Introduction
Upon cellular stress, the p53 protein is stabilized
to regulate the expression, cellular location and activity of key
effectors of cellular processes, such as DNA repair, cell cycle
arrest, senescence and apoptosis. The p53 tumor-suppressor pathway
plays a central role in reducing cancer frequency and mediating
response to commonly used cancer therapies. The most frequently
studied single nucleotide polymorphisms (SNPs) in the p53 pathway
have been identified in the p53, MDM2 and p21 genes (p53 codon 72,
rs1042522, G/C; MDM2 SNP309, rs2279744, T/G; p21 codon 31,
rs1801270, C/A). However, meta-analyses are required to further
highlight the predictive power of these SNPs. The present study was
designed to evaluate the predictive value of SNPs in the p53
pathway in response to radiotherapy using biopsy specimens of
locoregionally advanced nasopharyngeal carcinoma (NPC) obtained
before treatment.
Different alleles of proline (p53-codon 72-Pro) and
arginine (p53-codon 72-Arg) were initially reported in 1988 by
Buchman and colleagues (1). The
amino acid encoded by codon 72 resides in a polyproline region of
p53 located between the transactivation and DNA-binding domains.
This proline-rich region has been shown to be important for p53
function, in particular, its ability to induce apoptosis. Several
studies to date have provided evidence that the two different p53
isoforms encoded by codon 72 SNPs are not functionally equivalent
(2–6). For example, Vannini et
al(7) showed that metastatic
breast cancer patients homozygous for Arg have a significantly
shorter time to progression and overall survival (OS) than those
with heterozygous Arg/Pro tumors. Kim et al(8) reported that the Arg/Pro and Pro/Pro
genotypes of TP53 codon 72 are significantly correlated with a
lower response rate to combination chemotherapy, compared to the
Arg/Arg genotype, in advanced gastric cancer patients. Moreover,
knowledge of individual genotypes at the p53 codon 72 locus and p53
mutational status of tumors may facilitate subclassification of
patients according to their ability to respond to specific
chemotherapeutic agents, allowing determination of the optimal
therapeutic strategy. In patients retaining wild-type p53,
wtp53-codon 72-Arg was associated with the best response rates as
well as OS and progression-free survival (PFS), concordant with
higher apoptotic potential. In patients with mutant p53,
mtp53-codon 72-Pro was associated with prolonged OS and PFS, in
keeping with its relatively high apoptotic potential, compared with
mtp53-codon 72-Arg (9). The p53
gene is rarely mutated in NPC (10), and a meta-analysis (11) showed that individuals with the
homozygous Arg/Arg genotype have decreased risk of NPC, compared
with those carrying the Pro/Pro genotype. However, the relationship
between the TP53 codon 72 polymorphism and clinical outcomes of NPC
remains to be elucidated.
MDM2 binds directly to p53 and consequently
regulates its transcriptional activation ability, cellular
localization and targeting for proteasomal degradation. These
observations allow for the possibility that substituting a few (or
even a single) base pair(s) in the regulatory regions of the gene
alters MDM2 activity sufficiently to affect the p53 pathway, and
therefore, cancer in humans (12).
Extensive analysis of a heritable, human genetic variant, in
particular, a polymorphism in the MDM2 promoter (MDM2 SNP309 T/G)
has lent support to this hypothesis (12–14).
Tu et al(15) reported that
the MDM2 SNP309 G/G polymorphism is associated with poor OS in
advanced oral squamous cell carcinoma (OSCC), and a combination of
MDM2 SNP309 G/G and p53 codon 72 Arg/Arg polymorphisms was linked
to the poorest OS and PFS. Chaar et al(16) additionally showed a significantly
more favorable clinical outcome of the wild-type SNP 309 genotype
(T/T) than the genotype (T/G, G/G) in colorectal cancer.
Furthermore, a significant association of the MDM2 SNP309 G/G
allele with favorable outcomes in female glioblastoma patients was
reported by Zawlik et al(17). Zhou et al(18) demonstrated that, compared with the
TT genotype, the G allele (GT + GG genotype) is associated with
markedly increased susceptibility to NPC and advanced lymph node
metastasis. Similarly, Sousa et al(19) reported that the MDM2 SNP309 GG
homozygote confers increased risk of NPC development. However, few
studies have investigated the predictive or prognostic value of
MDM2 SNP309 polymorphisms in response to radiotherapy in patients
with local regionally advanced NPC.
The cyclin-dependent kinase (CDK) inhibitor, p21
(CDKN1A), mediates the induction of cell cycle arrest in response
to a variety of stimuli, mainly through its ability to inhibit the
kinase activities of CDK2 and CDK1 (20–23).
The role of p21 in promoting DNA damage-induced G1 growth arrest
relies significantly on its well-characterized transcriptional
activation by p53 (22,23). The nonsynonymous codon 31 (C/A) SNP
in the CDKN1A gene (rs1801270) results in an amino acid change from
serine (p21-Ser) to arginine (p21-Arg) in a highly conserved
region. Similar to p53 codon 72 and MDM2 SNP309, the allelic
frequency at this locus varies greatly among populations, from a 4%
prevalence of the Arg allele in a Swedish population to 50% in
Chinese (24). Moreover, the
different alleles encoding these variants have been shown to vary
considerably in terms of transcriptional efficiency (25,26).
Tan et al(27) reported that
in TP53 72 Pro breast cancer carriers with the p21 Ser/Ser
genotype, the occurrence of acute toxicity induced by radiotherapy
is reduced in normal weight, but not overweight patients. Tsai
et al(28) showed that the
serine form of p21 codon 31 is more prominent in smokers than
non-smokers among NPC patients. The difference between smokers and
non-smokers suggests the involvement of an environmental factor in
association with the p21 gene in NPC formation. In the present
study, upon analysis of the polymorphisms of p53 pathway genes and
their impact on response to radiotherapy in patients with
locoregionally advanced NPC, smoking was also consistently
identified as an important factor.
Materials and methods
Patient selection
Between January 2008 and October 2009, 75
consecutive patients with locoregionally advanced NPC at the
Department of Radiotherapy, Affiliated Tumor Hospital of Xiangya
Medical School (Central South University, Changsha, China), were
enrolled retrospectively in this study. Patients with
biopsy-confirmed previously untreated NPC with American Joint
Committee on Cancer (AJCC)/International Union Against Cancer
(UICC) stage II, III and IV(A–B) disease were eligible. Other
criteria included age >18 years, ethnic Han Chinese individuals,
and an Eastern Cooperative Oncology Group performance status of 0
or 1. The exclusion criteria included the presence of distant
metastasis and other concomitant malignant disease. The study was
approved by the Clinical Research Ethics Committee of the Hunan
Province Cancer Center, and written informed consent was obtained
from all of the patients. The patient characteristics are
summarized in Table I. The
participants included 57 male and 18 female patients with a
male-to-female ratio of 3.2:1 and a median age of 45 years (range,
22–72 years). All patients were diagnosed with World Health
Organization (WHO) grade 2–3 NPC. Overall, 16 patients had stage
II, 44 had stage III, and 15 had stage IV(A–B) disease.
