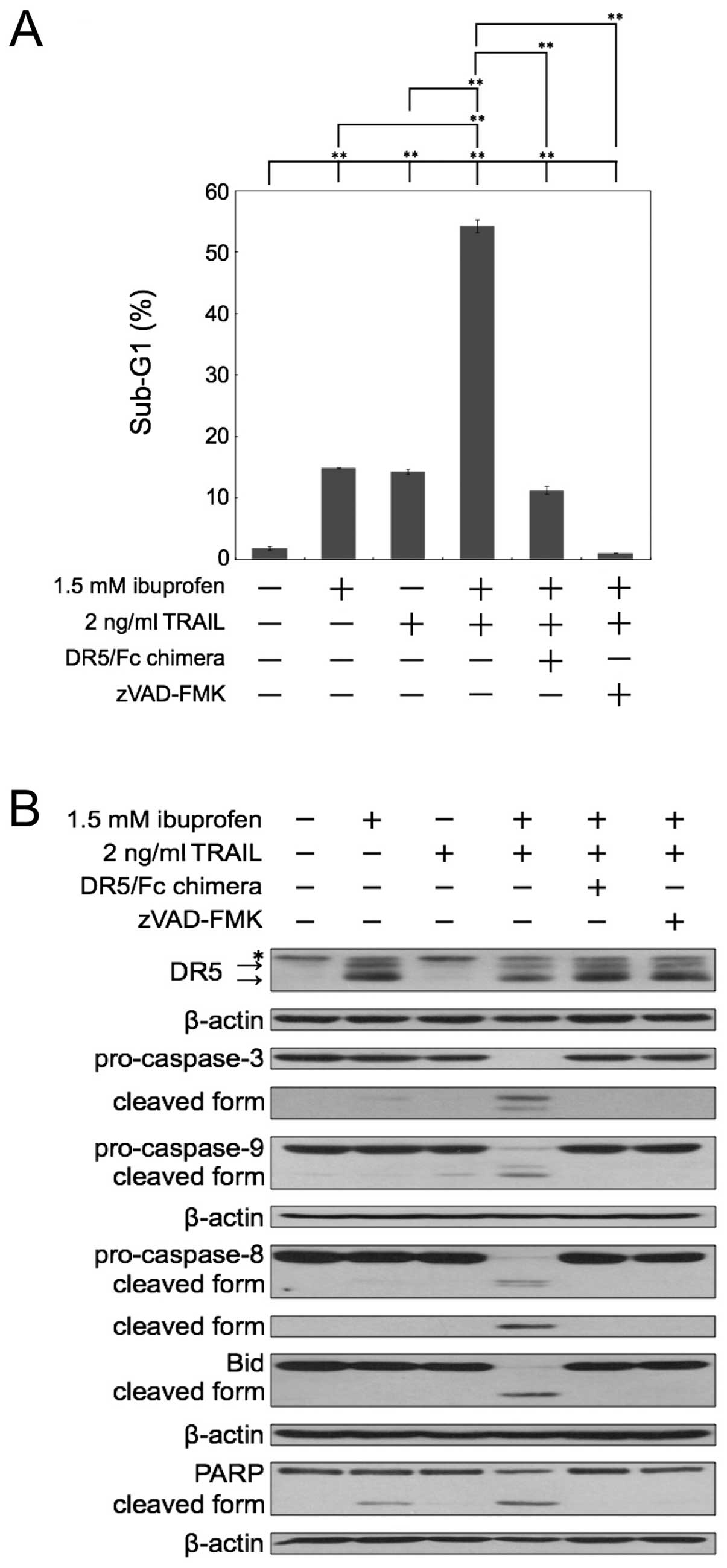
Combination of ibuprofen and TRAIL
cleaves caspases and a substrate of caspase PARP
We next investigated whether the sub-G1 population
reflects caspase-dependent apoptosis by inhibitors of caspase. The
apoptosis induced by the combination of ibuprofen and TRAIL was
almost completely inhibited by the pan-caspase inhibitor zVAD-FMK
(Fig. 3A). Next, we used a
recombinant human DR5/Fc chimeric protein, which has a
dominant-negative function against DR5. As shown in Fig. 3A, the apoptosis induced by the
combination of ibuprofen and TRAIL was effectively blocked by
DR5/Fc chimera. Thus, these results demonstrated that the
enhancement of apoptosis caused by the combination occurred in a
caspase-dependent manner and via the interaction between TRAIL and
DR5. Furthermore, we performed western blot analysis of caspase-3,
−8, −9, Bid and PARP in cells treated with ibuprofen and/or TRAIL
(Fig. 3B). Bid is a substrate of
caspase-8 and is cleaved during the activation of TRAIL signaling.
