Introduction
Osteopontin (OPN), a secreted non-collagenous,
sialic-acid-rich, chemokine-like extracellular matrix (ECM)
protein, has been implicated as an important mediator and a
potential therapeutic target of cancer metastasis (1). OPN could inhibit the apoptosis of
adherent endothelial cells induced by growth factors and cytokine
depletion by enhancing Bcl-xL expression (2). Bcl-2 has been demonstrated to play
important roles in the development and progression of several types
of cancer (3–5), and can be an independent predictor of
cancer outcome (6,7). Bcl-2 has also been found to play an
important role in resistance to 5-fluorouracil-caused cell death of
hepatocellular carcinoma (HCC) (8).
In a previous report, OPN silencing reduced the expression of
integrin αv, β1 and β3, blocked NF-κB activation, suppressed
Bcl-2/Bcl-xL and XIAP expression, increased Bax expression, and
induced a mitochondria-mediated apoptosis (9). However, the correlation between OPN
and BCL-2 in HCC and their potential roles in prognosis remain
unclear.
In the present study, we examined the expression
levels of OPN and Bcl-2 in HCC cell lines and tumor tissues from
454 cases of HCC patients to investigate the correlation between
OPN and Bcl-2 and its prognostic values for HCC patients. We found
that the combination of OPN and Bcl-2 could provide prognostic
information for HCC patients. Bcl-2 levels may be regulated by OPN
in the HCC microenvironment.
Materials and methods
Patients and cell lines
Four hundred and fifty-four patients undergoing
curative resection for HCC at the Liver Cancer Institute, Zhongshan
Hospital, Fudan University, between 2004 and 2006, were enrolled in
this study. For each patient, the diagnosis of HCC was confirmed by
pathologic examination and complete follow-up data were available.
The clinicopathologic characteristics of patients in the tumor
tissue microarray (TMA) are summarized in Table I. Follow-up was completed in March
2012. The mean survival was 61.6±1.9 months. Follow-up after
surgery included serum α-fetoprotein (AFP), ultrasonography and
chest X-ray every 2–3 months according to the postoperative time
(8,10). The diagnosis of recurrence was based
on typical imaging appearance in computed tomography and/or
magnetic resonance imaging scan and an elevated AFP level. Overall
survival (OS) was defined as the interval between the date of
surgery and mortality. Time to recurrence (TTR) was defined as the
time between the start of surgery and the first report of
recurrence (excluding patients who succumbed to non-liver cancer
causes before recurrence) (11,12).
For patients who had not experienced a recurrence at the time of
mortality or last follow-up, TTR was censored at the date of
mortality or the last follow-up.
 | Table IAssociations between OPN/BCL-2 density
as detected by immunohistochemical staining of tissue microarrays
and clinicopathological characteristics of HCC patients
(n=454). |
Table I
Associations between OPN/BCL-2 density
as detected by immunohistochemical staining of tissue microarrays
and clinicopathological characteristics of HCC patients
(n=454).
| Tumoral OPN density,
n | | Tumoral BCL-2
density, n | |
|---|
|
| |
| |
|---|
| Variable | High (n=227) | Low (n=227) | P-value | High (n=227) | Low (n=227) | P-value |
|---|
| Gender |
| Female | 35 | 42 | 0.381 | 39 | 38 | 0.901 |
| Male | 192 | 185 | | 188 | 189 | |
| Age, years |
| ≤51 | 123 | 111 | 0.260 | 117 | 117 | 1.000 |
| >51 | 104 | 116 | | 110 | 110 | |
| Preoperative AFP
(ng/ml) |
| ≤20 | 86 | 79 | 0.495 | 78 | 87 | 0.380 |
| >20 | 141 | 148 | | 149 | 140 | |
| HBsAg |
| Negative | 26 | 10 | 0.006 | 19 | 17 | 0.728 |
| Positive | 201 | 217 | | 208 | 210 | |
| Liver
cirrhosis |
| No | 22 | 25 | 0.664 | 27 | 20 | 0.281 |
| Yes | 205 | 202 | | 200 | 207 | |
| ALT (units/l) |
| ≤75 | 200 | 200 | 1.000 | 200 | 200 | 1.000 |
| >75 | 27 | 27 | | 27 | 27 | |
| Tumor size
(cm) |
| ≤5 | 157 | 174 | 0.073 | 158 | 173 | 0.113 |
| >5 | 70 | 53 | | 69 | 54 | |
| Tumor
encapsulation |
| Complete | 122 | 114 | 0.452 | 108 | 128 | 0.060 |
| None | 105 | 113 | | 119 | 99 | |
| Vascular
invasion |
| No | 161 | 145 | 0.109 | 152 | 154 | 0.841 |
| Yes | 66 | 82 | | 75 | 73 | |
| BCLC stage |
| A | 151 | 143 | 0.432 | 147 | 147 | 1.000 |
| B/C | 76 | 84 | | 80 | 80 | |
| Tumor
differentiation |
| I–II | 190 | 156 | <0.001 | 169 | 177 | 0.378 |
| III–IV | 37 | 71 | | 58 | 50 | |
The study was approved by the Research Ethics
Committee of Zhongshan Hospital, Fudan University (Shanghai,
China). Snap-frozen or paraffin-embedded tissue specimens were
obtained from these patients after receiving written informed
consent. Fresh tissues for western blotting were collected
immediately after resection, frozen in liquid nitrogen, and stored
at −80°C.
MHCC97-H and MHCC97-L (human HCC cell lines with
different metastatic potentials established by authors’ institute
from the same parent cell line), SMMC-7721, HepG2 cells
(low-metastatic; American Type Culture Collection) were used in
this study. Cells were cultured in Dulbecco’s Modified Eagle’s
Medium (DMEM) (HyClone, Logan, UT, USA) supplemented with 10%
(vol/vol) fetal bovine serum (FBS; Gibco-BRL, New York, NY, USA) at
37°C in a humidified incubator containing 5% CO2.
Tissue microarray immunohistochemistry
analysis for OPN and Bcl-2
Tissue microarrays were constructed as previously
described (13). Briefly, duplicate
1-mm-diameter cylinders were obtained from 2 different areas of the
donor blocks and transferred to the recipient paraffin block at
defined array positions (Shanghai Biochip Co., Ltd.). Consecutive
4-μm-thick sections were obtained on
3-aminopropyltriethoxysilane-coated slides (14).
