Introduction
Prostate cancer (PCa) is the most frequently
diagnosed male cancer in the developed world with increasing rates
in the developing countries (1).
DNA methylation of the promoter region is one of the regulatory
mechanisms of gene expression in prostate cancer (2). Not only tumor-suppressor genes, but
also numerous miRNAs can be silenced by methylation of the relavant
promoters in prostate cancer (3).
microRNAs (miR) are a conserved class of small
non-coding RNAs (approximately 22 nucleotides) which usually cause
gene silencing via translational repression or degradation of
specific mRNA (4). microRNA genes
are frequently located in cancer-associated genomic regions or at
certain fragile sites. Their aberrant expression has been shown to
be significantly involved in human cancer (5–7). In
particular, miR-375 was initially believed to be a tumor
suppressor, as it targets certain oncogenes and its expression
levels are significantly low in most tumors, including esophageal
squamous cell carcinoma (8), oral
squamous cell carcinoma (9),
gastric carcinomas (10),
pancreatic cancer (11),
hepatocellular carcinoma (12),
melanoma (13), squamous cervical
cancer (14) and head and neck
squamous cell carcinomas (15).
However, recently studies have indicated that
miR-375 is overexpressed in prostate and breast cancers, suggesting
that it might exert an oncogene function in these two cancer types
(16,17). Both breast cancer and prostate
cancer are sex hormone-dependent for their growth and progression,
and are also remarkably similar in some physiological and
pathological phenomena (18). For
example, previous studies have shown that estrogen receptor α
(ERα), a female hormone recentor, is involved in the DNA
hypomethylation and the expression of miR-375 in breast cancer
cells (17). Androgen receptor (AR)
is a male hormone receptor, which plays a crucial role in the
initiation and growth of prostate cancer (19). However, the relationship between
androgen receptor and DNA methylation and the expression of miR-375
in prostate cancer cells is not yet known and therefore the subject
of the present study. We report that AR is negatively correlated
with the methylation-mediated transcriptional repression of miR-375
in human prostate cancer cells.
Materials and methods
Cell lines
LNCaP, 22Rv1, PC-3 and DU145 cell lines were
obtained from the American Type Culture Collection (ATCC, Manassas,
VA, USA). C4-2 cells were obtained from UroCor (Oklahoma City, OK,
USA). PC3/AR cells are a stable cell line which were transfected
with a plasmid containing the AR cDNA sequence; PC3/neo cells
contained the identical vector lacking the AR cDNA sequence
(20). Cells were maintained
according to the protocols of the manufacturer and provider. In the
NCBI/GEO website, the GDS1699 database is from AR-positive PCa
cells (LAPC-4, MDA2a, MDA2b, LNCaP, 22RV1 cells) and AR-negative
PCa cells (PPC-1, PC3 and DU145 cells).
Quantitative real-time PCR analysis of
miRNA expression
Total RNA were extracted using the RNeasy Plus Mini
kit (Qiagen, Valencia, CA, USA) from various PCa cell lines.
Reverse transcription was carried out using the TaqMan MicroRNA
Reverse Transcription kit (Applied Biosystems, Foster City, CA,
USA). Relative quantities of miR-375 were performed with a 7500
Real-Time PCR System (Applied Biosystems) by using
TaqMan® microRNA Assays (Applied Biosystems). Gene
expression was normalized by the endogenous control RNU44, and the
Ct values were calculated using the ΔΔCt method.
RNAi
Specific AR shRNA sequence (5-GGTGTCACTATG
GAGCTCTCA-3) was designed as previously described (21). AR shRNA was cloned into the pLKO.1-
scramble cloning vector (Addgene plasmid 10879). Procedures of
vector construct and lentiviral production and infection were
according to the online protocol: http://www.addgene.org/tools/protocols/plko/#B. At
72-h post-infection, total RNA and bisulfite conversion DNA were
prepared from LNCaP AR RNAi cells (LNCaP-sh-AR) and RNAi control
cells (LNCaP-sh-control).
Cytospin and immunofluorescence
For immunocytochemical analysis of LNCaP AR RNAi
cells (LNCaP-sh-AR) and RNAi control cells (LNCaP-sh-control),
1×104 infected cells (72 h) were washed and resuspended
in 200 μl of phosphate-buffered saline (PBS). The preparation cells
were spun down onto coated slides using a ThermoShandon Cytospin 4
apparatus (Thermo Shandon Inc., Pittsburgh, PA, USA) at 1500 rpm
for 5 min. The slides were then air-dried for 5 min and fixed for
10 min in 4% paraformaldehyde (Dingguo Biotechnology Co., Ltd.,
Beijing, China) at room temperature. The fixed cells were washed
three times with PBS and incubated for 10 min in 0.5% Triton X-100.
