Introduction
Metastatic spread of tumor cells from the original
site to distant organs via blood or lymph vessels results in a high
mortality rate in cancer patients and remains a challenge in cancer
treatment (1). Metastasis involves
a complex multi-step process and various cytophysiological changes,
including invasion by degradation of the environmental barriers
surrounding cancer cells such as the extracellular matrix (ECM) and
basement membranes; entrance into vasculature; migration to distant
organs; adhesion to endothelial cells; extravasation leading to
infiltration into the underlying tissue; and establishment of
metastatic foci at the secondary site (2). Degradation of the ECM by cancer cells
mediated through a variety of proteolytic enzymes, such as matrix
metalloproteinases (MMPs), serine proteinase, cathepsins and
plasminogen activators (PAs), plays a critical role in tumor
invasion and metastasis. MMPs, a family of zinc-dependent
endopeptidases, is composed of 4 groups according to their
substrates, including collagenases, gelatinases, stromelysins and
membrane-associated MMPs. Among them, MMP-2 and -9 are particularly
elevated in malignant tumors and may be closely associated with the
progression and metastasis due to their capacity to degrade type IV
collagen, a major component of basement membranes. In addition,
MMP-9 may be critical in the process of tumor angiogenesis by
increasing the availability of vascular endothelial cell growth
factor (VEGF) in malignant tumors (3–6). Thus,
agents capable of modulating MMP-2 and -9 activity by targeting the
upstream regulatory pathways may have therapeutic potential for
controlling cancer metastasis (7–9).
Numerous studies have demonstrated that natural
herbal medicines have the potential to treat a wide range of human
diseases including cancer. A herbal cocktail, a multi-herb mixture
in a single formula, may act in concert to amplify the therapeutic
efficacies of each single herb, leading to maximal therapeutic
efficacy with minimal adverse effects (10,11).
Our group has developed a novel herbal medicine, KIOM-C, which is
composed of herbal medicinal plants including Radix Scutellariae,
Radix Glycyrrhizae, Radix Paeoniae Alba, Radix Angelicae Gigantis,
Platycodon grandiflorum, Zingiber officinale and
Lonicera japonica Thunb., among others. Recently, KIOM-C was
shown to improve the overall growth performance and restore
viability in pigs suffering from porcine circovirus-associated
disease (PCVAD) by increasing body weight and stimulating immune
responses (12). Furthermore, oral
administration of KIOM-C was clearly shown to reduce the influenza
virus titer in the lungs and protect mice from a lethal challenge
with A/Korea/maCJ01/2009 virus (13).
In the present study, we evaluated the effect of
KIOM-C on the metastatic potential of the highly metastatic
malignant tumor cells lines, human fibrosarcoma HT1080 and murine
melanoma B16F10 in an in vitro system and investigated
whether KIOM-C administration inhibits pulmonary metastasis of
B16F10 melanoma after intravenous injection in mice. Furthermore,
we investigated the detailed mechanism of the antimetastatic
activity of KIOM-C.
Materials and methods
Mice and cell cultures
B16F10 murine melanoma cells, which are highly
metastatic to the lungs of C57BL/6J mice, and HT1080 human
fibrosarcoma cells were purchased from the American Type Culture
Collection (ATCC, Manassas, VA, USA) and maintained in Dulbecco’s
modified Eagle’s medium (DMEM; Lonza, Walkersville, MD, USA)
supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum
(FBS) and 100 U/ml penicillin/100 μg/ml streptomycin (both from
Gibco, Invitrogen, Carlsbad, CA, USA) at 37°C in a humidified 5%
CO2 incubator. For animal experiments, specific
pathogen-free female C57BL/6J mice were purchased from Taconic
Farms lnc. (Samtako Bio Korea, O-San, Korea) and maintained in our
animal facility for 1 week before use. Mice were housed under
specific pathogen-free conditions at a temperature of 24±1°C and
humidity of 55±5% in a barrier facility with a 12-h light-dark
cycle. Animal experimental procedures were approved by the Korea
Institute of Oriental Medicine Care and Use Committee (reference
nos. 12-094 and 12-111) and performed in accordance with the Korea
Institute of Oriental Medicine Care Committee Guidelines.
Antibodies and chemicals
Anti-IκBα, anti-phospho-IκBα (Ser32/36), anti-NF-κB
p65, anti-MMP-9 and anti-tubulin antibodies were purchased from
Cell Signaling Technology, Inc. (Danvers, MA, USA). Anti-TATA
sequence-binding protein (TBP) was obtained from Lifespan
Biosciences, Inc. (Seattle, WA, USA). A cytotoxicity detection
lactate dehydrogenase (LDH) kit and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were purchased from Roche Diagnostics GmbH (Mannheim, Germany) and
Sigma Chemical Co. (St. Louis, MO, USA), respectively.
Herbal materials and preparation of
KIOM-C
The herbs for preparing KIOM-C, as mentioned above,
were purchased from Korea Medicine Herbs Association (Yeongcheon,
Korea). The identification of all herbs was confirmed by Professor
Ki Hwan Bae of the College of Pharmacy, Chungnam National
University (Daejeon, Korea), and all voucher specimens were
deposited in the herb bank of the Korea Institute of Oriental
Medicine (KIOM, Daejeon, Korea). A total of 2456.5 g KIOM-C formula
was placed in 15 liters distilled water and then heat-extracted for
3 h at 115°C in an extractor (Cosmos-600 Extractor; Gyeonseo Co.,
Inchon, Korea), filtered using standard testing sieves (150-μm;
Retsch, Haan, Germany) and then concentrated to dryness in a
lyophilizer. Freeze-dried KIOM-C powder (50 mg) was dissolved in 1
ml distilled water, filtered through a 0.22-μm disk filter, and
stored at −20°C until use.
Cytotoxicity assay
Cytotoxicity was evaluated using MTT and LDH release
assays. Briefly, cells seeded in 96-well culture plates at a
density of 5×103 cells/well were incubated with specific
KIOM-C concentrations between 10 and 250 μg/ml. After a 48-h
treatment, cells were incubated with 10 μl MTT solution (5 mg/ml in
PBS) for an additional 4 h. Formazan precipitates were dissolved
with dimethyl sulfoxide (DMSO), and then the absorbance was
measured at 570 nm with the Infinite® M200 microplate
reader (Tecan Group Ltd., Switzerland). In addition, LDH release
into the culture supernatant from KIOM-C-treated cells was
determined by a commercial cytotoxicity detection kit according to
the manufacturer’s instructions.
Soft agar colony formation assay
To determine anchorage-independent cell growth,
cells (1×104) suspended in 3 ml of medium containing
0.3% agar and 10% FBS were applied to the solidified bottom agar
containing 0.6% agar and 10% FBS. During 3 weeks of incubation,
colonies on soft agar were observed under a phase-contrast
microscope and photographed.
Colony formation assay
Two hundred cells were seeded in a 12-well culture
plate in 1 ml 10% FBS/DMEM and incubated to allow attachment. After
adding KIOM-C at the specified concentrations, cells were incubated
for 10 days, and colonies were stained with 0.2% crystal violet/20%
methanol (wt/vol) solution.
Wound healing assay
Cells were pre-incubated with 25 μg/ml mitomycin C
(Sigma Chemical Co.) for 30 min, and injury lines were drawn on a
confluent monolayer of cells. After washing with DMEM, cells were
allowed to migrate in the presence of KIOM-C, and migration was
observed under a phase-contrast microscope at specific time
points.
