Introduction
Lung cancer is the leading cause of cancer-related
mortality worldwide with only a 15% overall 5-year patient survival
rate (1). It is estimated that more
than 1,600,000 new lung cancer cases are diagnosed each year.
Non-small cell lung cancer (NSCLC) which includes adenocarcinomas,
large cell carcinomas, and squamous cell carcinomas, contribute to
~85% of the total lung cancer cases (2). Although recent advances have been made
in clinical diagnosis and therapeutic treatment, the overall 5-year
mortality rate has remained unfavorable since the 1970s (3). Further investigation of the underling
mechanisms of NSCLC development and progression is thus important
for improving the diagnosis, prevention and therapy of this
disease.
microRNAs (miRNAs) are a class of endogenous
noncoding RNAs which suppress gene expression through base pairing
with the 3′-untranslated region (3′-UTR) of target mRNAs, leading
to translational repression or mRNA decay (4). Emerging evidence has revealed that
aberrant expression of miRNAs may induce alterations in a variety
of biological processes, including proliferation, differentiation
and migration. Numerous deregulated miRNAs, such as miR-21,
miR-29b, miR-100, miR-155, miR-138, miR-449c and miR-847, have been
shown to regulate NSCLC cell proliferation, migration and/or
invasion (5–10). These findings indicate that
dysfunctions of miRNAs may be associated with NSCLC. Recent studies
have identified a series of miRNAs that are deregulated in NSCLC,
including miR-205 (11). It was
shown that ectopic expression of miR-205 in endometrial cancer
promoted tumor proliferation and invasion (12). Xie et al reported that
miR-205 overexpression increased cell proliferation and promoted
migration of human cervical cancer cells (13). These data suggest a potential tumor
promotive function of miR-205. However, the role and the molecular
mechanisms of miR-205 in NSCLC remain poorly understood.
In the present study, we confirmed that upregulation
of miR-205 was frequently found in NSCLC cell lines and tissue
samples. Furthermore, downregulation of miR-205 by an miR-205
inhibitor markedly inhibited the migration, invasion and reversed
the chemoresistance of NSCLC cells partially through targeting the
tumor-suppressor gene, phosphatase and tensin homolog (PTEN). Our
findings suggest a new therapeutic strategy for NSCLC.
Materials and methods
Cell culture and tissue samples
NSCLC cell lines A549, SPC-A1, SK-MES-1, and the
normal human bronchial epithelial cell line 16HBE were obtained
from the Institute of Biochemistry and Cell Biology (Shanghai,
China) and were maintained in RPMI-1640 containing 10% fetal bovine
serum, 100 μg/ml streptomycin, and 100 IU/ml penicillin at 37°C
with 5% CO2. Twenty-eight human NSCLC tissue samples and
corresponding non-tumor tissues were collected from Xiangyang
Central Hospital. Tissues were snap-frozen in liquid nitrogen
immediately after surgery and stored at 80°C. Informed consent was
obtained from all patients prior to surgery. This study was
approved by the Xiangyang Central Hospital Ethics Committee.
Constructs and transfection
miR-205 mimics (catalog# miR10000266-1-2), miR-205
inhibitor (catalog# miR10000266-1-2) and the corresponding controls
were purchased from RiboBio Co., Ltd. (Guangzhou, China). For the
luciferase activity assay, the wild-type (WT) or mutated (Mut)
human PTEN 3′-UTR sequences containing the potential binding sites
(nucleotides 759–765 of PTEN 3′-UTR) were amplified and inserted
into the psiCHECK-2 vector (Promega, Beijing, China) between the
XhoI and NotI restriction sites using the following
primers: sense, 5′-CTCGAGCCGCTGTCACTGCTTGT-3′ and antisense,
5′-GCGGCCGCAGGCAGCACATGAAGCA-3′. Mutation was performed using a
fast mutation kit from Agilent (Santa Clara, CA, USA). The human
PTEN overexpression construct was generated by amplifying the PTEN
sequences using the following primers: sense,
5′-GGATTCCCACAGGCTCCCAGACA-3′ and antisense,
5′-GAATTCTTGCCACAAGTGCAAAGG-3′, and were inserted into pcDNA3.0
between the BamHI and EcoRI restriction sites. PTEN
shRNA was designed and generated by GeneChem (Shanghi, China).
The transfection was performed using Lipofectamine
2000 (Invitrogen, Calsbad, CA, USA) according to the manufacturer’s
instructions. The transfected cells were cultured in regular
culture medium for 48–72 h before analysis.
Quantitative real-time PCR
RNA containing small RNA was isolated from the cell
lines with a microRNA extraction kit and reverse transcribed using
a reverse transcription kit (Tiangen, Beijing, China), and
subjected to SYBR Green real-time PCR (Takara, Dalian, China).
Reactions were performed using StepOnePlus instrument in
triplicate. Primers for miR-205 and U6 were purchased from
GeneCopoeia, Inc. (Rockville, MD, USA). Expression level of miR-205
was normalized to U6. Total RNA was extracted with TRIzol
(Invitrogen) and reversely transcribed into cDNA using the reverse
transcription kit. Expression of PTEN was normalized with GAPDH.
PTEN primers included sense, 5′-CCAGGACCAGAGGAAACCT-3′ and
antisense, 5′-GCTAGCCTCTGGATTTGA-3′. GAPDH primers were sense,
5′-CATCTTCTTTTGCGTCGCC-3′ and antisense,
5′-AAAAGCAGCCCTGGTGAC-3′.