Cells were washed carefully and incubated for 30 min in 10% goat
serum and then for overnight at 4°C with a primary rabbit
anti-androgen receptor antibody (Epitomics, Burlingame, CA, USA)
and subsequently with secondary Alexa Fluor 488-conjugated donkey
anti-rabbit IgG (Molecular Probes, Eugene, OR, USA). Coverslips
were mounted with VectaShield mounting media containing
4,6-diamidino-2-phenyindole, dilactate (DAPI) (Vector, Burlingame,
CA, USA). Epifluorescence microscopy was performed with a Nikon
microscope (Nikon, Melville, NY, USA).
Sodium bisulfite modification and
sequencing
Cells were directly subjected to bisulfite
conversion by using the EZ DNA methylation-direct kit (Zymo
Research, Irvine, CA, USA) as per manufacturer's protocol. The
forward primer: 5-GGGGATTGAATAGGTAGTATAAG-3 and reverse primer:
5-ATAATCTCCTAATCCTAATCTTCC-3 were used for miR-375 bisulfite PCR.
Ex Taq HS DNA polymerase (Takara Biotechnology Co., Ltd., Dalian,
China) were employed in PCR amplification, and the conditions were
95°C, 5 min, 39 cycles (95°C, 30 sec; 58°C, 30 sec; 72°C, 30 sec),
72°C, 7 min. The PCR products were then ligated into the pMD19-T
Simple Vector (Takara Biotechnology Co.). Colonies (n=10) were
selected per PCa cell line and sequenced by Shanghai Shenggong
Biotech (Shanghai, China).
Treatment of cells with
5-Aza-2′-deoxycytidine
The DU-145 and PC-3 cells were plated in T-25 flask.
Subconfluent cells (60–70% confluent) were maintained in medium
supplemented with 5 μM of 5-Aza-2′-deoxycytidine (5-Aza-dC)
(Sigma-Aldrich, St. Louis, MO, USA) and cells treated with vehicle