Immunohistochemistry was carried out using a
two-step protocol according to the manufacturer’s instructions.
Briefly, the sections were dewaxed in xylene and graded alcohols,
hydrated, and washed in PBS. After endogenous peroxidase was
inhibited by 3% H2O2 for 30 min, the sections
were pretreated in a microwave oven (14 min in sodium citrate
buffer; pH=6), nonspecific binding sites were blocked with Protein
Block (Novocastra Laboratories, Newcastle upon Tyne, UK), and then
the tissues were incubated with primary antibodies for 12 h in a
moist chamber at 4°C. Primary antibodies used for detecting the
expression levels of OPN and Bcl-2 were mouse anti-human OPN
antibody (1:100) and rabbit anti-human Bcl-2 antibody (1:100; both
from Abcam, Cambridge, UK). The components of the Envision-plus
detection system were applied (EnVision+/HRP/Mo; Dako, Carpinteria,
CA, USA). Reaction products were visualized by incubation with
3,3-diaminobenzidine solution. Negative controls were treated
identically but with the primary antibodies omitted (12).
The images of 4 representative fields were captured
by the Leica QWin Plus version 3 software and identical settings
were used for each image under high-power magnification (x200). The
density of positive staining was evaluated using a Leica CCD camera
DFC420 connected to a Leica DMIRE2 microscope (Leica Microsystems
Imaging Solutions, Cambridge, UK) and a computer. The OPN and Bcl-2
densities were determined by Image-Pro Plus version 5.0 software
(Media Cybernetics, Bethesda, MD, USA) as previously described
(10). Uniform settings were used
for each antibody staining. The IOD of all positive staining in
each image was measured and the density of each antibody was
calculated as the product of IOD/total area.
Western blot analysis
The protein levels of OPN and Bcl-2 in cell lines
and tumor tissues were evaluated via western blot analysis. Total
protein was extracted in lysis buffer for 45 min on ice. Equal
amounts of protein of each sample (50 μg) were denatured by heating
at 100°C for 5 min with Laemmli loading buffer, and subsequently
loaded on 10% SDS-polyacrylamide gel electrophoresis, then
transferred onto polyvinylidene fluoride membranes (Millipore).
Primary antibodies against OPN (1:1,000) and Bcl-2 (1:1,000)
(Abcam) were used. A monoclonal antibody against GAPDH (1:1,000;
Santa Cruz Biotechnology, Inc.) was used as the internal control.
The bands were quantified using Gel-Pro Analyzer 4.0 (Media
Cybernetics). The OPN/GAPDH and Bcl-2/GAPDH ratios were calculated
for relative quantization. Each experiment was repeated at least 3
times.
Statistical analyses
Statistical analysis was performed with SPSS 15.0
for Windows (SPSS, Chicago, IL, USA). The Kaplan-Meier method was
used to calculate the survival and recurrence curves and to
estimate OS and TTR. The log-rank test was used to compare TTR and
OS between patients in different groups. The Spearman’s rank and
Fisher’s exact tests were applied to demonstrate
clinicopathological correlations. Univariate and multivariate
analyses were performed with Cox proportional hazards model.
Student’s t-test was used for the comparison of data between
groups; if variances within groups were not homogeneous, the
nonparametric Mann-Whitney U test and Kruskal-Wallis H test were
used. Values are expressed as the means ± standard deviation.
p<0.05 was considered to indicate a statistically significant
difference. For OPN and Bcl-2 density, the cutoff for the
definition of subgroups was the median value.
Results
OPN and Bcl-2 expression in HCC cell
lines
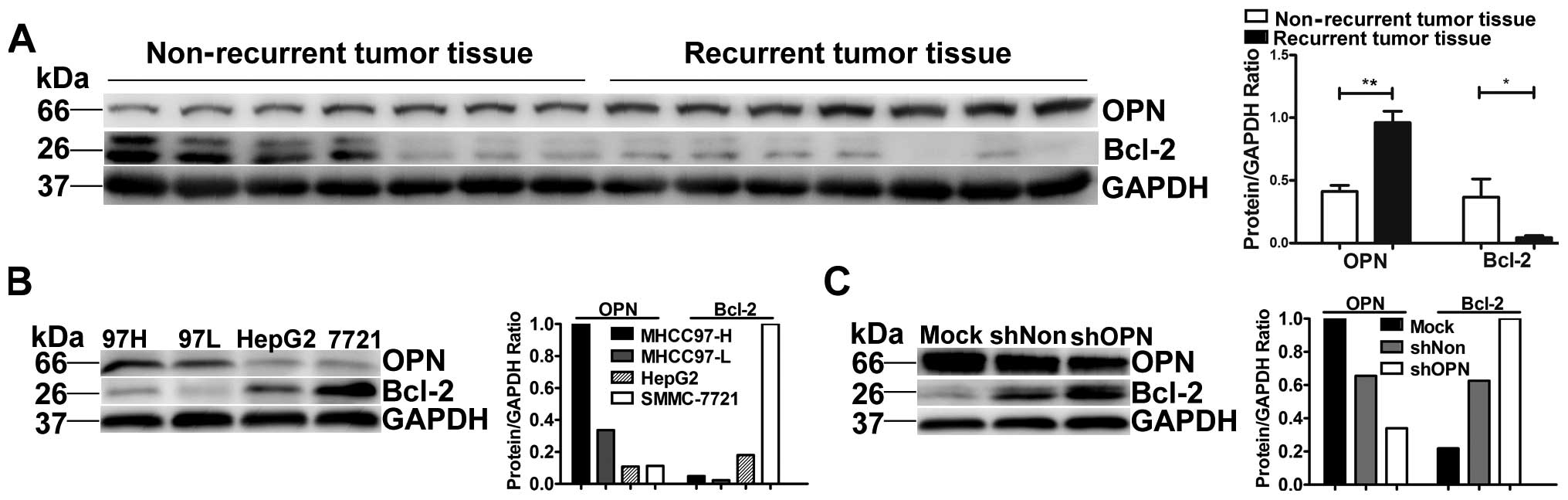
After assessing OPN and Bcl-2 expression levels in
MHCC97-H, MHCC97-L, HepG2 and SMMC-7721 cell lines by western blot
analysis, we found that OPN expression increased with increasing
metastatic potential in human HCC cell lines while Bcl-2 decreased
with increasing metastatic potential (Fig. 1B).