Combined treatment with ibuprofen and TRAIL markedly induced the
cleavage of caspases, Bid and PARP. Moreover, the DR5/Fc chimeric
protein and zVAD-FMK effectively blocked the cleavage of caspases,
Bid and PARP induced by the combination treatment. These results
also indicate that the combination of ibuprofen and TRAIL induces
apoptosis dependent on caspases and TRAIL-DR5 interaction.
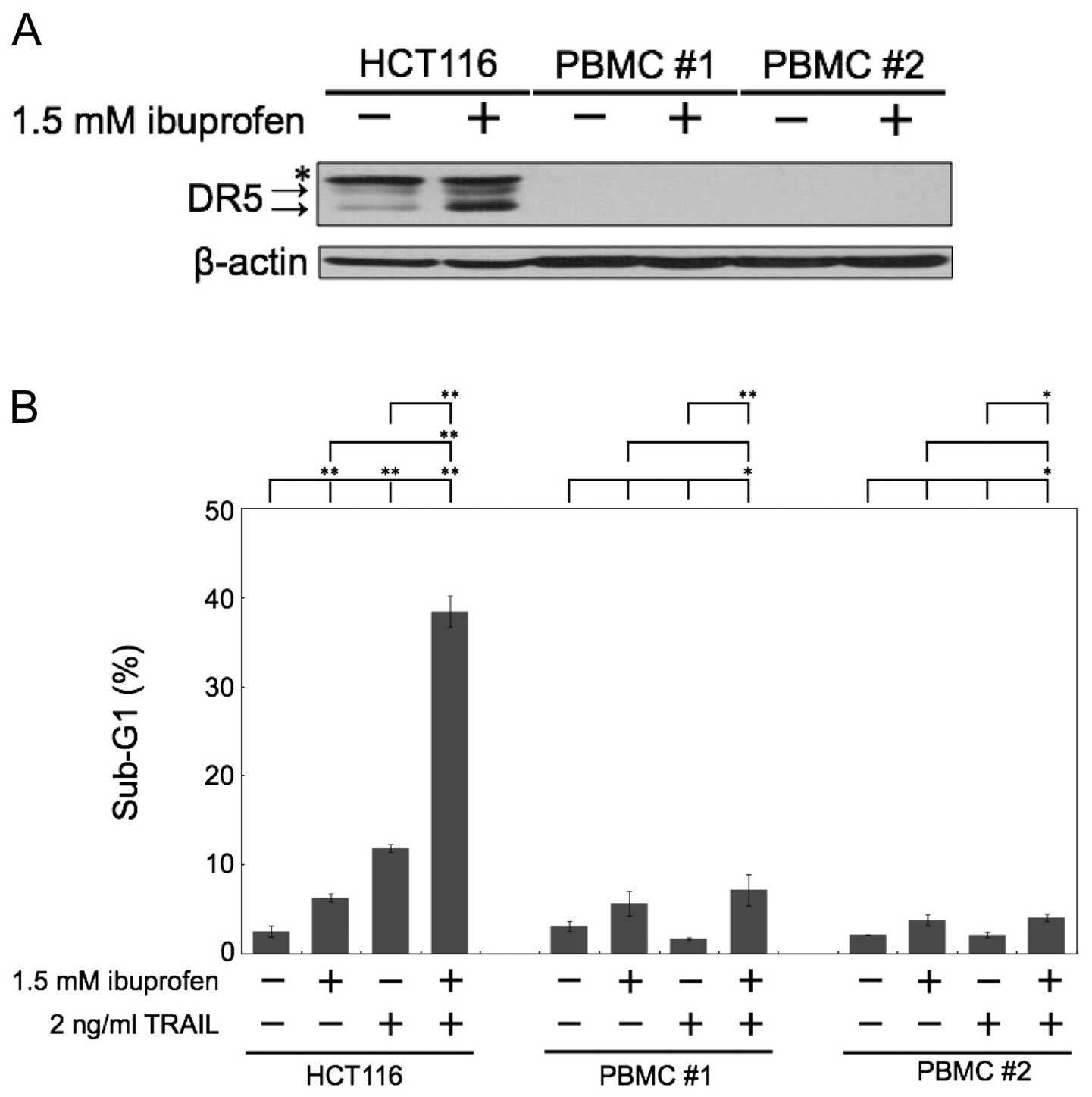
Combination of ibuprofen and TRAIL does
not induce apoptosis in normal human peripheral blood mononuclear
cells (PBMCs)
We next examined the effect of ibuprofen treatment
on normal human cells. The major side effect of chemotherapeutic
agents is pancytopenia, the dysfunction of hematopoietic cells
during clinical treatment. Thus, we used normal human PBMCs as a
comparison subject. In normal human PBMCs, the level of DR5
expression was barely expressed, and ibuprofen did not induce DR5
protein expression (Fig. 4A).
Noteworthy, the combination of ibuprofen and TRAIL did not induce
apoptosis in normal human PBMCs, although it markedly induced
apoptosis in HCT116 cells (Fig.
4B).
Discussion
There is epidemiological and experimental evidence
that NSAIDs confer a chemopreventive effect against various types
of tumors. Although NSAIDs exert anticancer effects on colorectal
cancer, their precise mechanisms are still controversial.
Moreover, several studies suggest that various
NSAIDs, such as aspirin, sulindac sulfide and diclofenac, sensitize
cancer cells to TRAIL-induced apoptosis. Ibuprofen, an NSAID, has
been widely used as a safe OTC drug, but it has not been reported
whether it enhances the cytocidal effect of TRAIL. In the present
study, we showed for the first time that ibuprofen with TRAIL
synergistically induced apoptosis. As the mechanism inducing
sensitivity to TRAIL, ibuprofen upregulated the expression of a
TRAIL receptor, DR5 and also decreased the expression of survivin
and XIAP (Fig. 2). In previous