Gelatin zymography
Cells were pre-incubated for 12 h in serum-free DMEM
with KIOM-C at the specified concentrations and then stimulated
with 5 nM PMA for an additional 24 h. The equivalent volumes of the
conditioned medium were electrophoresed on an 8% sodium dodecyl
sulfate-polyacrylamide gel (SDS-PAGE) containing 0.1% gelatin. Gels
were washed thoroughly with washing buffer (50 mM Tris-HCl, pH 7.5,
100 mM NaCl, 2.5% Triton X-100) and then incubated in activation
buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 10 mM
CaCl2, 0.02% NaN3, 1 μM ZnCl2) at
37°C. The gels were stained with Coomassie Brilliant Blue R-250
staining solution (Bio-Rad Laboratories, Hercules, CA, USA) and
destained (10% isopropanol, 10% acetic acid). MMPs were detected as
clear bands against a dark blue background.
Transwell migration and Matrigel invasion
assays
The in vitro migration and invasion assays
were performed using a Transwell chamber with a 10-mm diameter and
an 8-μm pore size polycarbonate membrane (Corning Costar,
Cambridge, MA, USA). In brief, after filling the lower chamber with
600 μl 10% FBS/DMEM, cells (1×105/100 μl) in serum-free
DMEM were added to each upper chamber and incubated for 12–36 h at
37°C. Cells attached to the upper surface of the filters were
removed by wiping with a cotton swab, and the filters were stained
with 0.2% crystal violet/20% methanol (wt/vol) solution. The
invasion assay was conducted using the Transwell chamber after
coating with 20 μl of a 1:2 mixture of Matrigel:DMEM (Matrigel; BD
Biosciences, Bedford, MA, USA) as the intervening invasive
barrier.
Western blot analysis
Whole cell lysates and nuclear/cytosolic extracts
were prepared using M-PER Mammalian Protein Extraction Reagent and
NE-PER Nuclear and Cytosolic Extraction Reagent (Pierce
Biotechnology, Inc., Rockford, IL, USA), respectively, according to
the manufacturer’s instructions. Protein concentration was
determined using the bicinchoninic acid (BCA) assay. After
immunoblotting, proteins were visualized using a Power Opti-ECL
Western blotting detection reagent (Animal Genetics, Inc., Korea)
and an ImageQuant LAS 4000 mini (GE Healthcare, Piscataway, NJ,
USA). Band intensities were calculated using ImageJ software
(National Institutes of Health, USA).
RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCT)
Total RNA was extracted using PureHelix™ RNA
extraction solution and reverse transcribed to cDNA using
HelixCript™ 1st Strand cDNA Synthesis kit (NanoHelix Co., Daejeon,
Korea). cDNA aliquots corresponding to 1 μg RNA were analyzed by
semi-quantitative PCR using the following primers; hMMP-9,
5′-TCTTCCCTGGAGACTGAGAA-3′ and 5′-GGCAAGTCTTCCGAGTAGTTT-3′; GAPDH,
5′-TCATGACCACAGTCCATGCC-3′ and 5′-TCCACCACCCTGTTGCTGTA-3′.
In vivo experimental pulmonary metastasis
assay
The female C57BL/6J mice were divided into 3 groups
(n=5 for each group), and B16F10 cells (3×105 cells/0.2
ml) were injected via the tail veins. The amount of KIOM-C for
human adults with an average body weight of 60 kg is ~50–150 g/day,
and the yield of powdered extraction is ~20.7% (wt/wt). Therefore,
KIOM-C at doses of 170 or 510 mg/day/kg of body weight and saline
(controls) were orally administered to mice for 17 days. The mice
were sacrificed, their lungs were fixed in Bouin’s solution (Sigma)
and the number of B16F10 colonies on the surface of the lung was
determined by visual inspection.
Statistical analysis
Statistical significance of the difference between
groups was analyzed using the Student’s t-test with the SigmaPlot
8.0 software, and a p-value <0.05 was considered to indicate a
significant result. Data are presented as the means ± standard
deviation (SD), and all experiments were repeated at least 3
times.
Results
KIOM-C suppresses colony-forming activity
and anchorage-independent growth of B16F10 melanoma cells
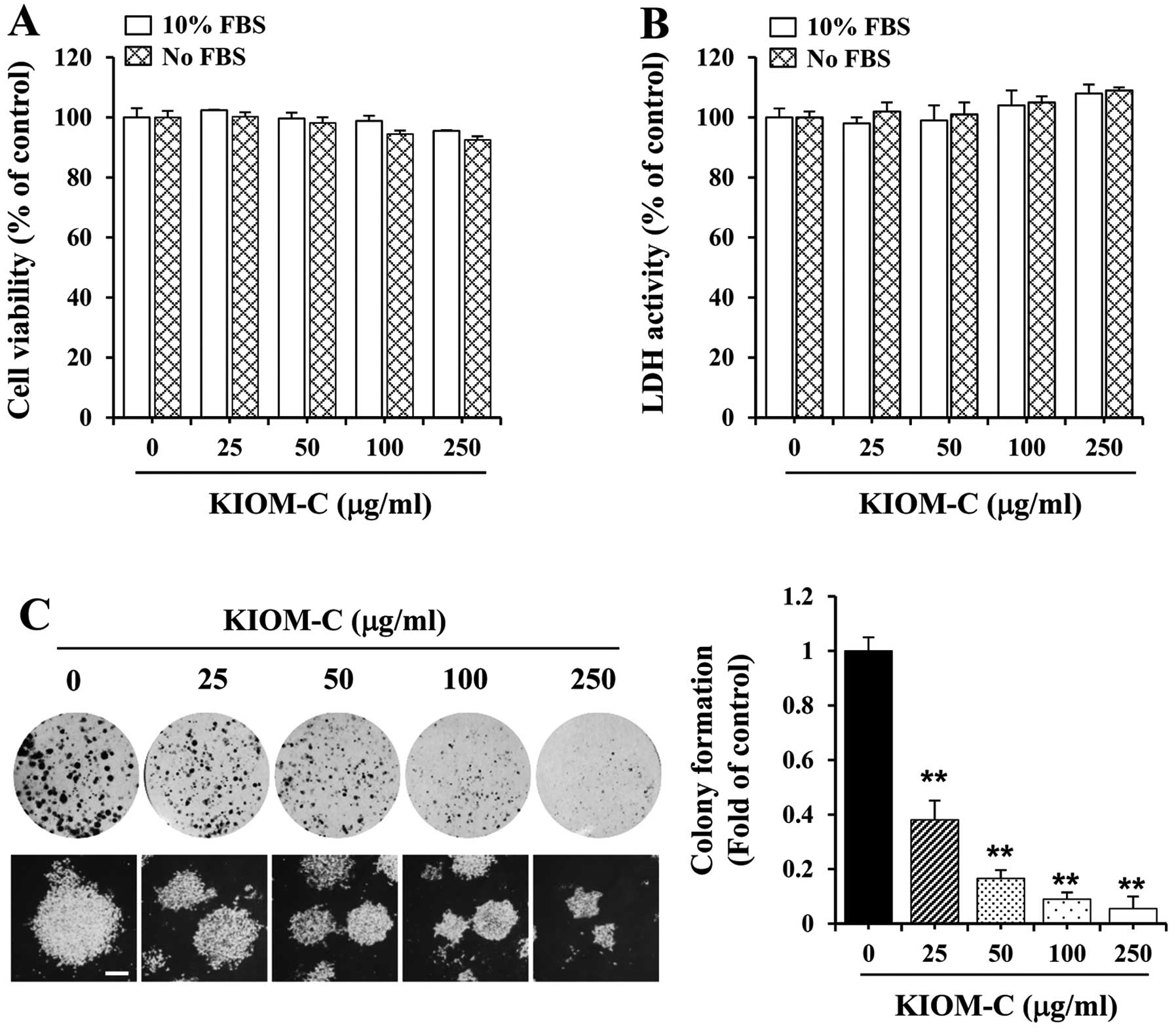
To determine the non-cytotoxic concentration of
KIOM-C, B16F10 cells were treated with various KIOM-C
concentrations for 48 h followed by MTT and LDH assays. Compared to
the control cells, the viability and morphology did not
significantly change in response to KIOM-C, suggesting that KIOM-C
at a concentration ranging from 25 to 250 μg/ml was non-cytotoxic
to B16F10 cells (Fig. 1A and B);
thus we used this concentration range for KIOM-C in all subsequent
experiments. We further investigated whether KIOM-C at
non-cytotoxic doses influences the malignant phenotype in terms of
anchorage-dependent colony formation at low density. As shown in
Fig. 1C (upper panel), untreated
control B16F10 cells displayed rapid proliferation and formed
sizable colonies from one cell, whereas KIOM-C treatment suppressed
colony-forming activity involving a lower number of sizable
colonies and a reduced colony size in a dose-dependent manner.