To investigate the association between OPN and BCL2,
we analyzed the expression level of BCL2 in HCCLM3 cells
transfected with lentiviral vectors with specific miRNAs for OPN,
whose OPN levels were previously analyzed (9); Bcl-2 expression increased
significantly in HCCLM3 cells when OPN was knocked down (Fig. 1C).
Expression of OPN and Bcl-2 in HCC
OPN expressed highly in recurrent tumor tissue but
low in non-recurrent ones by western blotting. Bcl-2 was expressed
low in recurrent tumor tissue and low in non-recurrent ones. In the
relative quantitative analysis below, OPN was significantly higher
in recurrent tumor tissue than in non-recurrent ones
(**p<0.001). Bcl-2 was significantly higher in
non-recurrent than in recurrent tumor tissue (*p=0.046)
(Fig. 1A).
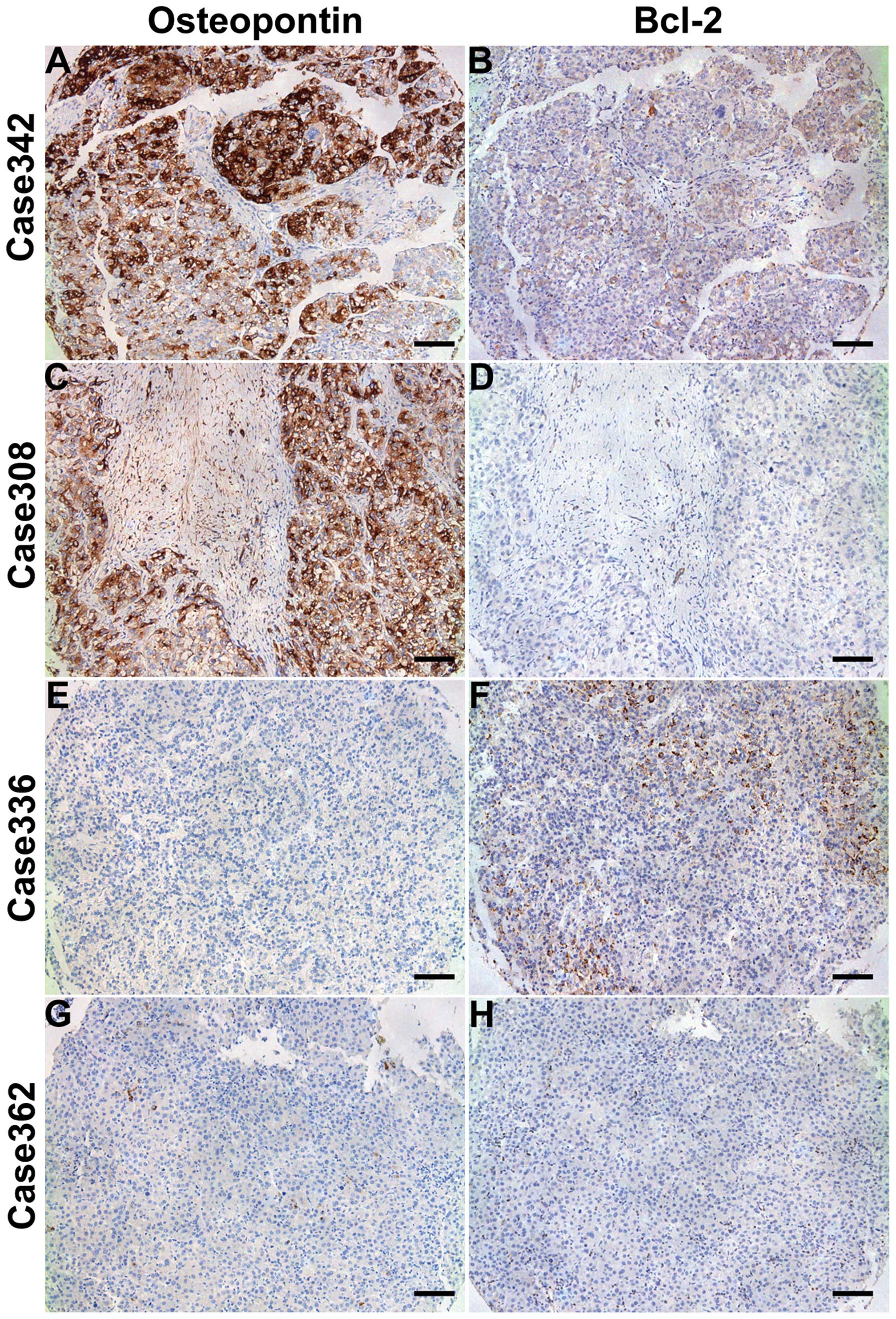
The positive staining for OPN was mainly found in
HCC cells in the peripheral region of cancer nodules, rather than
in the noncancerous hepatocytes. OPN staining was localized in the
cytoplasm of HCC cells. Immunoreactivity for Bcl-2 was observed
both in the cytoplasm and the nucleus of HCC cells (Fig. 2).
As shown in Table I,
high OPN expression in tumor tissue was significantly correlated
with tumor differentiation (p<0.001) and HBsAg level (p=0.006).
However, no statistically significant association was found between
the Bcl-2 expression and these clinical characteristics.
Prognostic value of OPN and Bcl-2 in 454
HCC patients
Using IOD median value as the cutoff value, 454 HCC
patients were divided into 2 groups. The 1-, 3-, 5- and 7-year OS
rates of high-OPN patients (75.9, 54.6, 46.9 and 42.7%,
respectively) were significantly lower than those of low-OPN
patients (87.1, 72.4, 64 and 55.2%, respectively; p=0.002)
(Fig. 3A). The 1-, 3-, 5- and
7-year recurrence rates of high-OPN patients (41.1, 46.6, 51.9 and
57.3%, respectively) were much higher than those of low-OPN
patients (23.8, 33.9, 42.2 and 48.4%, respectively; p=0.025)
(Fig. 3B). The 1-, 3-, 5- and
7-year OS rates of high-Bcl-2 patients (84.3, 68.9, 63.4 and 57.8%,
respectively) were significantly lower than those of low-Bcl-2
patients (78.7, 58.3, 47.8 and 40.5%, respectively; p=0.001)
(Fig. 3C). The 1-, 3-, 5- and
7-year tumor recurrence rates of high Bcl-2 patients (24.9, 32.7,
42.0 and 48.9%, respectively) were much higher than those of low
Bcl-2 patients (39.6, 46.0, 51.9 and 57.0%, respectively; p=0.019)
(Fig. 3D).