(dimethylsulfoxide, DMSO) served as control. The cells were
incubated for 5 days with a change of culture medium every 24
h.
DNA methyltransferase analysis of PCa
cells
PCa cell nuclear extracts were prepared using the
nuclear and cytoplasmic protein extraction kit (Bioteke Corp.,
China) in accordance with the manufacturer's protocol. Total DNA
methyltransferase activity was assayed using an EpiQuik DNA
Methyltransferase Activity/Inhibition Assay kit (Epigentek,
Brooklyn, NY, USA), according to the manufacturer's protocol. The
relative expression values of DNMT1 (GDS1699/38832), DNMT3A
(GDS1699/21176), DNMT3B (GDS1699/16487) were obtained from the NCBI
GEO website.
Data analysis
Data were analyzed using the SPSS 13.0 software
(SPSS Inc., Chicago, IL, USA) and Prism GraphPad 5 (GraphPad
Software, La Jolla, CA, USA). Statistical analysis was performed
using, paired and unpaired sample t-test. p-values <0.05 were
considered significant.
Results
Expression levels of miR-375 are higher
in PCa AR-positive cell lines than in PCa AR-negative cell
lines
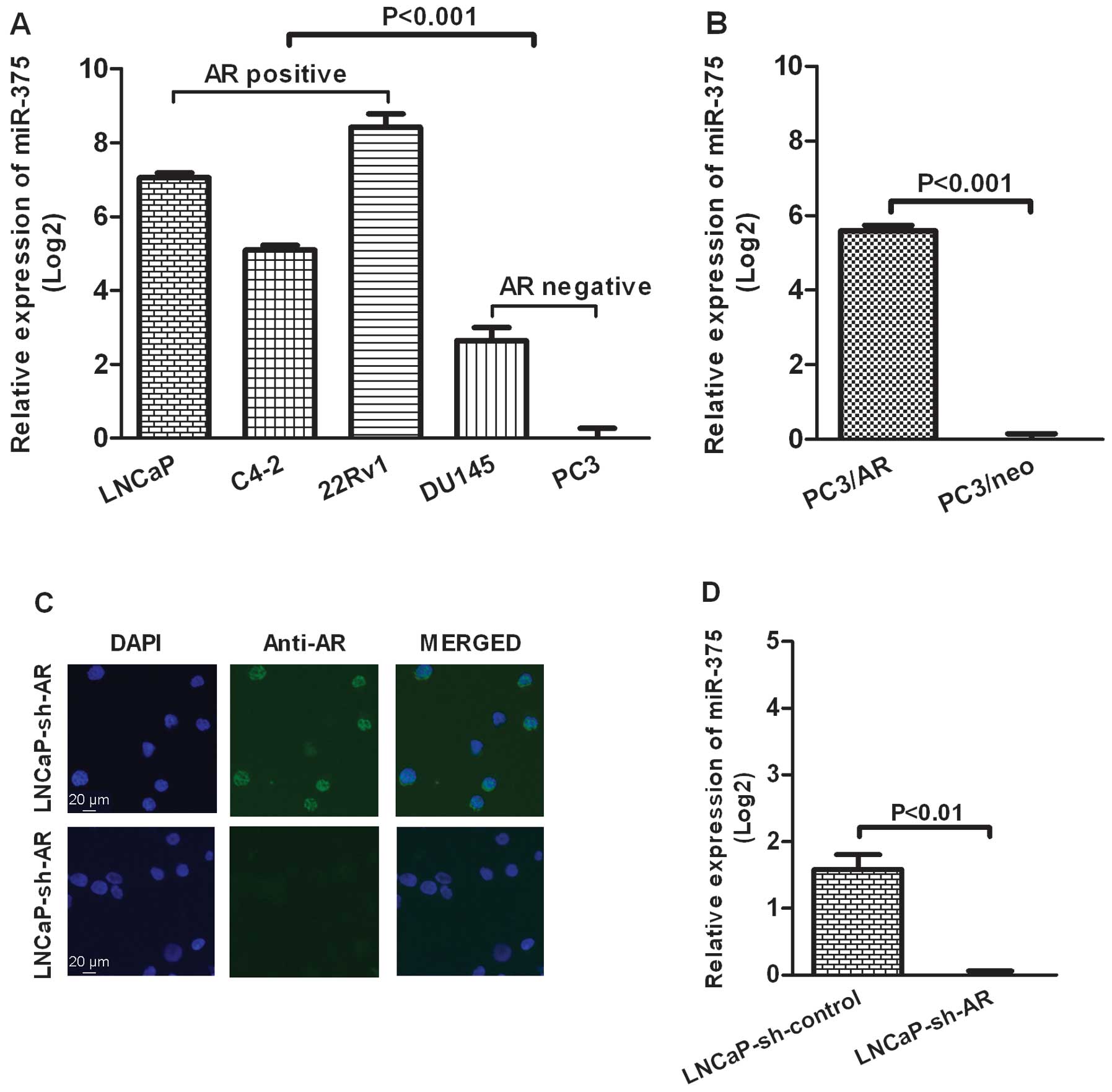
To examine the expression levels of miR-375 in PCa
cell lines, several AR-positive cell lines (LNCaP, C4-2, 22Rv1) and
AR-negative cell lines (PC3 and DU 145) were analyzed by real-time
PCR. The results revealed that the expression levels of miR-375
were significantly higher in PCa AR-positive cell lines than in PCa
AR-negative cell lines (p<0.001, Fig. 1A). Additionally, PC3/AR cells, which
are PC3 cells engineered to express AR (20), showed a high expression level of
miR-375 (p<0.001, Fig. 1B).