studies, aspirin or diclofenac increased sensitivity to TRAIL
without upregulation of DR4 and DR5 (33,34),
whereas sulindac sulfide increased DR5 expression in human colon
cancer cells, resulting in enhanced TRAIL sensitivity (35–37).
Therefore, we hypothesized that the behavior of ibuprofen may be
similar to that of sulindac sulfide against colon cancer cells. In
order to confirm this hypothesis, further verification experiments
are necessary and are in preparation.
Although NSAIDs exert anticancer effects on
colorectal cancer by COX-dependent and -independent mechanisms
(38), we used HCT116 cells to
examine the effect of ibuprofen on stimulating TRAIL sensitivity in
this study. HCT116 cells lack expression of COX-2 protein with very
low expression of COX-1 (39);
therefore, we considered that the effect of ibuprofen on HCT116
cells was a COX-independent mechanism.
Side effects caused by therapeutic drugs are crucial
problems in antitumor therapy. NSAIDs are frequently prescribed
worldwide, and their tolerance is better than other anticancer
drugs. Above all, the most serious adverse effects of NSAIDs occur
in the gastrointestinal tract. Thus, many trials have been
conducted to compare the tolerability of aspirin, ibuprofen and
other NSAIDs. Consequently, ibuprofen was shown to be remarkably
well tolerated at OTC doses in a number of studies (40–42).
Additionally, ibuprofen had the lowest incidence of significant
adverse events as compared to aspirin and acetaminophen (43); however, in all studies describing
the adverse effects of ibuprofen, the observation period was
relatively short in order to conclude that ibuprofen is definitely
safer than other NSAIDs. Therefore, long-term examination of
ibuprofen is warranted. Nevertheless, in the present study, the
combination of ibuprofen and TRAIL did not induce apoptosis in
normal human PBMCs (Fig. 4);
therefore, we believe that ibuprofen is a promising candidate as a
safe chemopreventive agent against colorectal tumors.