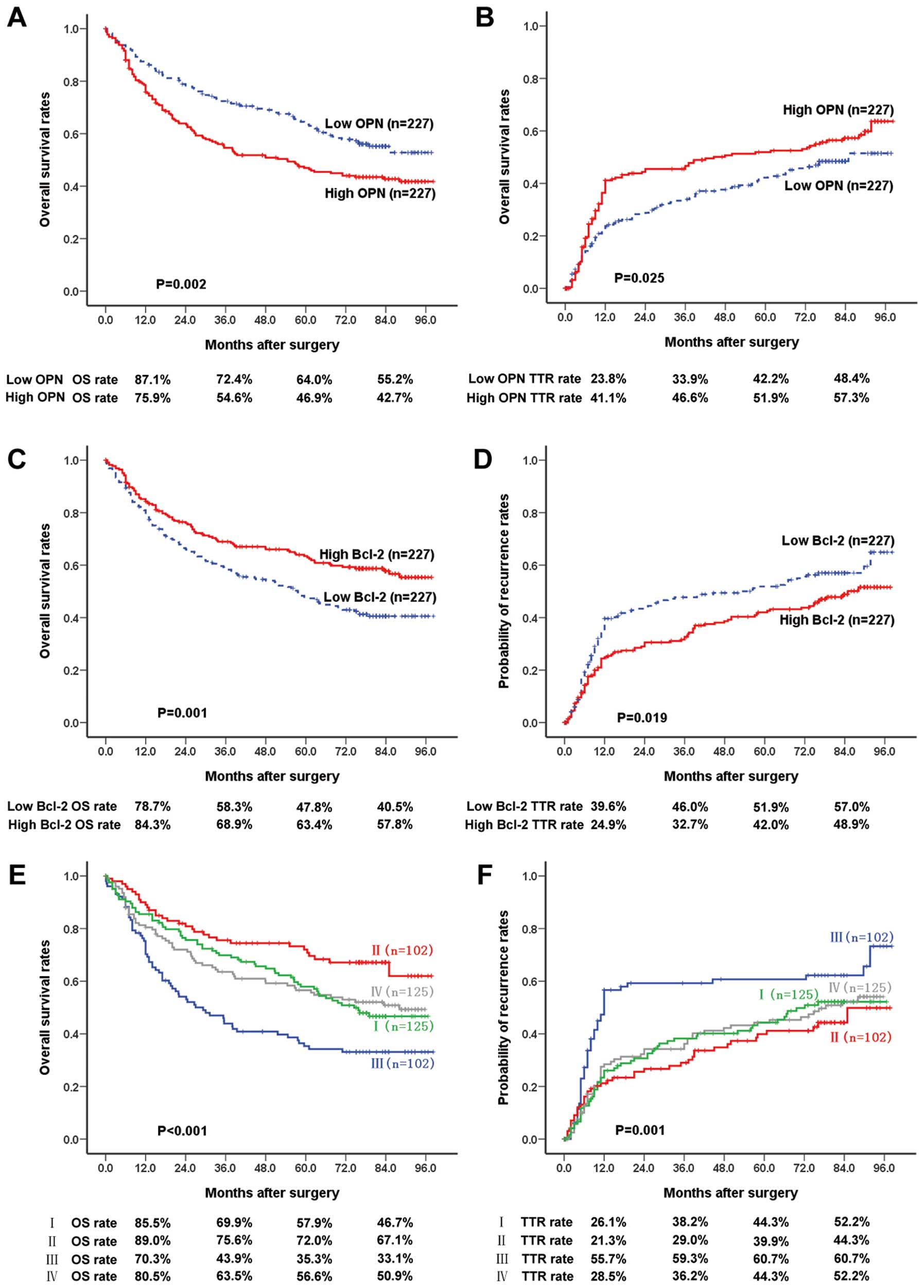 | Figure 3Prognostic significance assessed by
Kaplan-Meier analysis and log-rank tests. (A and B) The 1-, 3-, 5-
and 7-year overall survival rates (log-rank test, p=0.002; 87.1,
72.4, 64 and 55.2%, respectively for low OPN patients, vs. 75.9,
54.6, 46.9 and 42.7%, respectively for high OPN patients) and
cumulative recurrence rates (p=0.025; 23.8, 33.9, 42.2 and 48.4%,
respectively for low OPN patients, vs. 41.1, 46.6, 51.9 and 57.3%,
respectively for high OPN patients) of low OPN patients were
significantly different from those of high OPN patients. (C and D)
The 1-, 3-, 5- and 7-year overall survival rates (p=0.001; 78.7,
58.3, 47.8 and 40.5%, respectively for low Bcl-2 patients, vs.
