Introduction
The insulin-like growth factor receptor (IGF-1R) is
a receptor tyrosine kinase that is widely expressed in normal human
tissues and upregulated in a number of human cancers including
colorectal cancer (CRC) (1–3). IGF-1R is comprised of three
components, two extracellular α-chains, that are involved in ligand
binding, two transmembrane spanning β-chains and an intracellular
tyrosine kinase (2,4,5). Both
IGF1 and IGF2 are ligands for IGF-1R and their binding induces
receptor autophosphorylation at the tyrosine kinase domain,
resulting in its activation by a conformational change leading to
stimulation of signaling cascades, including PI3K/Akt and MAPK
pathways (4–8). Activation of IGF-1R has been reported
to stimulate oncogenic cellular processes, including aberrant cell
survival mechanisms, transformation, motility, angiogenesis and
metastasis (2,3,9).
Previous work from our laboratory as well as other groups has shown
that inhibition of IGF-1R has been shown to impede tumorigenesis in
several human xenograft models (2,3,9,10).
IGF-IR plays a multifunctional role in human CRC
growth and is widely regarded as an attractive target for
anticancer drug treatment based on the observation that inhibition
of IGF-1R function results in apoptosis and inhibition of tumor
growth. Several pharmacological strategies are currently being
adopted in clinical trials to disrupt the IGF-IF signaling pathway.
This includes anti-receptor antibodies to reduce receptor
expression, small-molecule IGF-1R kinase inhibitors, and targeting
downstream IGF-1R signaling pathways with agents, such as Akt or
mTOR inhibitors (9). In this study,
we characterized the in vivo and in vitro effects of
MK-0646, a novel IGF-1R recombinant humanized monoclonal antibody.
It has been reported that MK-0646 binds to IGF-1R and triggers
receptor internalization and degradation thereby blocking IGF-1 and
II mediated cellular proliferation and survival (11). MK-0646 specifically targets IGF-1R
and does not cross-react with the insulin receptor (12). It is in phase II clinical trial at
present (13–16).
OSI-906 is a potent and highly selective small
molecule tyrosine kinase inhibitor which binds dually to IGF-1R and
IR and inhibits autophosphorylation (6,7). It is
also in phase II clinical trials at present (16). Initiation of apoptosis and
inhibition of cell proliferation following OSI-906 treatment
appears to be directly linked to Akt inhibition in various tumor
cell lines including lung, pancreatic and CRC cell lines (6,17). In
addition, OSI-906 has shown potent antitumor activity in
vivo in several xenograft models (18). Buck et al has shown that
OSI-906 reduces tumorigenicity in GEO CRC xenografts (18). However, the signaling mechanisms
associated with OSI-906-mediated cell death are poorly
understood.
The goal of the present study was to compare the
antagonistic effects of MK-0646 and OSI-906 in vivo and
in vitro and characterize mechanisms associated with
drug-induced cell death. We report for the first time the antitumor
activity of MK-0646 in IGF-1R-dependent CRC cells and demonstrate
that inhibition of IGF-1R leads to control of aberrant cell
survival signaling through the downregulation of XIAP and induction
of cell death.
Materials and methods
Cell lines
GEO and CBS cell lines used in this study were
originally developed from primary CRC tumors and have been
extensively characterized (19).
Cells were maintained at 37°C in humidified atmosphere of 5%
CO2 in a chemically defined serum-free medium consisting
of McCoy's 5A medium (Sigma-Aldrich, St. Louis, MO, USA)
supplemented with amino acids, pyruvate, vitamins, antibiotics and
growth factors transferring (4 μg/ml; Sigma-Aldrich), insulin (20
μg/ml; Sigma-Aldrich), and EGF (10 ng/ml; R&D Systems) as
previously described (20).
Supplemented McCoy's medium (‘SM’) is McCoy's 5A medium
supplemented with antibiotics and nutrients but lacking any growth
factors. Cells were routinely subcultured with a 0.25% trypsin
(Invitrogen, Carlsbad, CA, USA) in Joklik's medium (Invitrogen)
containing 0.1% EDTA. When cells were under growth factor
deprivation status (GFDS), they were cultured in SM medium without
growth factor or serum supplements for the indicated time periods
without medium change in between.
Antibodies
IGF-1Rβ, pIGF-1Rβ (Y1135) and p21
antibodies were obtained from Cell Signaling Technology Inc.
(Beverly, MA, USA). XIAP antibody was obtained from abcam. β-actin
and GAPDH antibodies were from Sigma-Aldrich (St. Louis, MO,
USA).
Pharmacological antagonists
MK-0646 was provided by Merck & Co. (Whitehouse
Station, NJ, USA) and OSI-906 was purchased from Chemitek,
Indianapolis, IN, USA.
Xenograft experiments
All experiments involving animals were approved by
the University of Nebraska Medical Center Institutional Animal Care
and Use Committee. The GEO and CBS cells were transfected with
green fluorescence protein (GFP). Exponentially growing GFP-labeled
GEO and CBS cells (~7 million cells/ml SF media) were inoculated
subcutaneously onto the dorsal surfaces of athymic nude male mice
and the growth of the tumor was monitored by biweekly measurements
using a caliper. Once xenografts were established (~50–100
mm3), MK-0646 or OSI-906 treatment was initiated and
continued for two weeks. MK-0646 was given by intraperitoneal (IP)
injection weekly (20 mg/kg) on both GEO and CBS xenografted mice
for three doses and formulation buffer was the vehicle. OSI-906 was
given by daily oral gavage (40 mg/kg) on GEO xenografted mice and
tartaric acid was the vehicle. Xenografts were harvested after 14
days of treatment for assessment of molecular effects by the two
agents.
Xenograft lysate preparation
Xenografts were harvested and snap frozen in liquid
nitrogen and stored at −80°C. Xenografts were first washed in cold
5% PBS and collected in lysis buffer [50 mmol/l Tris (pH 7.4), 100
mmol/l NaCl, 1% NP40, 2 mmol/l EDTA, 0.1% SDS, 50 mmol/l NaF, 10
mmol/l Na3VO4, 1 mmol/l phenylmethylsulfonyl
fluoride, 25 μg/ml β-glycerophosphate, and one protease inhibitor
cocktail tablet from Roche]. Crude xenograft lysates were
homogenized to shear DNA and lysed for 30 min on ice. Xenograft
lysates were then cleared by centrifugation at 13000 rpm for 20 min
at 4°C. Protein concentrations were determined by the Pierce
bicinchonimic acid protein assay (Pierce Biotechnology, Inc.,
Rockford, IL, USA).
Cell lysate preparation
GEO CRC cells were allowed to grow until 70–80%
confluent in 100-mm culture plates and were treated with different
concentrations of either MK-0646 or OSI-906 under growth factor
deprivation status (GFDS) for 48 h. Cells were washed in cold 5%
PBS and collected in lysis buffer. Crude cell lysates were
homogenized using a 21-gauge needle to shear DNA and lysed for 30
min on ice. Cell lysates were then cleared by centrifugation at
13000 rpm for 20 min at 4°C. Protein concentrations were determined
by the Pierce bicinchonimic acid protein assay (Pierce
Biotechnology, Inc.).