Recently, endogenous TRAIL has been expected to be
important for cancer prevention (31). From this point of view, we aimed to
identify a TRAIL inducer for the application of TRAIL in cancer
prevention. We previously reported that a type of
lactobacillus markedly induced TRAIL expression in normal
human PBMCs, thereby inducing the cytotoxic effects of PBMCs
against human prostate cancer PC3 cells (44). We expect that the combination of
ibuprofen and a TRAIL inducer such as lactobacillus may have
a beneficial effect in cancer prevention.
In conclusion, the present findings suggest that
ibuprofen is a safer chemopreventive agent than aspirin against
colorectal tumors. We, therefore, confirm that ibuprofen is a safe
NSAID and suggest that ibuprofen be used in clinical
chemopreventive studies against colorectal adenoma or cancer.
Acknowledgements
This research was partly supported by the Japanese
Ministry of Education, Culture, Sports, Science and Technology.
Abbreviations:
|
NSAIDs
|
non-steroidal anti-inflammatory
drugs
|
|
TRAIL
|
tumor necrosis factor-related
apoptosis-inducing ligand
|
|
PARP
|
poly(ADP-ribose) polymerase
|
|
DR5
|
death receptor 5
|
|
BAX
|
Bcl2-associated X protein
|
|
XIAP
|
X-linked inhibitor of apoptosis
|
|
cIAP1
|
cellular inhibitor of apoptosis 1
|
|
PBMCs
|
peripheral blood mononuclear cells
|
References
|
1
|
Weitz J, Koch M, Debus J, Höhler T, Galle
PR and Büchler MW: Colorectal cancer. Lancet. 365:153–165. 2005.
View Article : Google Scholar
|
|
2
|
Brasky TM, Bonner MR, Moysich KB, et al:
Non-steroidal anti-inflammatory drugs (NSAIDs) and breast cancer
risk: differences by molecular subtype. Cancer Causes Control.
22:965–975. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Walter RB, Milano F, Brasky TM and White
E: Long-term use of acetaminophen, aspirin, and other nonsteroidal
anti-inflammatory drugs and risk of hematologic malignancies:
results from the prospective Vitamins and Lifestyle (VITAL) study.
J Clin Oncol. 29:2424–2431. 2011. View Article : Google Scholar
|
|
4
|
Thun MJ, Namboodiri MM and Heath CW Jr:
Aspirin use and reduced risk of fatal colon cancer. N Engl J Med.
325:1593–1596. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Baron JA, Cole BF, Sandler RS, et al: A
randomized trial of aspirin to prevent colorectal adenomas. N Engl
J Med. 348:891–899. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rothwell PM, Wilson M, Elwin CE, et al:
Long-term effect of aspirin on colorectal cancer incidence and
mortality: 20-year follow-up of five randomised trials. Lancet.
376:1741–1750. 2010.PubMed/NCBI
|
|
7
|
Rothwell PM, Wilson M, Price JF, et al:
Effect of daily aspirin on risk of cancer metastasis: a study of
incident cancers during randomised controlled trials. Lancet.
379:1591–1601. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ishikawa H, Nakamura T, Kawano A, Gondo N
and Sakai T: Chemoprevention of colorectal cancer in Japan: a brief
introduction to current clinical trials. J Gastroenterol. 44:77–81.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ishikawa H, Wakabayashi K, Suzuki S, et
al: Preventive effects of low-dose aspirin on colorectal adenoma
growth in patients with familial adenomatous polyposis:
double-blind, randomized clinical trial. Cancer Med. 2:50–56. 2013.
View Article : Google Scholar
|
|
10
|
Antithrombotic Trialists’ Collaboration.
Collaborative meta-analysis of randomised trials of antiplatelet
therapy for prevention of death, myocardial infarction, and stroke
in high risk patients. BMJ. 324:71–86. 2002. View Article : Google Scholar
|
|
11
|
Adams SS, McCullough KF and Nicholson JS:
The pharmacological properties of ibuprofen, an anti-inflammatory,
analgesic and antipyretic agent. Arch Int Pharmacodyn Ther.