MTT assay
Cell viability was examined by MTT assay. Briefly,
the transfected cells were plated into 96-well plates. After 12 h
of incubation, cells were treated with different concentrations of
chemotherapeutic drugs. Following 24 h of incubation, cell growth
was determined after addition of 0.5 mg/ml MTT solution (Sigma,
USA). After 4 h, the culture medium was replaced with 100 μl DMSO
and vortexed for 10 min. Absorbance at 490 nm was recorded using a
microplate reader (Bio-Rad, USA).
Flow cytometric analysis of
apoptosis
The level of apoptosis was determined by flow
cytometry. Cells were collected, washed with cold PBS twice, fixed
in ice cold 70% ethanol. Cells were then stained with FITC-Annexin
V and propidium iodide (PI), and analyzed by Cell Quest software
(Becton Dickinson, Franklin Lakes, NJ, USA). The percentage of
apoptotic cells was calculated.
In vitro migration and invasion
assays
For in vitro migration assays, 24 h after
transfection, cells in serum-free medium were seeded into the upper
chamber of an insert (8-μm pore size). For in vitro invasion
assays, cells in serum-free media were seeded into the upper
chamber coated with Matrigel (Sigma). Medium containing 10% FBS was
added to the lower chamber. After 24 h of incubation, cells
remaining on the upper chamber were removed with scraping, whereas
cells that migrated or invaded into the lower chamber were stained
with 0.05% crystal violet, imaged and counted under a microscope
(Olympus, Tokyo, Japan).
Luciferase assay
A549 cells were co-transfected with a mixture
including 0.02 μg of wild-type or mutated PTEN 3′-UTR and 0.08 μg
of miR205 mimic. Renilla luciferase or the control mimic was
used as the negative control. Luciferase activities were analyzed
using the Dual-Luciferase reporter assay system (Promega, Madison,
WI, USA).
Statistical analysis
Statistical analysis was performed using SPSS 16.0
software. Data are presented as means ± SD. Statistical analyses
were carried out by one-way ANOVA or the Student’s t-test.
P<0.05 was considered to indicate a statistically significant
result.
Results
miR-205 is increased in the NSCLC cell
lines and tissue samples
To investigate the biological role of miR-205 in
NSCLC, quantitative real-time PCR was initially performed to
measure miR-205 expression levels in three NSCLC cell lines and 28
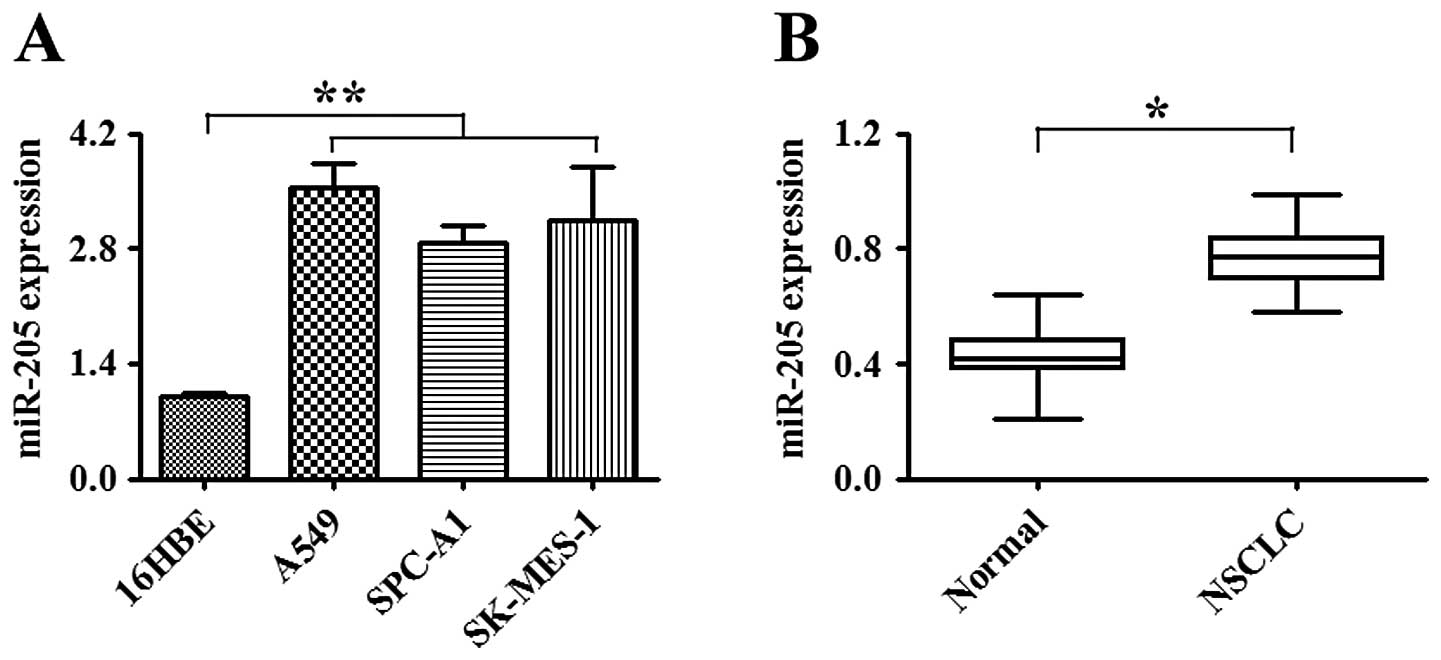
NSCLC tissue samples. As shown in Fig.
