Introduction
Lung cancer, of which 80–85% of cases are non-small
cell lung cancer (NSCLC), is at present the leading cause of
cancer-related mortality in both men and women worldwide (1). Adequate therapeutic regimens are
largely dependent on the early diagnosis and surgery remains the
preferred treatment for early-stage patients (2). However, it has been reported that ~70%
patients have advanced local invasion and/or distant metastasis
when they are diagnosed and lose the opportunity for surgery. Thus,
cisplatin-based doublet chemotherapy has commonly been recommended
as the standard regimen for these advanced patients (3). However, the 5-year relative survival
rate in the past 30 years has remained at 11–17% for these lung
cancer patients (4), in which the
low efficacy of chemotherapy (20–30%) is the major cause. Hence,
understanding the mechanism underlying chemotherapy and
radiotherapy failure is of great importance.
Tumor stem cell theory has provided us new insight
into developing strategies for the treatment of malignancies.
Emerging evidence indicates that cancer stem cells (CSCs)
contribute to tumor initiation, maintenance, metastasis and drug
resistance (5–8). To date, CSCs have been identified in
several types of malignancies including leukemia (9), brain tumor (10), breast (11) and prostate cancer (12,13–15).
CSCs have been characterized in lung cancer by using a variety of
stem cell markers (13,16–18),
including CD133 (16). However, a
recent study demonstrated that both CD133+ and
CD133− cells contain CSCs in the lung cancer cell lines
A549 and H446 (19), indicating
that new markers with higher specificity and accuracy in
identifying lung CSCs should be further explored.
CD90 (Thy-1) is a 25–37 kDa
glycosylphosphatidylinositol (GPI)-anchored glycoprotein expressed
mainly in leukocytes and is involved in cell-cell and cell-matrix
interactions (20). CD90 expression
was identified in murine breast CSCs (21), primary high-grade glioma CSCs
(22), and in liver malignancy
(23). Our previous study (24) initially demonstrated that the A549
tumor sphere cells had a stronger capacity for proliferation and
self-renewal, and expressed higher levels of stem cell markers Sox2
and Oct4 than A549 adherent cells. In the present study, we carried
out flow cytometry analysis and other experiments to explore
whether CD90 could be a marker for identification of lung CSCs.
Materials and methods
Cell and tumor sphere culture
Human lung cancer cell lines A549 and H446 (Shanghai
Institute of Cell Biology, China) were used in this study. The
cells were cultured in RPMI-1640 medium (Gibco, USA) supplemented
with 10% fetal bovine serum (FBS) (Gibco). The tumor spheres were
cultured in serum-free conditioned medium that contained DMEM/F12
medium (Gibco), 20 μl/ml B27 supplement (Gibco), 20 ng/ml basic
fibroblast growth factor (FGF) and 20 ng/ml epidermal growth factor
(EGF) (both from PeproTech, USA). All cells were incubated at 37°C
with 5% CO2 and 100% humidity. The third-generation
sphere cells were used for further experiments.
Colony and sphere formation
Colony and sphere formation were used to compare the
proliferation capability between the CD90+ and
CD90− cells. Briefly, cells were resuspended in singular
form from both A549 and H446 cell lines and were seeded in 6-well
plates with different density: 200 cells/well in A549 cells and
1,000 cells/well in H446 cells, respectively. Two microliters
RPMI-1640 culture medium containing 10% FBS were added to each
well, and the cells were incubated for 2 weeks at 37°C with 5%
CO2 and 100% humidity. Then, the medium was aspirated
off, the wells were washed 3 times with phosphate-buffered saline
(PBS), fixed with 4% paraformaldehyde for 15 min. After staining
with Giemsa for 5 min, the number of colonies in each group was
calculated to compare the colony formation ability (each colony
should contain at least 50 cells).
The method of sphere formation was described above.
CD90+ and CD90− cells were firstly sorted by
fluorescence-activated cell sorting assay (FACS) and then cultured
in serum-free medium. One week later, the number of spheres (each
sphere containing at least 50 cells) in each group was calculated
under an inverted microscope (BX-40; Olympus, Japan).
FACS analysis
The cells and tumor spheres were dissociated as
single cell suspension, and washed by PBS and then labeled with
antibodies, including CD133 antibody (Miltenyi Biotec, Germany),
mouse anti-human CD90 (BD Biosciences, San Jose, CA, USA). The
cells were labeled with these antibodies at 10 μl/1×106
cells at 4°C in the dark for 30 min followed by washing with PBS,
and then the samples were acquired and analyzed by flow cytometry
(FACSAria II; BD Biosciences).
Scanning electron microscopy (SEM)
The cells or spheres were fixed using 2.5%
glutaraldehyde for 20 min and washed with PBS. Then, the samples
were immerged in ethanol to dehydrate and then tert-butyl
alcohol to displacement. The samples were mounted on aluminum stubs
with adhesive tabs and sputter coated with ~30 nm thickness of
gold. The samples were observed under SEM (S-3400N II; Hitachi,
Japan).
Immunofluorescent staining
Immunofluorescent staining was performed to compare
the expression of Sox2 and Oct4 in CD90+ and
CD90− cells from both A549 and H446 cell lines. The
CD90+ cells and CD90− cells were first
isolated by FACS followed by fixation in 4% paraformaldehyde for 10
min. The cells were then washed with PBS and permeabilized with
0.3% Triton X-100/PBS for 15 min at room temperature, and incubated
overnight at 4°C with mouse anti-Sox2 (Novus Biologicals, LLC) and
rabbit anti-Oct4 (Sigma, USA). Secondary antibodies used were
Cy3-conjugated goat anti-mouse IgG and Cy5-conjugated goat
anti-rabbit IgG (Beyotime, China) for 1 h at room temperature. The
cell nuclei were counterstained with Hoechst 33342. The cells were
visualized under a laser confocal microscope (SP-5; Leica
Microsystems, Germany).
RT-PCR
Total RNA was isolated by RNAiso reagent (Takara,
Japan). Reverse transcription and RT-PCR (Fermentas, USA) were used
to observe the expression of stem genes in all samples. β-actin
mRNA expression was taken as an internal control. The stem cell
genes, including Oct4 and Sox2, were: Oct4 forward,
5′-GCAGCGACTATGCACAACGA-3′ and reverse, 5′-CCAGAGTGGTGACGGAGACA-3′;
Sox2 forward, 5′-CATCACCCACAGCAAATGACA-3′ and reverse,
5′-GCTCCTACCGTACCACTAGAACTT-3′; β-actin forward,
5′-TCAAGATCATTGCTCCTCCTG-3′ and reverse,
5′-CTGCTTGCTGATCCACATCTG-3′. PCR was run for 30 cycles with 15
sec/95°C denaturation, 20 sec/58°C annealing and 20 sec/72°C
elongation. A melting curve analysis was performed after
amplification to confirm the accuracy of the amplification.