Western blot analysis
Protein (30–100 μg) was fractionated on an
acrylamide denaturing gel and transferred onto a nitrocellulose
membrane (Amersham Biosciences) by electroblotting. The membrane
was blocked with 5% nonfat dry milk in 1X TBST (50 mM Tris, pH 7.5,
150 mM NaCl, 0.05% Tween-20) for 1 h at room temperature or
overnight at 4°C. The membrane was then incubated with primary
antibodies for 1 h at room temperature or overnight at 4°C with 5%
nonfat dry milk in 1X TBST or 5% bovine serum albumin (BSA) in 1X
TBST according to the manufacturer's instructions. After washing
three times with 1X TBST for 10 min each, the membrane was
incubated with horseradish peroxidase-conjugated secondary antibody
(Amersham Biosciences) for 1 h at room temperature. After further
washing in 1X TBST three times for 10 min each, the proteins were
detected by the enhanced chemiluminescence system (Amersham
Biosciences).
Cell death assays
DNA fragmentation assays were performed on cells
treated with either MK-0646 or OSI-906 under GFDS. Cells were
seeded in 96-well plates and allowed to grow to 70–80% confluence.
The cells were then changed to Supplemented McCoy's medium (‘SM’)
and treated with various concentrations of either MK-0646 or
OSI-906 for 48 h. Assays were then performed using a cell death
ELISA kit (Roche Applied Science) according to the manufacturer's
protocol as previously described (21). The plate was read at 405 nm.
Inhibition of proliferation was assessed by the MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]
assay as previously described (22).
Cell cycle arrest analysis
GEO CRC cells were allowed to grow until 70–80%
confluent in 100-mm culture plates and were treated with different
concentrations of either MK-0646 or OSI-906 under growth factor
deprivation status (GFDS) for 48 h and cell cycle analysis was
performed as previously described (23).
Hematoxylin and eosin, TUNEL and Ki67
staining
Xenografts obtained from subcutaneous injection of
GEO cells were harvested and placed in 10% neutral buffer formalin
fixative for 12–24 h and then embedded in paraffin. Sections (4 μm)
were cut from paraffin-embedded blocks using a microtome and were
used for hematoxylin and eosin stains and immunohistochemical
characterizations. Serial sections were cut to complement the
hematoxylin and eosin sections and were stained with the Apo-tag
(Millipore) terminal nucleotidyl transferase mediated nick
end-labeling (TUNEL) kit following the manufacturer's protocol. The
apoptotic rate was determined semi-quantitatively by counting the
number of positively stained apoptotic bodies per 75-μm2
field at a magnification of ×20. Approximately 1000 total cells
were counted and the percentages of positively stained cells were
calculated. Four control and four treated slides for MK-0646 and
OSI-906 were analyzed, respectively. Staining was also performed
with IgG1 rabbit polyclonal antibody for Ki67 (Dako
Corp.). Ki67 is a non-histone nuclear antigen present in late
G1, G2, and S phases of the cell cycle but
not in G0. A 1:20 dilution was used and staining was
performed following the manufacturer's protocol. The proliferation
rate was determined semi-quantitatively by counting the number of
positively stained proliferative cells per 75-μm2 field
at a magnification of ×20. Approximately 1000 total cells were
counted and the percentages of positively stained cells were
calculated. Four control and four treated slides for MK-0646 and
OSI-906 were analyzed, respectively.
Immunohistochemistry
Sections (4 μm) were cut from the paraffin-embedded
xenograft tumor blocks, deparaffinized in histoclear, and
rehydrated in descending grades of ethanol. Endogenous peroxidase
activity was blocked with 3% hydrogen peroxide in water.
Immunostaining was performed for XIAP using an indirect detection
method (24). The staining was
accompanied by a negative control in which slides were incubated
with a matching blocking peptide to the primary antibody. Slides
were counterstained with hematoxylin. Specimens were processed on
the same day to eliminate any variability in conditions. Slides
were digitally photographed using the same settings.
Statistical analysis
Statistical significance was determined using
two-tailed Student's t-test with a p-value <0.05. All the
experiments were repeated three times independently to determine
consistency in the results. The results were expressed as mean ± SE
for three replicates for each treatment.
Results
Inhibition of IGF-1R is associated with
antitumor activity in vivo
The antitumor effects were determined in the
IGF-1R-dependent CRC sub-cutaneous xenograft tumors following
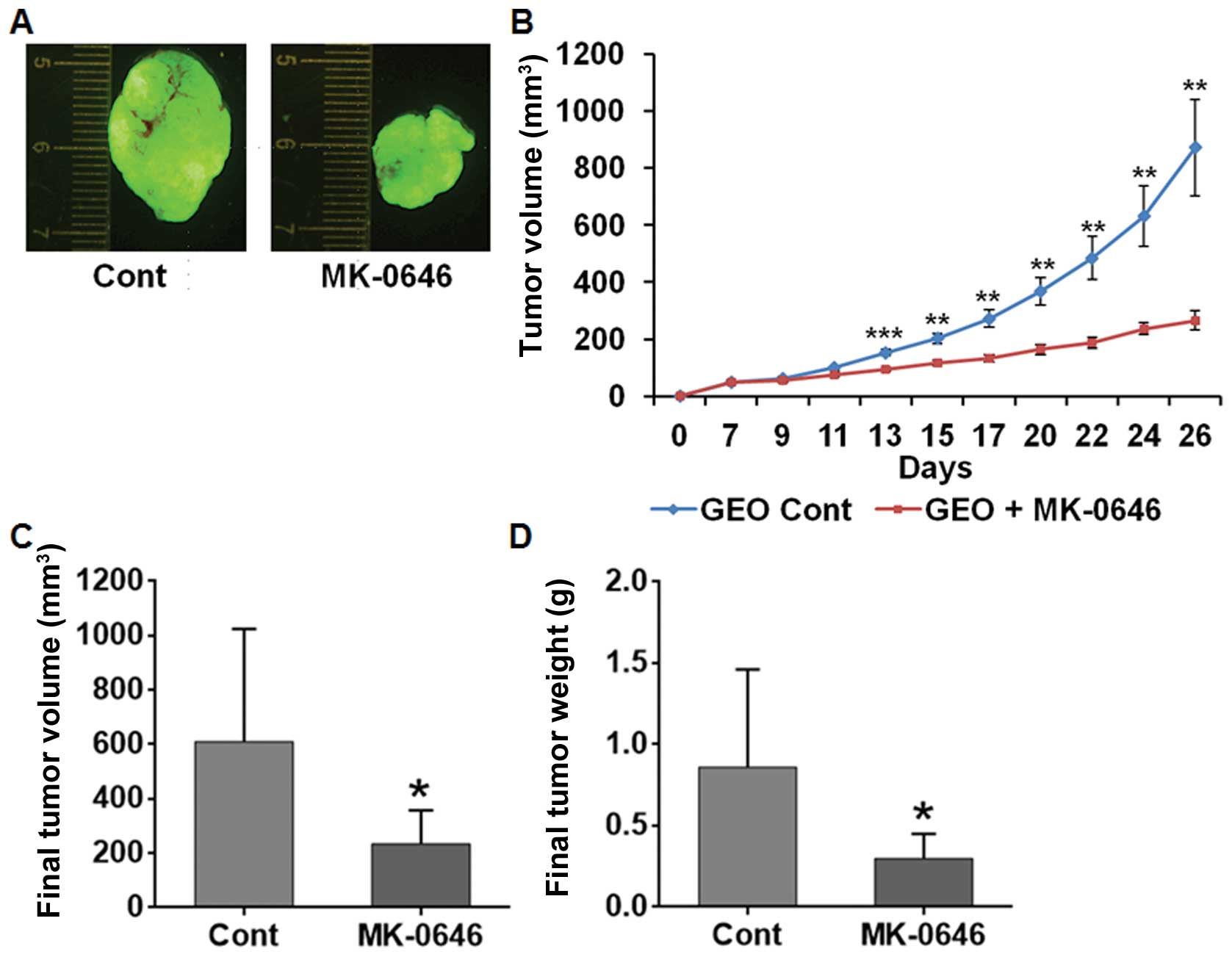
treatment with IGF-1R antagonist MK-0646 or OSI-906. MK-0646
treatment for three weeks at 20 mg/kg dose once weekly inhibited
the growth of the GEO xenograft tumors (Fig. 1A and B). Similar results were
obtained in MK-0646 treated CBS xenograft tumor (data not shown).