84.3, 68.9, 63.4 and 57.8%, respectively for high Bcl-2 patients)
and cumulative recurrence rates (p=0.019; 39.6, 46.0, 51.9 and
57.0%, respectively for low Bcl-2 patients, vs. 24.9, 32.7, 42.0
and 48.9%, respectively for high Bcl-2 patients) of low Bcl-2
patients were significantly different from those of high Bcl-2
patients. (E and F) By combining OPN and Bcl-2, the 1-, 3-, 5- and
7-year overall survival rates (p<0.001; 89.0, 75.6, 72.0 and
67.1%, respectively for group II, vs. 70.3, 43.9, 35.3 and 33.1%,
respectively for group III) and cumulative recurrence rates
(p=0.001; 21.3, 29.0, 39.9 and 44.3%, respectively for group II,
vs. 55.7, 59.3, 60.7 and 60.7%, respectively for group III) of
group II patients were significantly different from those of group
III patients. Group I, patients with high OPN and high Bcl-2
(n=125); group II, patients with high OPN and low Bcl-2 (n=102);
group III, patients with low OPN and high Bcl-2 (n=102); group IV,
low OPN and low Bcl-2 (n=125). OPN, osteopontin. |
Combined value of OPN and Bcl-2 to
predict OS or TTR
Based on the expression levels of OPN and Bcl-2, we
stratified the 454 HCC patients into four groups: Group I, patients
with high OPN and high Bcl-2 (n=125); Group II, high OPN and low
Bcl-2 (n=102); Group III, low OPN and high Bcl-2 (n=102); Group IV,
low OPN and low Bcl-2 (n=125). The patients of Group II (high
OPN/low Bcl-2) had much shorter OS and TTR compared with those of
Group III (low OPN/high Bcl-2). The 1-, 3-, 5- and 7-year OS rates
of Group II (89.0%, 75.6%, 72.0% and 67.1%, respectively) were
significantly lower compared with Group III (70.3%, 43.9%, 35.3%
and 33.1%, respectively; p<0.001) (Fig. 3E). The 1-, 3-, 5- and 7-year
recurrence rates of Group II (21.3%, 29.0%, 39.9% and 44.3%,
respectively) were significantly lower than those of patients in
Group III (55.7%, 59.3%, 60.7% and 60.7%, respectively; p=0.001)
(Fig. 3F).
Univariate and multivariate analyses of
the prognostic values of OPN and Bcl-2 in HCC patients
To further evaluate the prognostic value of OPN and
Bcl-2 for HCC patients, univariate and multivariate analyses were
performed with the clinicopathological characteristics as well as
the OPN and Bcl-2 levels (Tables
II and III). In the
univariate analysis, serum AFP level, tumor size, tumor number,
vascular invasion, BCLC stage, tumor differentiation and tumor
encapsulation were shown to be associated with OS of HCC patients,
and serum AFP, tumor size, vascular invasion, BCLC stage with TTR.
The OPN and Bcl-2 expression levels in HCC tissues were also
significantly associated with both OS and TTR.
 | Table IIUnivariate analyses of factors
associated with overall survival and time to recurrence. |
Table II
Univariate analyses of factors
associated with overall survival and time to recurrence.
| Overall
survival | Time to
recurrence |
|---|
|
|
|
|---|
| Variable | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Gender (male vs.
female) | 0.974
(0.687–1.382) | 0.882 | 1.066
(0.735–1.545) | 0.737 |
| Age, years (>52
vs. ≤52) | 1.057
(0.812–1.377) | 0.679 | 1.056
(0.806–1.382) | 0.694 |
| AFP (ng/ml; >20
vs. ≤20) | 1.597
(1.196–2.131) | 0.001 | 1.411
(1.061–1.875) | 0.018 |
| HBsAg (positive vs.
negative) | 1.766
(0.999–3.122) | 0.050 | 2.224
(1.188–4.162) | 0.012 |
| Liver cirrhosis
(yes vs. no) | 1.464
(0.892–2.401) | 0.131 | 1.625
(0.976–2.706) | 0.062 |
| ALT (units/l;
>75 vs. ≤75) | 1.062
(0.710–1.590) | 0.768 | 0.910
(0.595–1.393) | 0.666 |
| Tumor size (cm;
>5 vs. ≤5) | 1.758
(1.327–2.329) | <0.001 | 1.639
(1.220–2.202) | 0.001 |
| Tumor number
(multiple vs. single) | 1.468
(0.917–2.350) | 0.110 | 0.919
(0.524–1.612) | 0.769 |
| Vascular invasion
(yes vs. no) | 2.041
(1.560–2.671) | <0.001 | 1.590
(1.197–2.112) | 0.001 |
| BCLC stage (B/C vs.
A) | 2.055
(1.573–2.684) | <0.001 | 1.527
(1.153–2.021) | 0.003 |
| Tumor
differentiation (III–IV vs. I–II) | 1.521
(1.132–2.045) | 0.005 | 1.338
(0.980–1.827) | 0.067 |
| Tumor encapsulation
(none vs. complete) | 1.414
(1.085–1.844) | 0.010 | 1.268
(0.968–1.660) | 0.084 |
| OPN (high vs.
low) | 1.530
(1.171–1.999) | 0.002 | 1.359
(1.036–1.782) | 0.027 |
| Bcl-2 (high vs.
low) | 0.639
(0.489–0.836) | 0.001 | 0.727
(0.554–0.953) | 0.021 |
| Combination of OPN
and Bcl-2 |
| Overall | | <0.001 | | 0.002 |
| II vs. III | 2.726
(1.784–4.164) | <0.0001 | 1.922
(1.285–2.876) | 0.001 |
 | Table IIIMultivariate analyses of factors
associated with overall survival and time to recurrence. |
Table III
Multivariate analyses of factors
associated with overall survival and time to recurrence.
| Overall
survival | Time to
recurrence |
|---|
|
|
|
|---|
| Variable | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| AFP (ng/ml; >20
vs. ≤20) | 1.397
(1.038–1.880) | 0.027 | 1.263
(0.941–1.694) | NS |
| Tumor size (cm;
>5 vs. ≤5) | 1.826
(1.364–2.444) | <0.001 | 1.697
(1.253–2.299) | 0.001 |
| HBsAg (positive vs.
negative ) | 1.373
(0.767–2.458) | NS | | |
| Vascular invasion
(yes vs. no ) | 1.648
(1.246–2.181) | <0.001 | 1.381
(1.029–1.855) | 0.032 |
| Tumor encapsulation
(none vs. complete) | 1.421
(1.084–1.863) | 0.011 | | |
| Tumor
differentiation (III–IV vs. I–II) | 1.187
(0.873–1.615) | NS | | |
| OPN (high vs.
low) | 1.652
(1.250–2.183) | <0.001 | 1.478
(1.115–1.959) | 0.007 |
| Bcl-2 (high vs.