1A, the expression of miR-205 in NSCLC cell lines was
significantly increased when compared to that in the normal human
bronchial epithelial cell line (16HBE) (P<0.01). The expression
of miR-205 was also substantially elevated in NSCLC tissues when
compared to that in the corresponding non-tumor tissues (P<0.05,
Fig. 1B). These results suggest
that the upregulation of miR-205 contributes to NSCLC
carcinogenesis.
miR-205 promotes the growth of the NSCLC
cell lines
To study the effect of miR-205 on the growth of
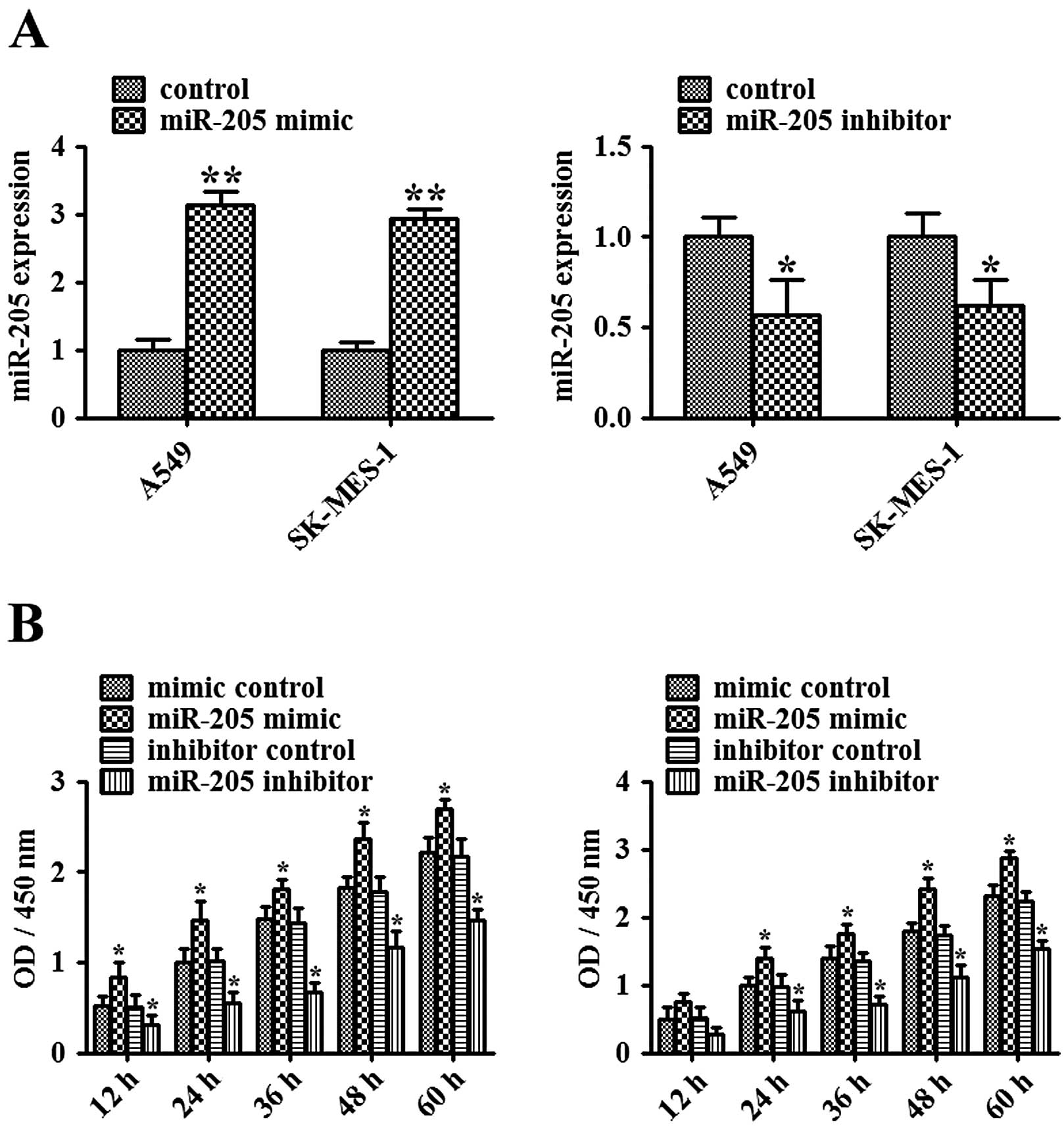
NSCLC cells, we initially examined the effect of miR-205
mimic/inhibitor on miR-205 expression. miR-205 mimics or the
inhibitor was transiently transfected into A549 and SK-MES-1 cells.
After 48 h of transfection, expression of miR-205 was determined by
quantitative real-time PCR (Fig.
2A). Compared with the mimic control-transfected A549 or
SK-MES-1 cells, expression of miR-205 in the miR-205
mimic-transfected cells was markedly increased (P<0.01).
Compared with the inhibitor control-transfected A549 or SK-MES-1
cells, expression of miR-205 in the miR-205 inhibitor-transfected
cells was significantly decreased (P<0.05). Next, MTT assay was
used to examine the effect of miR-205 on the growth of NSCLC cells.