Xenografts
To explore the tumorigenicity of A549 adherent cells
and A549 sphere cells, theses 2 types of cells were injected into
the different sides of 4-week-old nude mice (Laboratory Animal
Center, The Third Hospital of Third Military Medical University) at
doses of 1×106, 1×105, 1×104 and
5×103 cells. After 8 weeks, the tumors were removed and
measured. The same method was applied to compare the tumorigenicity
of CD90+ and CD90− cells from A549 cell
lines. However, the doses of CD90+ cells or
CD90− cells injected into nude mice were
5×104, 1×104 and 5×103 cells.
Results
SEM reveals different features in A549
adherent and sphere cells
Tumor spheres formed in serum-free medium were
considered to be feasible and effective in the enrichment of CSCs
(25–27), which had also been used to enrich
lung CSCs in our previous study (24).
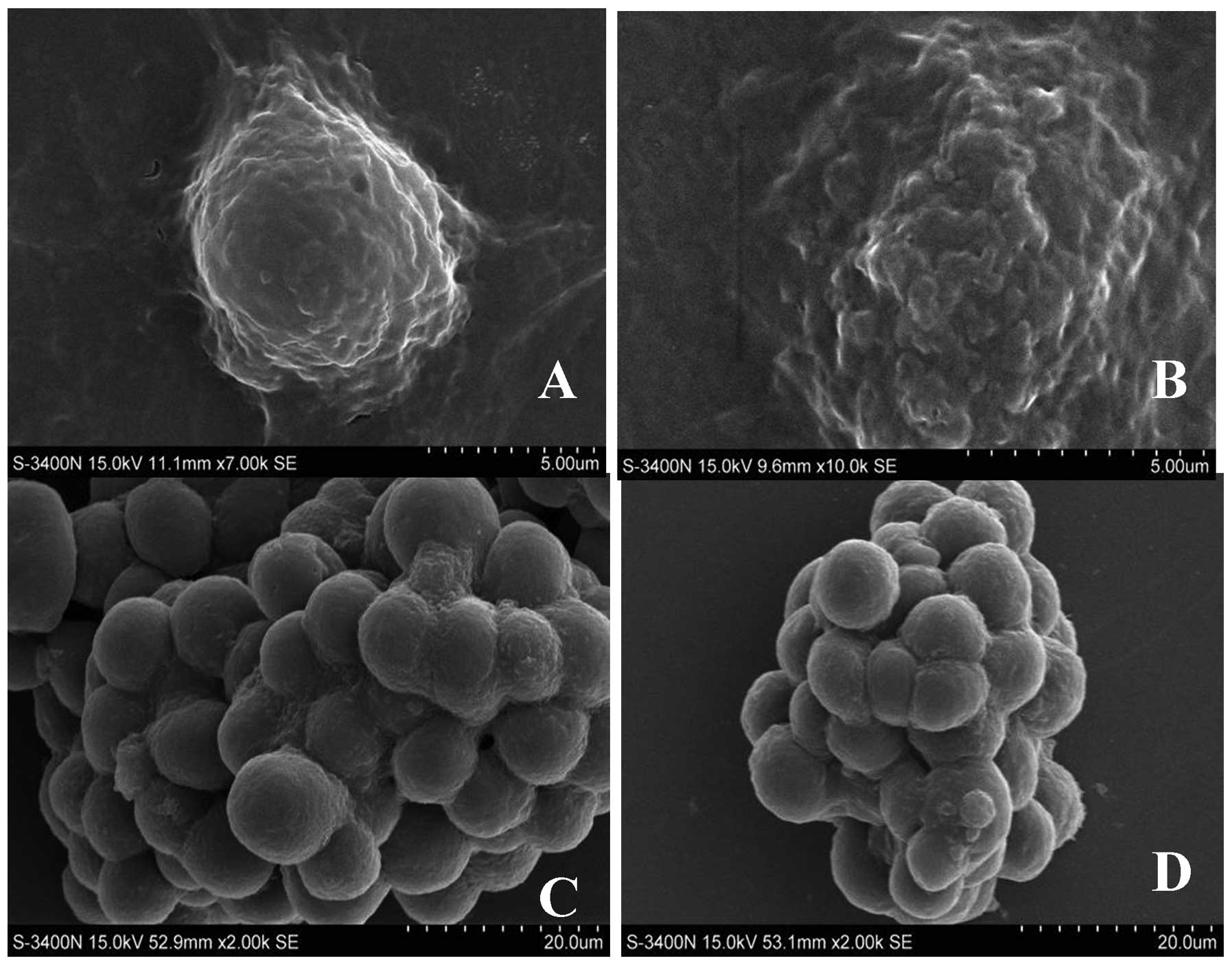
In the present study, SEM was used to directly
observe and compare the surface morphology of both A549 adherent
and sphere cells. As shown in Fig.
1, there were several protrusions on the surface of A549
adherent cells which also exhibited high proliferation ability
(Fig. 1A and B), while the surface
of A549 sphere cells was smooth (Fig.
1C and D), and most of these cells were undifferentiated.
Therefore, morphological features between these 2 types of cells
suggest that the sphere cells exhibit stem-cell
characteristics.
The percentages of CD90 are higher in
tumor sphere than adherent cells
Our previous study suggested that sphere cells
exhibited stem-cell characteristics, but the markers identifying
these lung CSCs needed to be further explored. It is highly
controversial whether CD133 is a molecular marker for lung CSCs,
and CD90 received increasing attention as a new CSC marker in
several types of malignancies. Thus, we speculated that CD90 may
present a new marker for lung CSCs.