The final tumor volume and weight were significantly reduced in
MK-0646 treated xenografts compared with the control (Fig. 1C and D). Results obtained by daily
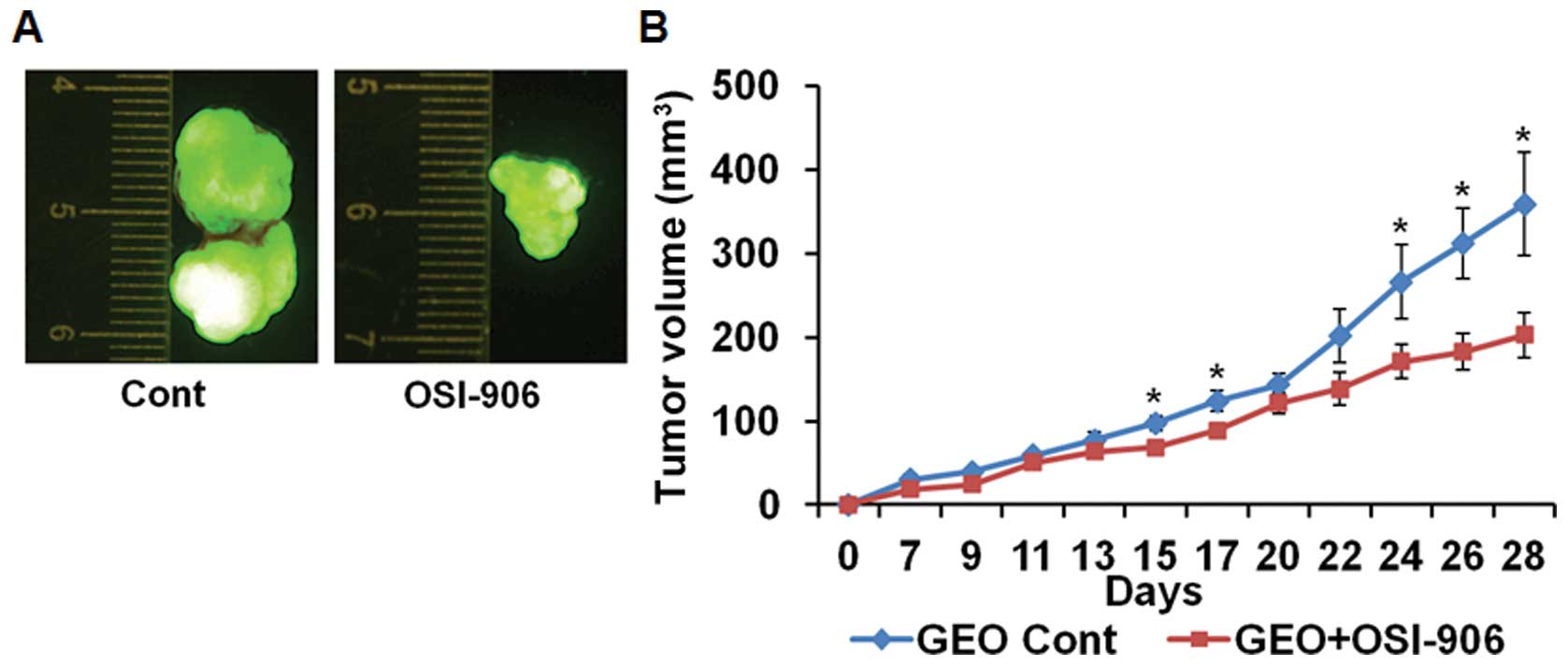
treatment of OSI-906 (40 mg/kg) for two weeks orally (Fig. 2A and B) were comparable to the
MK-0646 treated tumor xenografts showing decrease in tumor growth.
However, we observed ~10% body weight reduction in OSI-906 treated
mice compared with the MK-0646 treated mice (data not shown), which
may be attributed to inhibition of insulin receptor by the dual
kinase inhibitor.