Questionnaires on smoking habits collected prior to treatment
contained information on smoking status, number of cigarettes/day,
and number of years of smoking. Pack-year was defined as years of
smoking 20 cigarettes/day.
 | Table IPatient demographics and treatment
characteristics. |
Table I
Patient demographics and treatment
characteristics.
| Characteristics | No. of patients
(%) |
|---|
| Age (years) |
| Range | 22–72 |
| Median | 45 |
| Gender |
| Male | 57 (76.0) |
| Female | 18 (24.0) |
| Overall stage
(AJCC)a |
| II | 16 (21.3) |
| III | 44 (58.7) |
| IVa–b | 15 (20.0) |
| T
classificationa |
| T1–2 | 42 (56.0) |
| T3–4 | 33 (44.0) |
| N
classificationa |
| N0 | 16 (21.3) |
| N1–3 | 59 (78.7) |
| Concurrent
chemotherapy |
| No | 36 (48.0) |
| Yes | 39 (52.0) |
| Adjuvant
chemotherapy |
| No | 35 (46.7) |
| Yes | 40 (53.3) |
| Smoking status
(pack-years) |
| 0 | 38 (50.7) |
| >0 –
<20 | 16 (21.3) |
| ≥20 | 21 (28.0) |
| ≥30 | 16 (21.3) |
Pretreatment evaluation
Evaluations for all patients included complete
physical examination, fiber optic nasopharyngoscopy, magnetic
resonance imaging (MRI) of the head and neck, chest X-ray,
abdominal imaging with ultrasound and bone scan. All patients were
prospectively included in a disease-specific database.
Treatment
Megavoltage photons (6 MV) were used to treat the
primary tumor and neck lymph nodes. Radiotherapy was administered
five times a week at a dose of 2 Gy/day. The accumulated dose of
radiation was 68–72 Gy to the primary tumor, 60–62 Gy to the
involved areas of the neck, and 50 Gy to the uninvolved areas.
Concurrent chemoradiotherapy was administered to 39 patients and
adjuvant chemoradiotherapy to 40 patients. As concurrent
chemoradiotherapy, DDP (100 mg/m2) was administered on
days 1, 22 and 43 during radiotherapy.
Endpoints
The primary endpoint for the study was PFS, defined
as the time from the day of enrollment to the date of first
documented relapse, categorized as locoregional (primary site or
regional node) failure or distant metastases, or the final
follow-up visit.
Sample preparation, DNA extraction and
quantification of DNA yield
From each sample, 5 10-μm tissue sections were
deparaffinized using xylene and ethanol. Specimens were digested
with proteinase K for 24 h at 55°C. Subsequently, the enzyme was
inactivated by boiling samples for 10 min prior to mixing with
absolute ethyl alcohol. After purification using ion-exchange
columns, genomic DNA was incubated at −20°C before use.
DNA amplification and genotyping
procedures
For each assessed polymorphism, the nested
polymerase chain reaction (PCR) method was used to amplify specific
fragments with the primers listed in Table II. The amplification reaction was
performed in three steps: 5 min at 95°C, followed by 35 cycles of
30 sec at 95°C, 30 sec at 55°C and 40 sec at 72°C, and 10 min at
72°C. PCR products were identified by electrophoresis before Sanger
sequencing using BigDye Terminator v3.1 chemistry (Life
Technologies, Carlsbad, CA, USA) on an Applied Biosystems 3130xl
Genetic Analyzer. All PCR reactions and sequencing analyses were
performed twice to confirm the results.
 | Table IIPrimers for nested PCR amplification
of the p53 pathway genes. |
Table II
Primers for nested PCR amplification
of the p53 pathway genes.
| SNPs | Initial
primers | Second primers |
|---|
| rs1042522 |
5′-GCAAGAAGCCCAGACGG-3′ (267 bp) |
5′-GGGAAGGGACAGAAGATG-3′ (222 bp) |
|
5′-CTCTTTTCACCCATCTACAGTC-3′ |
5′-CTCTTTTCACCCATCTACAGTC-3′ |
| rs2279744 |
5′-GTCGCCGCCAGGGAGGA-3′ (338 bp) |
5′-GAGTTCAGGGTAAAGGTCAC-3′ (165 bp) |
|
5′-GGGAAAATGCATGGTTTAAATAGCC-3′ |
5′-TCAAGAGGAAAAGCTGAGTC-3′ |
| rs1801270 |
5′-AGGTAACATAGTGTCTAATCTCCG-3′ (343
bp) |
5′-AGGTAACATAGTGTCTAATCTCCG-3′ (246
bp) |
|
5′-CCTGCCTCCTCCCAACTC-3′ |
5′-CCCTCCAGTGGTGTCTCG-3′ |
Follow-up
The follow-up period ended on October 31, 2012, with
a median follow-up of 25 months (range, 5–46). After completion of
treatment, patients were followed up at least every 3 months during
the first year, and every 6 months thereafter until disease
progression (recurrence or distant metastases). All local
recurrences were diagnosed via fiberoptic endoscopy and biopsy
and/or MRI of the nasopharynx and the skull base showing
progressive bone erosion and/or soft tissue swelling. Regional
recurrence was diagnosed based on clinical examination of the neck,
and in doubtful cases, fine needle aspiration or MRI of the neck.
Distant metastases were diagnosed based on clinical symptoms,
physical examination and imaging methods, including chest
radiography, abdominal sonography, whole body bone scan, computed
tomography (CT) scan and MRI. During follow-up, 22 (29.3%) patients
had locoregional relapse while 21 (28.0%) displayed distant
metastasis. No patients succumbed to the disease during follow-up.
The 3-year PFS rate was 42.7%.
Statistical analysis
Demographic and clinical information was compared
across genotypes using Pearson’s χ2 test (for
categorical variables) and one-way ANOVA (for continuous
variables), where appropriate. Hardy-Weinberg equilibrium was
tested using a goodness-of-fit χ2 test with one degree
of freedom. Each genotype was independently analyzed for
correlation with survival times. The Kaplan-Meier method was
adopted to estimate survival curves, and the log-rank test to
compare patient survival times between subgroups. Multivariate
analyses using Cox regression were employed to assess the
significance of genotypes with adjustment for age, gender, T
classification, N classification, overall stage and chemotherapy.