As shown in Fig. 2B, miR-205
mimic-mediated elevation of miR-205 significantly promoted the
growth of NSCLC cells, while miR-205 inhibitor-mediated suppression
of miR-205 substantially inhibited the growth of NSCLC cells
(P<0.05). These data imply that miR-205 acts as an oncogene in
human NSCLC.
miR-205 promotes the migration and
invasion of the NSCLC cell lines
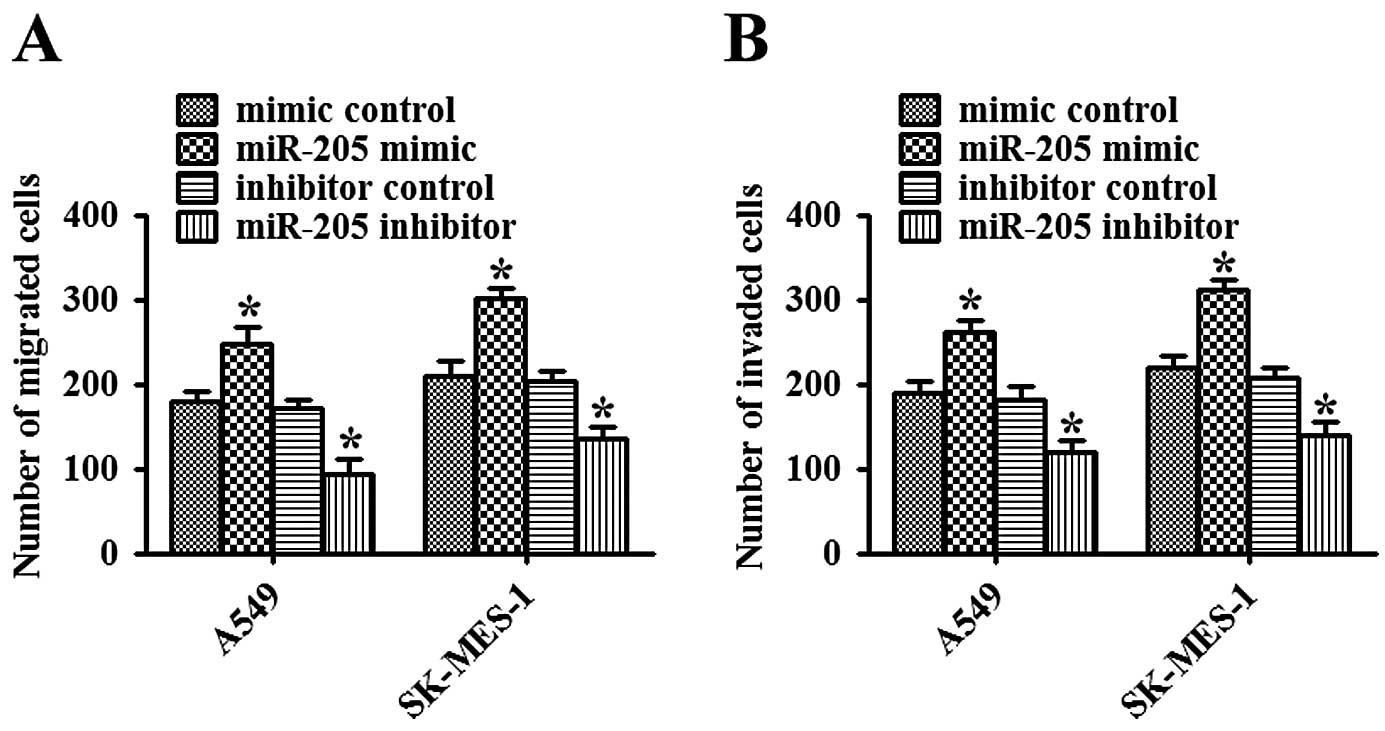
Cell invasion is an important aspect of cancer
progression, and involves the migration and dissolution of
extracellular matrix proteins of cancer cells into contiguous
tissues. To investigate whether miR-205 has a direct functional
role in promoting NSCLC cell migration and invasion, we assessed
cancer cell invasion by Matrigel and migration through a transwell.
As shown in Fig. 3A, overexpression
of miR-205 promoted the migration of NSCLC cells, while inhibition
of miR-205 significantly impeded the migration of NSCLC cells
(P<0.05). Consistent with the results of the migration assay,
upregulation of miR-205 promoted invasion, while downregulation of
miR-205 inhibited invasion of NSCLC cells (P<0.05, Fig. 3B). Therefore, these data suggest
that miR-205 facilitates both migration and invasion of NSCLC
cells.
