Introduction
Brain-derived neurotrophic factor (BDNF), a member
of the neurotrophin family, is known to regulate cell growth,
differentiation, migration and apoptosis in the nervous system
(1,2). There are two forms of BDNF, a
precursor and a mature form, in the central nervous system (CNS)
(3). The precursor of BDNF
(proBDNF) is synthesised and subsequently cleaved either
intracellularly by prohormone convertases (PCs) and/or furin, or
extracellularly by plasmin and matrix metalloproteases (MMPs) to
release the mature homodimeric protein (mature BDNF) (4,5). Their
diverse actions are mediated through two different transmembrane
receptor signalling systems: Trk tyrosine kinase B (TrkB) receptor
and p75 neurotrophin receptor (NTR). Mature BDNF activates the high
affinity TrkB receptor, promoting cell survival while proBDNF binds
to both p75NTR and the co-receptor sortilin with a high affinity,
to modulate cell apoptosis (6–8).
Increasing evidence suggests that altered signalling
through TrkB promotes tumour formation and metastasis. TrkB, in
conjunction with its primary ligand BDNF, is often overexpressed in
a variety of human cancers, ranging from neuroblastomas to
pancreatic ductal adenocarcinomas, where it may allow tumour
expansion and contribute to resistance to antitumour agents
(9). Neurotrophin genes were found
to be expressed in 24 cell lines derived from human malignant
gliomas, and the BDNF gene was most abundantly expressed
(10). Expression of Trk receptors
(TrkA, TrkB and TrkC) has been detected in human astrocytomas and
promotes tumour growth. Furthermore, activation of the JNK pathway
may contribute to progression towards malignancy (11). Expression of BDNF and TrkB is also
observed in human gangliogliomas (12). However, histological data in
previous studies were unable to distinguish mature BDNF from
proBDNF due to the lack of specific antibodies. We generated
specific antibodies to mature BDNF and proBDNF, respectively. In
our recent study, the proBDNF/p75NTR pathway appeared to inhibit
malignant glioma cell growth and migration (13). A recent report also showed that
proBDNF suppressed the growth of colorectal cancer cells (14). Therefore, we postulated that the
mature form of BDNF plays a supportive role in the growth and
migration of tumour cells. In the present study, specific
anti-mature BDNF antibodies were used. We examined the role of
mature BDNF in glioma using the C6 glioma cell line in
vitro.
Materials and methods
C6 cell culture
C6 glioma cells were grown in low-glucose Dulbecco’s
modified Eagle’s medium (DMEM; Gibco) supplemented with 10% fetal
bovine serum (FBS; Gibco) or otherwise specified, 1% glutamate and
1% penicillin/streptomycin at 37°C in a humidified atmosphere of 5%
CO2 and 95% air.
Fluorescence double-labelling of mature
BDNF and TrkB in C6 cells
C6 cells were cultured on coverslips and fixed with
4% paraformaldehyde in PBS at room temperature for 20 min.
Co-expression of mature BDNF/TrkB was assayed by immunofluorescence
double-labelling as previously described (13). After blocking, the sections were
first incubated with sheep anti-mature BDNF (2 μg/ml; laboratory of
X.-F. Zhou) and rabbit anti-TrkB (1:1000; Millipore) at 4°C
overnight, and then with donkey anti-goat Alexa 488 (green)
(1:1,000) and anti-rabbit Alexa 546 (red) (1:1,000) (both from
Invitrogen) secondary antibodies for 1 h. The sections were mounted
with mounting media containing 4′,6′-diamidino-2-phenylindole
(DAPI; Vector Laboratories) and observed and photographed using a
Leica confocal microscope.
Western blot analysis
The C6 cells were harvested with lysis buffer
containing 50 mM Tris HCl, (pH 7.4), 150 mM NaCl, 1%
nonyl-phenoxylpolyethoxylethanol (NP)-40, 1% sodium deoxycholate,
0.1% sodium dodecyl sulfate (SDS) and protease inhibitor cocktail
(Roche), vortexed and centrifuged at 4°C at 13,000 rpm for 20
min.