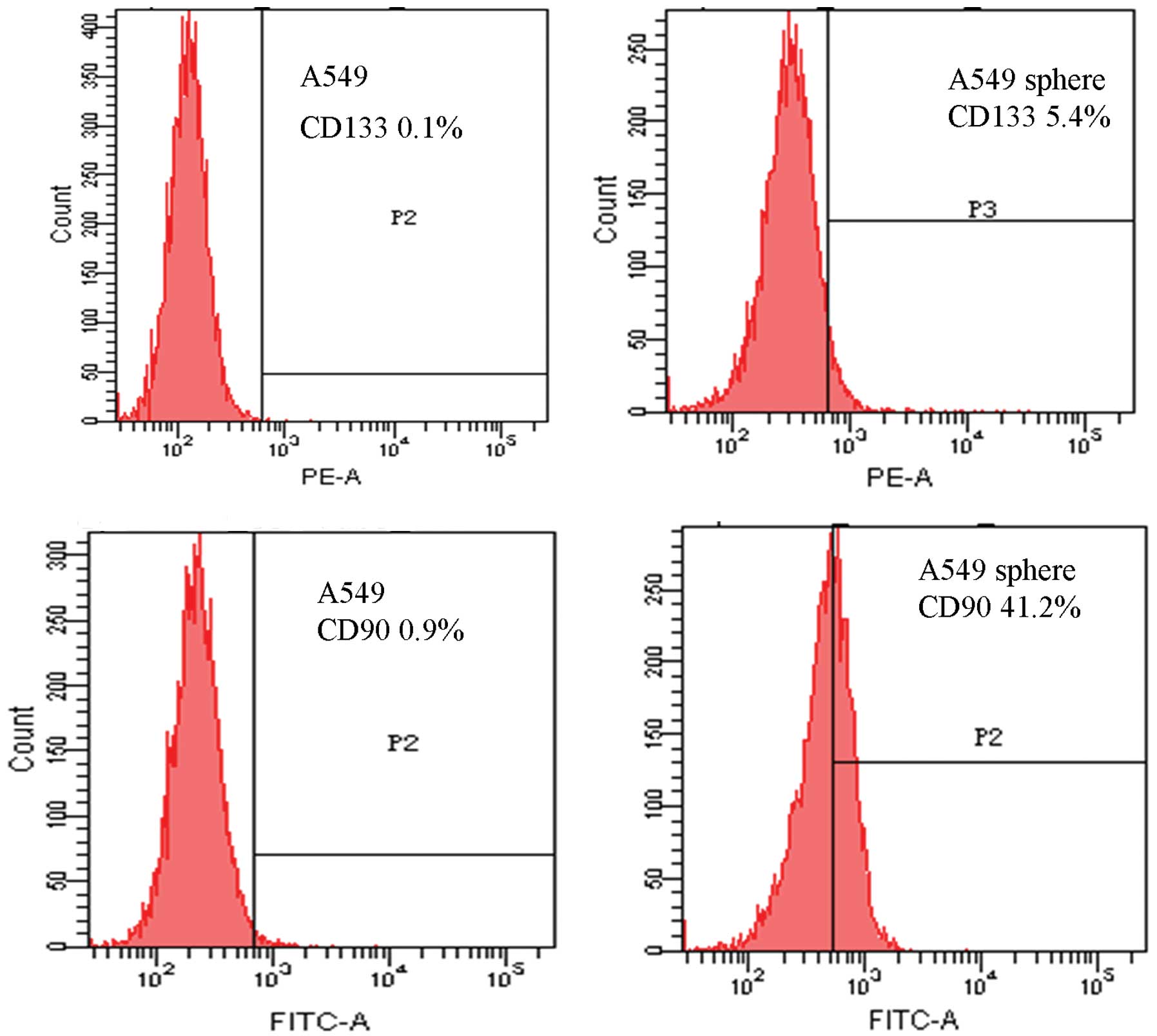
To verify this hypothesis, FACS assay was first
performed to detect the expression of CD133 and CD90 in both A549
adherent cells and A549 sphere cells. As shown in Fig. 2, the fractions of CD133+
cells in tumor spheres and adherent cells were 5.4 and 0.1%,
respectively (Fig. 2A and B).
Correspondingly, we also found that the percentages of
CD90+ cells were 41.2 and 0.9% in tumor spheres and
adherent cells, respectively (Fig. 2C
and D). As CD90 was highly expressed in A549 sphere cells, but
low in A549 adherent cells, a percentage considerably higher than
CD133, CD90 may present a potential marker for A549 sphere cells,
rich in lung CSCs.
The colony and sphere formation of
CD90+ and CD90− cells from A549 and H446 cell
lines
We previously tested the proliferation ability
between A549 adherent cells and A549 sphere cells using colony
formation and MTT assay, by which we showed the A549 sphere cells
have a stronger proliferation ability (P<0.05) (24). In the present study, the colony
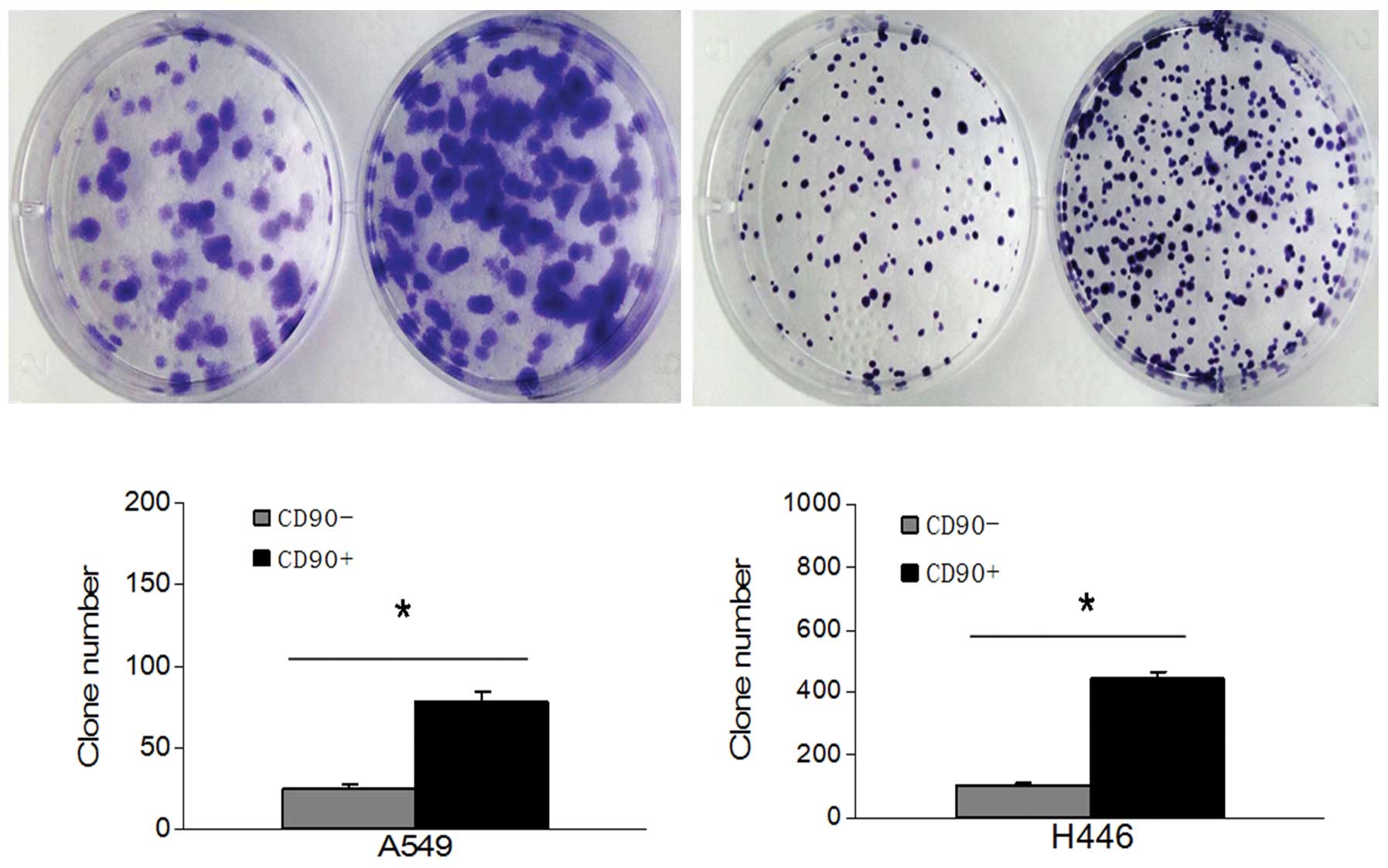
formation assay was used to detect the proliferation ability of
both CD90+ and CD90− cells isolated from both
A549 and H446 cell lines. The number of CD90+ cell
colonies was higher and the size was larger than CD90−
cells both in the A549 and the H446 cell line (P<0.05) (Fig. 3).