low) | 0.532
(0.404–0.701) | <0.001 | 0.632
(0.478–0.835) | 0.001 |
| Combination of OPN
and Bcl-2 |
| Overall | | <0.001 | | 0.001 |
| II vs. III | 3.725
(2.330–5.954) | <0.0001 | 2.244
(1.469–3.429) | 0.0002 |
Individual characteristics that showed significance
by univariate analysis were adopted as covariates in a multivariate
Cox proportional hazards model, and then combined variables were
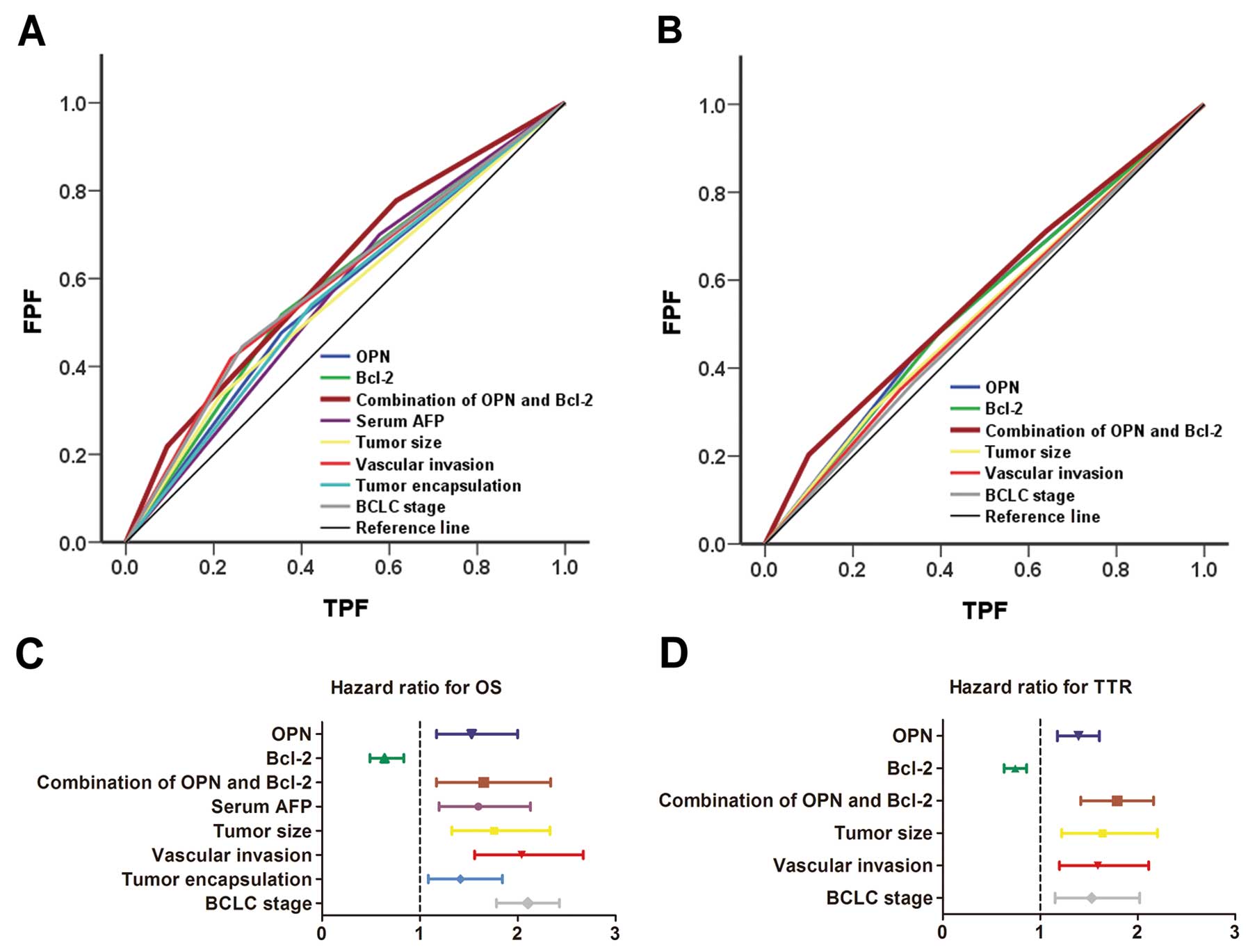
further analyzed. Both OPN and Bcl-2 were demonstrated to be
independent prognostic indicators for OS (p<0.001 both for OPN
and Bcl-2) and TTR (p=0.004 for OPN, p=0.002 for Bcl-2). Moreover,
combination of OPN with Bcl-2 was an even more powerful independent
prognostic indicator for both OS (p<0.001) and TTR (p<0.001).
Hazard ratio analysis showed Bcl-2 was a protective factor in HCC
prognosis (Fig. 4C and D).
ROC analysis for OPN and Bcl-2
Clinicopathological factors showing significance by
multivariate survival analysis and the combination of OPN and Bcl-2
were adopted for ROC analysis. For both mortality and recurrence,
the predictive value of the combination of OPN and Bcl-2 was the
best. The area under the curve of this combination was 0.622 (95%
CI, 0.570–0.673; p<0.001) for mortality and 0.569 (95% CI,
0.516–0.622; p=0.011) for recurrence (Fig. 4A and B).
Discussion
The poor prognosis of HCC is often the result of
late diagnosis or high rate of recurrence after surgery. Metastasis
is a major complication of HCC pathogenesis that typifies a poor
prognosis. Current approaches are hindered by a lack of clinically
useful biomarkers. OPN has been implicated as an important mediator
of tumor metastasis and has been investigated for use as a
biomarker for advanced disease and as a potential therapeutic
target in the regulation of cancer metastasis. It is a secreted
multifunctional glycoprotein expressed at high levels in tumors and
the surrounding stroma of numerous types of cancer, including liver
(15–17). Increased serum and plasma OPN levels
are associated with advanced-stage lung, breast (18), colon and prostate carcinomas
(19,20). OPN expression can predict
high-grade, late-stage and early-recurrence HCC (21).
Our previous study identified OPN as a predictive
marker for HCC and a molecular target of the HCC metastatic
phenotype. The data demonstrated a correlation between OPN mRNA
expression and primary HCC metastasis (22). Furthermore, increased OPN levels
were associated with increased aggressiveness and metastatic
potential of HCC and positively correlated with poor prognosis and
early tumor recurrence in HCC patients (23,24).
In vitro, we found that OPN expression
increased with increasing metastatic potential in human HCC cell
lines while Bcl-2 decreased with increasing metastatic potential.