Western blot analysis was conducted as described in
our previous report (13). In
brief, after blocking, the membranes were incubated with sheep
anti-mature BDNF (2 μg/ml) and rabbit anti-TrkB (1:1,000;
Millipore) at 4°C overnight. After several washes in TBST, the
membranes were incubated with HRP-conjugated secondary antibodies
(mouse anti-goat or goat anti-rabbit, 1:1,000; both from Santa Cruz
Biotechnology) for 2 h at room temperature. Immunoreactive bands
were detected using an enhanced chemiluminescence kit (CWBio
Technology).
Cell viability assay
The cell viability assay was conducted as previously
described in detail (13). Cells
were plated and treated with recombinant mature BDNF protein (1, 3,
10 and 30 ng/ml) or anti-mature BDNF (1, 3 and 10 μg/ml) dissolved
in serum-free media. The control remained untreated. The treated
cells were incubated for 24–48 h. The
3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT)
(Sigma) assays were performed at 0, 24 and 48 h. To minimise the
variation among different assays, the data were corrected against
the control and were plotted using the optical density of the
control wells as 100% survival. The experiments were performed in
triplicate and repeated at least three times.
Cell apoptosis assay
C6 cells were plated (15,000/well) in 96-well plates
and cultured to 60–70% confluency. On the day of the experiment,
the cells were treated and prepared as above in serum-free medium.
After 48 h, C6 cells were fixed using 4% paraformaldehyde for 20
min and then stained with DAPI as previously reported (15). Cell images were collected (at least
five fields/well) using a fluorescence microscope (Leica) for each
sample. The apoptotic and total number of nuclei were counted. The
ratio of apoptotic nuclei was calculated against the total number
of nuclei counted. To minimise the variation among different
assays, the data were corrected against the control.
Scratch assay
The details of the scratch assay were previously
described (13). Confluent
monolayer cells were scratched with a 10-μl pipette tip, washed,
photographed (0 h) and treated with recombinant mature BDNF protein
(1, 3, 10 and 30 ng/ml) or mature BDNF antibodies (1, 3 and 10
μg/ml). The cells were cultured in serum-free medium for another 24
h and were then photographed at the same position. The relative
migration distance was calculated using the following formula:
Relative migration distance = (A – B)/A, where A represents the
mean width of the cell scratch before treatments and B represents
the mean width of the cell scratch after treatments. Results are
expressed as the means ± SE.
Cell invasion assay
C6 cell invasion was examined using 24-well Boyden
chemotaxis chamber (BD Biosciences) as previously described
(13). Cells
(3×105/well) were seeded into the upper compartment
individually and incubated in 100 μl serum-free media containing
recombinant mature BDNF (1, 3, 10 and 30 ng/ml) or antibodies to
mature BDNF (1, 3 and 10 μg/ml), while 750 μl/well of media
containing 20% FBS was placed in the bottom wells. Controls
remained untreated. After a 24-h incubation at 37°C in a
CO2 incubator, the cells on the upper surface of the
inserts were gently wiped off with a cotton swab. The cells on the
lower surface of the inserts were fixed, stained with DAPI and
sampled. The data are presented as means ± SE. In additional
experiments, the cells on the bottom of the insert were stained
with Cresyl violet solution (0.2%) for 15 min. The dye was
extracted with 10% acetic acid, and dye levels were directly
proportional to the number of cells (16). The absorbance was measured at 570 nm
using an EIA microplate reader.
Statistical analysis
The data of the cell experiments from different
groups were analysed by one-way analysis of variance (ANOVA)
followed by post-hoc analysis of multiple comparisons. P<0.05
was considered to indicate a statistically significant result.