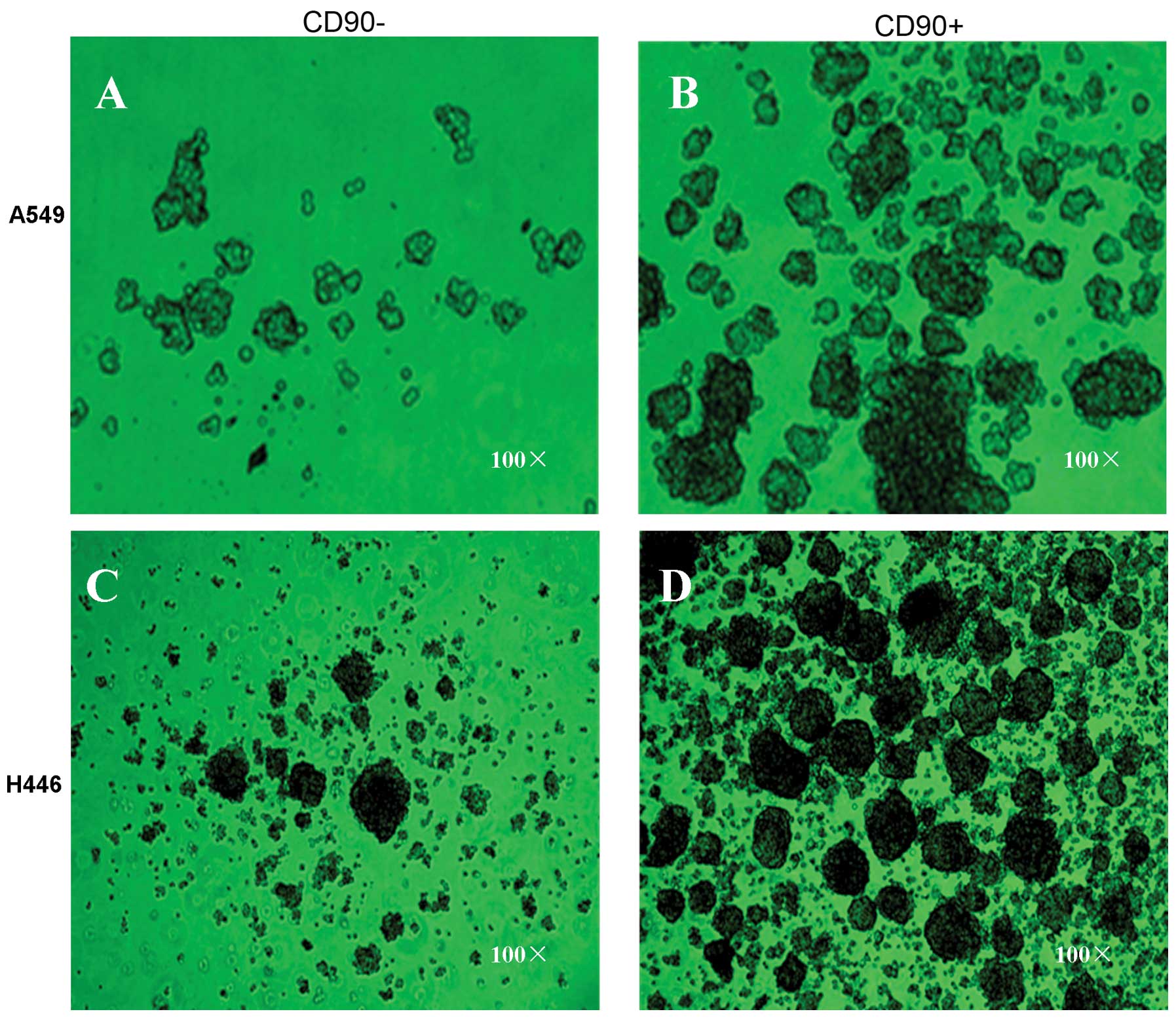
To further determine whether the CD90+
cells had stronger sphere formation capacity, both CD90+
and CD90− cells were firstly sorted from A549 and H446
cell lines with FACS assay and then cultured in the serum-free
conditioned medium. The results showed that the number and volume
of spheres formed by CD90+ cells were superior to those
formed by CD90− cells in both A549 and H446 cell lines.
It is worth mentioning that the cells in the spheres formed by
CD90+ cells clustered more closely with regular shape
than those in the spheres formed by CD90− cells
(Fig. 4). Thus, the cell spheres
formed by CD90− cells are inferior to those formed by
CD90+ cells both in quality and quantity, indicating
that CD90+ cells displayed stem-cell
characteristics.