Anchorage-independent growth, the ability of cells to form colonies
in a semi-solid medium, which is closely correlated with metastatic
potential (14), was also
significantly decreased in the KIOM-C-treated cells when compared
to the untreated control cells by ~60–95% at concentrations of
25–250 μg/ml (Fig. 1C, lower
panel). The inhibition of colony formation was not due to KIOM-C
cytotoxicity, as shown in Fig. 1A and
B.
KIOM-C prevents in vitro migration and
invasion of B16F10 melanoma cells
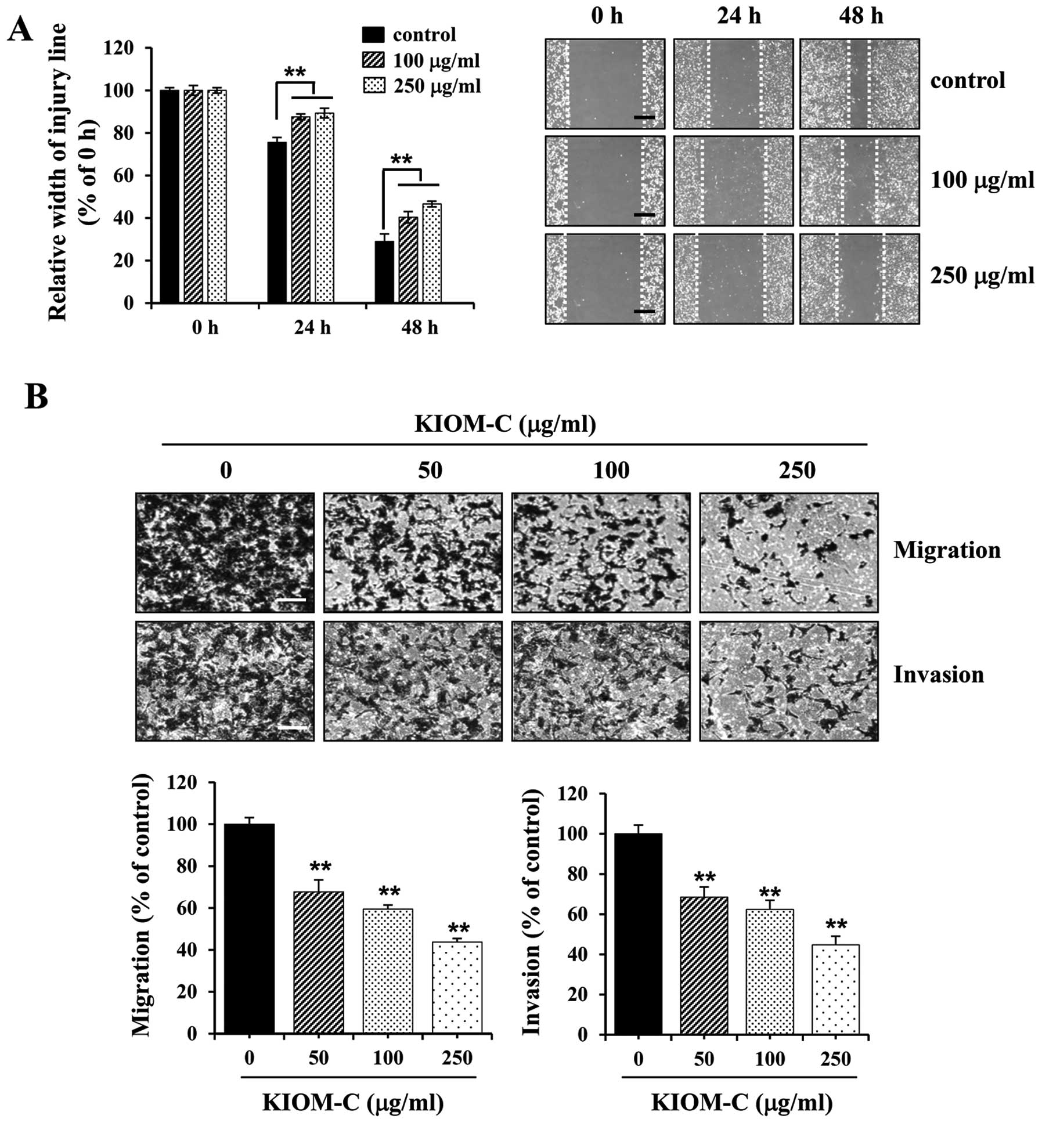
To investigate the effect of KIOM-C on metastatic
activity, we first assessed migration activity using a wound
healing assay. Control B16F10 cells migrated across the wound area,
leading to ~25 and 70% healing at 24 and 48 h, respectively,
whereas KIOM-C treatment at 100 and 250 μg/ml significantly
suppressed wound migration to 60 and 55% at 48 h, respectively
(Fig. 2A). Next, we performed
Transwell migration and invasion assays. As shown in Fig. 2B, the migration activity of
serum-induced B16F10 cells was significantly decreased following
KIOM-C treatment in a dose-dependent manner. In addition, the
invasiveness, which is determined by the ability of cells to invade
a Matrigel barrier, was also considerably suppressed in
KIOM-C-treated cells. Treatment with KIOM-C at 250 μg/ml prevented
serum-induced migration and invasion by ~55% compared to the
untreated control cells (Fig.
2B).