IGF-1R inhibition of apoptosis in vivo
and in vitro
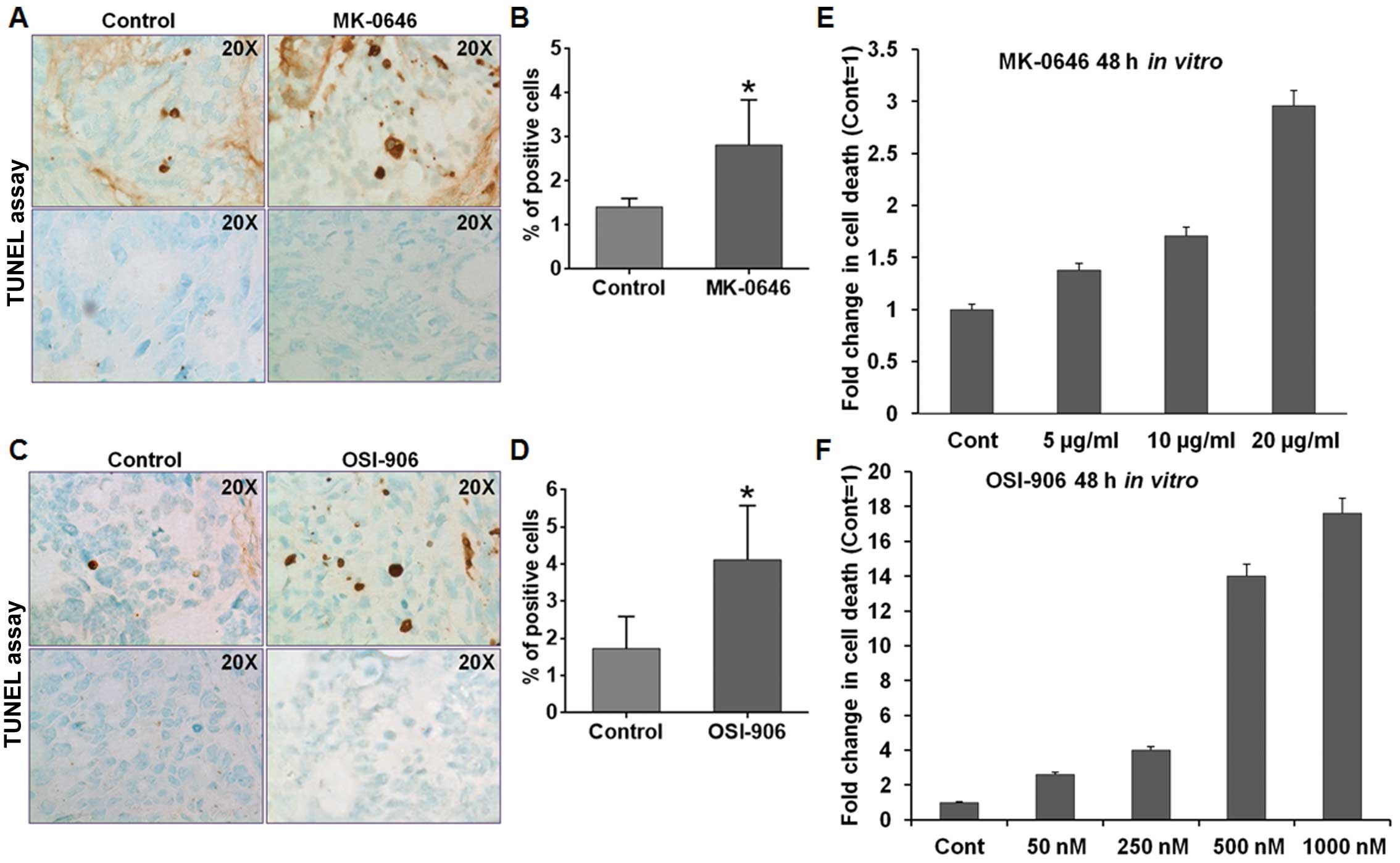
We assessed the apoptosis level of control and
drug-treated xenografts using TUNEL assays. Comparable results were
obtained for both IGF-1R antagonists. Both MK-0646 and OSI-906
treated GEO xenografts had statistically significant increase in
apoptosis (p<0.05) as compared with the control tumors (Fig. 3A–D). Based on the pro-apoptotic
effects of both MK-0646 and OSI-906 in vivo, DNA
fragmentation was performed in vitro on GEO CRC cells to
determine cell death following IGF-1R antagonist treatment
(Fig. 3E and F). In accordance with
the in vivo study, both MK-0646 and OSI-906 treatment showed
significant increases in apoptosis demonstrating that IGF-1R
inhibition elicits pro-apoptotic effects on CRC cells.
IGF-1R inhibition of cell proliferation
in vivo and in vitro
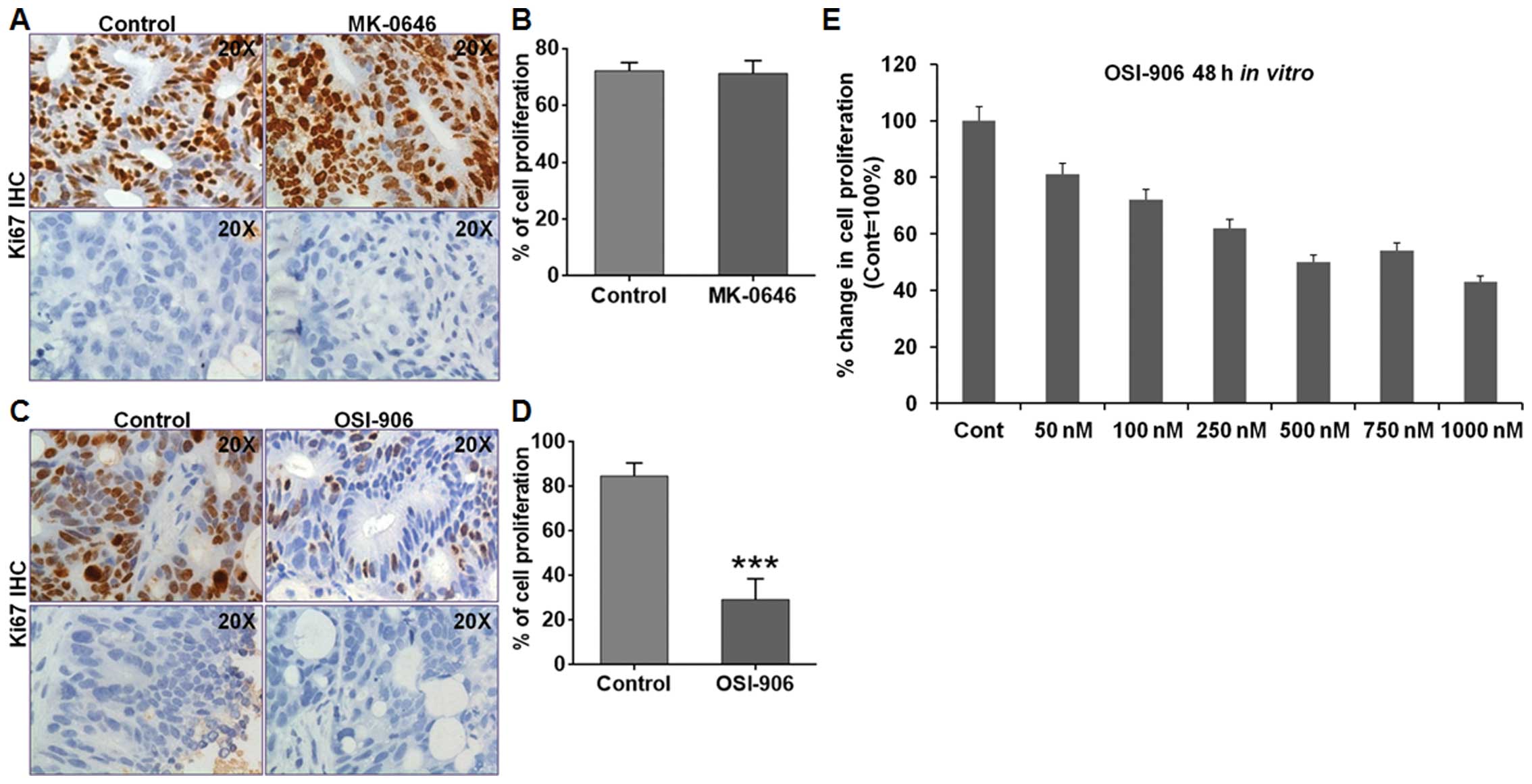
Ki67 staining was performed to assess the
proliferation level on MK-0646 and OSI-906 treated GEO xenografts.