Analyses were carried out using the statistical software package
SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). All statistical tests
were two-sided, and a value of P<0.05 was considered to indicate
a statistically significant result. The Bonferroni correction was
applied to adjust primary analysis.
Results
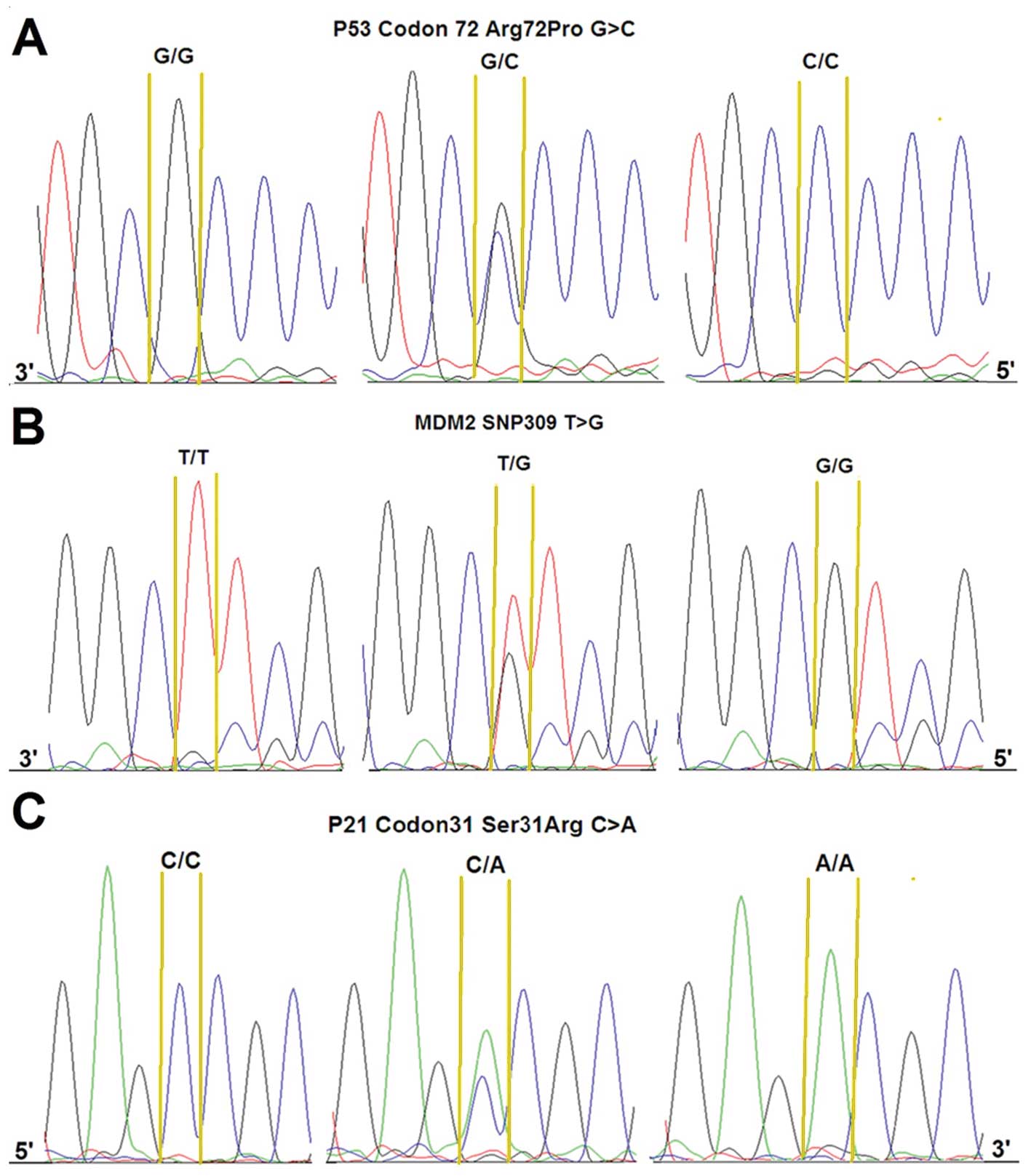
p53 codon 72 SNP, p21 codon 31 SNP and MDM2 SNP309
were clearly distinguished by sequencing. The typical sequencing
peak diagrams that were obtained are depicted in Fig. 1. The Arg allelic frequencies were
0.23 and 0.37 for p53 codon 72 SNP and p21 codon 31 SNP,
respectively, and G allelic frequency was 0.55 for MDM2 SNP309.
These frequencies fulfill the Hardy-Weinberg distribution. Table III shows the distribution of SNPs
according to clinical variables. Tumor stage was not significantly
associated with any of the polymorphisms analyzed. However, the
frequency of MDM2 SNP309 G allele was higher in more advanced
disease (T3–4) (P=0.001).
 | Table IIIAssociation between clinical
variables and SNPs of the p53 pathway (χ2 analysis) [n
(%)]. |
Table III
Association between clinical
variables and SNPs of the p53 pathway (χ2 analysis) [n
(%)].