178:115–129. 1969.PubMed/NCBI
|
|
12
|
Chan AT, Ogino S and Fuchs CS: Aspirin and
the risk of colorectal cancer in relation to the expression of
COX-2. N Engl J Med. 356:2131–2142. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ogino S, Kirkner GJ, Nosho K, et al:
Cyclooxygenase-2 expression is an independent predictor of poor
prognosis in colon cancer. Clin Cancer Res. 14:8221–8227. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kawamori T, Rao CV, Seibert K and Reddy
BS: Chemopreventive activity of celecoxib, a specific
cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer
Res. 58:409–412. 1998.PubMed/NCBI
|
|
15
|
Solomon SD, McMurray JJ, Pfeffer MA, et
al: Cardiovascular risk associated with celecoxib in a clinical
trial for colorectal adenoma prevention. N Engl J Med.
352:1071–1080. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baron JA, Sandler RS, Bresalier RS, et al:
A randomized trial of rofecoxib for the chemoprevention of
colorectal adenomas. Gastroenterology. 131:1674–1682. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Harris RE, Beebe-Donk J and Alshafie GA:
Similar reductions in the risk of human colon cancer by selective
and nonselective cyclooxygenase-2 (COX-2) inhibitors. BMC Cancer.
14:2372008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schönthal AH, Chen TC, Hofman FM, et al:
Celecoxib analogs that lack COX-2 inhibitory function: preclinical
development of novel anticancer drugs. Expert Opin Investig Drugs.
17:197–208. 2008.PubMed/NCBI
|
|
19
|
Bonelli P, Tuccillo FM, Calemma R, et al:
Changes in the gene expression profile of gastric cancer cells in
response to ibuprofen: a gene pathway analysis. Pharmacogenomics J.
11:412–428. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Greenspan EJ, Madigan JP, Boardman LA and
Rosenberg DW: Ibuprofen inhibits activation of nuclear β-catenin in
human colon adenomas and induces the phosphorylation of GSK-3β.
Cancer Prev Res. 4:161–171. 2011.
|
|
21
|
Harris RE, Beebe-Donk J, Doss H and Burr
Doss D: Aspirin, ibuprofen, and other non-steroidal
anti-inflammatory drugs in cancer prevention: A critical review of
non-selective COX-2 blockade (Review). Oncol Rep. 13:559–583.
2005.
|
|
22
|
Taniguchi H, Horinaka M, Yoshida T, et al:
Targeting the glyoxalase pathway enhances TRAIL efficacy in cancer
cells by downregulating the expression of antiapoptotic molecules.
Mol Cancer Ther. 11:2294–2300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Taniguchi H, Yoshida T, Horinaka M, et al:
Baicalein overcomes tumor necrosis factor-related
apoptosis-inducing ligand resistance via two different
cell-specific pathways in cancer cells but not in normal cells.
Cancer Res. 68:8918–8927. 2008. View Article : Google Scholar
|
|
24
|
Yoshida T, Maoka T, Das SK, et al:
Halocynthiaxanthin and peridinin sensitize colon cancer cell lines
to tumor necrosis factor-related apoptosis-inducing ligand. Mol
Cancer Res. 5:615–625. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Horinaka M, Yoshida T, Shiraishi T, et al:
The dietary flavonoid apigenin sensitizes malignant tumor cells to
tumor necrosis factor-related apoptosis-inducing ligand. Mol Cancer
Ther. 5:945–951. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Walczak H, Miller RE, Ariail K, et al:
Tumoricidal activity of tumor necrosis factor-related
apoptosis-inducing ligand in vivo. Nat Med. 5:157–163.