MK-0646 treated GEO xenografts showed no change in the cell
proliferation compared with the control (Fig. 4A and B). However, OSI-906 treated
GEO xenografts showed a statistically significant reduction
(p<0.05) in cell proliferation compared with the control
(Fig. 4C and D). We also assessed
cell proliferation of GEO CRC cells in vitro by MTT assay
after treating with different concentrations of MK-0646 or OSI-906.
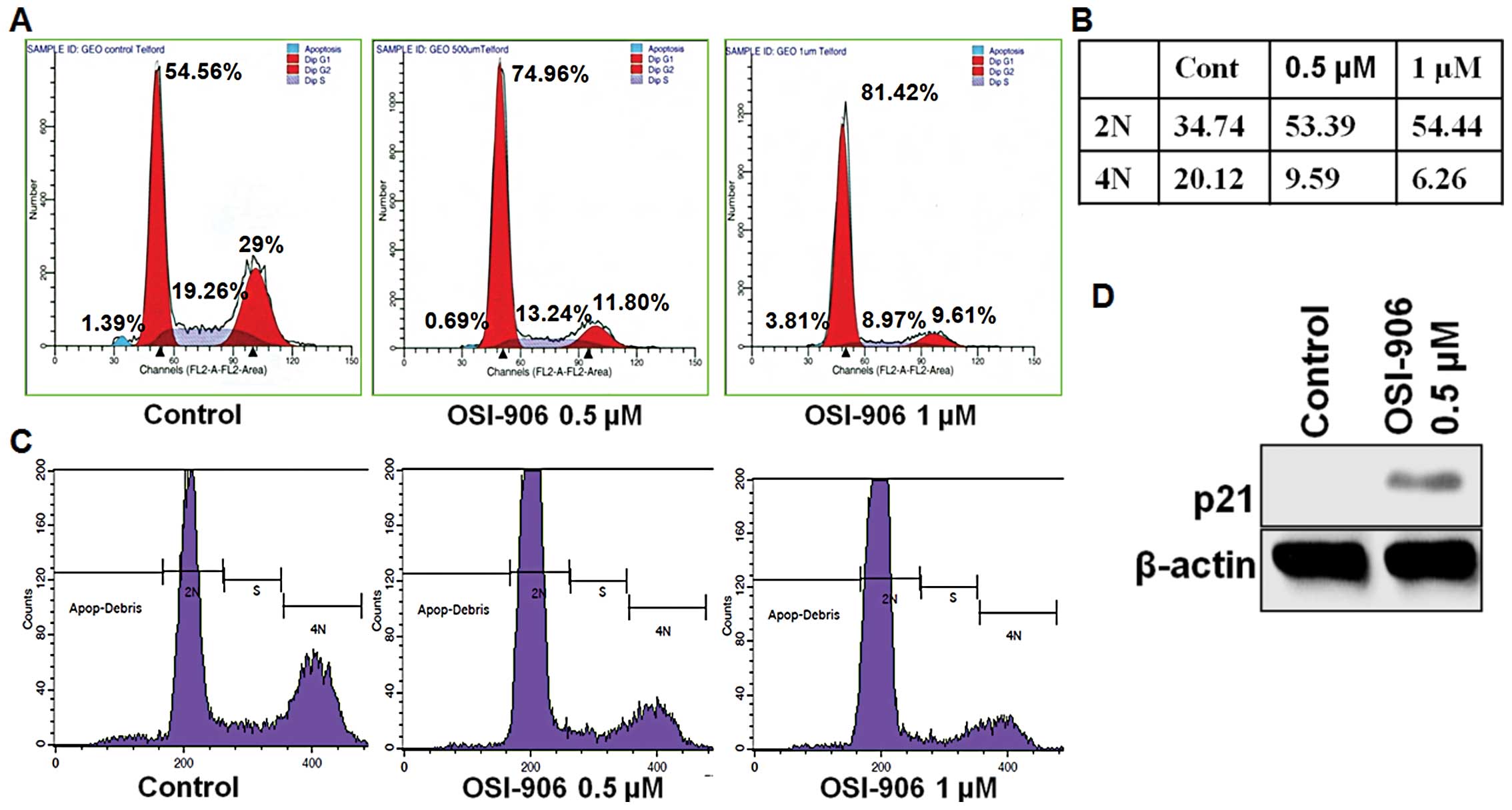
OSI-906 treatment showed a decrease in cell proliferation (Fig. 4E), altered cell cycle in
G0/G1 phase (Fig.
5A) and showed a decrease in the 4N DNA content (Fig. 5B and C). However, MK-0646 treatment
showed no effect on cell cycle (data not shown). OSI-906 treatment
also led to increase in p21 expression (Fig. 5D).
IGF-1R treatment in vivo and in vitro
decreases downstream substrates
Previous studies have shown that IGF-1R signaling
exerts its anti-apoptotic effect through the IRS1/IRS2/PI3K/Akt
pathway (25–28). We determined the effects of MK-0646
and OSI-906 on the IGF-1R and its downstream signaling pathways on
control and treated GEO CRC xenografts. The xenograft tumor samples
were analyzed for the expression of molecules associated with the
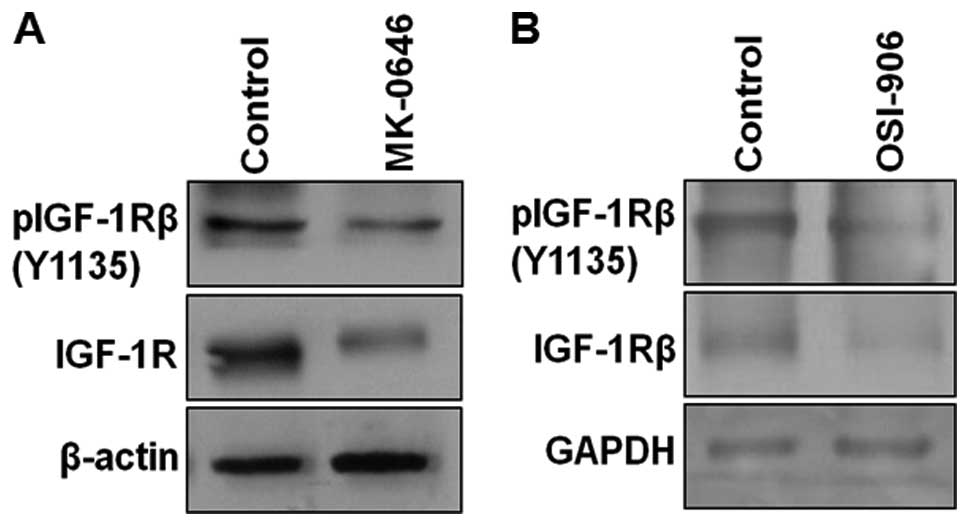
IGF-1R signaling pathway, including IGF-1Rβ (Y1135), Akt
and pAkt (S473). Inhibition of IGF-1R by MK-0646 and
OSI-906 led to the downregulation of IGF-1R and its phosphorylation
(Fig. 6A and B), confirming the
inhibitory effect of both antagonists on IGF-1R signaling. IGF-1R
inhibition by MK-0646 or OSI-906 showed dephosphorylation of Akt at
S473 site (data not shown). We next determined the
effects of IGF-1R inhibition in vitro, using GEO cells
following treatment with either MK-0646 or OSI-906. GEO cells were
treated with MK-0646 or OSI-906 under GFDS conditions. Both MK-0646
and OSI-906 showed similar response on IGF-1R and p-IGF-1R in
vitro (data not shown). Additionally, marked reduction in pAkt
(S473) was also observed in MK-0646 (20 μg/ml) or
OSI-906 (0.5 μM) treated cells (data not shown). These results
showed that both antagonists MK-0646 and OSI-906 effectively
inhibited IGF-1R and its downstream signaling.