| p53 codon 72
SNP | P-value |
|---|
|
|---|
| ArgArg | Arg/Pro | Pro/Pro | |
|---|
|
|
| |
| T1–2 | T3–4 | T1–2 | T3–4 | T1–2 | T3–4 | 0.810 |
| 17 (40.48) | 16 (48.48) | 19 (45.24) | 11 (33.33) | 6 (14.29) | 6 (18.18) | |
| N0 | N+ | N0 | N+ | N0 | N+ | 0.842 |
| 8 (50) | 25 (42.37) | 5 (31.25) | 25 (42.37) | 3 (18.75) | 9 (15.25) | |
| Stage II | Stage III–IV | Stage II | Stage III–IV | Stage II | Stage III–IV | 0.842 |
| 7 (43.75) | 26 (447) | 7 (43.75) | 23 (38.98) | 2 (12.5) | 10 (16.95) | |
|
| MDM2 SNP309 | |
|
| T/T | G/T | G/G | |
|
|
| |
| T1–2 | T3–4 | T1–2 | T3–4 | T1–2 | T3–4 |
0.001a |
| 10 (23.80) | 5 (15.15) | 27 (64.29) | 10 (30.30) | 5 (11.90) | 18 (54.55) | |
| N0 | N+ | N0 | N+ | N0 | N+ | 0.192 |
| 3 (18.75) | 12 (20.34) | 5 (31.25) | 32 (54.24) | 8 (50) | 15 (25.42) | |
| Stage II | Stage III–IV | Stage II | Stage III–IV | Stage II | Stage III–IV | 0.061 |
| 5 (31.25) | 10 (12.5) | 9 (56.25) | 28 (47.46) | 2 (12.5) | 21 (35.59) | |
|
| p21 codon31 | |
|
| SerSer | SerArg | ArgArg | |
|
|
| |
| T1–2 | T3–4 | T1–2 | T3–4 | T1–2 | T3–4 | 0.091 |
| 10 (25) | 16 (53.33) | 23 (57.5) | 9 (30) | 7 (17.5) | 5 (16.67) | |
| N0 | N+ | N0 | N+ | N0 | N+ | 0.131 |
| 9 (56.25) | 17 (31.48) | 5 (31.25) | 27 (50) | 2 (12.5) | 10 (18.52) | |
| Stage II | Stage III–IV | Stage II | Stage III–IV | Stage II | Stage III–IV | 0.419 |
| 4 (26.67) | 22 (40) | 8 (53.33) | 24 (43.64) | 3 (20) | 9 (16.36) | |
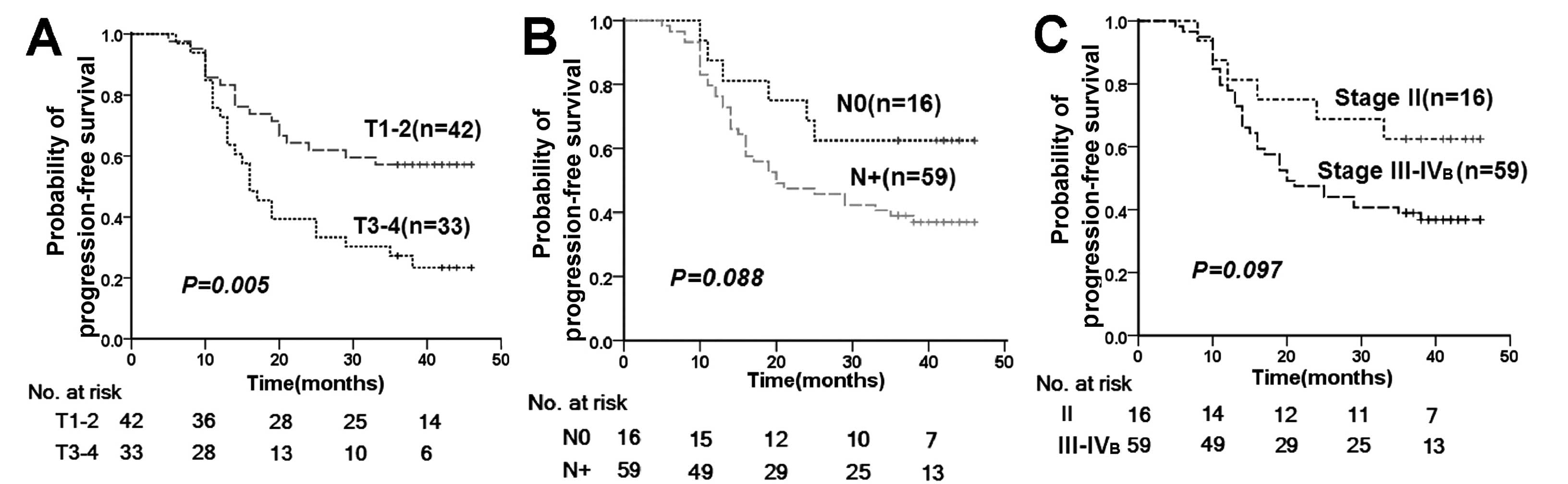
For tumor stage, only T classification was
significantly associated with the time to disease progression
(Fig. 2). However, both T and N
classification were significantly associated with PFS rates
(Table IV) after adjustment for
age (<45 and ≥45 years), gender (male and female) and concurrent
chemotherapy (whether or not administered).
 | Table IVMultivariate analysis of
progression-free survival by the Cox proportional hazards
model. |
Table IV
Multivariate analysis of
progression-free survival by the Cox proportional hazards
model.
| Variables | Subgroups | HR | 95% CI | P-value |
|---|
| T
classification | T3–4 vs. T1–2 | 2.683 | 1.430–5.032 | 0.002 |
| N
classification | N+ vs.
N0 | 1.778 | 1.196–2.644 | 0.004 |
| Smoking status
(pack-years) | Ever vs. never | 2.356 | 1.032–5.375 | 0.042 |
| ≥20 vs. <20 | 2.153 | 1.049–4.416 | 0.037 |
| ≥30 vs. <30 | 2.899 | 1.349–6.229 | 0.006 |
| p53 codon 72
SNP | Pro/Pro vs.
Arg/Pro+Arg/Arg | 0.300 | 0.092–0.983 | 0.047 |
| p21 codon 31
SNP | Ser/Ser vs.
Ser/Arg+Arg/Arg | 1.411 | 0.697–2.854 | 0.339 |
| MDM2 SNP309 | T/T vs.
T/G+G/G | 0.719 | 0.349–1.479 | 0.37 |
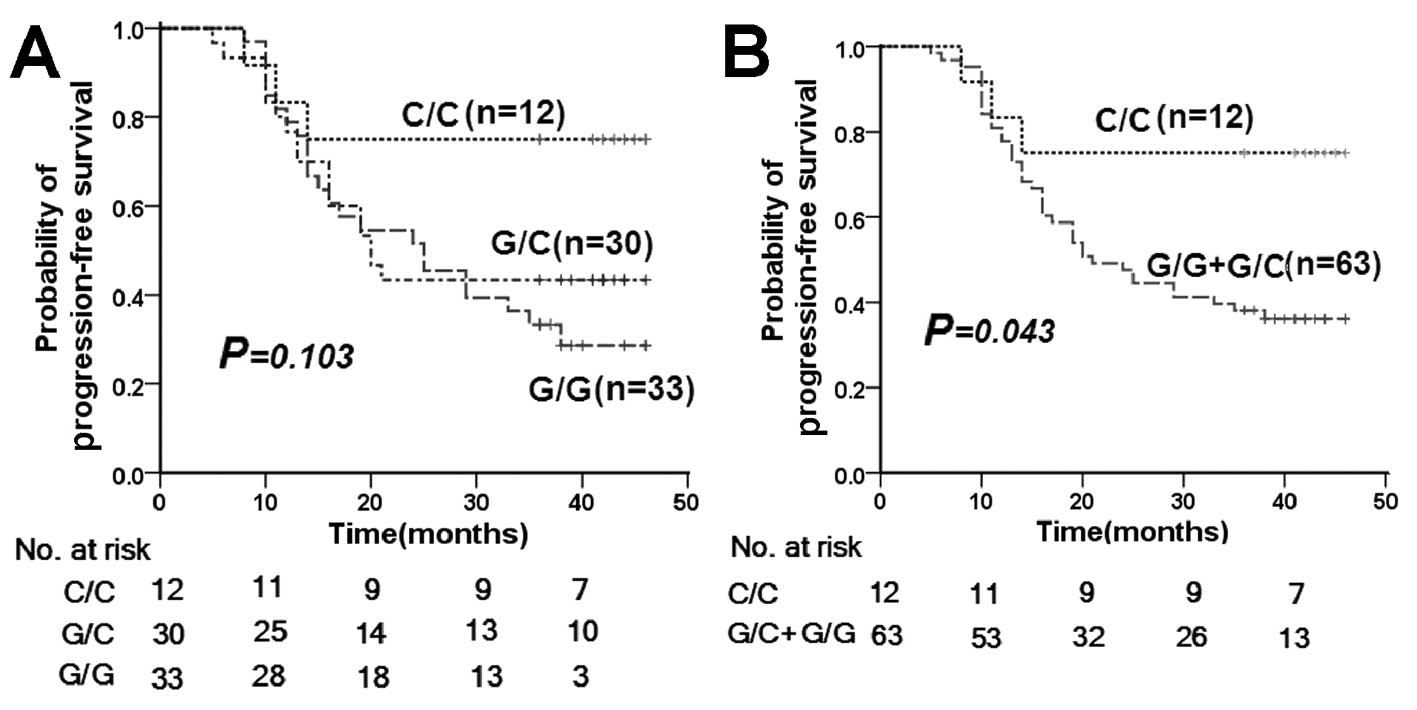
Patients carrying the Pro/Pro p53 codon 72 SNP
displayed prolonged PFS when compared with patients with other
polymorphic variants (Fig. 3).