KIOM-C administration dramatically
inhibits in vivo pulmonary metastasis of B16F10 cells
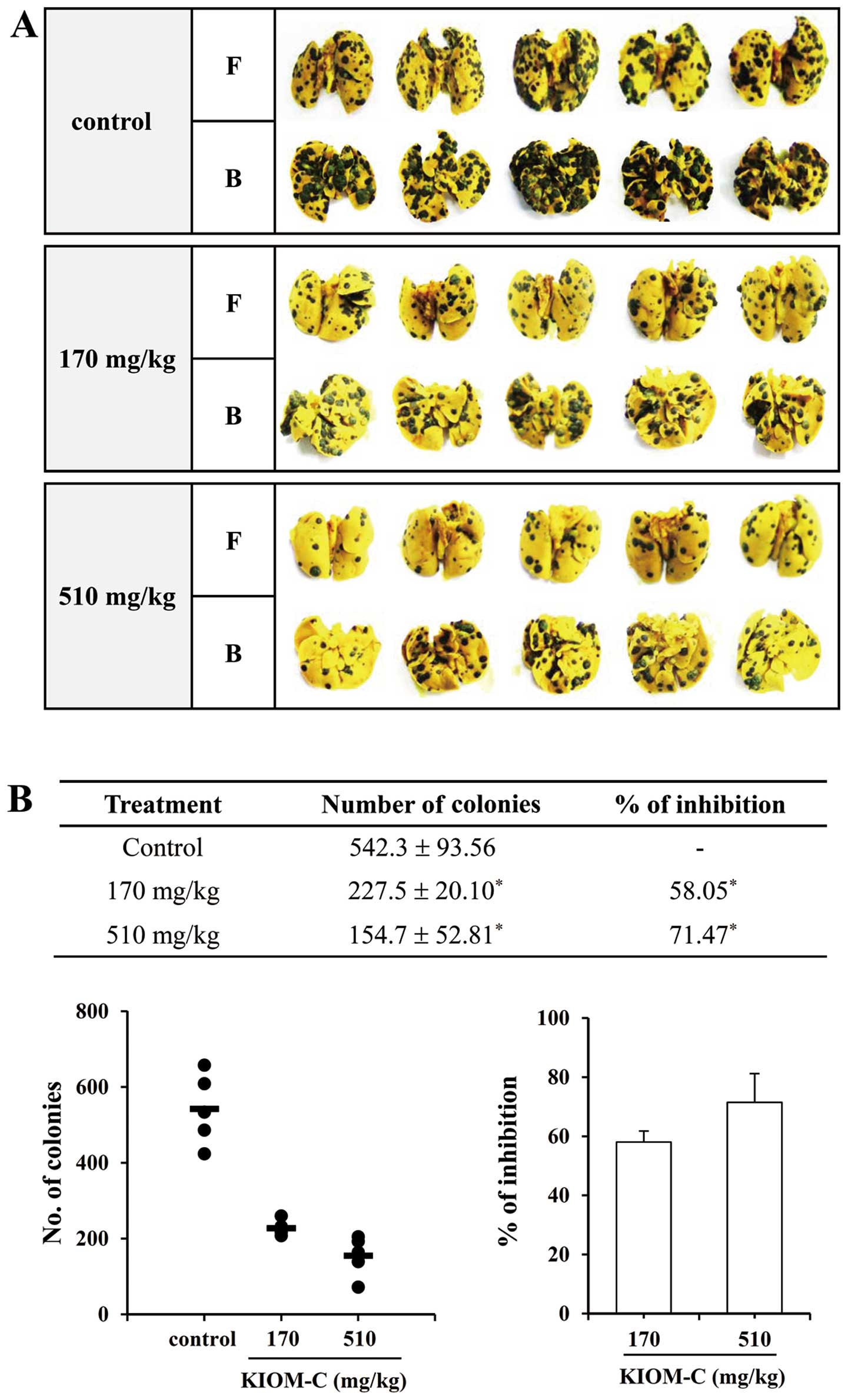
To confirm the inhibitory effect of KIOM-C on tumor
metastasis in vivo, we evaluated the ability of B16F10 cells
to colonize the lungs of C57BL/6J mice after intravenous injection.
As shown in Fig. 3, the incidence
of metastatic black colonies was significantly decreased by KIOM-C
administration in a dose-dependent manner. In particular,
administration with 510 mg/kg KIOM-C resulted in ~71.47% inhibition
of lung metastases as compared to the control group. To evaluate
whether repeated administration of KIOM-C elicits systemic
toxicity, mice were medicated with saline only (control) and KIOM-C
at doses of 170 and 510 mg/kg. During the 2-week experimental
period, the administration of KIOM-C did not cause death or
abnormal behavior and did not affect weight gain in mice (Table I). Organ weight changes were not
significantly different between the KIOM-C-treated groups and the
controls (Table II). In addition,
no critical differences in the serological parameters between
KIOM-C-treated and control groups were observed (Table III). The ratios of GOT/GPT and
BUN/CRE were not significantly altered in the KIOM-C-treated group
when compared to levels in the control, suggesting that KIOM-C
administration did not cause hepatic and renal damage. The
hematological parameters of KIOM-C-treated mice were also similar
to the control mice (Table IV).
The numbers of RBCs and the Hb level, an indicator of RBC count
balance and anemia, were not altered by KIOM-C. The numbers of WBCs
and other parameters were within the normal ranges. These data
indicate that KIOM-C administration efficiently suppressed
pulmonary metastasis of melanoma cells when compared to the
metastasis noted in the controls, but without any adverse effect
during treatment based on serological and hematological
findings.
 | Table IMean body weights of mice
administered 170 or 510 mg/kg of KIOM-C. |
Table I
Mean body weights of mice
administered 170 or 510 mg/kg of KIOM-C.
| Body weight
(g) |
|---|
|
|
|---|
| Days after
treatment |
|---|
|
|
|---|
| Treatment | 0 | 3 | 7 | 10 | 14 |
|---|
| Control | 14.61±0.31 | 14.93±0.29 | 15.33±0.51 | 15.71±0.36 | 16.43±0.22 |
| 170 mg/kg | 15.09±0.13 | 15.37±0.01 | 15.64±0.31 | 16.12±0.25 | 16.96±0.16 |
| 510 mg/kg | 14.82±0.27 | 15.19±0.45 | 15.50±0.07 | 15.89±0.06 | 16.65±0.46 |
 | Table IIMean organ weights of mice
administered 170 or 510 mg/kg of KIOM-C. |
Table II
Mean organ weights of mice
administered 170 or 510 mg/kg of KIOM-C.
| Weight of organs
(g) |
|---|
|
|
|---|
| Treatment | Liver | Heart | Lung | Spleen | Kidney (L) | Kidney (R) |
|---|
| Control | 1.05±0.07 | 0.10±0.01 | 0.13±0.00 | 0.09±0.01 | 0.11±0.01 | 0.12±0.00 |
| 170 mg/kg | 0.90±0.01 | 0.09±0.00 | 0.13±0.01 | 0.08±0.00 | 0.11±0.00 | 0.11±0.02 |
| 510 mg/kg | 0.89±0.01 | 0.09±0.00 | 0.14±0.00 | 0.08±0.00 | 0.10±0.00 | 0.11±0.01 |
 | Table IIIChemical analysis of serum sample
obtained from mice administered 170 or 510 mg/kg of KIOM-C. |
Table III
Chemical analysis of serum sample
obtained from mice administered 170 or 510 mg/kg of KIOM-C.
| Treatment | GOT (IU/l) | GPT (IU/l) | ALP (IU/l) | BUN (mg/dl) | CRE (mg/dl) |
|---|
| Control | 55.0±0.0 | 20.0±0.0 | 185.0±21.2 | 9.5±1.4 | 1.0±0.0 |
| 170 mg/kg | 55.0±0.0 | 22.5±3.5 | 212.5±3.54 | 10.5±1.4 | 1.0±0.0 |
| 510 mg/kg | 55.0±0.0 | 20.0±0.0 | 202.5±3.54 | 11.5±4.2 | 1.0±0.0 |
 | Table IVHematological analysis of blood
obtained from mice administered 170 or 510 mg/kg of KIOM-C. |
Table IV
Hematological analysis of blood
obtained from mice administered 170 or 510 mg/kg of KIOM-C.
| Parameter | Control | 170 mg/kg | 510 mg/kg |
|---|
| WBCP
(×103 cells/μl) | 2.38±0.41 | 2.11±0.27 | 2.33±0.39 |
| WBCB
(×103 cells/μl) | 2.62±0.63 | 2.36±0.25 | 2.38±0.41 |
| RBC
(×106 cells/μl) | 9.43±0.69 | 9.17±0.09 | 9.47±0.04 |
| Mean HGB
(g/dl) | 13.9±1.13 | 13.4±0.07 | 13.9±0.07 |
| HCT (%) | 50.8±2.55 | 50.0±1.20 | 50.9±0.92 |
| MCV (fl) | 53.9±1.27 | 54.6±0.78 | 53.8±0.78 |
| MCH (pg) | 14.8±0.14 | 14.6±0.00 | 14.6±0.00 |
| MCHC (g/dl) | 27.4±0.85 | 26.8±0.42 | 27.2±0.42 |
| PLT (×104
cells/μl) | 34.3±1.54 | 29.8±1.04 | 31.9±1.88 |
| % NEUT | 7.11±0.99 | 8.40±0.00 | 8.42±0.37 |
| % LYM | 88.0±3.54 | 85.7±0.35 | 84.9±5.94 |
| % MONO | 0.50±0.14 | 0.57±0.09 | 0.65±0.15 |
KIOM-C suppresses in vitro PMA-induced
migration and invasion of HT1080 fibrosarcoma cells
We first examined the cytotoxicity of KIOM-C on
HT1080 cells by MTT and determined that the non-cytotoxic
concentrations ranged from 25 to 250 μg/ml which were used for
subsequent experiments (Fig. 4A).