Results
Expression of mature BDNF and its
receptor TrkB in C6 cells
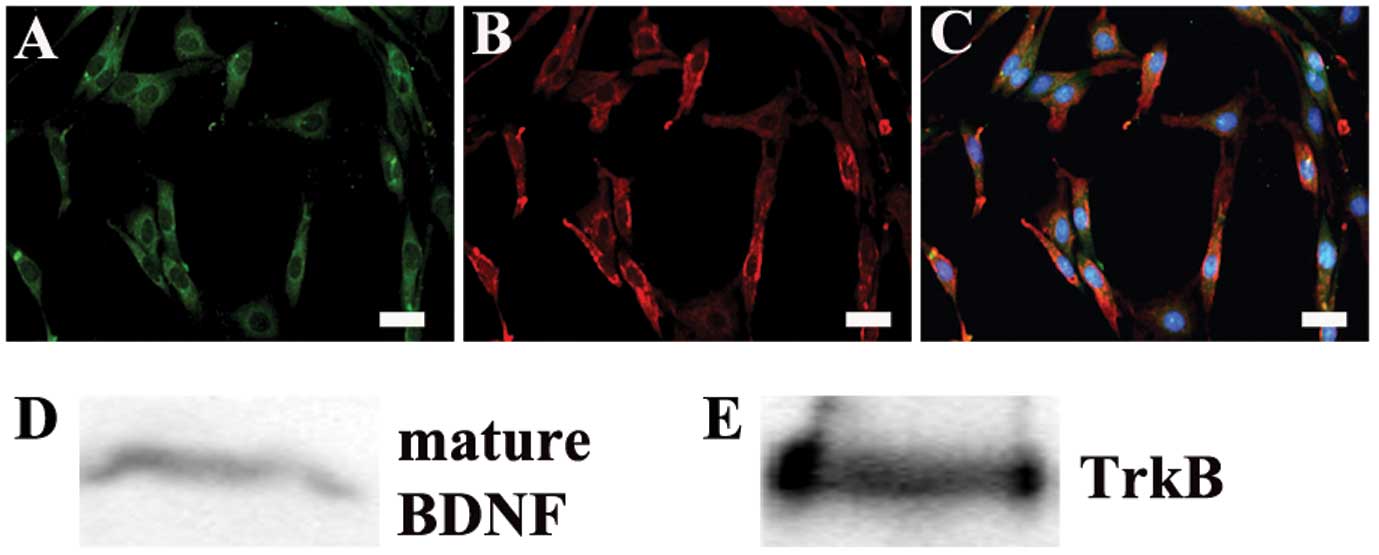
To examine the effect of mature BDNF, the C6 cell
line was used in the present study. Immunostaining and western
blots showed the expression of mature BDNF and its main receptor
TrkB in C6 cells (Fig. 1). These
results indicate that mature BDNF may activate its receptors by an
autocrine mechanism in the behavior of C6 glioma cells.
Mature BDNF promotes C6 cell growth
Next, we investigated whether mature BDNF promotes
the growth of glioma cells. As shown in Fig. 2A and C, MTT assays revealed that
exogenous mature BDNF significantly increased cell proliferation at
a dose of 30 ng/ml. Blocking endogenous mature BDNF by using mature
BDNF antibodies inhibited cell proliferation in a dose-dependent
manner (Fig. 2B and C), supporting
the specific roles of endogenous mature BDNF signalling in
promoting glioma cell growth.