Multivariate analysis of PFS with Cox proportional hazards model is
presented in Table IV. Hazard
ratios (HRs) (0.300; 95% CI, 0.092–0.983; P=0.047) of Pro/Pro p53
codon 72 type for distant metastases and local recurrence were
significantly lower after adjustment for age (<45 and ≥45
years), gender, T/N classification and concurrent chemotherapy
(whether or not administered). No significant association was
evident between MDM2 SNP309 or the p21 codon 31 SNP and
susceptibility to PFS, and no combined effects of p53 codon 72, p21
codon 31 and MDM2 309SNP genotypes on risk of progression
(recurrence or distant metastases) were observed (data not
shown).
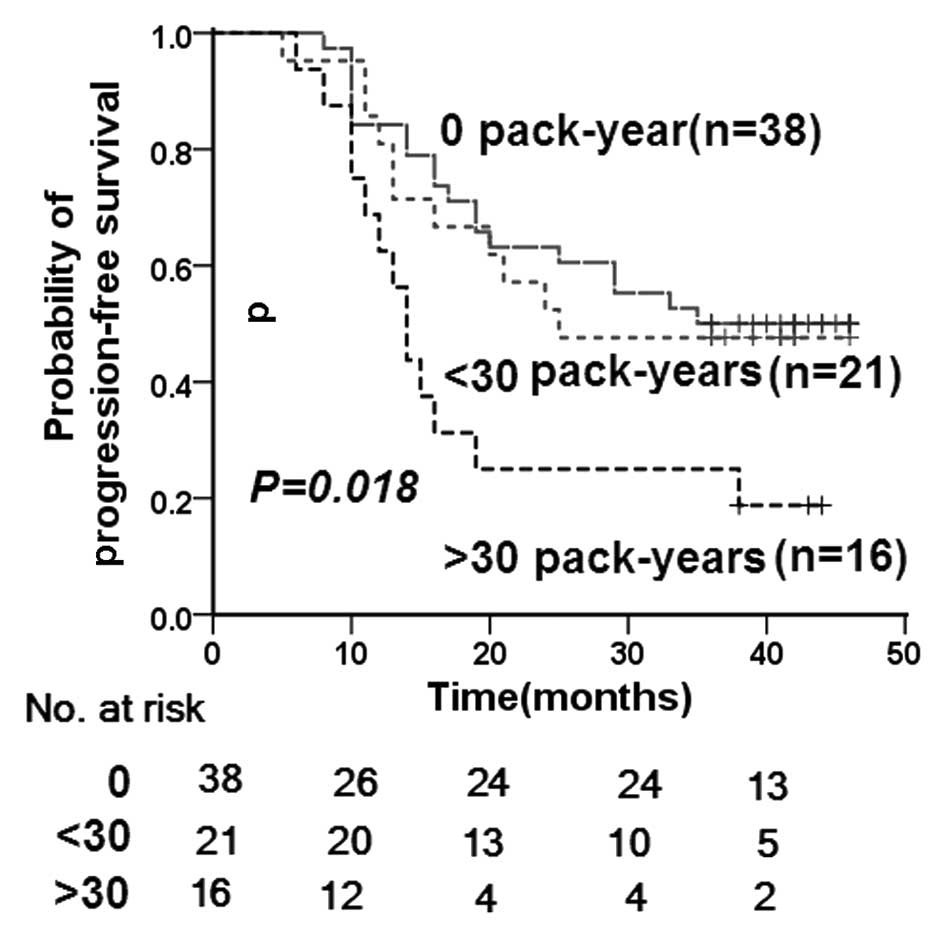
Compared with cumulative cigarette smoking of <20
pack-years as the reference, the multivariate-adjusted HR (95% CI)
after adjustment for age, gender, T and N classification and
chemotherapy was 2.153 (1.049–4.416; P=0.037) for ≥20 pack-years of
cumulative cigarette smoking. Comparison of the cumulative
cigarette smoking of ≥30 pack-years with <30 pack-years revealed
an increase in the multivariate-adjusted hazard ratio (95% CI) to
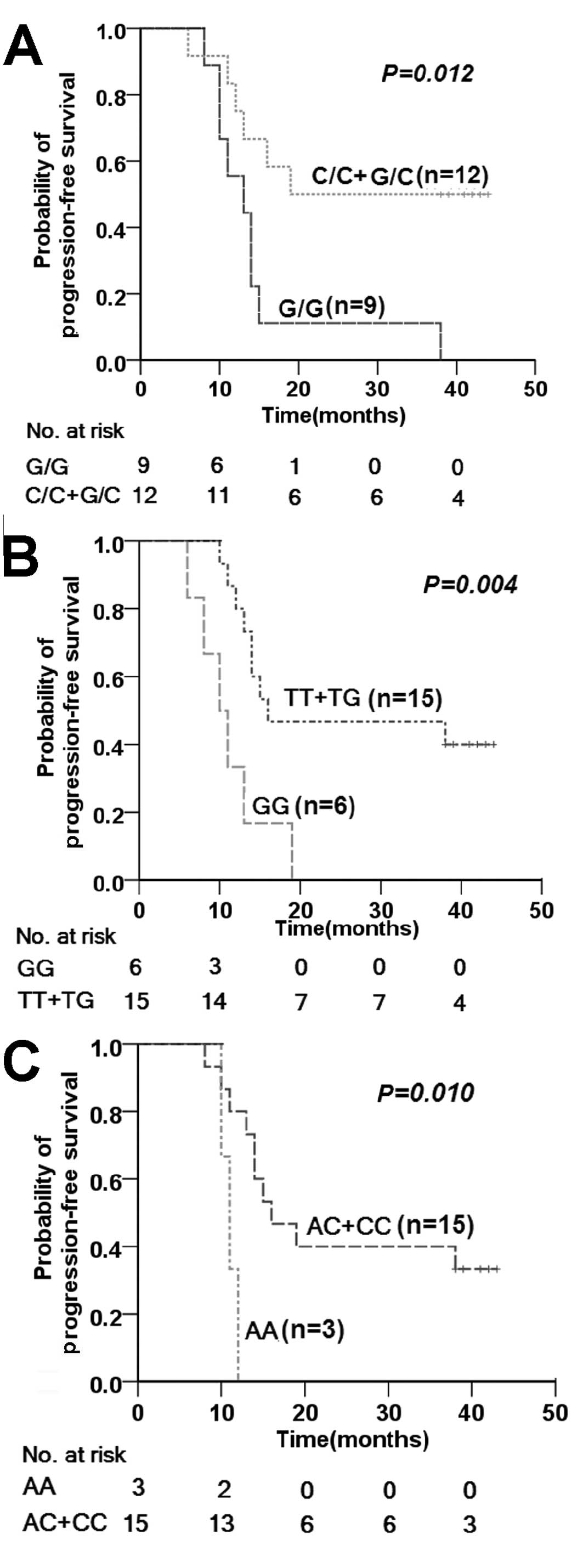
2.899 (1.349–6.229; P=0.006). As shown in Fig. 4, smoking status was significantly
associated with the time to disease progression (P=0.018).