While knocking down of AR by lentivious AR shRNA interference in
LNCaP cells (Fig. 1C) reversed the
high level of miR-375 (p<0.01, Fig.
1D).
microRNA-375 promoter shows a
hypermethylation phenotype in AR-negative PCa cells
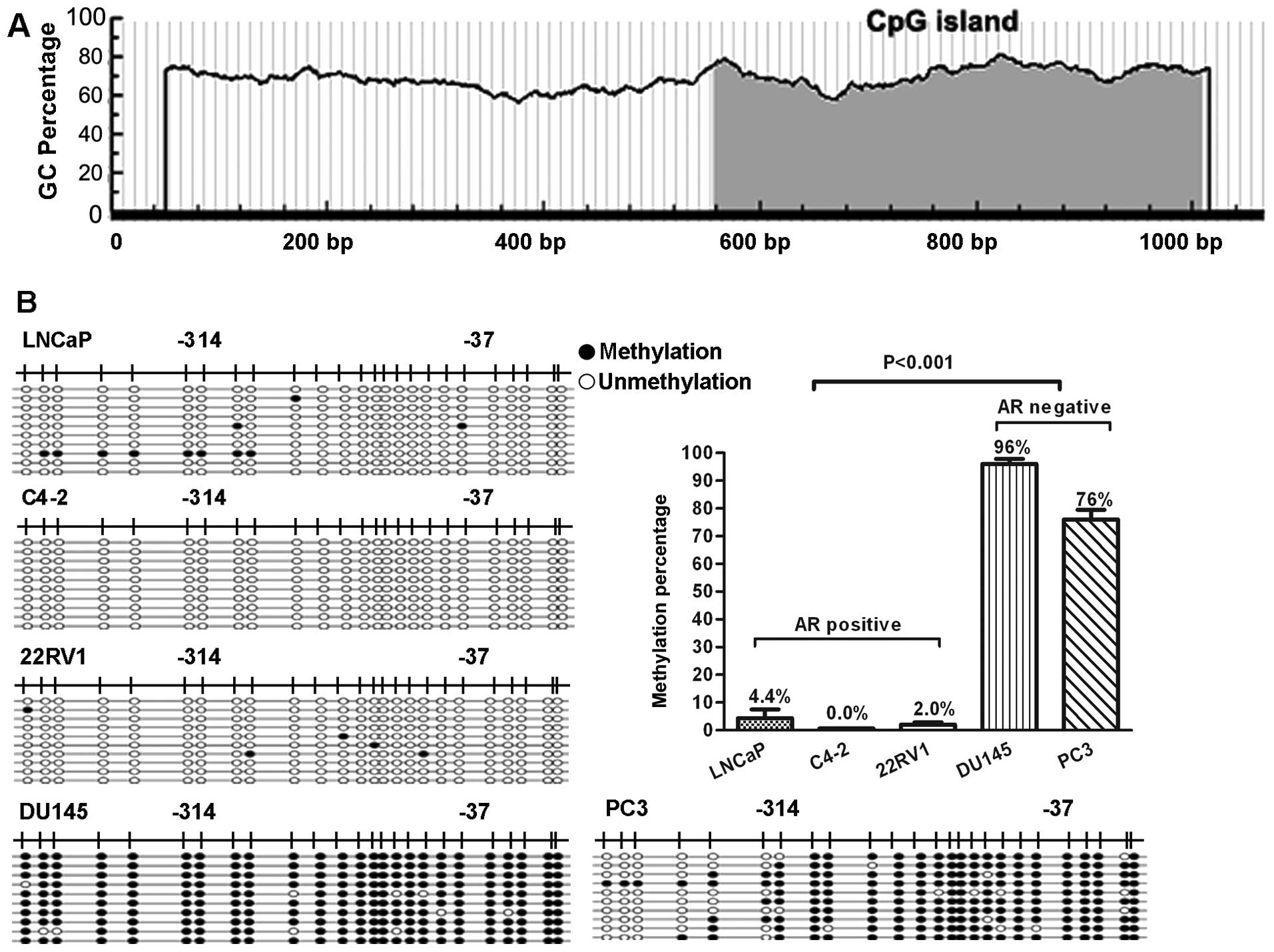
To determine whether DNA methylation was involved in
the regulation of miR-375 gene expression, the 1 kb upstream of
miR-375 gene was scanned for potential CpG islands using the Li Lab
program at http://www.urogene.org/cgi-bin/methprimer/methprimer.cgi
(22).
This analysis revealed a CpG island in the region of
miR-375 promoter (Fig. 2A). To
investigate the methylation status of the island, the genomic DNAs
of PCa cells were isolated and pyrosequenced using bisulfite
sequencing (BSP). We found that the CpG islands in the miR-375
promoter region were hypermethylated (76–96%) in AR-negative PCa
cells (PC3 and DU145), but only very limited methylation (0.0–4.4%)
was seen in AR-positive PCa cells (LNCaP, C4-2, 22Rv1) (p<0.001,
Fig. 2B). In addition, PC3/AR cells
also showed a very low methylation (0.4%) status in the miR-375
promoter region, while the PC3-neo cells remained hypermethylation
(78%) status in the miR-375 promoter region (p<0.001, Fig. 2C). However, knocking down AR by
lentivious shRNA interference in LNCaP cells did not change the
hypomethylation status of miR-375 promoter (p>0.05, Fig. 2D).
5-Aza-dC reversed the expression of
miR-375 in AR-negative DU145 and PC3 cell lines
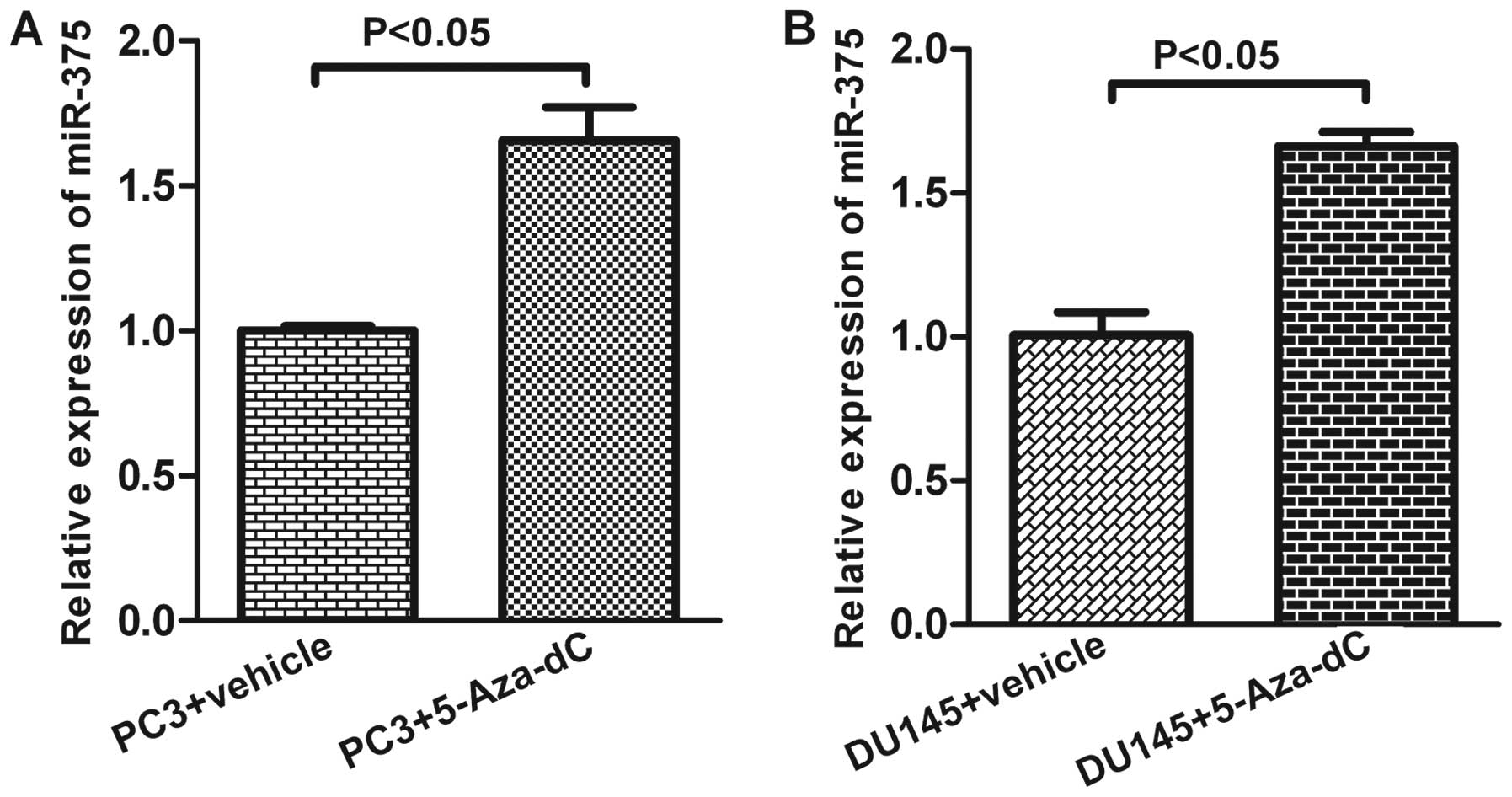
To verify whether the low expression level of the
miR-375 resulted in the promoter hypermethylation status of the