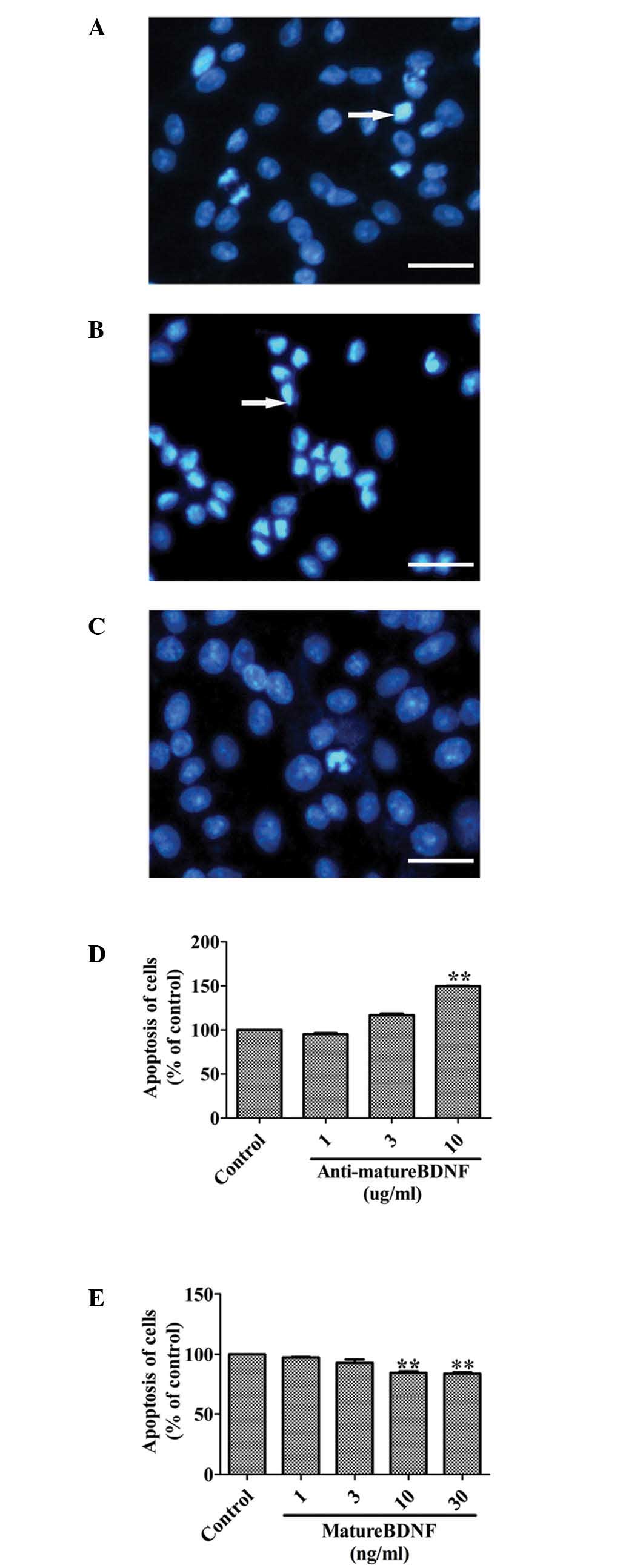
Mature BDNF inhibits apoptosis of C6
cells in vitro
To support the notion that mature BDNF promotes the
growth of glioma cells, we further investigated whether this
molecule is involved in protecting against apoptosis. Following the
addition of anti-mature BDNF antibodies (1, 3 and 10 μg/ml) into
the cultured C6 cells, the apoptosis of C6 cells was significantly
promoted in a dose-dependent manner (Fig. 3A, B and D). In contrast, apoptosis
was significantly decreased in a dose-dependent manner when C6
glioma cells were treated with mature BDNF (1, 3, 10 and 30 ng/ml)
in serum-free medium for 48 h (Fig. 3A,
C and E).