Results of the analysis of the combined effects of
p53 pathway SNPs and smoking status on the risk of progression
(recurrence and distant metastases) are shown in Table V. Subjects with the p53 codon 72
Arg/Arg genotype were at higher risk of disease progression
compared with the other genotypes (HR, 3.590; 95% CI, 1.219–10.576;
P=0.020) among the heavy smoker group, as assessed with Cox
proportional hazards model (pack-years, ≥20). Similar results were
observed with the p21 codon 31 Arg/Arg (HR, 6.151; 95% CI,
1.216–21.116; P=0.028) and MDM2 309 SNP G/G (HR, 2.899; 95% CI,
4.174–12.336; P=0.010) genotypes. However, among moderate smokers
(pack-years, <20), no association was evident between these
genotypes and 3-year PFS rates. According to log-rank analysis,
mean times to progression for heavy smokers (pack-years, ≥20)
carrying p53 codon 72 Arg/Arg, p21 codon 31 Arg/Arg, and MDM2 309
SNP G/G genotypes were only 14.78±3, 11.00±0.58 and 11.17±1.85
months, respectively. These time scales were less than half of
those recorded for patients with other genotypes and moderate
smokers (pack-years, <20) (Fig.
5).
 | Table VLog-rank and proportional hazards
analysis (Cox method) for progression-free survival related to the
genotype of the p53 pathway and smoking status. |
Table V
Log-rank and proportional hazards
analysis (Cox method) for progression-free survival related to the
genotype of the p53 pathway and smoking status.
| Genotypes | Smoking status
(pack-years) | Log-rank
analysis | Cox-regression |
|---|
|
|
|---|
| Mean survival time
(months) | P-value | HR | 95% CI | P-value |
|---|
| p53 codon 72 |
| C/C+G/C | <20 | 30.97±3.00 | | 1 | | |
| G/G | <20 | 31.58±2.79 | 0.748 | 1.127 | 0.537–2.366 | 0.752 |
| C/C+G/C | ≥20 | 28.42±4.57 | | 1 | | |
| G/G | ≥20 | 14.78±3.00 | 0.012 | 3.590 | 1.219–10.576 | 0.020 |
| p21 codon 31 |
| C/C+C/A | <20 | 29.72±2.35 | | 1 | | |
| A/A | <20 | 38.44±4.07 | 0.197 | 0.768 | 0.307–1.919 | 0.572 |
| C/C+C/A | ≥20 | 24.87±3.71 | | 1 | | |
| A/A | ≥20 | 11.00±0.58 | 0.010 | 6.151 | 1.216–31.116 | 0.028 |
| MDM2 309 SNP |
| T/T+T/G | <20 | 29.81±2.48 | | 1 | | |
| G/G | <20 | 34.35±3.69 | 0.329 | 0.659 | 0.280–1.552 | 0.340 |
| T/T+T/G | ≥20 | 27.13±3.90 | | 1 | | |
| G/G | ≥20 | 11.17±1.85 | 0.004 | 4.174 | 1.412–12.336 | 0.010 |
Discussion
Multivariate analysis showed that the p53 codon 72
SNP is a useful prognostic factor in patients with locoregionally
advanced NPC (P=0.047). Analysis stratified by smoking status
revealed a more significant association between p53 codon 72 SNP
and PFS (P=0.020). Patients with p53 codon 72 Arg/Arg genotype had
poorer PFS than those containing other polymorphisms within the
heavy smoker group. Two earlier studies proposed that several
common mutants of mtp53-codon 72-Arg bind with greater affinity to
a p53 family member, the tumor suppressor protein p73, and inhibit
its ability to induce apoptosis (9,29).
Among patients with wild-type p53, wtp53-codon 72-Arg was
associated with best PFS, while among those containing mutant p53,
mtp53-codon 72-Pro was associated with best PFS (9). A mutation was distinguished when the
mutant signal was >30% that of the wild-type allele. Although
the p53 gene is rarely mutated in NPC (10), the p53 codon 72 Arg/Arg genotype
presented as an independent predictor of poorer outcomes in the
present study, and therefore, the underlying mechanisms require
further investigation. Vannini et al(7) provided a molecular explanation for
association of the Arg allele with tumor aggressiveness and
treatment resistance in advanced breast cancer. The group showed
that lower cell death is induced under hypoxia upon transfection of
the Arg allele relative to the Pro allele in vitro, which
was explained by the finding that the Arg allele upregulates
BCRP-I, a hypoxia response gene, which increased treatment
resistance.