IGF-1R inhibition downregulates the IAP
molecule XIAP in vivo and in vitro
XIAP, an IAP (inhibitor of apoptosis) molecule and a
key cell survival protein for inhibition of caspases, is a
physiological substrate of Akt (29). Akt phosphorylates XIAP at
Ser87 and regulates its autoubiquitination and
degradation, and thereby stabilizes XIAP (29). Chowdhury et al have shown
that XIAP is associated with pAKT and this association is disrupted
following TGFβ treatment leading to XIAP degradation (30). Moreover, XIAP and survivin form a
complex in the cytosol and this complex inhibits caspase activity
as well as cell death and promotes tumor growth in
vivo(31). Inhibition of the
aberrant cell survival signaling of XIAP through destabilization of
XIAP/survivin complexes leads to caspase reactivation and cell
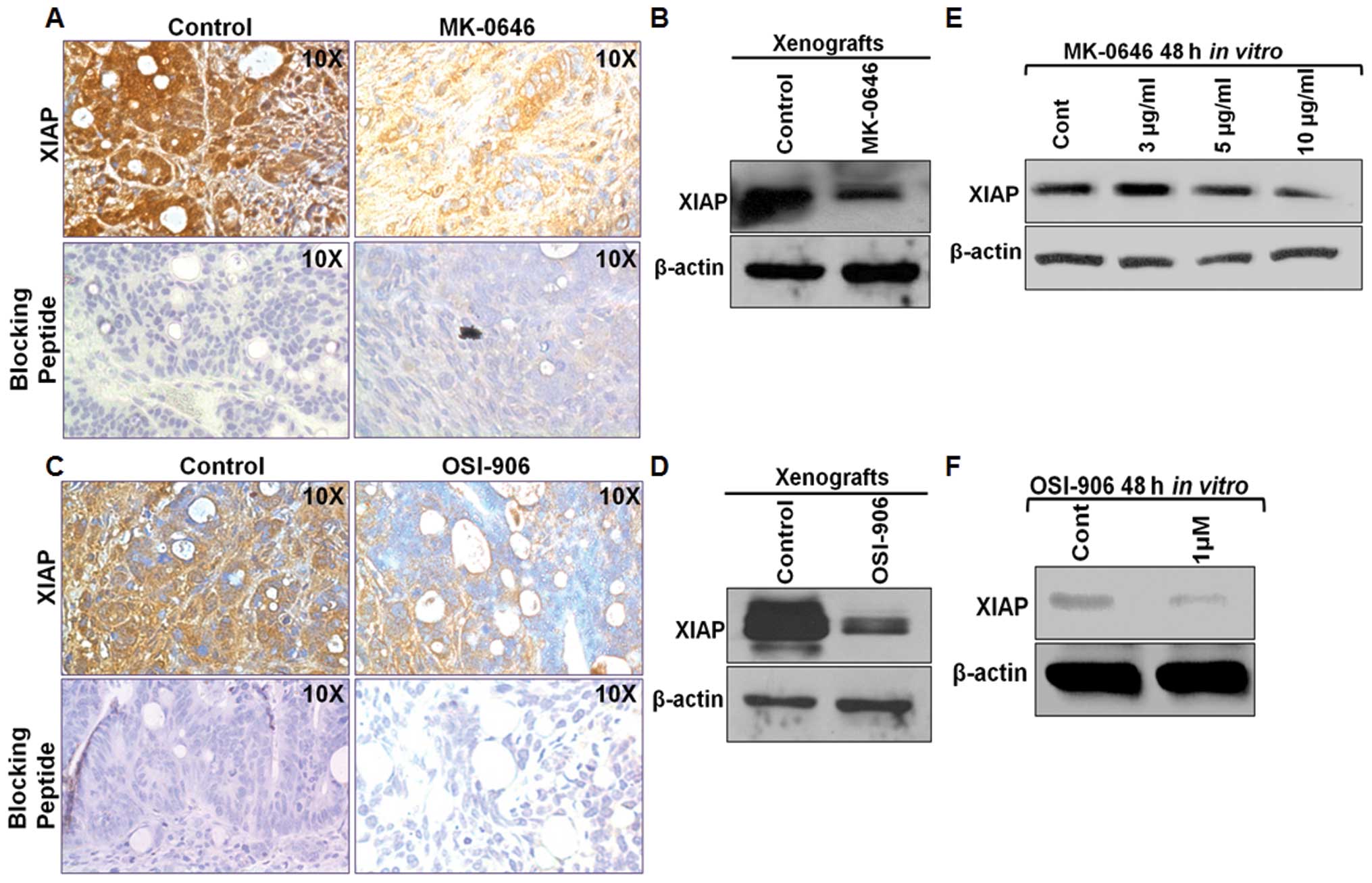
death (30,32). We demonstrated that IGF-1R signaling
pathway inhibition either by MK-0646 or OSI-906 both in vivo
and in vitro downregulated Akt signaling, and this
inhibition in turn leads to the downregulation of XIAP by
immunohistochemical and western blot analysis (Fig. 7A–D). Next, we treated GEO cells with
the IGF-1R antagonist in vitro for 48 h. Similar to the
in vivo results, MK-0646 and OSI-906 both showed XIAP
downregulation in the treated lysates compared to the control
(Fig. 7E and F). These results
showed that both antagonists exhibited their pro-apoptotic
mechanism through inhibition of XIAP, an important downstream cell
survival pathway molecule required for the survival of the
IGF-1R-dependent CRC cells.
Discussion
Aberrant regulation of growth factors and their
corresponding receptors play important roles in malignant
progression (33–38). IGF-1R signaling pathway is prevalent
in many cancers, including CRC (39–41).
The IGF-IR gene has been reported to be overexpressed in human CRC
(36) with ~30–40% of all CRC being
IGF-1R-dependent (22,42). Therefore, IGF-1R signaling pathway
is under intense investigation as an attractive candidate for the
development of novel therapeutic strategies for anticancer
treatment.