AR-negative PCa cells, a demethylating agent 5-Aza-dC was used to
treat AR-negative PC-3 and DU145 cells. Real-time PCR analysis
revealed an elevation in the expression of miR-375 in these cells 5
days post 5-Aza-dC (5 μM) treatment (p<0.05, Fig. 3).
Expression of DNA methyltransferase
enzymes in PCa cell lines
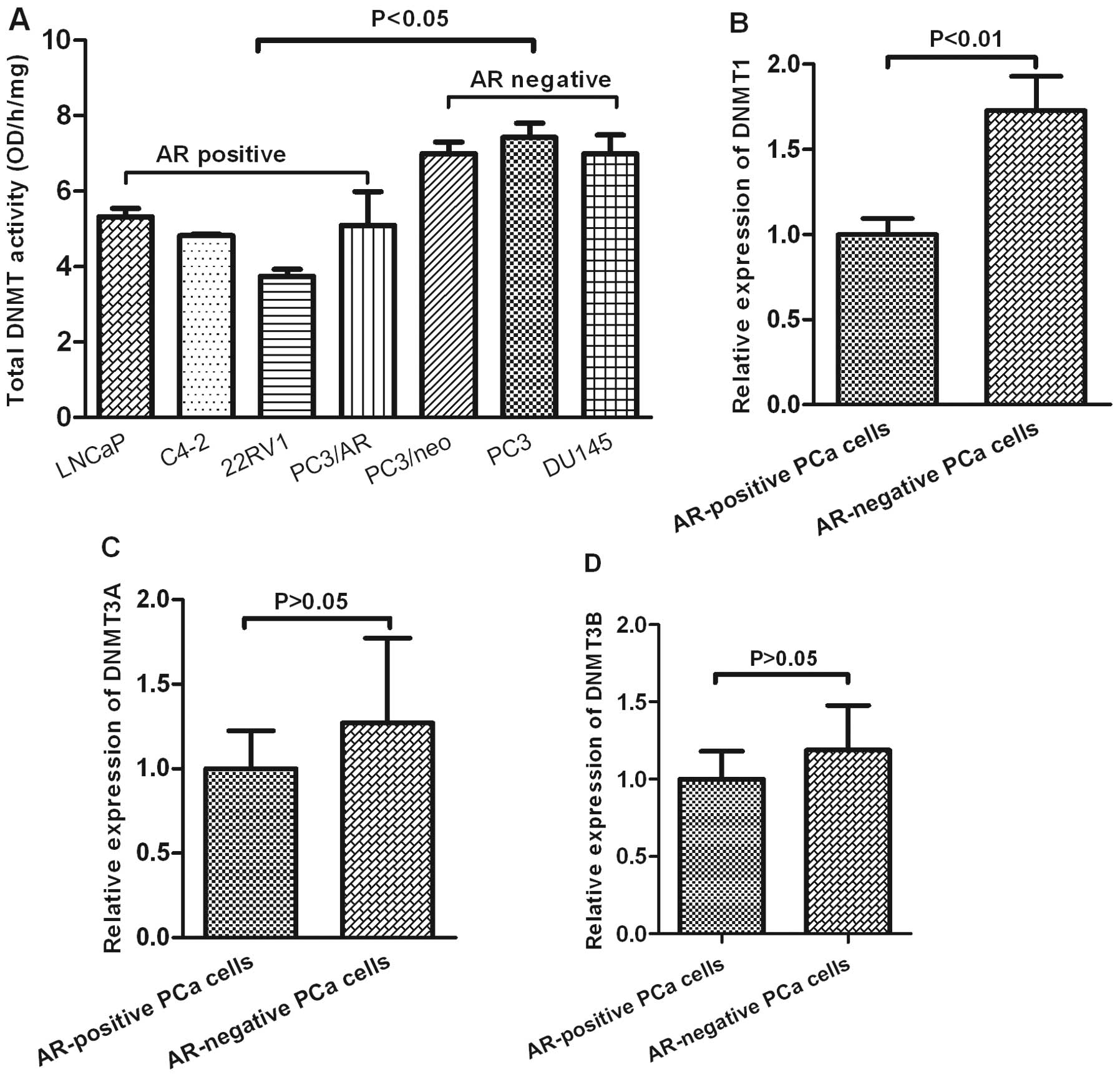
To study whether the DNA methyltransferase enzymes
were involved in the promoter methylation status of miR-375, the
total activity levels of DNMTs were measured by an ELISA-based DNMT
activity assay. As shown in Fig.
4A, total DNMT activity was higher in AR-negative PCa cells
(PC3/neo, PC3 and DU145) than in AR-positive PCa cells (LNCaP,
C4-2, 22Rv, PC3/AR) (p<0.05, Fig.
4A). We further analyzed the expression of DNMT1, DNMT3A and
DNMT3B in PCa cell lines based on the study GDS1699 from the
NCBI/GEO database. This analysis revealed that the levels of DNMT1
were much higher in AR-negative PCa cells than in AR-positive PCa
cells (p<0.01). However, there was no significant difference in
the expression of DNMT3A and DNMT3B between AR-positive PCa and
AR-negative PCa cells.
Discussion
We have presented evidence that AR-negative PCa
cells have high activity levels of total DNMT, hypermethylation of
the miR-375 promoter and lower expression levels of miR-375. Yet,
these results were exactly reversed in AR-positive PCa cells.
Hence, we propose that the total DNMT activity is negatively
regulated by the AR, which results in hypomethylation or
hypermethylation of the miR-375 promoter in AR-positive PCa cells