Incubation with specified concentrations of KIOM-C substantially
inhibited colony formation (Fig.
4B) and wound migration (Fig.
4C) in a dose-dependent manner. In particular, KIOM-C at 250
μg/ml almost completely suppressed colony formation and wound
migration. As shown in Fig. 4D, the
ability of HT1080 cells to migrate and invade across the Transwell
was markedly increased following PMA stimulation to ~3.5- and
4.5-fold, respectively. In contrast, treatment with KIOM-C
attenuated migration and invasion by ~50% when compared to the
control cells under the PMA-stimulated conditions.
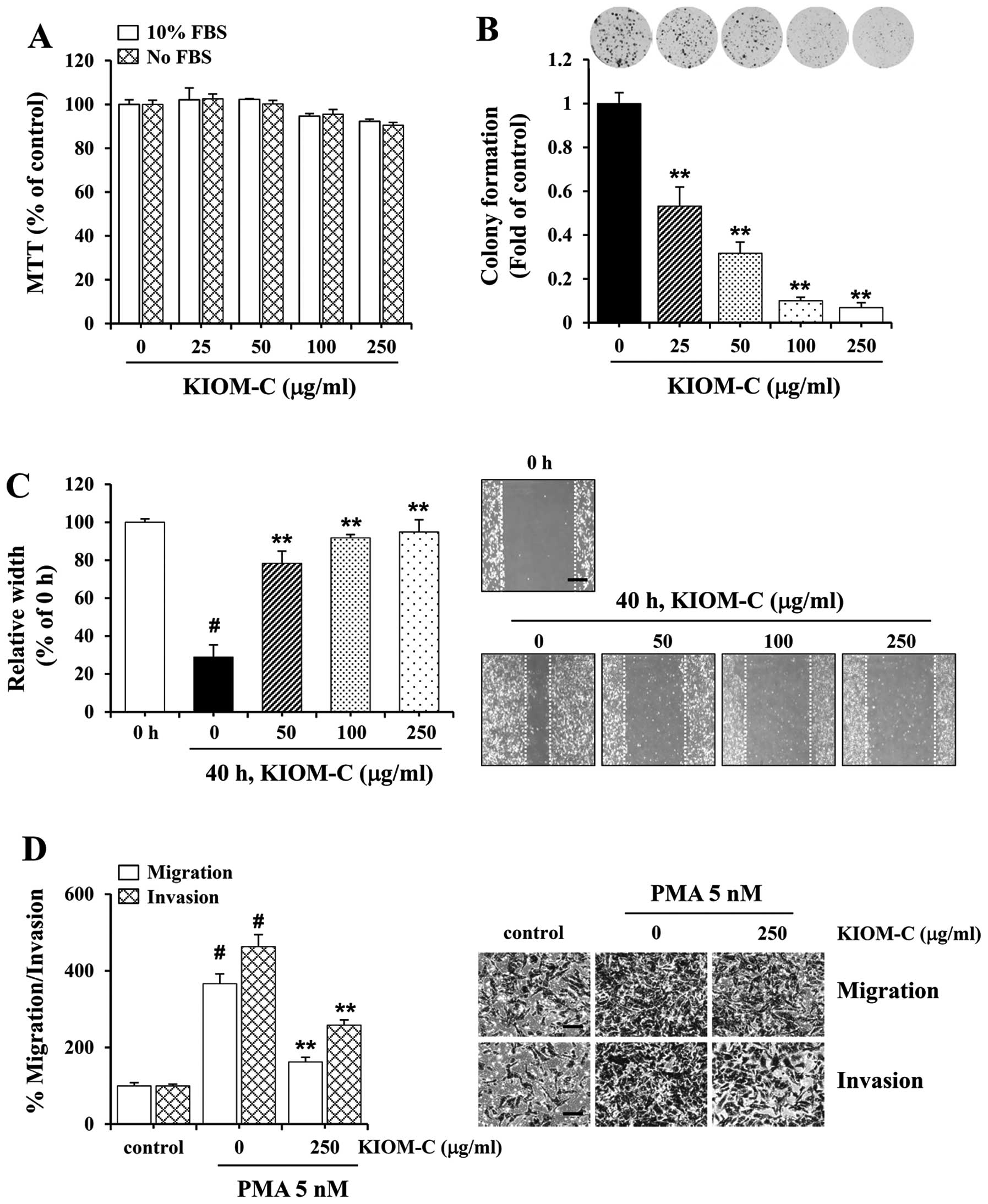 | Figure 4Effect of KIOM-C on colony formation,
migration and invasion of HT1080 cells. (A) The non-cytotoxic
concentration of KIOM-C in the HT1080 cells was determined by MTT
assay. (B) Colony formation and (C) wound migration (magnification,
×40; scale bar, 0.5 mm) of HT1080 cells in the presence or absence
of KIOM-C at the specified concentrations were examined.
#p<0.01 vs. 0 h, **p<0.01 vs. control
at 40 h. (D) PMA-induced migration and invasion were measured using
the Transwell system. Cells were pretreated with or without 250
μg/ml KIOM-C for 12 h, harvested, counted and plated on the
Transwell as described in Fig. 2B.
After incubation with PMA (5 nM) as stimulator for 12 h (migration)
and 24 h (invasion), cells that migrated and invaded were stained
and images were captured. Magnification, ×200; scale bar, 0.5 mm.
Relative migration and invasion were calculated using ImageJ.