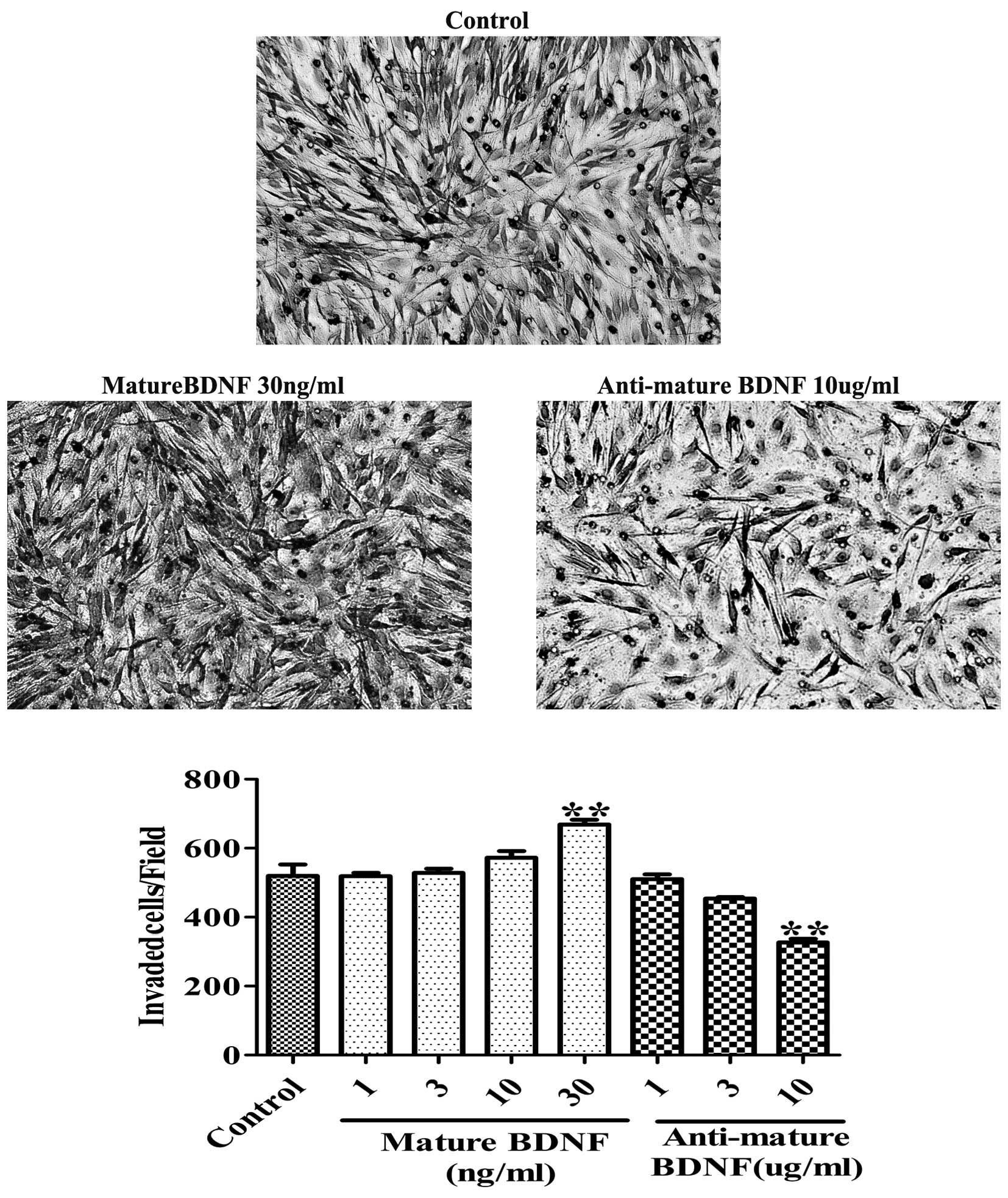
Mature BDNF increases motility and
invasion of C6 glioma cells
Next, we investigated the effect of mature BDNF on
motility and invasion by performing a scratch assay and a Boyden
chamber assay on glioma cells. The scratch assay showed that the
wound closure activity was increased in C6 glioma cells following
treatment with mature BDNF. In contrast, anti-mature BDNF
antibodies decreased the migratory activity after a 24-h culture
(Fig. 4). Furthermore, the Boyden
chamber assay revealed that cell invasion was significantly
increased by mature BDNF (30 ng/ml; 28.65±2.69%; P<0.001;
Fig. 5) and was inhibited by
anti-mature BDNF antibodies (10 μg/ml; 47.12±2.11%; P<0.001;
Fig. 5). Moreover, exogenous BDNF
stimulation increased cell invasion in a dose-dependent manner
in vitro after 24 h of culture, whereas anti-mature BDNF
antibodies decreased the activity in a dose-dependent manner
(Fig. 5). These data demonstrate
that mature BDNF plays a role in promoting motility and invasion of
C6 glioma cells.