or AR-negative PCa cells, respectively. The methylation-mediated
transcriptional repression then determines the different expression
level of miR-375 in these PCa cells.
The growth and progression of prostate cancer depend
largely on AR signaling (21). AR
is a key transcription factor which is activated by androgens and
transduces androgen signaling in prostate cells (21). In prostate cancer, many oncogenic
genes such as prostate-specific antigen (PSA) (23), Cdk1 and Cdk2 (24), PMEPA1 (25), FGF8 (26) as well as TMPRSS2 (27) are closely associated with AR. A
recent study has shown that miR-375 is an oncogenic miRNA and
targets the Sec23A tumor suppressor gene in prostate cancer
(16). However, it is unclear how
AR interacts with miR-375. Our results demonstrate that the AR
status can influence the expression of miR-375. While AR-negative
PCa cells show a low level of miR-375, AR-positive PCa cells
display a high level of miR-375. Overexpression of AR in PC3 cells
upregulates the level of miR-375, while knockdown of AR in LNCaP
cells downregulates the level of miR-375.
Given that the hypermethylation of DNA is one of the
important mechanism to silence gene expression, we further
investigated whether the low expression level of miR-375 in
AR-negative PCa cells was due to the effect of methylation. Our
findings indicate that the promoter of miR-375 is hypermethylated
in AR-negative PCa cell lines. In contrast, the promoter of miR-375
is hardly methylated in AR-positive PCa cells. Overexpression of AR
in PC3 cells reversed the hypermethylation of the miR-375 promoter.