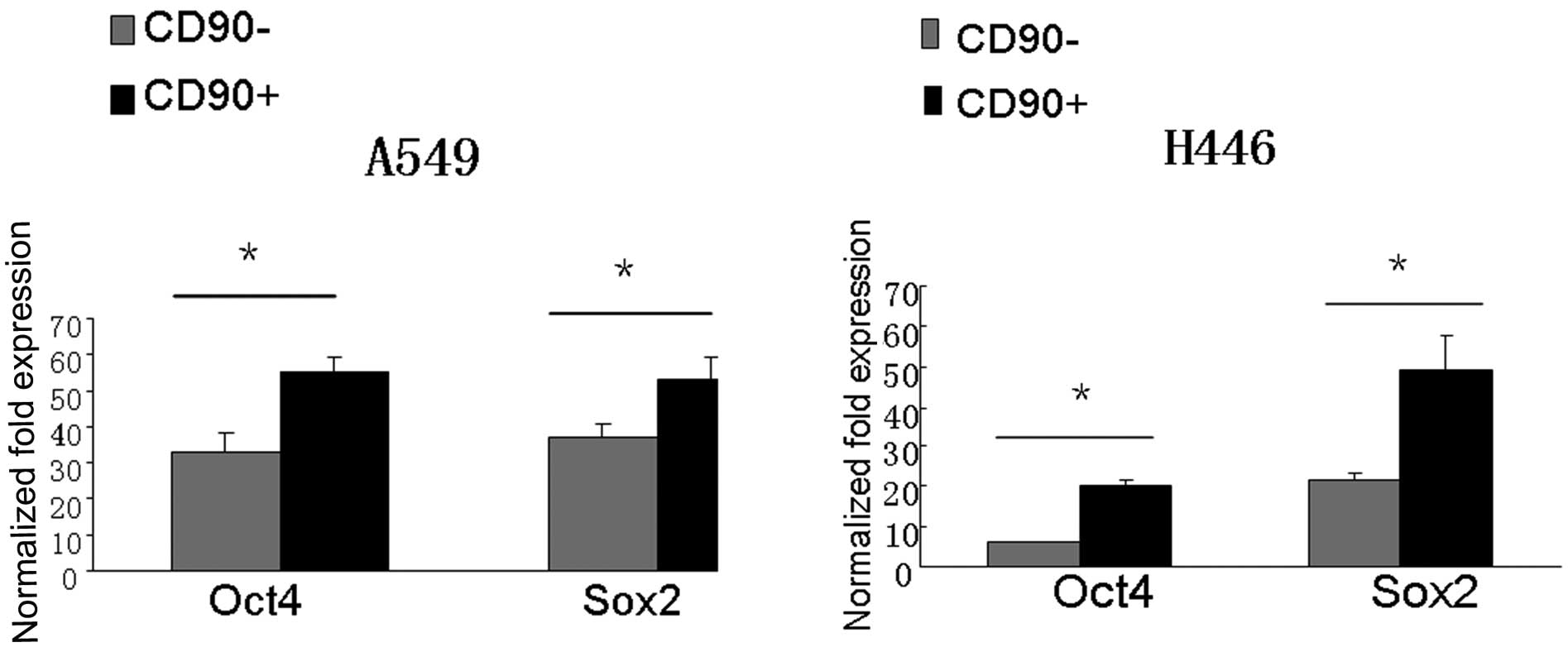
CD90+ cells express higher
levels of stem cell genes Oct4 and Sox2
The embryonic markers, Sox2 and Oct4, play an
important role in the growth and metastasis of lung cancer
(28,29), and are candidate stem cell-related
genes to study lung CSCs. Our previous study demonstrated that both
Sox2 and Oct4 are highly expressed in A549 sphere cells than A549
adherent cells (24). In the
present study, quantitative reverse transcriptase-polymerase chain
reaction (qRT-PCR) was employed to analyze stem-cell related gene
expression profiles of CD90+ and CD90− cells
isolated from both A549 and H446 cell lines. Results showed that
the mRNA expressions of either Oct4 and Sox2 in CD90+
cells were markedly higher than those in CD90− cells
(P<0.05) (Fig. 5).
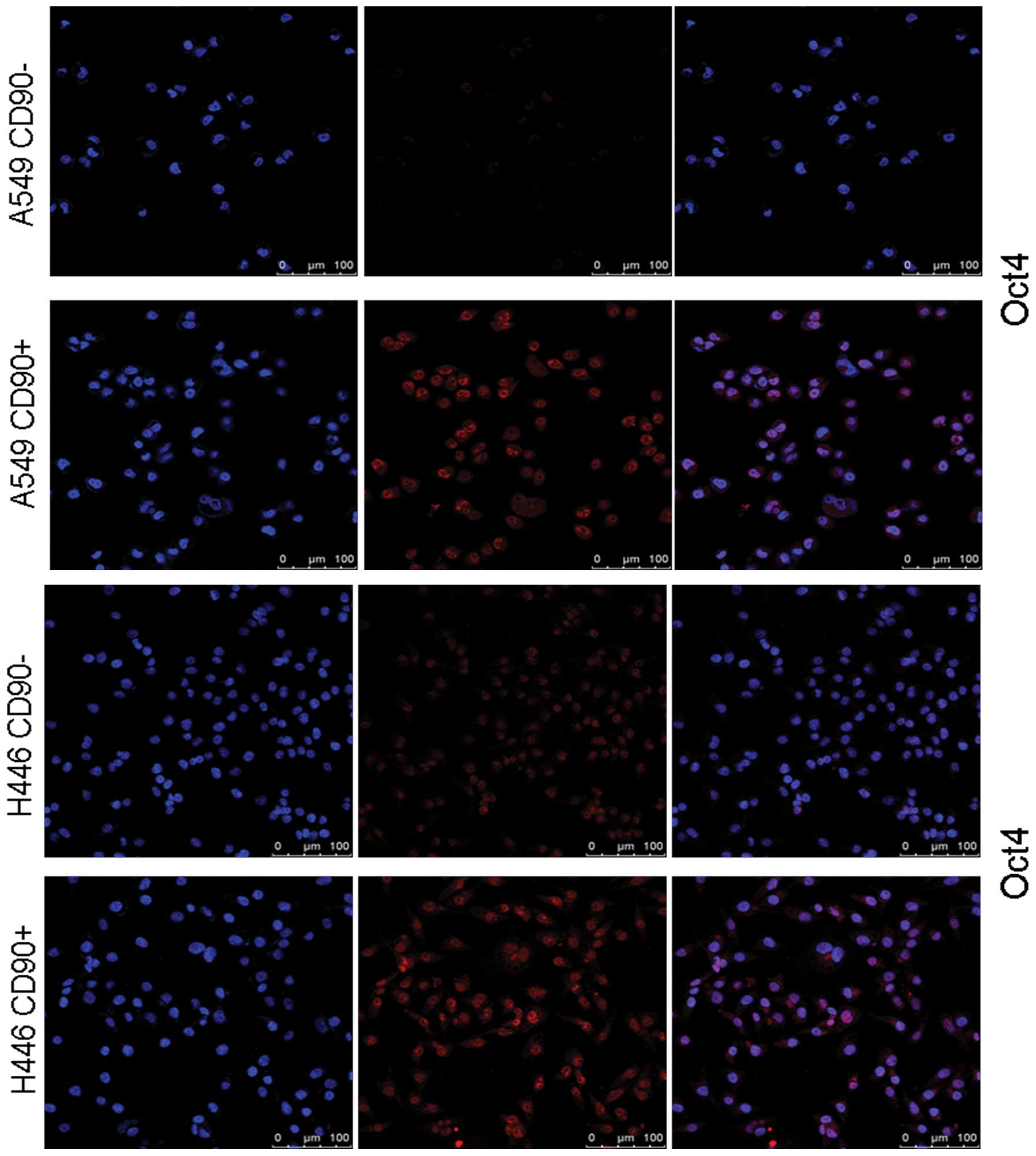
Immunofluorescent staining was also performed to
further confirm the protein levels of Oct4 in both CD90+
and CD90− cells sorted from A549 or H446 cell lines. The
results demonstrated that the Oct4 protein level was markedly
higher in CD90+ cells (Fig.