These data support the findings in HCC TMA and demonstrated that
OPN may interact with Bcl-2 in HCC tumors and cells. Thus, we
performed miRNAs for OPN in MHCC97-H cells which have a high
expression level of OPN. We found that OPN decreased in shOPN
MHCC97-H cells significantly compared with mock and shNon ones.
Bcl-2 expression increased significantly in shOPN MHCC97-H cells.
This discovery appears to be in contrast to previous reports. For
example, in malignant gliomas, OPN siRNA induced clear upregulation
of Bax expression, downregulation of Bcl-2, Bcl-xL (25). Our findings are based on large
cohort of HCC patients. Hung et al(8) reported that HBV pre-S2D increased
Bcl-2 expression, indicating that the cohort of HCC patients, who
had 92.1% HBV infection, will have some difference compared with
other types of tumors. Furthermore, patients with higher OPN levels
had significantly shorter median survival time and recurrence time
than the lower ones. It was confirmed that OPN levels are also
significantly higher in recurrent tumor tissues than in the
non-recurrent ones by western blotting (p<0.001). In previous
reports, an elevated plasma level of OPN was regarded as a
potential prognostic biomarker, and overexpression of OPN was
closely correlated with intrahepatic metastasis, early recurrence,
and a poorer prognosis; this result is similar to ours in tumor
tissue (21,22,24,26).
Improving OPN predicting efficiency for HCC patients
needs to be elucidated. It is necessary to combine OPN and Bcl-2 to
deal with this issue. Therefore, we used TMA contained 454 HCC
cases to examine both OPN and Bcl-2 expression levels. Inversely,
the higher BCL-2 levels were associated with longer median survival
time and recurrence time in HCC patients. The co-index of OPN/Bcl-2
was an independent prognostic factor for both overall survival
(p<0.001) and time to recurrence (p=0.001).
Bcl-2 overexpression has since been reported in
several tumor types and is often correlated with poor survival
(27). Bcl-2 is best known for its
ability to suppress apoptosis and belongs to a group of related
proteins that are key regulators of apoptosis or programmed cell
death (28). The phenomenon of
Bcl-2 expression in our study seems to deviate from the common
notion that genes are ‘vicious’ factors. Furthermore, numerous
studies have suggested that the gene may undergo conversion from
protector to killer under some circumstances. Dawson et
al(7) performed a study on
breast cancer in 11,212 women and found that increasing expression
levels of Bcl-2 predict better survival in early breast cancer and
Bcl-2 is a suitable time-independent prognostic marker in early
breast cancer.
Several possible mechanisms have been founf
regarding this seemingly contradictory phenomenon. First, mutations
in translocated Bcl-2 alleles have been identified in lymphomas
which ablate the aspartic acid residue required for caspase
cleavage (29). Of note,
proteolytic removal of N-terminal sequences by caspase-mediated
cleavage could reverse the phenotype of Bcl-2 (30). Second, the orphan nuclear receptor
Nur77 can be induced to translocate from nucleus to cytosol,
binding Bcl-2, and inducing a conformational change in Bcl-2 that
probably mimics what happens during caspase cleavage, exposing the
normally buried BH3 domain of Bcl-2 and causing it to function as a
pro-apoptotic protein (31).
Another hypothesis (28) considers
increased expression of Bcl-2 protein may also disrupt the balance
with other members of the Bcl-2 family, including the expression of
pro-apoptotic proteins. The molecular nature of such pores and how
anti-apoptotic Bcl-2 family proteins may regulate them remains to
be clarified. A potentially similar mechanism identified for Bcl-xL
showed that lipid modifications of K-Ras can promote its
association with Bcl-xL in mitochondria and induce apoptosis
(32). Thus, depending on which
proteins Bcl-2 and Bcl-xL interact with, their phenotypes can be
converted from anti- to pro-apoptotic (33), providing a potential explanation for
why high levels of Bcl-2 expression are sometimes associated with
improved patient prognosis (34).
Most studies have demonstrated that HCC tissues do
not express or have only a low positive rate of Bcl-2 protein.
Sometimes the positive rate of Bcl-2 in HCC tissues was lower than
that in the non-tumor liver tissues immediately adjacent to HCC
tissues (35). The mechanism of
this phenomenon remains unclear. There may be specific
characteristics of the regulation of Bcl-2 in HCC.
In conclusion, we showed that OPN/Bcl-2 expression
is a promising independent predictor of recurrence and survival in
HCC that may aid in the therapy of HCC patients. Bcl-2 levels may
be regulated by OPN in the HCC microenvironment. The HBV background
of the patients may have some unique influence on Bcl-2 expression
(8). Related mechanisms should be
investigated in future studies.
References
|
1
|
Wai PY and Kuo PC: Osteopontin: regulation
in tumor metastasis. Cancer Metastasis Rev. 27:103–118. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Khan SA, Lopez-Chua CA, Zhang J, Fisher
LW, Sørensen ES and Denhardt DT: Soluble osteopontin inhibits
apoptosis of adherent endothelial cells deprived of growth factors.
J Cell Biochem. 85:728–736. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Frenzel A, Grespi F, Chmelewskij W and
Villunger A: Bcl2 family proteins in carcinogenesis and the
treatment of cancer. Apoptosis. 14:584–596. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yoo SH, Yoon YG, Lee JS, et al: Etoposide
induces a mixed type of programmed cell death and overcomes the
resistance conferred by Bcl-2 in Hep3B hepatoma cells. Int J Oncol.
41:1443–1454. 2012.PubMed/NCBI
|
|
5
|
Spampanato C, De Maria S, Sarnataro M, et
al: Simvastatin inhibits cancer cell growth by inducing apoptosis
correlated to activation of Bax and downregulation of BCL-2 gene
expression. Int J Oncol. 40:935–941. 2012.PubMed/NCBI
|
|
6
|
Callagy GM, Pharoah PD, Pinder SE, et al:
Bcl-2 is a prognostic marker in breast cancer independently of the
Nottingham Prognostic Index. Clin Cancer Res. 12:2468–2475. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dawson SJ, Makretsov N, Blows FM, et al:
BCL2 in breast cancer: a favourable prognostic marker across
molecular subtypes and independent of adjuvant therapy received. Br
J Cancer. 103:668–675. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hung JH, Teng YN, Wang LH, et al:
Induction of Bcl-2 expression by hepatitis B virus pre-S2 mutant
large surface protein resistance to 5-fluorouracil treatment in
Huh-7 cells. PLoS One. 6:e289772011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao J, Dong L, Lu B, et al:
Down-regulation of osteopontin suppresses growth and metastasis of
hepatocellular carcinoma via induction of apoptosis.