miR-205 promotes the chemoresistance of
NSCLC cells
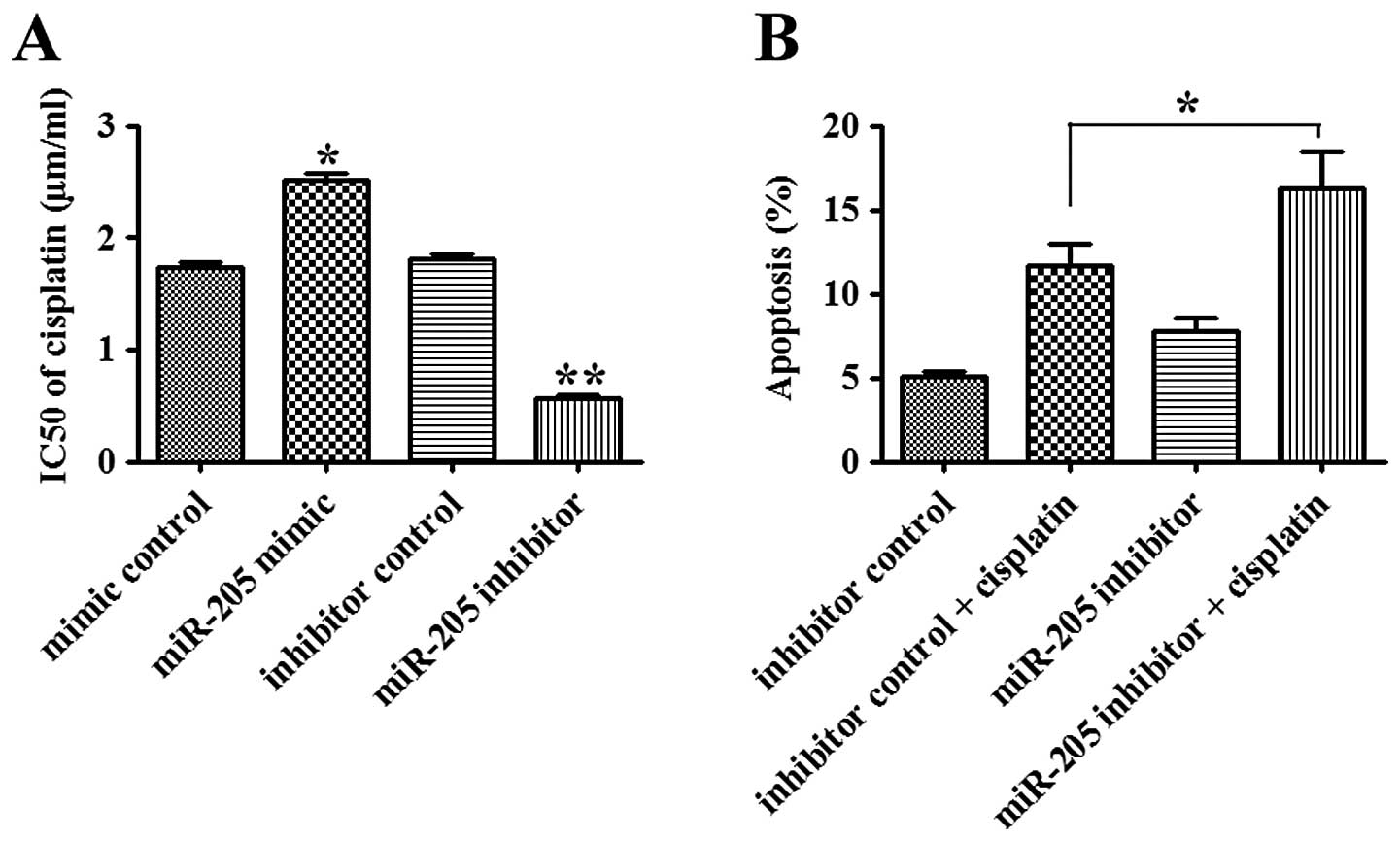
The effect of miR-205 on the sensitivity of NSCLC
cells to chemotherapeutic agent, cisplatin, was investigated.
Overexpression of miR-205 led to a significant increase in the
IC50 value of cisplatin (1.50 μg/ml) in A549 cells when
compared with that in the control group (P<0.05). In contrast,
inhibition of miR-205 decreased the IC50 value of
cisplatin in the A549 cells when compared with that in the control
group (P<0.01, Fig. 4A). When
combined with cisplatin treatment, the apoptosis of A549 cells
transfected with the miR-205 inhibitor was markedly enhanced when
compared with that in the inhibitor control-transfected cells
(P<0.05; Fig. 4B). Our data
suggest that miR-205 promotes chemoresistance of NSCLC cells via
inhibition of apoptosis.