This differential methylation patterns in AR-negative and
AR-positive cells were correlated with the expression of miR-375.
These results suggest that DNA methylation maybe one of the
important factors to silence the miR-375 gene expression in
AR-negative PCa cells. The conclusion is further confirmed by our
finding that the low expression level of miR-375 could be reversed
through using a demethylating agent 5-Aza-dC in AR-negative DU145
and PC3 cells that otherwise show a hypermethylation pattern.
It should be pointed out that unexpectedly,
knockdown of AR in LNCaP cells did not change the methylation
status of miR-375. The cells still retain a hypomethylation status
after AR knockdown. This might have been due to the lentivious AR
shRNA that could be only transient in LNCaP cells, which are highly
dependent on AR; yet, the PC3/AR is a stable cell line that has
been developed for a long time (20). Additional studies are needed to
clarify this point.
DNA methylation is mediated by a family of DNA
methyltransferase enzymes (DNMTs). It is well known that DNMT
overexpression induces aberrant hypermethylation, which contributes
to the silence of genes in various cancer cells (28–31).
Our studies also show that AR-negative PCa cells, with a
hypermethylator status of the miR-375, have a high level of total
DNMT activity. Among these multiple DNMT isoforms, DNMT3A and
DNMT3B are responsible for creating global DNA methylation patterns
during embryogenesis and gametogenesis (32), yet Dnmt1 is in charge of maintaining
the established DNA methylation patterns (33). From the NCBI/GEO database, we
further found that the DNMT1, but not DNMT3A and DNMT3B, was
overexpressed in AR-negative PCa cells. According to the above
results, we propose that elevated total DNMT, especially DNMT1, may
play an important role in the hypermethylator phenotype in human
AR-negative PCa cell lines. Nevertheless, the PCa cell lines used
in NCBI/GEO database are not exactly the same as in our
experiments. Hence, this hypothesis needs further confirmation.
In conclusion, this study indicates that the AR
regulated expression level of DNMTs is likely one of the mechanisms
to influence the methylation status of the miR-375 promoter, which
in turn regulates the expression of miR-375.
Acknowledgements
The study was supported by funds to W.-Q. Gao from
the National Natural Science Foundation of China (81130038), the
Chinese Ministry of Science and Technology (2012CB966800), Science
and Technology Commission of Shanghai Municipality (Pujiang
program), Shanghai Health Bureau Key Disciplines and Specialties
Foundation, Shanghai Education Committee Key Discipline and
Specialties Foundation (J50208) and K.C. Wong Foundation, and to M.
Chu from Shanghai Postdoctoral Scientific Program of China
(12R21415100).
References
|
1
|
Baade PD, Youlden DR and Krnjacki LJ:
International epidemiology of prostate cancer: geographical
distribution and secular trends. Mol Nutr Food Res. 53:171–184.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Majumdar S, Buckles E, Estrada J and
Koochekpour S: Aberrant DNA methylation and prostate cancer. Curr
Genomics. 12:486–505. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Formosa A, Lena AM, Markert EK, et al: DNA
methylation silences miR-132 in prostate cancer. Oncogene.
32:127–134. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen K and Rajewsky N: The evolution of
gene regulation by transcription factors and microRNAs. Nat Rev
Genet. 8:93–103. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Calin GA, Sevignani C, Dumitru CD, et al:
Human microRNA genes are frequently located at fragile sites and
genomic regions involved in cancers. Proc Natl Acad Sci USA.
101:2999–3004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sotiropoulou G, Pampalakis G, Lianidou E
and Mourelatos Z: Emerging roles of microRNAs as molecular switches
in the integrated circuit of the cancer cell. RNA. 15:1443–1461.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fang YX and Gao WQ: Roles of microRNAs
during prostatic tumorigenesis and tumor progression. Oncogene. Mar
4–2013.(Epub ahead of print).
|
|
8
|
Komatsu S, Ichikawa D, Takeshita H, et al:
Circulating microRNAs in plasma of patients with oesophageal
squamous cell carcinoma. Br J Cancer. 105:104–111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lajer CB, Nielsen FC, Friis-Hansen L, et
al: Different miRNA signatures of oral and pharyngeal squamous cell
carcinomas: a prospective translational study. Br J Cancer.
104:830–840. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takeshita K, Suetake I, Yamashita E, et
al: Structural insight into maintenance methylation by mouse DNA
methyltransferase 1 (Dnmt1). Proc Natl Acad Sci USA. 108:9055–9059.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Basu A, Alder H, Khiyami A, Leahy P, Croce
CM and Haldar S: MicroRNA-375 and microRNA-221: potential noncoding
RNAs associated with antiproliferative activity of benzyl
isothiocyanate in pancreatic cancer. Genes Cancer. 2:108–119. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu AM, Poon RT and Luk JM: MicroRNA-375
targets Hippo-signaling effector YAP in liver cancer and inhibits
tumor properties. Biochem Biophys Res Commun. 394:623–627. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mazar J, DeBlasio D, Govindarajan SS,
Zhang S and Perera RJ: Epigenetic regulation of microRNA-375 and
its role in melanoma development in humans. FEBS Lett.