Discussion
ProBDNF and mature BDNF play opposing roles in
neuronal function (6–8,17–21).
Their diverse actions are mediated through two different
transmembrane receptor signalling systems: p75NTR and TrkB. ProBDNF
is a high-affinity, functional ligand for the pro-apoptotic p75NTR,
whereas the proteolytically cleaved mature BDNF is the preferred
ligand for TrkB. The important roles of the BDNF/TrkB signalling
system in tumour cell proliferation and survival have been
demonstrated (22–25). Previous studies have demonstrated
that TrkB frequently exhibits robust expression in highly invasive
tumours (11) and is a key
regulator of tumour malignancy (26,27).
BDNF has also been found to be expressed in a series of tumours,
including tumors in the CNS (28)
and enhances tumour cell survival and resistance to chemotherapy
(29). However, few studies were
able to distinguish mature BDNF from proBDNF due to the limitation
of specific antibodies. Recent studies have shown that proBDNF is a
potent tumour suppressor (13,14).
We previously generated specific antibodies to mature BDNF and
proBDNF, respectively. Our present study demonstrated that proBDNF
negatively regulates the growth and migration of glioma cells
through p75NTR (13).
In the present study, we found that mature BDNF and
its receptor TrkB are highly expressed by glioma cells. Using the
C6 glioma cell model, we found that exogenous recombinant mature
BDNF increased growth and decreased apoptosis. Anti-mature BDNF
treatment decreased proliferation and increased the apoptosis of C6
glioma cells in a dose-dependent pattern, indicating the ability of
endogenous mature BDNF to promote cell proliferation and survival.
BDNF/TrkB signalling is a key pathway which regulates infiltration
of malignant tumours (30).
High-grade glioma is notorious for its invasiveness and rapid
penetration to normal tissues (31). Our results also indicate that mature
BDNF promotes C6 cell infiltration and migration in a
dose-dependent manner. These findings suggest that mature BDNF
contributes to glioma growth and migration and may be a potential
prognostic marker in glioma. Suppression of the mature BDNF/TrkB
signalling pathway by either mature BDNF neutralising antibodies or
TrkB-receptor antibodies may have therapeutic significance in
high-grade glioma.
The ratio of proBDNF to mature BDNF levels was found
to be decreased in high grade glioma tissues and was negatively
correlated with tumour grade (13).
In the present study, mature BDNF promoted glioma cell growth in
vitro. Therefore, it is likely that increased levels of mature
BDNF contribute mainly to tumourigenesis, as observed in previous
reports. Both proBDNF and mature BDNF are present in gliomas. These
findings suggest that the proBDNF-p75-sortilin pathway may be a
balancing signal to tumour growth by the mature BDNF-TrkB pathway.
The balance between tumour cell survival and death could depend
upon the proportions of mature and proBDNF available to cells
expressing TrkB and p75NTR.
Given the opposing functions of proBDNF and mature
BDNF in glioma, it would be of great interest to study the precise
mechanisms controlling the relative expression levels of proBDNF
and mature BDNF to limit or amplify distinct neurotrophin
activities in the development of glioma.
In conclusion, using C6 cells as a model, we
demonstrated that mature BDNF induces tumour cell proliferation and
invasion in vitro. Our observations provide the framework
for novel therapeutic strategies through targeting mature BDNF/TrkB
signalling cascades in glioma.