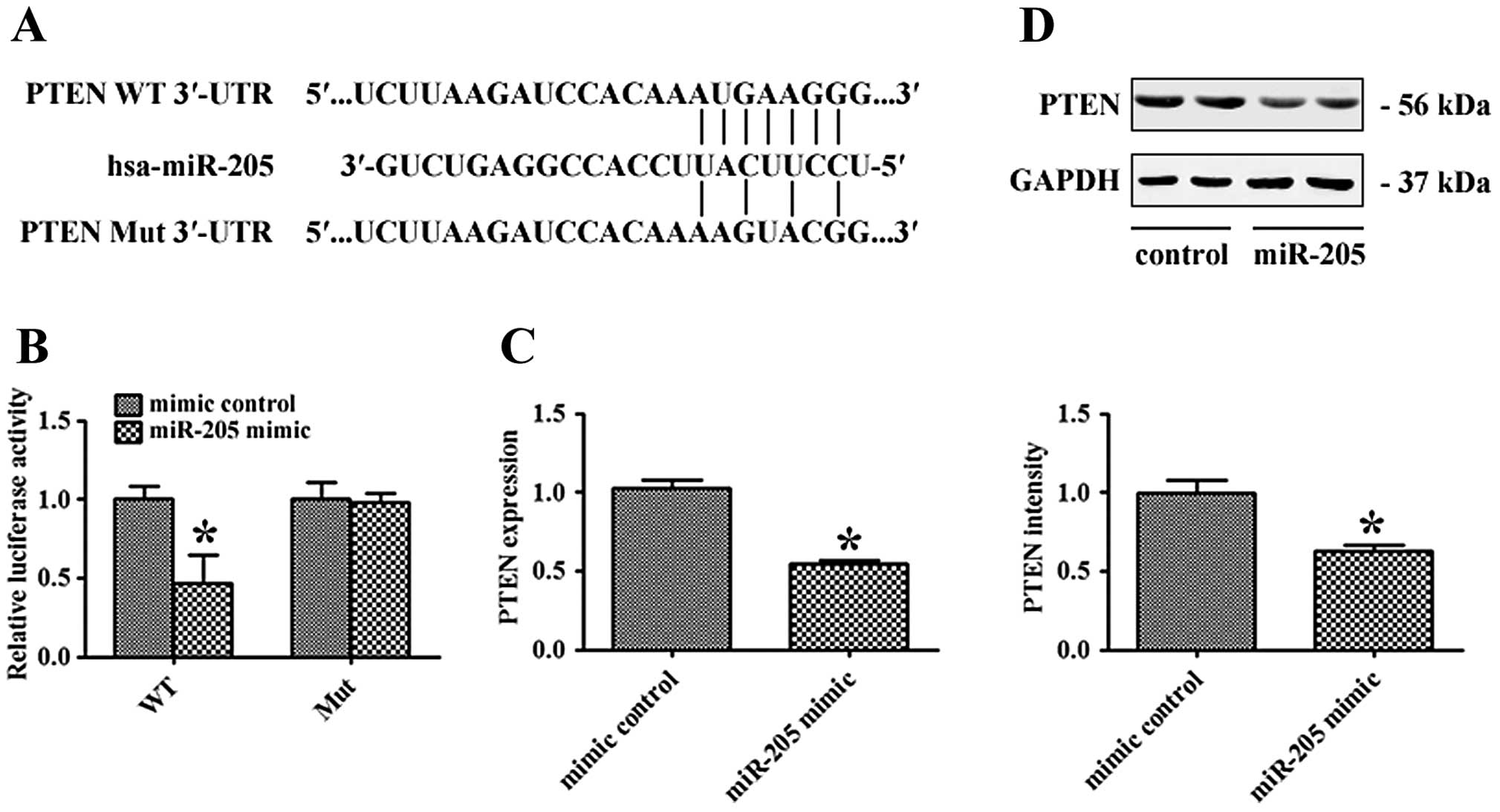
PTEN is a direct target of miR-205
To explore the molecular mechanism of miR-205, we
adopted bioinformatic algorithm, TargetScan 6.2, to predict many
potential miR-205 target genes. Among them, PTEN was found to have
a putative miR-205 binding site within its 3′-UTR. To verify
whether PTEN is a direct target of miR-205, WT or Mut 3′-UTR of
PTEN was inserted into the downstream of the firefly luciferase
gene (Fig. 5A). Then, miR-205 mimic
or the mimic control-transfected A549 cells were co-transfected
with WT or Mut 3′-UTR plasmids. As shown in Fig. 5B, miR-205 mimic decreased WT 3′-UTR
luciferase activity (P<0.05), while it had no significant effect
on Mut 3′-UTR luciferase activity. In addition, quantitative
real-time PCR and western blot analyses showed that miR-205 mimic
significantly decreased PTEN mRNA and protein levels in A549 cells
(P<0.05; Fig. 5C and D). These
results indicate that PTEN is a direct target of miR-205 in NSCLC
cells.
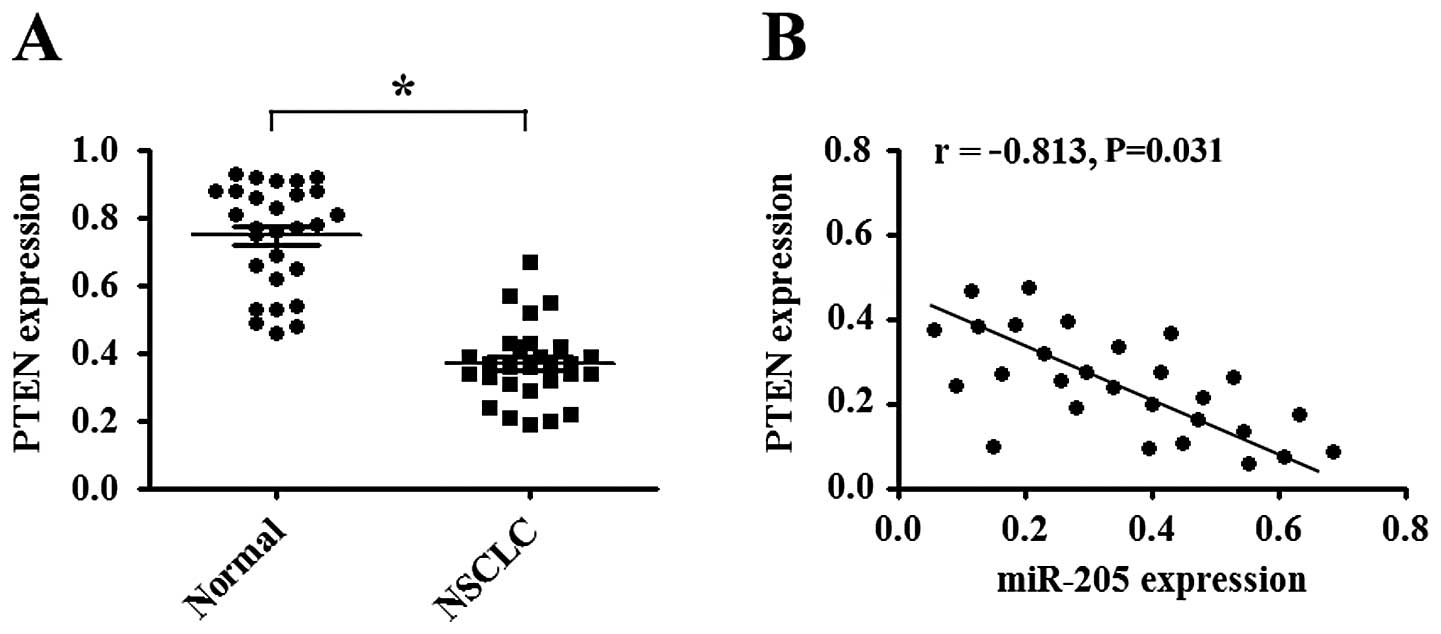
miR-205 is inversely correlated with PTEN
expression
Quantitative real-time PCR was performed to detect
the expression of PTEN in 28 NSCLC tissue samples and the
corresponding normal tissues. Data showed that the average level of
PTEN mRNA was significantly decreased in NSCLC tissues when
compared with that in the corresponding normal tissues (P<0.05;
Fig. 6A). Moreover, we correlated
PTEN with miR-205 expression in the same NSCLC tissues and found
that PTEN mRNA level was inversely correlated with miR-205 level
(P<0.05; Fig. 6B).