The MDM2 SNP309 G allele frequency varies among
different races. In keeping with other studies, our results showed
a ~0.5 frequency for the G allele in a healthy Chinese population
(12), compared with <0.4 in
Caucasians (30). Although the
sample size in our study was small, this difference may partly
account for the discrepancies in data in regards to the
relationship between tumor progression and MDM2 SNP309. Our
experiments demonstrated that MDM2 SNP309 is significantly
associated with T classification, but not p53 codon 72 SNP and age
at onset of NPC (data not shown). The association of MDM2
overexpression with tumor invasiveness and poor survival has been
reported in various cancer types, including leiomyosarcoma,
astrocytoma, soft tissue sarcoma, pancreatic cancer, melanoma,
medulloblastoma and clear cell renal carcinoma (12,31–34).
Since the G/G polymorphism is associated with increased MDM2
transcription (30), it is
postulated that patients with this polymorphism have poor
prognosis. Our data support this hypothesis in the subgroup of
heavy smokers (≥20 pack-years). Specifically, patients with the G/G
polymorphism had reduced PFS when compared to those with other
polymorphisms (P=0.010). The G allele of SNP309 was shown to alter
the affinity of a well-characterized co-transcriptional activator
for multiple hormone receptors, including ER, namely Sp1,
consistent with the theory that MDM2 SNP309 may be responsible for
increased risk of tumorigenesis, especially in young women
(13). However, our overall data
showed no association between MDM2 SNP309 and outcomes in NPC,
whether or not adjusted for age (<45 and ≥45 years) and
gender.
P21 plays a direct role in mediating
irradiation-induced G1 arrest, with p53 as the transcription factor
in this process. Correlation of the gene p21 codon 31 polymorphism
with NPC has been rarely demonstrated. Tsai et al(28) reported that the serine form is
predominant in smokers within NPC groups, and the polymorphism may
therefore be utilized as a candidate genetic marker for screening
NPC risk in association with smoking. We did not observe a
significant association between p21 Ser31Arg polymorphism and PFS,
either with univariate or multivariate analysis, in overall data.
However, as with p53 codon 72 and MDM2 SNP309, the p21 Ser31Arg/Arg
genotype may be a predictive factor of poorer outcome of NPC
treated with radiotherapy in the heavy smoker group.
Smoking influences the hemoglobin content, owing to
the formation of carboxyhemoglobin (COHb) via binding of carbon
monoxide (CO). The formation of carboxyhemoglobin causes a left
shift in the hemoglobin-oxygen dissociation curve. If other factors
remain unchanged, this causes the pO2 to drop to lower
levels than normal to release a normal amount of O2 to
tissues. Low pO2 values result in decreased diffusion
distance from blood vessels and an increase in the fraction of
hypoxic cells in tumors. In addition to carboxyhemoglobin
formation, smoking may influence the amount of oxygen carried to
tissues due to a vasoconstrictive effect of nicotine (35). Owing to the theoretical importance
of the tumor oxygenation status on the effectiveness of
radiotherapy, there is a compelling biologic rationale for
concluding that smoking affects the response to radiotherapy. In
the present study, persistent smokers showed higher risk of disease
progression, compared to non-smokers (HR, 2.356; 95% CI,
1.032–5.375; P=0.042). Further comparison of groups of smokers
between ≥20 and <20 pack-years, and ≥30 and <30 pack-years
revealed an association of poorer PFS of locoregionally advanced
NPC with longer and heavier cigarette smoking habit (Table IV and Fig. 4). The most intriguing finding was
that associations between p53 pathway SNPs and PFS were more or
only significant in the subgroup of heavy smokers (≥20 pack-years)
(Table V and Fig. 5). Our data strongly advocate that
smoking should be avoided to improve the therapeutic efficacy of
radiotherapy, particularly for patients carrying SNPs correlated
with high risk of local relapse or distant metastasis.
In conclusion, this preliminary study demonstrates
for the first time that p53 codon 72 SNP contributes to
locoregionally advanced NPC response to radiotherapy, particularly
in patients smoking ≥20 pack-years. Based on the collective
findings, we suggest that p53 codon 72 SNP is an independent
predictor of outcome for locoregionally advanced NPC treated with
radiotherapy. Our data confirmed that smoking has a negative effect
on treatment outcomes of cancers. We further showed that the MDM2
SNP309 G/G and p21 codon 31 Arg/Arg genotype are correlated with
poorest PFS in heavy smokers (≥20 pack-years) among patients with
locoregionally advanced NPC treated with radiotherapy.
Acknowledgements
This study was supported by the Science Foundation
for Post Doctorate Research from the China Hunan Provincial Science
and Technology Department (no. 2012RS4011), as well as by a
Collaboration Project between the Biomedical Engineering Center of
Hunan University (China) and Emory-Georgia Tech Nanotechnology
Center (USA) (no. 2010DFB30300). We would like to thank Professor
Xuping Xi and Professor Junming Luo for their role in the
completion of this research.
References
|
1
|
Buchman VL, Chumakov PM, Ninkina NN,
Samarina OP and Georgiev GP: A variation in the structure of the
protein-coding region of the human p53 gene. Gene. 70:245–252.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thomas M, Kalita A, Labrecque S, Pim D,
Banks L and Matlashewski G: Two polymorphic variants of wild-type
p53 differ biochemically and biologically. Mol Cell Biol.
19:1092–1100. 1999.PubMed/NCBI
|
|
3
|
Dumont P, Leu JI, Della Pietra AC III,
George DL and Murphy M: The codon 72 polymorphic variants of p53
have markedly different apoptotic potential. Nat Genet. 33:357–365.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sullivan A, Syed N, Gasco M, Bergamaschi
D, Trigiante G, Attard M, et al: Polymorphism in wild-type p53
modulates response to chemotherapy in vitro and in vivo. Oncogene.
23:3328–3337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pim D and Banks L: p53 polymorphic
variants at codon 72 exert different effects on cell cycle
progression. Int J Cancer. 108:196–199. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bergamaschi D, Samuels Y, Sullivan A,
Zvelebil M, Breyssens H, Bisso A, et al: iASPP preferentially binds
p53 proline-rich region and modulates apoptotic function of codon
72-polymorphic p53. Nat Genet. 38:1133–1141. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vannini I, Zoli W, Tesei A, Rosetti M,
Sansone P, Storci G, et al: Role of p53 codon 72 arginine allele in
cell survival in vitro and in the clinical outcome of patients with
advanced breast cancer. Tumor Biol. 29:145–151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim JG, Sohn SK, Chae YS, Song HS, Kwon
KY, Do YR, et al: TP53 codon 72 polymorphism associated with
prognosis in patients with advanced gastric cancer treated with
paclitaxel and cisplatin. Cancer Chemother Pharmacol. 64:355–360.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bergamaschi D, Gasco M, Hiller L, Sullivan
A, Syed N, Trigiante G, et al: p53 polymorphism influences response
in cancer chemotherapy via modulation of p73-dependent apoptosis.