Acknowledgements
The present study was supported by grants to X.F.Z.
from the Chinese MST 2011CB944200 and Australian NHMRC (595937);
and to Z.C.X. from the Talent Program, Yunnan, China and the Monash
Professorial Fellowship. We wish to thank Ms Kate Rees from UniSA
for her critical reading of the manuscript.
References
|
1
|
Barde YA: Trophic factors and neuronal
survival. Neuron. 2:1525–1534. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bartkowska K, Turlejski K and Djavadian
RL: Neurotrophins and their receptors in early development of the
mammalian nervous system. Acta Neurobiol Exp. 70:454–467.
2010.PubMed/NCBI
|
|
3
|
Zhou XF, Song XY, Zhong JH, Barati S, Zhou
FH and Johnson SM: Distribution and localization of
pro-brain-derived neurotrophic factor-like immunoreactivity in the
peripheral and central nervous system of the adult rat. J
Neurochem. 91:704–715. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Seidah NG and Chretien M: Proprotein and
prohormone convertases: a family of subtilases generating diverse
bioactive polypeptides. Brain Res. 848:45–62. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barker PA: Whither proBDNF? Nat Neurosci.
12:105–106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Teng HK, Teng KK, Lee R, Wright S, Tevar
S, Almeida RD, Kermani P, Torkin R, Chen ZY, Lee FS, Kraemer RT,
Nykjaer A and Hempstead BL: ProBDNF induces neuronal apoptosis via
activation of a receptor complex of p75NTR and sortilin. J
Neurosci. 25:5455–5463. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kenchappa RS, Zampieri N, Chao MV, Barker
PA, Teng HK, Hempstead BL and Carter BD: Ligand-dependent cleavage
of the P75 neurotrophin receptor is necessary for NRIF nuclear
translocation and apoptosis in sympathetic neurons. Neuron.
50:219–232. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fan YJ, Wu LL, Li HY, Wang YJ and Zhou XF:
Differential effects of pro-BDNF on sensory neurons after sciatic
nerve transection in neonatal rats. Eur J Neurosci. 27:2380–2390.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Desmet CJ and Peeper DS: The neurotrophic
receptor TrkB: a drug target in anti-cancer therapy? Cell Mol Life
Sci. 63:755–759. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hamel W, Westphal M, Szonyi E, Escandon E
and Nikolics K: Neurotrophin gene expression by cell lines derived
from human gliomas. J Neurosci Res. 34:147–157. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Assimakopoulou M, Kondyli M, Gatzounis G,
Maraziotis T and Varakis J: Neurotrophin receptors expression and
JNK pathway activation in human astrocytomas. BMC Cancer.
7:2022007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aronica E, Leenstra S, Jansen GH, van
Veelen CW, Yankaya B and Troost D: Expression of brain-derived
neurotrophic factor and tyrosine kinase B receptor proteins in
glioneuronal tumors from patients with intractable epilepsy:
colocalization with N-methyl-D-aspartic acid receptor. Acta
Neuropathol. 101:383–392. 2001.
|
|
13
|
Xiong J, Zhou L, Yang M, Lim Y, Zhu YH, Fu
DL, Li ZW, Zhong JH, Xiao ZC and Zhou XF: ProBDNF and its receptors
are upregulated in glioma and inhibit the growth of glioma cells in
vitro. Neuro Oncol. 15:990–1007. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Akil H, Perraud A, Melin C, Jauberteau MO
and Mathonnet M: Fine-tuning roles of endogenous brain-derived
neurotrophic factor, TrkB and sortilin in colorectal cancer cell
survival. PloS One. 6:e250972011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kelly KJ, Sandoval RM, Dunn KW, Molitoris
BA and Dagher PC: A novel method to determine specificity and
sensitivity of the TUNEL reaction in the quantitation of apoptosis.