6). Taken together, we concluded that CD90+ cells,
but not CD90− cells, are characterized as stem-like
cells.
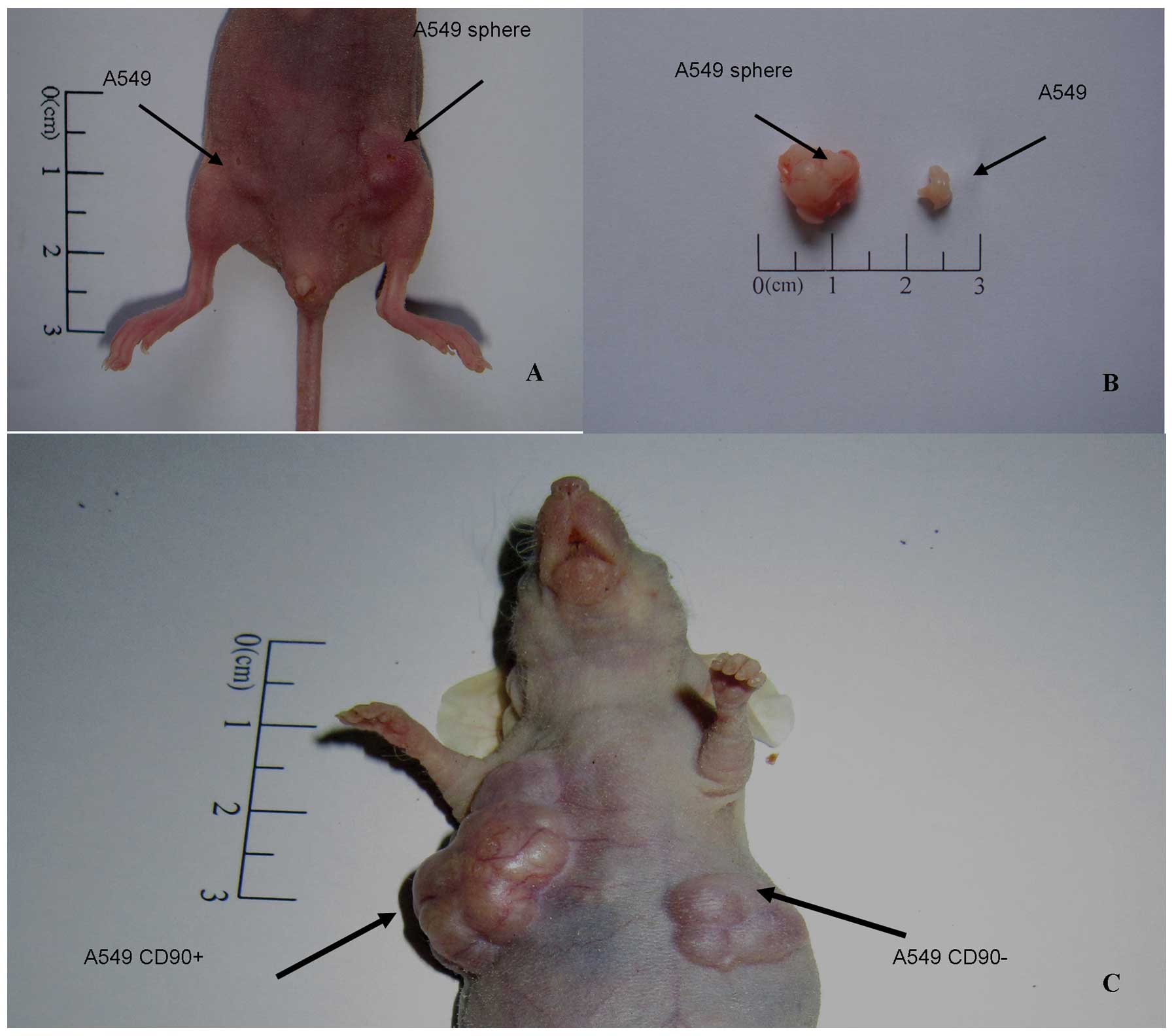
Xenograft experiments reveal
CD90+ cells have a higher tumorigenicity than
CD90− cells
The effect of CD90+ and CD90−
cells on tumor formation was further investigated in vivo.