#p<0.01 vs. no PMA control, **p<0.01
vs. PMA + no KIOM-C. |
KIOM-C suppresses PMA-induced MMP-9
expression and activity through blockage of NF-κB activation
Since MMP-2 and -9 play essential roles in
facilitating cancer metastasis by degrading the surrounding ECM
(2,6), we examined whether KIOM-C modulates
the expression and activity of these MMPs in order to elucidate the
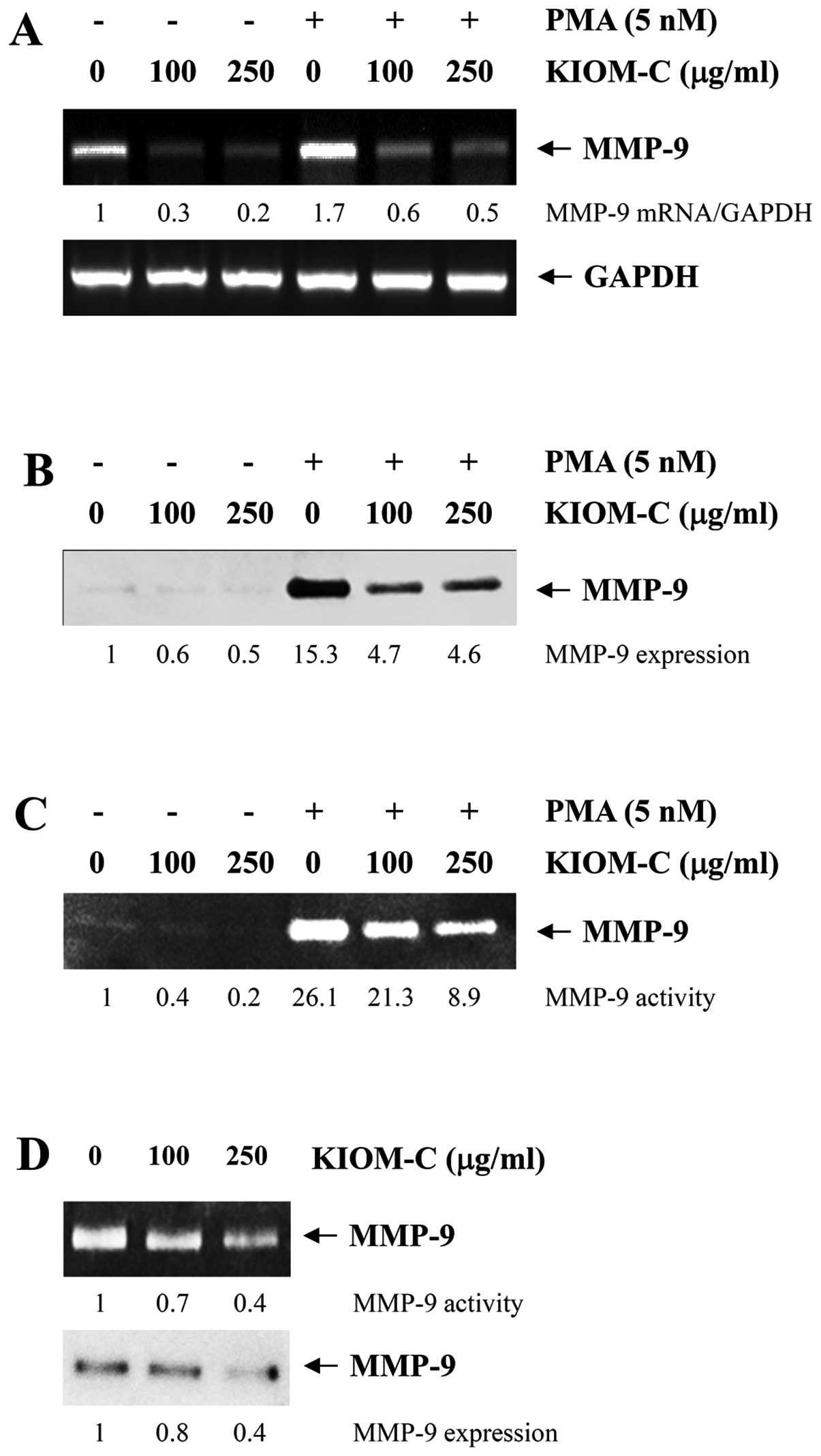
inhibitory mechanism of KIOM-C on migration and invasion. As shown
in Fig. 5A, mRNA expression of
MMP-9 was significantly decreased by KIOM-C treatment in HT1080
cells both under a resting and PMA-stimulated condition. In
addition, KIOM-C treatment significantly decreased secreted MMP-9
expression and gelatinolytic activity in the resting state. PMA, a
potent inducer for MMP-9 activation (15), increased MMP-9 expression and
activity 15.3- and 26.1-fold, respectively, in the control cells.
In the KIOM-C-treated cells, the increase in MMP-9 expression and
activity in response to PMA was significantly lower than these
values in the control cells (Fig. 5B
and C). PMA stimulation also increased the secretion of active
MMP-2 in the control cells, which was confirmed by western blotting
and gelatin zymography (15);
however, KIOM-C did not prevent the PMA-induced conversion of
latent pro-MMP-2 into its active form (data not shown). In
addition, in B16F10 cells, KIOM-C treatment also significantly
decreased MMP-9 activity and MMP-9 expression (Fig. 5D). Previous reports have
demonstrated that transcription factors such as NF-κB and activator
protein-1 (AP-1) are significantly involved in the regulation of
the MMP-9 gene expression and enhanced tumor invasion in various
types of cells (15–17). To elucidate whether the inhibitory
effect of KIOM-C on MMP-9 expression and invasion is linked to
NF-κB activity, we examined the levels of IκBα and phospho-IκBα,
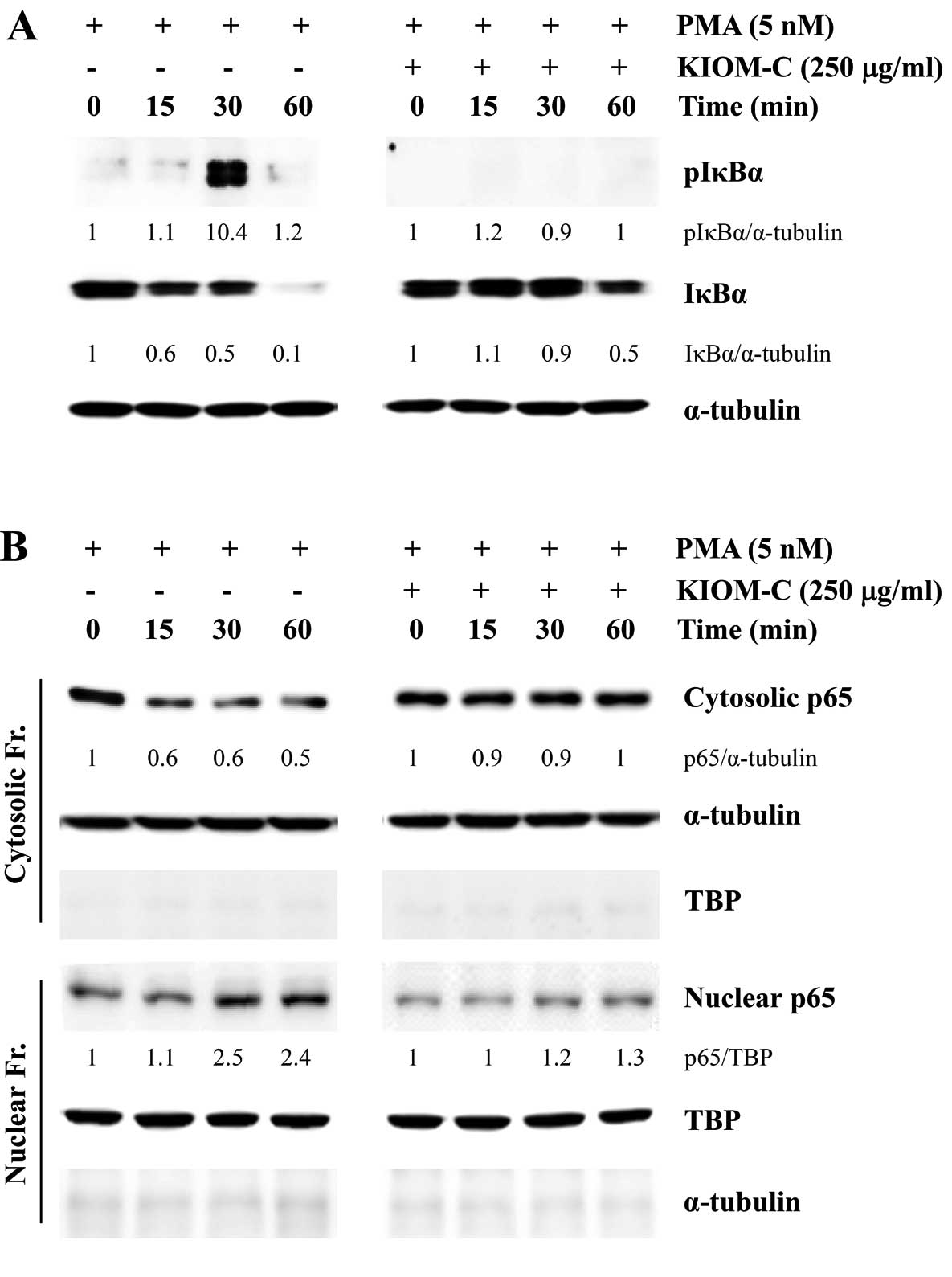
along with p65 nuclear translocation. As shown in Fig. 6A, PMA stimulation in control HT1080
cells immediately increased the level of IκBα phosphorylation,
accompanied by IκBα degradation. In addition, the p65 subunit was
rapidly translocated from the cytosol to the nucleus following PMA
stimulation (Fig. 6B). However, in
the KIOM-C-treated HT1080 cells, the increase in IκBα
phosphorylation and p65 nuclear translocation in response to PMA
stimulation was insignificant when compared to the control cells,
collectively suggesting that KIOM-C inhibits migration and invasion
by reducing MMP-9 activity via suppression of NF-κB activation.