Buck et al inhibited the IGF-1R signaling
pathway using OSI-906 in GEO xenograft tumors (18). It was reported that OSI-906
inhibited the growth of the GEO xenografts (18). Recently, we demonstrated that IGF-1R
kinase inhibitor PQIP causes marked antitumor activity in these
colon cancer cell lines by abrogating the IGF-1R mediated
activation of IRS1/Akt to inhibit survival signaling, and inducing
apoptosis (10). In the present
study, we analyzed the antitumor activity of a novel recombinant
humanized monoclonal antibody, MK-0646 in CRC cells both in
vivo and in vitro in comparison to OSI-906. Monoclonal
antibodies against IGF-1R share a common mechanism of action,
involving blockade of ligand-receptor interactions and decreased
cell surface receptor through receptor internationalization and
downregulation of the receptor (11,43–45).
This leads to blockade of the PI3K/Akt signaling pathway (43–46).
However, mechanisms associated with IGF-1R antagonist-mediated cell
death are poorly understood. Our data demonstrated that MK-0646
decreased tumor growth in CRC xenografts in vivo and is
supported by downregulation of IGF-1R and pIGF-1Rβ in western blot
analysis. MK-0646 demonstrated similar antitumor activity when
compared to OSI-906.
IGF-1R signaling exerts its anti-apoptotic effect
through IRS1/IRS2/PI3K/Akt pathway (10,22,25–28).
We observed downregulation of IRS-1/2 and pAkt (S473)
and significant increase in tumor cell apoptosis after MK-0646 or
OSI-906 treatment. Akt and its downstream molecular targets
constitute a major cell survival pathway (47). XIAP, a pro-survival IAP, is a
physiological substrate of Akt (29). Akt phosphorylates XIAP at
Ser87 and reduces its degradation conferring resistance
to caspase activation and apoptosis (29). Deregulation of IAP functions
aberrantly prolonging cancer cell viability, and XIAP and survivin
have been recognized for their role in tumor formation and are
targets for cancer therapeutics (31). We made the novel observation that
XIAP, a critical cell survival molecule that counteracts caspase
activation and induction of apoptosis (29,31) is
downregulated with both MK-0646 and OSI-906 treatments
demonstrating that XIAP is downstream of IGF-1R/Akt mediated
control of aberrant cell survival responses. XIAP has been linked
to cell survival and metastasis (48). XIAP/survivin complexes that mediate
caspase inhibition have been shown to be a key cell survival
mechanism for supporting the metastatic process (31). Previous studies in our laboratory
have shown that destabilization of XIAP/survivin complexes by the
TGFβ tumor suppressor signaling leads to inhibition of aberrant
cell survival resulting in cell death (30,32).
Therefore, the IGF-1R signaling pathway and its downstream cell
survival mediator XIAP may be potential dual targets for anticancer
therapy.
In conclusion, this study demonstrated that MK-0646,
a novel humanized IGF-1R monoclonal antibody, has comparable
antitumor effects to IGF-1R small molecule inhibitor OSI-906, and
may be a potential novel targeted therapy against IGF-1R-dependent
subset of human CRC. Therefore, the results obtained in this study
utilizing IGF-1R antagonists provide a rationale for further
pre-clinical studies in order to dissect the IGF-1R signaling
pathway to obtain full benefit from this receptor targeted therapy
as a single agent or in combination against CRC as well as other
solid tumors dependent upon IGF-1R signaling.
Acknowledgements
This work was supported by the NIH grants, CA 72001,
CA 34432, CA 54807, CA 38173, to M.G.B.
References
|
1
|
Weber MM, Fottner C, Liu SB, Jung MC,
Engelhardt D and Baretton GB: Overexpression of the insulin-like
growth factor I receptor in human colon carcinomas. Cancer.
95:2086–2095. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maloney EK, McLaughlin JL, Dagdigian NE,
et al: An anti-insulin growth factor I receptor antibody that is a
potent inhibitor of cancer cell proliferation. Cancer Res.
63:5073–5083. 2003.PubMed/NCBI
|
|
3
|
Peters G, Gongoll S, Langner C, et al:
IGF-1R, IGF-1 and IGF-2 expression as potential prognostic and
predictive markers in colorectal-cancer. Virchows Arch.
443:139–145. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ewing GP and Goff LW: The insulin-like
growth factor signaling pathway as a target for treatment of
colorectal carcinoma. Clin Colorectal Cancer. 9:219–223. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Asghar U, Hawkes E and Cunningham D:
Predictive and prognostic biomarkers for targeted therapy in
metastatic colorectal cancer. Clin Colorectal Cancer. 9:274–281.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McKinley ET, Bugaj JE, Zhao P, et al:
18FDG-PET predicts pharmacodynamic response to OSI-906, a dual
IGF-1R/IR inhibitor, in preclinical mouse models of lung cancer.
Clin Cancer Res. 17:3332–3340. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goetsch L, Gonzalez A, Leger O, et al: A
recombinant humanized anti-insulin-like growth factor receptor type
I antibody (h7C10) enhances the antitumor activity of vinorelbine
and anti-epidermal growth factor receptor therapy against human
cancer xenografts. Int J Cancer. 113:316–328. 2005. View Article : Google Scholar
|
|
8
|
Allison AS, McIntyre MA, McArdle C and
Habib FK: The insulin-like growth factor type 1 receptor and
colorectal neoplasia: insights into invasion. Hum Pathol.
38:1590–1602. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pollak MN, Schernhammer ES and Hankinson
SE: Insulin-like growth factors and neoplasia. Nat Rev Cancer.
4:505–518. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chowdhury S, Dominguez I, Sharratt E,
Spernyak J, Brattain MG and Rajput A: Anti-tumor activity of IGF-1R
kinase inhibitor PQIP in colon cancer. Clin Exp Pharmacol.
S4:0052013.
|
|
11
|
Donovan EA and Kummar S: Role of
insulin-like growth factor-1R system in colorectal carcinogenesis.
Crit Rev Oncol Hematol. 66:91–98. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Atzori F, Traina TA, Ionta MT and Massidda
B: Targeting insulin-like growth factor type 1 receptor in cancer
therapy. Target Oncol. 4:255–266. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reidy-Lagunes DL, Vakiani E, Segal MF, et
al: A phase 2 study of the insulin-like growth factor-1 receptor
inhibitor MK-0646 in patients with metastatic, well-differentiated
neuroendocrine tumors. Cancer. 118:4795–4800. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reichert JM: Antibody-based therapeutics
to watch in 2011. MAbs. 3:76–99. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
King ER and Wong KK: Insulin-like growth
factor: current concepts and new developments in cancer therapy.