Am J Physiol Cell Physiol. 284:C1309–C1318. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu ZQ, Sun Y, Li HY, Lim Y, Zhong JH and
Zhou XF: Endogenous proBDNF is a negative regulator of migration of
cerebellar granule cells in neonatal mice. Eur J Neurosci.
33:1376–1384. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Woo NH, Teng HK, Siao CJ, Chiaruttini C,
Pang PT, Milner TA, Hempstead BL and Lu B: Activation of p75NTR by
proBDNF facilitates hippocampal long-term depression. Nat Neurosci.
8:1069–1077. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu B: Pro-region of neurotrophins: role in
synaptic modulation. Neuron. 39:735–738. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu B, Pang PT and Woo NH: The yin and yang
of neurotrophin action. Nat Rev Neurosci. 6:603–614. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koshimizu H, Hazama S, Hara T, Ogura A and
Kojima M: Distinct signaling pathways of precursor BDNF and mature
BDNF in cultured cerebellar granule neurons. Neurosci Lett.
473:229–232. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun Y, Lim Y, Li F, Liu S, Lu JJ,
Haberberger R, Zhong JH and Zhou XF: ProBDNF collapses neurite
outgrowth of primary neurons by activating RhoA. PLoS One.
7:e358832012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang YT, Lai PC, Wu CC, Hsu SH, Cheng CC,
Lan YF and Chiu TH: BDNF mediated TrkB activation is a survival
signal for transitional cell carcinoma cells. Int J Oncol.
36:1469–1476. 2010.PubMed/NCBI
|
|
23
|
Lam CT, Yang ZF, Lau CK, Tam KH, Fan ST
and Poon RT: Brain-derived neurotrophic factor promotes
tumorigenesis via induction of neovascularization: implication in
hepatocellular carcinoma. Clin Cancer Res. 17:3123–3133. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pearse RN, Swendeman SL, Li Y, Rafii D and
Hempstead BL: A neurotrophin axis in myeloma: TrkB and BDNF promote
tumor-cell survival. Blood. 105:4429–4436. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thiele CJ, Li Z and McKee AE: On Trk - the
TrkB signal transduction pathway is an increasingly important
target in cancer biology. Clin Cancer Res. 15:5962–5967. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Haapasalo A, Sipola I, Larsson K, Akerman
KE, Stoilov P, Stamm S, Wong G and Castren E: Regulation of TRKB
surface expression by brain-derived neurotrophic factor and
truncated TRKB isoforms. J Biol Chem. 277:43160–43167. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Haapasalo A, Saarelainen T, Moshnyakov M,
Arumae U, Kiema TR, Saarma M, Wong G and Castren E: Expression of
the naturally occurring truncated trkB neurotrophin receptor
induces outgrowth of filopodia and processes in neuroblastoma
cells. Oncogene. 18:1285–1296. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Edsjo A, Lavenius E, Nilsson H, Hoehner
JC, Simonsson P, Culp LA, Martinsson T, Larsson C and Pahlman S:
Expression of trkB in human neuroblastoma in relation to MYCN
expression and retinoic acid treatment. Lab Invest. 83:813–823.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ho R, Eggert A, Hishiki T, Minturn JE,
Ikegaki N, Foster P, Camoratto AM, Evans AE and Brodeur GM:
Resistance to chemotherapy mediated by TrkB in neuroblastomas.
Cancer Res. 62:6462–6466. 2002.PubMed/NCBI
|
|
30
|
Han L, Zhang Z, Qin W and Sun W:
Neurotrophic receptor TrkB: is it a predictor of poor prognosis for
carcinoma patients? Med Hypotheses. 68:407–409. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Goebell E, Paustenbach S, Vaeterlein O,
Ding XQ, Heese O, Fiehler J, Kucinski T, Hagel C, Westphal M and
Zeumer H: Low-grade and anaplastic gliomas: differences in
architecture evaluated with diffusion-tensor MR imaging. Radiology.
239:217–222. 2006. View Article : Google Scholar : PubMed/NCBI
|