Discussion
Early-stage primary tumors can generally be
controlled by conventional treatment such as surgery, radiation and
chemotherapy. However, tumors retaining invasive and metastatic
potential are relatively resistant to current chemotherapeutic
agents, accounting for poor prognoses and high mortality rates
(18). Although many anticancer
drugs that target cell proliferation and/or induce apoptosis are
believed to block or hinder the progression of cancer cells, these
agents are also associated with undesirable and severe side-effects
due to the non-selective killing of proliferating cells, which
limit clinical applications (1,19,20).
Thus, identification of agents with little or no toxicity to normal
cells that can interrupt one or more steps of metastasis and limit
the spread of cancer cells to a new site may be a valid strategy by
which to improve the efficacy of cancer treatment and to enhance
the survival of cancer patients. The metastatic cascade occurs in a
sequential order including cell adhesion, invasion, proliferation
and vessel formation. In particular, degradation of the basement
membrane and stromal ECM is essential for invasion and metastasis
of malignant cancer cells, and type IV collagen-degrading enzymes,
mainly MMP-2 and -9, participate in these initial steps. Recent
studies have revealed that invasive cells have higher expression
levels of MMP-9 and that its expression is closely correlated with
vascular invasion and aggressive malignant phenotypes (4,6). In
addition, MMP-2 has been found to be associated with adverse
prognosis and relapse in breast cancer patients (21–23).
Recently, natural plant products have received considerable
attention for their potential use in the treatment of malignant
invasive progression, and several herbal medicines are frequently
used as a supplemental therapy for many chronic diseases including
cancer due to the possible beneficial effect of flavonoids
(24–30).
In the present study, we demonstrated that KIOM-C, a
novel herbal medicine, abrogates the metastatic potential of
malignant HT1080 cells by reducing MMP activity via suppression of
NF-κB activation, and inhibits in vivo pulmonary metastasis
of B16F10 melanoma (Figs.
1–6). Long-term intake of
KIOM-C showed a dose-dependent reduction in the number of pulmonary
metastatic colonies, and intake of the most effective dose of 510
mg/kg, which corresponds to the human daily dose, did not result in
systemic toxicity throughout the experimental period (Table I–IV). Furthermore, oral administration of a
single dose of KIOM-C of up to 2,000 mg/kg in Sprague-Dawley rats
did not show acute toxicity or genotoxicity (12). These results suggest that KIOM-C can
potentially be used as an antimetastatic remedy.
An herbal cocktail may target multiple cellular
pathways in multifactorial diseases such as cancer, and may show
synergy and reciprocal action among the myriad of phytochemicals
present. The aqueous extract of Platycodon grandiflorum, one
of the constituents of KIOM-C, has been reported to strongly
suppress in vivo experimentally induced lung cancer, and to
prolong survival by possibly inhibiting the adhesion of B16F10
melanoma cells to the basement membrane and by activating NK cells
(31). Extracts of Lonicera
japonica Thunb. inhibit HepG2 cell motility by suppressing
MMP-2 activity and inducing G2/M cell cycle arrest via ERK
activation (32). In addition,
baicalein, baicalin, and woogonin isolated from Scutellariae Radix
exhibited strong antitumor activity by interruption of cell
proliferation, migration and invasion (33–35).
In HPLC analysis, baicalin was recovered as a dominant component
(~15.03–15.12%) in KIOM-C (13). In
previous studies, baicalin was demonstrated to inhibit basic
fibroblast growth factor (bFGF)-induced neovascularization in a
chicken chorioallantoic membrane (CAM) assay by reducing
cell-associated MMP-2 activity and inhibiting migration and
proliferation (34), indicating
that it plays a pivotal role in mediating anticancer activity. In
addition, baicalin was shown to inhibit migration and invasion of
MDA-MB-231 cells in vitro and suppress tumor growth and
pulmonary metastasis in a xenograft model of MDA-MB-231 cells in
vivo with no change in body weight or liver or kidney function,
which was explained by a decrease in MMP-2, MMP-9, uPA and uPAR
expression via the p38 mitogen-activated protein kinase (MAPK)
pathway (36). Decursin was shown
to inhibit the growth of cancer cells via apoptosis, cell cycle
arrest in the G1 phase and ERK activation (37). Furthermore, it reduced the
expression as well as the activity of MMP-9 in CT-26 murine colon
carcinoma via suppression of ERK and JNK phosphorylation, and
significantly reduced the formation of tumor nodules in the lung
and increased lung weight caused by CT-26 metastasis (38). In addition, 6-gingerol reduced
MMP-2/MMP-9 activities and inhibited the metastatic potential of
MDA-MB-231 human breast cancer cells (39). These results suggest that KIOM-C may
have potential antimetastatic effects via these active
components.
PMA reportedly stimulates MAPKs, including p38, ERK
and JNK, which leads to the activation of AP-1 and NF-κB
transcription factors. Activation of NF-κB and AP-1 downstream of
MAPKs or PI3K-Akt pathways is involved in many pathological
processes, such as inflammation, cancer cell adhesion, invasion,
metastasis and angiogenesis (2,40). In
particular, enhanced activation of MMP-9 by treatment with PMA in
HT1080 cells is mediated through the activation of NF-κB (15). In accordance with previous results,
KIOM-C suppressed PMA-induced MMP-9 expression through inhibition
of NF-κB activation in HT1080 cells (Figs. 5 and 6).
In summary, our results clearly demonstrated the
antimetastatic activity of KIOM-C via suppression of NF-κB
activation in highly malignant cancer cells. Moreover, oral
administration of KIOM-C considerably prevented pulmonary
metastasis of intravenously injected B16F10 melanoma cells with no
systemic toxicity, possibly through suppression of migration and
invasion. Collectively, these results suggest that KIOM-C may be a
safe herbal medicine for controlling metastatic cancer.
Acknowledgements
This study was supported by Grant K12050 awarded to
the Korea Institute of Oriental Medicine (KIOM) from the Ministry
of Education, Science and Technology (MEST), Republic of Korea.
References
|
1
|
Liotta LA, Steeg PS and Stetler-Stevenson
WG: Cancer metastasis and angiogenesis: an imbalance of positive
and negative regulation. Cell. 64:327–336. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arvelo F and Cotte C: Metalloproteinases
in tumor progression. Review Invest Clin. 47:185–205. 2006.(In
Spanish).
|
|
3
|
Pasco S, Brassart B, Ramont L, Maquart FX
and Monboisse JC: Control of melanoma cell invasion by type IV
collagen. Cancer Detect Prev. 29:260–266. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McCawley LJ and Matrisian LM: Matrix
metalloproteinases: multifunctional contributors to tumor
progression. Mol Med Today. 6:149–156. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiang ZQ, Zhu FC, Qu JY, Zheng X and You
CL: Relationship between expression of matrix metalloproteinase
(MMP-9) and tumor angiogenesis, cancer cell proliferation,
invasion, and metastasis in invasive carcinoma of cervix. Ai Zheng.