Gastroenterology. 135:956–968. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu XD, Zhang JB, Zhuang PY, et al: High
expression of macrophage colony-stimulating factor in peritumoral
liver tissue is associated with poor survival after curative
resection of hepatocellular carcinoma. J Clin Oncol. 26:2707–2716.
2008. View Article : Google Scholar
|
|
11
|
Llovet JM, Di Bisceglie AM, Bruix J, et
al: Design and endpoints of clinical trials in hepatocellular
carcinoma. J Natl Cancer Inst. 100:698–711. 2008. View Article : Google Scholar
|
|
12
|
Huang H, Zhang XF, Zhou HJ, et al:
Expression and prognostic significance of osteopontin and caspase-3
in hepatocellular carcinoma patients after curative resection.
Cancer Sci. 101:1314–1319. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao Q, Qiu SJ, Fan J, et al: Intratumoral
balance of regulatory and cytotoxic T cells is associated with
prognosis of hepatocellular carcinoma after resection. J Clin
Oncol. 25:2586–2593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ding ZB, Shi YH, Zhou J, et al:
Association of autophagy defect with a malignant phenotype and poor
prognosis of hepatocellular carcinoma. Cancer Res. 68:9167–9175.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Butler WT and Ritchie H: The nature and
functional significance of dentin extracellular matrix proteins.
Int J Dev Biol. 39:169–179. 1995.PubMed/NCBI
|
|
16
|
Coppola D, Szabo M, Boulware D, et al:
Correlation of osteopontin protein expression and pathological
stage across a wide variety of tumor histologies. Clin Cancer Res.
10:184–190. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rittling SR and Chambers AF: Role of
osteopontin in tumour progression. Br J Cancer. 90:1877–1881. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Singhal H, Bautista DS, Tonkin KS, et al:
Elevated plasma osteopontin in metastatic breast cancer associated
with increased tumor burden and decreased survival. Clin Cancer
Res. 3:605–611. 1997.PubMed/NCBI
|
|
19
|
Fedarko NS, Jain A, Karadag A, Van Eman MR
and Fisher LW: Elevated serum bone sialoprotein and osteopontin in
colon, breast, prostate, and lung cancer. Clin Cancer Res.
7:4060–4066. 2001.PubMed/NCBI
|
|
20
|
Hotte SJ, Winquist EW, Stitt L, Wilson SM
and Chambers AF: Plasma osteopontin: associations with survival and
metastasis to bone in men with hormone-refractory prostate
carcinoma. Cancer. 95:506–512. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pan HW, Ou YH, Peng SY, et al:
Overexpression of osteopontin is associated with intrahepatic
metastasis, early recurrence, and poorer prognosis of surgically
resected hepatocellular carcinoma. Cancer. 98:119–127. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ye QH, Qin LX, Forgues M, et al:
Predicting hepatitis B virus-positive metastatic hepatocellular
carcinomas using gene expression profiling and supervised machine
learning. Nat Med. 9:416–423. 2003. View
Article : Google Scholar
|
|
23
|
Sun J, Xu HM, Zhou HJ, et al: The
prognostic significance of preoperative plasma levels of
osteopontin in patients with TNM stage-I of hepatocellular
carcinoma. J Cancer Res Clin Oncol. 136:1–7. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang H, Ye QH, Ren N, et al: The
prognostic significance of preoperative plasma levels of
osteopontin in patients with hepatocellular carcinoma. J Cancer Res
Clin Oncol. 132:709–717. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yan W, Qian C, Zhao P, et al: Expression
pattern of osteopontin splice variants and its functions on cell
apoptosis and invasion in glioma cells. Neuro Oncol. 12:765–775.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xie H, Song J, Du R, et al: Prognostic
significance of osteopontin in hepatitis B virus-related
hepatocellular carcinoma. Dig Liver Dis. 39:167–172. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fesik SW: Promoting apoptosis as a
strategy for cancer drug discovery. Nat Rev Cancer. 5:876–885.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cory S, Huang DC and Adams JM: The Bcl-2
family: roles in cell survival and oncogenesis. Oncogene.
22:8590–8607. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tanaka S, Louie D, Kant J and Reed JC:
Application of a PCR-mismatch technique to the BCL-2 gene:
detection of point mutations in BCL-2 genes of malignancies with A
t(14,18). Leukemia. 6(Suppl 3): S15–S19. 1992.PubMed/NCBI
|
|
30
|
Cheng EH, Kirsch DG, Clem RJ, et al:
Conversion of Bcl-2 to a Bax-like death effector by caspases.
Science. 278:1966–1968. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin YP, Zhu BZ, Yang MC, et al: Bcl-2
overexpression inhibits tetrachlorohydroquinone-induced apoptosis
in NIH3T3 cells: a possible mechanism for tumor promotion. Mol
Carcinog. 40:24–33. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bivona TG, Quatela SE, Bodemann BO, et al:
PKC regulates a farnesyl-electrostatic switch on K-Ras that
promotes its association with Bcl-XL on mitochondria and induces
apoptosis. Mol Cell. 21:481–493. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schwartz PS and Hockenbery DM: Targeted
therapies for epithelial cancers: in vivo efficacy of the
BCL-2/BCL-XL inhibitor 2-MeAA. Cancer Biol Ther. 6:465–466. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Silvestrini R, Veneroni S, Daidone MG, et
al: The Bcl-2 protein: a prognostic indicator strongly related to
p53 protein in lymph node-negative breast cancer patients. J Natl
Cancer Inst. 86:499–504. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tsamandas AC, Thomopoulos K, Gogos C, et
al: Expresssion of bcl-2 oprotein in cases of acute and chronic
viral hepatitis type B and type C: a clinicopathologic study. Dig
Dis Sci. 47:1618–1624. 2002. View Article : Google Scholar : PubMed/NCBI
|