In the present study, tumorigenicity was defined as the capacity of
the cells with certain number, following serial dilution, to form
tumor nodules in immunodeficient mice within a certain time
interval (8 weeks). We first established the xenograft model to
assess the tumorigenic capacity of A549 sphere cells as compared to
A549 adherent cells as control. Nude mice were divided into 4
groups randomly and were subcutaneously inoculated under bilateral
axillaries with different doses of cell numbers as described in
Materials and methods. Eight weeks later, tumor nodules appeared in
one nude mouse from 5,000 inoculated A549 sphere cells, but there
were no nodules found in the control side. When the cell number was
increased to 1×105, all 5 nude mice generated tumor
nodules formed by A549 sphere cells, while the remaining 2 out of 5
nude mice generated tumor nodules in the control (Table I). In addition, the tumor nodules
induced by A549 sphere cells were considerably bigger than those
formed by A549 adherent cells (Fig. 7A
and B).
 | Table IComparison of tumorigenic capacity of
the A549 adherent and sphere cells. |
Table I
Comparison of tumorigenic capacity of
the A549 adherent and sphere cells.
| Cell no. |
|---|
|
|
|---|
| Cell type |
1×106 |
1×105 |
1×104 |
5×103 |
|---|
| A549 | 5/5 | 2/5 | 0/5 | 0/5 |
| A549 sphere | 5/5 | 5/5 | 2/5 | 1/5 |
Using the same method, we further compared the
tumorigenic capacity between CD90+ and CD90−
cells both isolated from A549 cell lines. Tumor nodules from
CD90+ cells appeared after 8 weeks from 5×103
inoculated cells. When the cell number was increased to
1×104, 4 out of 5 nude mice generated tumor nodules. By
contrast, the CD90− cells did not induce tumor formation
in nude mice, even after injecting 1×104 cells (Table II). Furthermore, the tumor nodules
induced by CD90+ cells were much bigger than those
formed by CD90− cells (Fig.
7C). These results strongly indicate that A549 sphere cells
have a higher tumorigenic capacity, and CD90+ cells but
not CD90− cells are stem-like cells.
 | Table IIComparison of tumorigenic capacity of
the CD90+ and CD90− cells from A549 cell
lines. |
Table II
Comparison of tumorigenic capacity of
the CD90+ and CD90− cells from A549 cell
lines.
| Cell no. |
|---|
|
|
|---|
| Cell type |
5×104 |
1×104 |
5×103 |
|---|
| A549
CD90− | 3/5 | 0/5 | 0/5 |
| A549
CD90+ | 5/5 | 4/5 | 1/5 |
Discussion
In our previous study, tumor spheres successfully
cultured in the serum-free medium were used to enrich and identify
the lung cancer stem cells (CSCs) in the A549 cell line. The
results showed that, compared with their adherent cells, tumor
sphere cells had higher expression of stem genes Oct4 and Sox2.
Therefore, we concluded that CSCs could be enriched in tumor sphere
and such an enrichment process was an effective and convenient
method for screening and identifying tumor stem cells. In the
present study, we identified CD90 as a novel unique surface marker
for further isolation and identification of CSCs from lung cancer
cell lines A549 and H446. Our results revealed that
CD90+ cells, but not CD90− cells, from lung
cancer cell lines highly expressed stem cell markers, Oct4 and
Sox2, and displayed higher proliferative and tumorigenic capacity.
Therefore, CD90 is a promising new marker for lung CSCs.
The CSC theory has been widely recognized due to the
continual emergence of new evidence. At present, the origin of the
CSCs remains unclear, but emerging evidence suggests that CSCs play
an important role in the occurrence and development of malignant
tumors. Tumor stem cells have also been considered as the major
cause of drug resistance to chemotherapy and radiotherapy failure
(7,30–33).
Surface molecular markers can be used for flow cytometry screening
of a unique subset in a heterogeneous population of cells such as
cancer cells. CD133 is expressed in a variety of tumors as a
recognized marker for tumor stem cells such as brain tumor
(10,34), breast cancer (11) prostate cancer (12), colon cancer (14), pancreatic cancer (35), hepatocellular (36) and renal carcinoma (37). However, CD133 remains highly
controversial as a tumor stem cell marker (38–40). A
previous study showed that both CD133+ and
CD133− subpopulations of A549 and H446 cells contain
cancer-initiating cells (19),
which indicated that CD133 may not be an ideal marker for lung
CSCs. Therefore, as the identification of lung CSCs is fundamental
and vital for further research, for example, the mechanism of lung
CSCs involved in the drug resistance to chemotherapy and
radiotherapy, more specific and accurate markers for identifying
lung CSCs are urgently required.
CD90 has been characterized as a surface molecular
marker of CSCs in various malignancies including glioblastoma and
liver cancer (22,23). In our previous experiments, we found
the proportion of CD90+ in lung cancer tumor sphere
cells higher than in the adherent cells. Therefore, we isolated the
CD90+ and CD90− cells from the lung cancer
cell lines A549 and H446. We found that the numbers of the clones
and spheres formed by CD90+ cells were more than those
formed by CD90− cells (P<0.05). The expressions of
stem cell-related genes Oct4 and Sox2 in CD90+ were
markedly higher than those in CD90− cells (P<0.05).
Moreover, the animal experiments also indicated that the A549
sphere cells had a higher tumorigenic capacity, and
CD90+ cells were more likely to be stem cells than
CD90− cells. Therefore, both in vitro and in
vivo experiments indicate that CD90+ cells have
higher proliferation, self-renewal and tumorigenic capacity, and
CD90 is a novel marker of lung CSCs.
In conclusion, we demonstrated that serum-free
conditioned culture can be effectively employed for enrichment of
CSCs, and tumor spheres are suitable for studying CSCs. Meanwhile,
our preliminary results revealed the CD90+ cells, but
not CD90− cells, can be characterized with stem-like
features. The expression of CD90+ in human lung cancer
tissues and primary cells from lung cancer patient specimens
require further investigation in future studies. Finally, the
identification of CSCs with CD90 will provide a cellular basis to
investigate the mechanism of tumorigenesis, drug resistance to
chemotherapy and, thus, favor the design of future therapeutic
strategies for lung cancer.
Acknowledgements
We thank Professor Xia Zhang, the PI of Institute of
Pathology and Southwest Cancer Center, for the valuable suggestions
during the manuscript writing. This study was supported by grants
from the National Basic Research Program of China (973 Program, no.
2010CB529402).