Recent Pat Anticancer Drug Discov. 7:14–30. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Heidegger I, Pircher A, Klocker H and
Massoner P: Targeting the insulin-like growth factor network in
cancer therapy. Cancer Biol Ther. 11:701–707. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mulvihill MJ, Cooke A, Rosenfeld-Franklin
M, et al: Discovery of OSI-906: a selective and orally efficacious
dual inhibitor of the IGF-1 receptor and insulin receptor. Future
Med Chem. 1:1153–1171. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Buck E, Gokhale PC, Koujak S, et al:
Compensatory insulin receptor (IR) activation on inhibition of
insulin-like growth factor-1 receptor (IGF-1R): rationale for
cotargeting IGF-1R and IR in cancer. Mol Cancer Ther. 9:2652–2664.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brattain MG, Levine AE, Chakrabarty S,
Yeoman LC, Willson JK and Long B: Heterogeneity of human colon
carcinoma. Cancer Metastasis Rev. 3:177–191. 1984. View Article : Google Scholar
|
|
20
|
Boyd DD, Levine AE, Brattain DE, McKnight
MK and Brattain MG: Comparison of growth requirements of two human
intratumoral colon carcinoma cell lines in monolayer and soft
agarose. Cancer Res. 48:2469–2474. 1988.PubMed/NCBI
|
|
21
|
Wang J, Yang L, Yang J, et al:
Transforming growth factor beta induces apoptosis through
repressing the phosphoinositide 3-kinase/AKT/survivin pathway in
colon cancer cells. Cancer Res. 68:3152–3160. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu YP, Patil SB, Panasiewicz M, et al:
Heterogeneity of receptor function in colon carcinoma cells
determined by cross-talk between type I insulin-like growth factor
receptor and epidermal growth factor receptor. Cancer Res.
68:8004–8013. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vanamala J, Reddivari L, Radhakrishnan S
and Tarver C: Resveratrol suppresses IGF-1 induced human colon
cancer cell proliferation and elevates apoptosis via suppression of
IGF-1R/Wnt and activation of p53 signaling pathways. BMC Cancer.
10:2382010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakane PK: Recent progress in the
peroxidase-labeled antibody method. Ann NY Acad Sci. 254:203–211.
1975. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Peruzzi F, Prisco M, Dews M, et al:
Multiple signaling pathways of the insulin-like growth factor 1
receptor in protection from apoptosis. Mol Cell Biol. 19:7203–7215.
1999.PubMed/NCBI
|
|
26
|
Ouban A, Muraca P, Yeatman T and Coppola
D: Expression and distribution of insulin-like growth factor-1
receptor in human carcinomas. Hum Pathol. 34:803–808. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Myers MG Jr, Grammer TC, Wang LM, et al:
Insulin receptor substrate-1 mediates phosphatidylinositol
3′-kinase and p70S6k signaling during insulin, insulin-like growth
factor-1, and interleukin-4 stimulation. J Biol Chem.
269:28783–28789. 1994.
|
|
28
|
Kennedy SG, Wagner AJ, Conzen SD, et al:
The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic
signal. Genes Dev. 11:701–713. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dan HC, Sun M, Kaneko S, et al: Akt
phosphorylation and stabilization of X-linked inhibitor of
apoptosis protein (XIAP). J Biol Chem. 279:5405–5412. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chowdhury S, Howell GM, Rajput A, et al:
Identification of a novel TGFbeta/PKA signaling transduceome in
mediating control of cell survival and metastasis in colon cancer.
PLoS One. 6:e193352011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dohi T, Xia F and Altieri DC:
Compartmentalized phosphorylation of IAP by protein kinase A
regulates cytoprotection. Mol Cell. 27:17–28. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chowdhury S, Howell GM, Teggart CA, et al:
Histone deacetylase inhibitor belinostat represses survivin
expression through reactivation of transforming growth factor beta
(TGFbeta) receptor II leading to cancer cell death. J Biol Chem.
286:30937–30948. 2011. View Article : Google Scholar
|
|
33
|
Watson DS, Brotherick I, Shenton BK,
Wilson RG and Campbell FC: Growth dysregulation and p53
accumulation in human primary colorectal cancer. Br J Cancer.
80:1062–1068. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gryfe R, Swallow C, Bapat B, Redston M,
Gallinger S and Couture J: Molecular biology of colorectal cancer.
Curr Probl Cancer. 21:233–300. 1997. View Article : Google Scholar
|
|
35
|
Moschos SJ and Mantzoros CS: The role of
the IGF system in cancer: from basic to clinical studies and
clinical applications. Oncology. 63:317–332. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sulkowski S, Kanczuga-Koda L, Koda M,
Wincewicz A and Sulkowska M: Insulin-like growth factor-I receptor
correlates with connexin 26 and Bcl-xL expression in human
colorectal cancer. Ann NY Acad Sci. 1090:265–275. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu KD, Zhou L, Burtrum D, Ludwig DL and
Moore MA: Antibody targeting of the insulin-like growth factor I
receptor enhances the anti-tumor response of multiple myeloma to
chemotherapy through inhibition of tumor proliferation and
angiogenesis. Cancer Immunol Immunother. 56:343–357.
2007.PubMed/NCBI
|
|
38
|
Hakam A, Yeatman TJ, Lu L, et al:
Expression of insulin-like growth factor-1 receptor in human
colorectal cancer. Hum Pathol. 30:1128–1133. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Reinmuth N, Liu W, Fan F, et al: Blockade
of insulin-like growth factor I receptor function inhibits growth
and angiogenesis of colon cancer. Clin Cancer Res. 8:3259–3269.
2002.PubMed/NCBI
|
|
40
|
Sachdev D and Yee D: The IGF system and
breast cancer. Endocr Relat Cancer. 8:197–209. 2001. View Article : Google Scholar
|
|
41
|
Surmacz E: Function of the IGF-I receptor
in breast cancer. J Mammary Gland Biol Neoplasia. 5:95–105. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Buck E, Eyzaguirre A, Rosenfeld-Franklin
M, et al: Feedback mechanisms promote cooperativity for small
molecule inhibitors of epidermal and insulin-like growth factor
receptors. Cancer Res. 68:8322–8332. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gualberto A and Karp DD: Development of
the monoclonal antibody figitumumab, targeting the insulin-like
growth factor-1 receptor, for the treatment of patients with
non-small-cell lung cancer. Clin Lung Cancer. 10:273–280. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shang Y, Mao Y, Batson J, et al:
Antixenograft tumor activity of a humanized anti-insulin-like
growth factor-I receptor monoclonal antibody is associated with
decreased AKT activation and glucose uptake. Mol Cancer Ther.
7:2599–2608. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zha J and Lackner MR: Targeting the
insulin-like growth factor receptor-1R pathway for cancer therapy.
Clin Cancer Res. 16:2512–2517. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chitnis MM, Yuen JS, Protheroe AS, Pollak
M and Macaulay VM: The type 1 insulin-like growth factor receptor
pathway. Clin Cancer Res. 14:6364–6370. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Agarwal E, Brattain MG and Chowdhury S:
Cell survival and metastasis regulation by Akt signaling in
colorectal cancer. Cell Signal. 25:1711–1719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mehrotra S, Languino LR, Raskett CM,
Mercurio AM, Dohi T and Altieri DC: IAP regulation of metastasis.
Cancer Cell. 17:53–64. 2010. View Article : Google Scholar
|