1993.PubMed/NCBI
|
|
27
|
Ashkenazi A, Pai RC, Fong S, et al: Safety
and antitumor activity of recombinant soluble Apo2 ligand. J Clin
Invest. 104:155–162. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fesik SW: Promoting apoptosis as a
strategy for cancer drug discovery. Nat Rev Cancer. 5:876–885.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Soria JC, Smit E, Khayat D, et al: Phase
1b study of dulanermin (recombinant human Apo2L/TRAIL) in
combination with paclitaxel, carboplatin, and bevacizumab in
patients with advanced non-squamous non-small-cell lung cancer. J
Clin Oncol. 28:1527–1533. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Leong S, Cohen RB, Gustafson DL, et al:
Mapatumumab, an antibody targeting TRAIL-R1, in combination with
paclitaxel and carboplatin in patients with advanced solid
malignancies: results of a phase I and pharmacokinetic study. J
Clin Oncol. 27:4413–4421. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zerafa N, Westwood JA, Cretney E, et al:
Cutting edge: TRAIL deficiency accelerates hematological
malignancies. J Immunol. 175:5586–5590. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nakata S, Yoshida T, Horinaka M, Shiraishi
T, Wakada M and Sakai T: Histone deacetylase inhibitors upregulate
death receptor 5/TRAIL-R2 and sensitize apoptosis induced by
TRAIL/APO2-L in human malignant tumor cells. Oncogene.
23:6261–6271. 2004. View Article : Google Scholar
|
|
33
|
Kim KM, Song JJ, An JY, Kwon YT and Lee
YJ: Pretreatment of acetylsalicylic acid promotes tumor necrosis
factor-related apoptosis-inducing ligand-induced apoptosis by
down-regulating BCL-2 gene expression. J Biol Chem. 49:41047–41056.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Braun FK, Al-Yacoub N, Plötz M, Möbs M,
Sterry W and Eberle J: Nonsteroidal anti-inflammatory drugs induce
apoptosis in cutaneous T-cell lymphoma cells and enhance their
sensitivity for TNF-related apoptosis-inducing ligand. J Invest
Dermatol. 132:429–439. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang Y, He Q, Hillman MJ, Rong R and
Sheikh MS: Sulindac sulfide-induced apoptosis involves death
receptor 5 and the caspase 8-dependent pathway in human colon and
prostate cancer cells. Cancer Res. 61:6918–6924. 2001.
|
|
36
|
Sinicrope FA and Penington RC: Sulindac
sulfide-induced apoptosis is enhanced by a small-molecule Bcl-2
inhibitor and by TRAIL in human colon cancer cells overexpressing
Bcl-2. Mol Cancer Ther. 4:1475–1483. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Heijink DM, Jalving M, Oosterhuis D, et
al: TNF-related apoptosis-inducing ligand cooperates with NSAIDs
via activated Wnt signalling in (pre)malignant colon cells. J
Pathol. 223:378–389. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ricchi P, Zarrilli R, Di Palma A and
Acquaviva AM: Nonsteroidal anti-inflammatory drugs in colorectal
cancer: from prevention to therapy. Br J Cancer. 88:803–807. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sheng H, Shao J, Kirkland SC, et al:
Inhibition of human colon cancer cell growth by selective
inhibition of cyclooxygenase-2. J Clin Invest. 99:2254–2259. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bjarnason I: Gastrointestinal safety of
NSAIDs and over-the-counter analgesics. Int J Clin Pract (Suppl).
178:37–42. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schnitzer TJ, Burmester GR, Mysler E, et
al: Comparison of lumiracoxib with naproxen and ibuprofen in the
Therapeutic Arthritis Research and Gastrointestinal Event Trial
(TARGET), reduction in ulcer complications: randomised controlled
trial. Lancet. 364:665–674. 2004. View Article : Google Scholar
|
|
42
|
Lugardon S, Lapeyre-Mestre M and
Montastruc JL: Upper gastrointestinal adverse drug reactions and
cyclo-oxygenase-2 inhibitors (celecoxib and rofecoxib): a
case/non-case study from the French Pharmacovigilance Database. Eur
J Clin Pharmacol. 60:673–677. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Van GE, Jones JK, Moore N, Parc JM, Wall R
and Schneid H: A large simple clinical trial prototype for
assessment of OTC drug effects using patient-reported data.
Pharmacoepidemiol Drug Saf. 14:249–255. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Horinaka M, Yoshida T, Kishi A, et al:
Lactobacillus strains induce TRAIL production and facilitate
natural killer activity against cancer cells. FEBS Lett.
584:577–582. 2010. View Article : Google Scholar : PubMed/NCBI
|