Abbreviations:
|
CSCs
|
cancer stem cells
|
|
NSCLC
|
non-small cell lung cancer
|
|
FGF
|
fibroblast growth factor
|
|
EGF
|
epidermal growth factor
|
|
SEM
|
scanning electron microscopy
|
|
FACS
|
fluorescence-activated cell sorting
assay
|
|
qRT-PCR
|
quantitative reverse transcriptase
polymerase chain reaction
|
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
3
|
Davidoff AJ, Tang M, Seal B and Edelman
MJ: Chemotherapy and survival benefit in elderly patients with
advanced non-small-cell lung cancer. J Clin Oncol. 28:2191–2197.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
5
|
Bautch VL: Cancer: Tumour stem cells
switch sides. Nature. 468:770–771. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wicha MS, Liu S and Dontu G: Cancer stem
cells: an old idea - a paradigm shift. Cancer Res. 66:1883–1890;
discussion 1895–1896. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar
|
|
8
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Singh SK, Clarke ID, Terasaki M, et al:
Identification of a cancer stem cell in human brain tumors. Cancer
Res. 63:5821–5828. 2003.PubMed/NCBI
|
|
11
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Collins AT, Berry PA, Hyde C, Stower MJ
and Maitland NJ: Prospective identification of tumorigenic prostate
cancer stem cells. Cancer Res. 65:10946–10951. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eramo A, Lotti F, Sette G, et al:
Identification and expansion of the tumorigenic lung cancer stem
cell population. Cell Death Differ. 15:504–514. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
et al: Identification and expansion of human
colon-cancer-initiating cells. Nature. 445:111–115. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Galli R, Binda E, Orfanelli U, et al:
Isolation and characterization of tumorigenic, stem-like neural
precursors from human glioblastoma. Cancer Res. 64:7011–7021. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tirino V, Camerlingo R, Franco R, et al:
The role of CD133 in the identification and characterisation of
tumour-initiating cells in non-small-cell lung cancer. Eur J
Cardiothorac Surg. 36:446–453. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang F, Qiu Q, Khanna A, et al: Aldehyde
dehydrogenase 1 is a tumor stem cell-associated marker in lung
cancer. Mol Cancer Res. 7:330–338. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hirschmann-Jax C, Foster AE, Wulf GG, et
al: A distinct ‘side population’ of cells with high drug efflux
capacity in human tumor cells. Proc Natl Acad Sci USA.
101:14228–14233. 2004.
|
|
19
|
Meng X, Li M, Wang X, Wang Y and Ma D:
Both CD133+ and CD133− subpopulations of A549
and H446 cells contain cancer-initiating cells. Cancer Sci.
100:1040–1046. 2009.
|
|
20
|
Rege TA and Hagood JS: Thy-1 as a
regulator of cell-cell and cell-matrix interactions in axon
regeneration, apoptosis, adhesion, migration, cancer, and fibrosis.
FASEB J. 20:1045–1054. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cho RW, Wang X, Diehn M, et al: Isolation
and molecular characterization of cancer stem cells in MMTV-Wnt-1
murine breast tumors. Stem Cells. 26:364–371. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
He J, Liu Y, Zhu T, et al: CD90 is
identified as a candidate marker for cancer stem cells in primary
high-grade gliomas using tissue microarrays. Mol Cell Proteomics.
11:M111.0107442012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang ZF, Ho DW, Ng MN, et al: Significance
of CD90+ cancer stem cells in human liver cancer. Cancer
Cell. 13:153–166. 2008.
|
|
24
|
Yan YP, Luo H and Zhou XD: Isolation and
identification of lung cancer stem like cells from human lung
cancer cell line A549. J Third Mil Med Univ. 34:1153–1157.
2012.
|
|
25
|
Liu ZZ, Chen P, Lu ZD, Cui SD and Dong ZM:
Enrichment of breast cancer stem cells using a keratinocyte
serum-free medium. Chin Med J (Engl). 124:2934–2936.
2011.PubMed/NCBI
|
|
26
|
Hong X, Chedid K and Kalkanis SN:
Glioblastoma cell line-derived spheres in serum-containing medium
versus serum-free medium: a comparison of cancer stem cell
properties. Int J Oncol. 41:1693–1700. 2012.PubMed/NCBI
|
|
27
|
Chase LG, Lakshmipathy U, Solchaga LA, Rao
MS and Vemuri MC: A novel serum-free medium for the expansion of
human mesenchymal stem cells. Stem Cell Res Ther. 1:82010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xiang R, Liao D, Cheng T, et al:
Downregulation of transcription factor SOX2 in cancer stem cells
suppresses growth and metastasis of lung cancer. Br J Cancer.
104:1410–1417. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen YC, Hsu HS, Chen YW, et al: Oct-4
expression maintained cancer stem-like properties in lung
cancer-derived CD133-positive cells. PLoS One. 3:e26372008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huss WJ, Gray DR, Greenberg NM, Mohler JL
and Smith GJ: Breast cancer resistance protein-mediated efflux of
androgen in putative benign and malignant prostate stem cells.
Cancer Res. 65:6640–6650. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bao S, Wu Q, McLendon RE, et al: Glioma
stem cells promote radioresistance by preferential activation of
the DNA damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Q, Shi S, Yen Y, Brown J, Ta JQ and
Le AD: A subpopulation of CD133(+) cancer stem-like cells
characterized in human oral squamous cell carcinoma confer
resistance to chemotherapy. Cancer Lett. 289:151–160. 2010.
|
|
33
|
Li HZ, Yi TB and Wu ZY: Suspension culture
combined with anticancer regimens for screening breast cancer stem
cells. Med Hypotheses. 68:988–990. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Singh SK, Hawkins C, Clarke ID, et al:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hermann PC, Huber SL, Herrler T, et al:
Distinct populations of cancer stem cells determine tumor growth
and metastatic activity in human pancreatic cancer. Cell Stem Cell.
1:313–323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Suetsugu A, Nagaki M, Aoki H, Motohashi T,
Kunisada T and Moriwaki H: Characterization of CD133+
hepatocellular carcinoma cells as cancer stem/progenitor cells.
Biochem Biophys Res Commun. 351:820–824. 2006.
|
|
37
|
Bruno S, Bussolati B, Grange C, et al:
CD133+ renal progenitor cells contribute to tumor
angiogenesis. Am J Pathol. 169:2223–2235. 2006.
|
|
38
|
Shmelkov SV, Butler JM, Hooper AT, et al:
CD133 expression is not restricted to stem cells, and both
CD133+ and CD133− metastatic colon cancer
cells initiate tumors. J Clin Invest. 118:2111–2120.
2008.PubMed/NCBI
|
|
39
|
Wang J, Sakariassen PO, Tsinkalovsky O, et
al: CD133 negative glioma cells form tumors in nude rats and give
rise to CD133 positive cells. Int J Cancer. 122:761–768. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Joo KM, Kim SY, Jin X, et al: Clinical and
biological implications of CD133-positive and CD133-negative cells
in glioblastomas. Lab Invest. 88:808–815. 2008. View Article : Google Scholar : PubMed/NCBI
|