Discussion
Recently, numerous studies have focused on
investigating the aberrant expression of miRNAs and their potential
roles in tumor development and malignant transformation (14). miRNA expression profiles appear to
be essential in cancer diagnosis, and strategies to modulate miRNA
expression and function are considered to offer new opportunities
for cancer therapy (15). In the
present study, we demonstrated that overexpression of miR-205
promoted the growth, metastasis and chemoresistance of NSCLC cells
partially by targeting PTEN, a tumor suppressor.
miR-205 was reported to be elevated in many types of
cancer including NSCLC (11,16).
It was found that miR-205 promoted tumor proliferation and invasion
in endometrial cancer (12). Xie
et al reported that miR-205 overexpression increased cell
proliferation and promoted migration of human cervical cancer cells
(13). In the present study, we
initially demonstrated that the expression of miR-205 in NSCLC cell
lines was much higher than that in a normal human bronchial
epithelial cell line. Similarly, the expression of miR-205 was
significantly elevated in NSCLC tissues when compared with that in
the corresponding normal tissues. These data imply that the
overexpression of miR-205 may play an essential role in NSCLC
tumorigenesis. We found that inhibition of miR-205 significantly
suppressed the growth of NSCLC cells. Herein, we also found that
inhibition of miR-205 markedly inhibited the migration and invasion
of NSCLC cells while overexpression of miR-205 promoted these
capacities in NSCLC cells. Acquisition of resistance to
chemotherapy or radiotherapy is a significant problem in cancer
treatment, increasing the morbidity and mortality of patients.
Thus, we next investigated whether miR-205 affects the
chemosensitivity of NSCLC cells. Our data showed that inhibition of
miR-205 significantly enhanced the sensitivity of NSCLC cells to
the chemotherapeutic agent, cisplatin.
Wang et al found that miR-205 induced the
radioresistance of human nasopharyngeal carcinoma by targeting the
PTEN-Akt pathway (17). We also
observed an inverse correlation between miR-205 and PTEN expression
in NSCLC tissues. Several studies found that miR-205 acts as a
tumor suppressor in several cancer types. For instance, Wu et
al showed that ectopic expression of miR-205 suppressed
proliferation and anchorage of breast cancer cell lines, MCF-7 and
MDA-MB-231, by targeting vascular endothelial growth factor A
(18). Boll et al found that
miR-130a, miR-203 and miR-205 jointly impeded the growth of
prostate cancer cells by induction of apoptosis and cell cycle
arrest (19). These observations of
increased or decreased miR-205 expression in different cancer types
suggest that miR-205 may have different functions in different
cancer types depending on the specific tumor context and target
genes accompanied by other miRNAs deregulated as well (20).
PTEN, first identified as a tumor suppressor, is an
important regulator of proliferation, differentiation and apoptosis
(21). PTEN has been reported to be
decreased in a wide variety of cancers including NSCLC (22,23).
Qu et al(24) reported that
miR-205 contributed to the radioresistance of human nasopharyngeal
carcinoma by targeting PTEN. Moreover, Karaayvaz et
al(25) observed that miR-205
was negatively correlated with PTEN expression in endometrial
cancer, and may act as a prognostic biomarker of endometrial
cancer. To the best of our knowledge, there is no report concerning
the associations of miR-205 and PTEN with the phenotypes of NSCLC
cells, particularly chemoresistance. In the present study, we
demonstrated that overexpression of miR-205 decreases both the mRNA
and protein levels of PTEN in NSCLC cells, consistent with previous
studies in other cancer types. These data suggest that miR-205 acts
as an oncogene by suppressing PTEN expression in NSCLC.
In conclusion, the present study demonstrated the
biological functions of miR-205, and its ability to promote growth,
migration, invasion and chemoresistance of NSCLC cells by targeting
PTEN. This finding suggests that inhibition of miR-205 may be a
useful therapeutic strategy for NSCLC treatment.