585:2467–2476. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang F, Li Y, Zhou J, et al: miR-375 is
down-regulated in squamous cervical cancer and inhibits cell
migration and invasion via targeting transcription factor SP1. Am J
Pathol. 179:2580–2588. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nohata N, Hanazawa T, Kikkawa N, et al:
Tumor suppressive microRNA-375 regulates oncogene AEG-1/MTDH in
head and neck squamous cell carcinoma (HNSCC). J Hum Genet.
56:595–601. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Szczyrba J, Nolte E, Wach S, et al:
Downregulation of Sec23A protein by miRNA-375 in prostate
carcinoma. Mol Cancer Res. 9:791–800. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Simonini PdSR, Breiling A, Gupta N, et al:
Epigenetically deregulated microRNA-375 is involved in a positive
feedback loop with estrogen receptor α in breast cancer cells.
Cancer Res. 70:9175–9184. 2010.PubMed/NCBI
|
|
18
|
Shibata A and Minn AY: Perinatal sex
hormones and risk of breast and prostate cancers in adulthood.
Epidemiol Rev. 22:239–248. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Heinlein CA and Chang C: Androgen receptor
in prostate cancer. Endocr Rev. 25:276–308. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Le Dai J, Maiorino CA, Gkonos PJ and
Burnstein KL: Androgenic up-regulation of androgen receptor cDNA
expression in androgen-independent prostate cancer cells. Steroids.
61:531–539. 1996.PubMed/NCBI
|
|
21
|
Guo Z and Qiu Y: A new trick of an old
molecule: androgen receptor splice variants taking the stage?! Int
J Biol Sci. 7:815–822. 2011.PubMed/NCBI
|
|
22
|
Li L-C and Dahiya R: MethPrimer: designing
primers for methylation PCRs. Bioinformatics. 18:1427–1431. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Whitbread AK, Veveris-Lowe TL, Lawrence
MG, Nicol DL and Clements JA: The role of kallikrein-related
peptidases in prostate cancer: potential involvement in an
epithelial to mesenchymal transition. Biol Chem. 387:707–714. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gregory CW, Hamil KG, Kim D, et al:
Androgen receptor expression in androgen-independent prostate
cancer is associated with increased expression of
androgen-regulated genes. Cancer Res. 58:5718–5724. 1998.PubMed/NCBI
|
|
25
|
Xu LL, Shi Y, Petrovics G, et al: PMEPA1,
an androgen-regulated NEDD4-binding protein, exhibits cell growth
inhibitory function and decreased expression during prostate cancer
progression. Cancer Res. 63:4299–4304. 2003.
|
|
26
|
Gnanapragasam VJ, Robson CN, Neal DE and
Leung HY: Regulation of FGF8 expression by the androgen receptor in
human prostate cancer. Oncogene. 21:5069–5080. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin B, Ferguson C, White JT, et al:
Prostate-localized and androgen-regulated expression of the
membrane-bound serine protease TMPRSS2. Cancer Res. 59:4180–4184.
1999.PubMed/NCBI
|
|
28
|
Yoo J and Medina-Franco JL: Discovery and
optimization of inhibitors of DNA methyltransferase as novel drugs
for cancer therapy. Drug Development - A Case Study Based Insight
into Modern Strategies. Rundfeldt C: InTech; pp. 3–22. 2011
|
|
29
|
Majumder S, Ghoshal K, Datta J, et al:
Role of de novo DNA methyltransferases and methyl CpG-binding
proteins in gene silencing in a rat hepatoma. J Biol Chem.
277:16048–16058. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ahluwalia A, Hurteau JA, Bigsby RM and
Nephew KP: DNA methylation in ovarian cancer: II. Expression of DNA
methyltransferases in ovarian cancer cell lines and normal ovarian
epithelial cells. Gynecol Oncol. 82:299–304. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jin B, Yao B, Li J-L, et al: DNMT1 and
DNMT3B modulate distinct polycomb-mediated histone modifications in
colon cancer. Cancer Res. 69:7412–7421. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Patra SK, Patra A, Zhao H and Dahiya R:
DNA methyltransferase and demethylase in human prostate cancer. Mol
Carcinogen. 33:163–171. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Okano M, Bell DW, Haber DA and Li E: DNA
methyltransferases Dnmt3a and Dnmt3b are essential for de novo
methylation and mammalian development. Cell. 99:247–257. 1999.
View Article : Google Scholar : PubMed/NCBI
|