Cancer Cell. 3:387–402. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lo KW and Huang DP: Genetic and epigenetic
changes in nasopharyngeal carcinoma. Semin Cancer Biol. 12:451–462.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhuo XL, Cai L, Xiang ZL, Zhuo WL, Wang Y
and Zhang XY: TP53 codon 72 polymorphism contributes to
nasopharyngeal cancer susceptibility: a meta-analysis. Arch Med
Res. 40:299–305. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bond GL, Hu W, Bond EE, Robins H, Lutzker
SG, Arva NC, et al: A single nucleotide polymorphism in the MDM2
promoter attenuates the p53 tumor suppressor pathway and
accelerates tumor formation in humans. Cell. 119:591–602. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bond GL and Levine AJ: A single nucleotide
polymorphism in the p53 pathway interacts with gender,
environmental stresses and tumor genetics to influence cancer in
humans. Oncogene. 26:1317–1323. 2007. View Article : Google Scholar
|
|
14
|
Hu Z, Jin G, Wang L, Chen F, Wang X and
Shen H: MDM2 promoter polymorphism SNP309 contributes to tumor
susceptibility: evidence from 21 case-control studies. Cancer
Epidemiol Biomarkers Prev. 16:2717–2723. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tu HF, Chen HW, Kao SY, Lin SC, Liu CJ and
Chang KW: MDM2 SNP 309 and p53 codon 72 polymorphisms are
associated with the outcome of oral carcinoma patients receiving
postoperative irradiation. Radiother Oncol. 87:243–252. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chaar I, Arfaoui TA, El Amine el HO,
Mahmoud LB, Khiari M, Sammoud S, et al: Impact of MDM2
polymorphism: increased risk of developing colorectal cancer and a
poor prognosis in the Tunisian population. Eur J Gastroenterol
Hepatol. 24:320–327. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zawlik I, Kita D, Vaccarella S,
Mittelbronn M, Franceschi S and Ohgaki H: Common polymorphisms in
the MDM2 and TP53 genes and the relationship between TP53 mutations
and patient outcomes in glioblastomas. Brain Pathol. 19:188–194.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou G, Zhai Y, Cui Y, Zhang X, Dong X,
Yang H, et al: MDM2 promoter SNP309 is associated with risk of
occurrence and advanced lymph node metastasis of nasopharyngeal
carcinoma in Chinese population. Clin Cancer Res. 13:2627–2633.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sousa H, Pando M, Breda E, Catarino R and
Medeiros R: Role of the MDM2 SNP309 polymorphism in the initiation
and early age of onset of nasopharyngeal carcinoma. Mol Carcinog.
50:73–79. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brugarolas J, Chandrasekaran C, Gordon JI,
Beach D, Jacks T and Hannon GJ: Radiation-induced cell cycle arrest
compromised by p21 deficiency. Nature. 377:552–557. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Deng C, Zhang P, Harper JW, Elledge SJ and
Leder P: Mice lacking p21CIP1/WAF1 undergo normal
development, but are defective in G1 checkpoint control. Cell.
82:675–684. 1995.
|
|
22
|
Macleod KF, Sherry N, Hannon G, Beach D,
Tokino T, Kinzler K, et al: p53-dependent and independent
expression of p21 during cell growth, differentiation, and DNA
damage. Genes Dev. 9:935–944. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abbas T and Dutta A: p21 in cancer:
intricate networks and multiple activities. Nat Rev Cancer.
9:400–414. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Birgander R, Själander A, Saha N, Spitsyn
V, Beckman L and Beckman G: The codon 31 polymorphism of the
p53-inducible gene p21 shows distinct differences between major
ethnic groups. Hum Hered. 46:148–154. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Su L, Sai Y, Fan R, Thurston SW, Miller
DP, Zhou W, et al: P53 (codon 72) and P21 (codon 31) polymorphisms
alter in vivo mRNA expression of p21. Lung Cancer. 40:259–266.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Johnson GG, Sherrington PD, Carter A, Lin
K, Liloglou T, Field JK and Pettitt AR: A novel type of p53 pathway
dysfunction in chronic lymphocytic leukemia resulting from two
interacting single nucleotide polymorphisms within the p21 gene.
Cancer Res. 69:5210–5217. 2009. View Article : Google Scholar
|
|
27
|
Tan XL, Popanda O, Ambrosone CB, Kropp S,
Helmbold I, von Fournier D, et al: Association between TP53 and p21
genetic polymorphisms and acute side effects of radiotherapy in
breast cancer patients. Breast Cancer Res Treat. 97:255–262. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsai MH, Chen WC and Tsai FJ: Correlation
of p21 gene codon 31 polymorphism and TNF-alpha gene polymorphism
with nasopharyngeal carcinoma. J Clin Lab Anal. 16:146–150. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Marin MC, Jost CA, Brooks LA, Irwin MS,
O’Nions J, Tidy JA, et al: A common polymorphism acts as an
intragenic modifier of mutant p53 behaviour. Nat Genet. 25:47–54.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alhopuro P, Ylisaukko-Oja SK, Koskinen WJ,
Bono P, Arola J, Järvinen HJ, et al: The MDM2 promoter polymorphism
SNP309T→G and the risk of uterine leiomyosarcoma, colorectal
cancer, and squamous cell carcinoma of the head and neck. J Med
Genet. 42:694–698. 2005.
|
|
31
|
Giordana MT, Duo D, Gasverde S, Trevisan
E, Boghi A, Morra I, et al: MDM2 overexpression is associated with
short survival in adults with medulloblastoma. Neuro Oncol.
4:115–122. 2002.PubMed/NCBI
|
|
32
|
Haitel A, Wiener HG, Baethge U, Marberger
M and Susani M: Mdm2 expression as a prognostic indicator in clear
cell renal cell carcinoma: comparison with p53 overexpression and
clinic pathological parameters. Clin Cancer Res. 6:1840–1844.
2000.PubMed/NCBI
|
|
33
|
Polsky D, Melzer K, Hazan C, Panageas KS,
Busam K, Drobnjak M, et al: HDM2 protein overexpression and
prognosis in primary malignant melanoma. J Natl Cancer Inst.
94:1803–1806. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ranuncolo SM, Varela M, Morandi A, Lastiri
J, Christiansen S, Bal de Kier Joffé E, et al: Prognostic value of
Mdm2, p53 and p16 in patients with astrocytomas. J Neurooncol.
68:113–121. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hoff CM, Grau C and Overgaard J: Effect of
smoking on oxygen delivery and outcome in patients treated with
radiotherapy for head and neck squamous cell carcinoma - a
prospective study. Radiother Oncol. 103:38–44. 2012. View Article : Google Scholar : PubMed/NCBI
|