Acknowledgements
This work was supported by the Natural Science
Foundation of Hubei Province of China (grant no. 2011CDA065).
References
|
1
|
Travis WD, Brambilla E, Noguchi M, et al:
International Association for the Study of Lung Cancer/American
Thoracic Society/European Respiratory Society international
multidisciplinary classification of lung adenocarcinoma. J Thorac
Oncol. 6:244–285. 2011. View Article : Google Scholar
|
|
2
|
Jemal A, Ma J, Rosenberg PS, Siegel R and
Anderson WF: Increasing lung cancer death rates among young women
in southern and midwestern States. J Clin Oncol. 30:2739–2744.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marshall E: Cancer research and the $90
billion metaphor. Science. 331:1540–1541. 2011.
|
|
4
|
Boeri M, Pastorino U and Sozzi G: Role of
microRNAs in lung cancer: microRNA signatures in cancer prognosis.
Cancer J. 18:268–274. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu Y, Crawford M, Mao Y, et al:
Therapeutic delivery of microRNA-29b by cationic lipoplexes for
lung cancer. Mol Ther Nucleic Acids. 2:e842013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kesanakurti D, Maddirela DR, Chittivelu S,
Rao JS and Chetty C: Suppression of tumor cell invasiveness and in
vivo tumor growth by microRNA-874 in non-small cell lung cancer.
Biochem Biophys Res Commun. 434:627–633. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miao LJ, Huang SF, Sun ZT, et al: MiR-449c
targets c-Myc and inhibits NSCLC cell progression. FEBS Lett.
587:1359–1365. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang H, Zhao M, Lv Z, et al: MiR-138
inhibits tumor growth through repression of EZH2 in non-small cell
lung cancer. Cell Physiol Biochem. 31:56–65. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu J, Lu KH, Liu ZL, Sun M, De W and Wang
ZX: MicroRNA-100 is a potential molecular marker of non-small cell
lung cancer and functions as a tumor suppressor by targeting
polo-like kinase 1. BMC Cancer. 12:5192012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang M, Shen H, Qiu C, et al: High
expression of miR-21 and miR-155 predicts recurrence and
unfavourable survival in non-small cell lung cancer. Eur J Cancer.
49:604–615. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mallick R, Patnaik SK and Yendamuri S:
MicroRNAs and lung cancer: biology and applications in diagnosis
and prognosis. J Carcinog. 9:2010. View Article : Google Scholar
|
|
12
|
Su N, Qiu H, Chen Y, Yang T, Yan Q and Wan
X: miR-205 promotes tumor proliferation and invasion through
targeting ESRRG in endometrial carcinoma. Oncol Rep. 29:2297–2302.
2013.PubMed/NCBI
|
|
13
|
Xie H, Zhao Y, Caramuta S, Larsson C and
Lui WO: miR-205 expression promotes cell proliferation and
migration of human cervical cancer cells. PLoS One. 7:e469902012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Du L and Pertsemlidis A: microRNA
regulation of cell viability and drug sensitivity in lung cancer.
Expert Opin Biol Ther. 12:1221–1239. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nana-Sinkam SP and Croce CM: Clinical
applications for microRNAs in cancer. Clin Pharmacol Ther.
93:98–104. 2013. View Article : Google Scholar
|
|
16
|
Vosa U, Vooder T, Kolde R, Vilo J,
Metspalu A and Annilo T: Meta-analysis of microRNA expression in
lung cancer. Int J Cancer. 132:2884–2893. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang D, Wang S, Liu Q, Wang M, Wang C and
Yang H: SZ-685C exhibits potent anticancer activity in both
radiosensitive and radioresistant NPC cells through the
miR-205-PTEN-Akt pathway. Oncol Rep. 29:2341–2347. 2013.PubMed/NCBI
|
|
18
|
Wu H, Zhu S and Mo YY: Suppression of cell
growth and invasion by miR-205 in breast cancer. Cell Res.
19:439–448. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boll K, Reiche K, Kasack K, et al:
MiR-130a, miR-203 and miR-205 jointly repress key oncogenic
pathways and are downregulated in prostate carcinoma. Oncogene.
32:277–285. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qin AY, Zhang XW, Liu L, et al: MiR-205 in
cancer: an angel or a devil? Eur J Cell Biol. 92:54–60. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamada KM and Araki M: Tumor suppressor
PTEN: modulator of cell signaling, growth, migration and apoptosis.
J Cell Sci. 114:2375–2382. 2001.PubMed/NCBI
|
|
22
|
Panagiotou I, Tsiambas E, Lazaris AC, et
al: PTEN expression in non small cell lung carcinoma based on
digitized image analysis. J BUON. 17:719–723. 2012.PubMed/NCBI
|
|
23
|
Li G, Zhao J, Peng X, Liang J, Deng X and
Chen Y: The mechanism involved in the loss of PTEN expression in
NSCLC tumor cells. Biochem Biophys Res Commun. 418:547–552. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qu C, Liang Z, Huang J, et al: MiR-205
determines the radioresistance of human nasopharyngeal carcinoma by
directly targeting PTEN. Cell Cycle. 11:785–796. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Karaayvaz M, Zhang C, Liang S, Shroyer KR
and Ju J: Prognostic significance of miR-205 in endometrial cancer.
PLoS One. 7:e351582012. View Article : Google Scholar : PubMed/NCBI
|