22:178–184. 2003.(In Chinese).
|
|
6
|
Kim TS and Kim YB: Correlation between
expression of matrix metalloproteinase-2 (MMP-2), and matrix
metalloproteinase-9 (MMP-9) and angiogenesis in colorectal
adenocarcinoma. J Korean Med Sci. 14:263–270. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
London CA, Sekhon HS, Arora V, Stein DA,
Iversen PL and Devi GR: A novel antisense inhibitor of MMP-9
attenuates angiogenesis, human prostate cancer cell invasion and
tumorigenicity. Cancer Gene Ther. 10:823–832. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lakka SS, Gondi CS, Yanamandra N, et al:
Inhibition of cathepsin B and MMP-9 gene expression in glioblastoma
cell line via RNA interference reduces tumor cell invasion, tumor
growth and angiogenesis. Oncogene. 23:4681–4689. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kong D, Li Y, Wang Z, Banerjee S and
Sarkar FH: Inhibition of angiogenesis and invasion by
3,3′-diindolylmethane is mediated by the nuclear factor-κB
downstream target genes MMP-9 and uPA that regulated
bioavailability of vascular endothelial growth factor in prostate
cancer. Cancer Res. 67:3310–3319. 2007.
|
|
10
|
Corson TW and Crews CM: Molecular
understanding and modern application of traditional medicines:
triumphs and trials. Cell. 130:769–774. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kiyohara H, Matsumoto T and Yamada H:
Combination effects of herbs in a multi-herbal formula: expression
of Juzen-taiho-to’s immuno-modulatory activity on the intestinal
immune system. Evid Based Complement Alternat Med. 1:83–91.
2004.PubMed/NCBI
|
|
12
|
Chung TH, Kang TJ, Lee GS, et al: Effects
of the novel herbal medicine, KIOM-C, on the growth performance and
immune status of porcine circovirus-associated disease (PCVAD)
affected pigs. J Med Plants Res. 6:4456–4466. 2012.
|
|
13
|
Kim EH, Pascua PN, Song MS, et al:
Immunomodulation and attenuation of lethal influenza A virus
infection by oral administration with KIOM-C. Antiviral Res.
98:386–393. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Price JE: Clonogenicity and experimental
metastatic potential of spontaneous mouse mammary neoplasms. J Natl
Cancer Inst. 77:529–535. 1986.PubMed/NCBI
|
|
15
|
Kim A, Kim MJ, Yang Y, Kim JW, Yeom YI and
Lim JS: Suppression of NF-κB activity by NDRG2 expression
attenuates the invasive potential of highly malignant tumor cells.
Carcinogenesis. 30:927–936. 2009.
|
|
16
|
Fong Y, Shen KH, Chiang TA and Shih YW:
Acacetin inhibits TPA-induced MMP-2 and u-PA expressions of human
lung cancer cells through inactivating JNK signaling pathway and
reducing binding activities of NF-κB and AP-1. J Food Sci.
75:30–38. 2010.PubMed/NCBI
|
|
17
|
Takahra T, Smart DE, Oakley F and Mann DA:
Induction of myofibroblast MMP-9 transcription in three-dimensional
collagen I gel cultures: regulation by NF-κB, AP-1 and Sp1. Int J
Biochem Cell Biol. 36:353–363. 2004.PubMed/NCBI
|
|
18
|
Weiss L: Metastatic inefficiency:
intravascular and intraperitoneal implantation of cancer cells.
Cancer Treat Res. 82:1–11. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Petrylak DP: The current role of
chemotherapy in metastatic hormone-refractory prostate cancer.
Urology. 65:3–7. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Evans T: Chemotherapy in advanced
non-small cell lung cancer. Semin Respir Crit Care Med. 26:304–313.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu LS, Chen L, Ding WX, Li K and Wu JJ:
Elevated expression of both MDR1 and MMP-2 genes in metastasized
lymph node of invasive ductal breast cancer. Eur Rev Med Pharmacol
Sci. 16:2037–2043. 2012.PubMed/NCBI
|
|
22
|
Jinga DC, Blidaru A, Condrea I, et al:
MMP-9 and MMP-2 gelatinases and TIMP-1 and TIMP-2 inhibitors in
breast cancer: correlations with prognostic factors. J Cell Mol
Med. 10:499–510. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakopoulou L, Tsirmpa I, Alexandrou P, et
al: MMP-2 protein in invasive breast cancer and the impact of
MMP-2/TIMP-2 phenotype on overall survival. Breast Cancer Res
Treat. 77:145–155. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee HJ, Lee EO, Rhee YH, et al: An
Oriental herbal cocktail, Ka-Mi-Kae-Kyuk-Tang, exerts anticancer
activities by targeting angiogenesis, apoptosis and metastasis.
Carcinogenesis. 27:2455–2463. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang SF, Chen MK, Hsieh YS, et al:
Antimetastatic effects of Terminalia catappa L. on oral
cancer via a down-regulation of metastasis-associated proteases.
Food Chem Toxicol. 48:1052–1058. 2010.
|
|
26
|
Ho ML, Hsieh YS, Chen JY, et al:
Antimetastatic potentials of Dioscorea nipponica on melanoma
in vitro and in vivo. Evid Based Complement Alternat Med.
2011:5079202011.PubMed/NCBI
|
|
27
|
Sun T, Chen QY, Wu LJ, Yao XM and Sun XJ:
Antitumor and antimetastatic activities of grape skin polyphenols
in a murine model of breast cancer. Food Chem Toxicol.
50:3462–3467. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Weng CJ and Yen GC: Chemopreventive
effects of dietary phytochemicals against cancer invasion and
metastasis: phenolic acids, monophenol, polyphenol, and their
derivatives. Cancer Treat Rev. 38:76–87. 2012. View Article : Google Scholar
|
|
29
|
Liu YH, Li ML, Hsu MY, et al: Effects of a
Chinese herbal medicine, Guan-Jen-Huang (Aeginetia indica
Linn.), on renal cancer cell growth and metastasis. Evid Based
Complement Alternat Med. 2012:9358602012.PubMed/NCBI
|
|
30
|
Kim SC, Magesh V, Jeong SJ, et al: Ethanol
extract of Ocimum sanctum exerts antimetastatic activity
through inactivation of matrix metalloproteinase-9 and enhancement
of anti-oxidant enzymes. Food Chem Toxicol. 48:1478–1482. 2010.
|
|
31
|
Lee KJ, Kim JY, Choi JH, et al: Inhibition
of tumor invasion and metastasis by aqueous extract of the radix of
Platycodon grandiflorum. Food Chem Toxicol. 44:1890–1896.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Park HS, Park KI, Lee DH, et al:
Polyphenolic extract isolated from Korean Lonicera japonica
Thunb. induces G2/M cell cycle arrest and apoptosis in HepG2 cells:
involvements of PI3K/Akt and MAPKs. Food Chem Toxicol.
50:2407–2416. 2012.PubMed/NCBI
|
|
33
|
Chiu YW, Lin TH, Huang WS, et al:
Baicalein inhibits the migration and invasive properties of human
hepatoma cells. Toxicol Appl Pharmacol. 255:316–326. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu JJ, Huang TS, Cheng WF and Lu FJ:
Baicalein and baicalin are potent inhibitors of angiogenesis:
inhibition of endothelial cell proliferation, migration and
differentiation. Int J Cancer. 106:559–565. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee SO, Jeong YJ, Yu MH, et al: Wogonin
suppresses TNF-α-induced MMP-9 expression by blocking the NF-κB
activation via MAPK signaling pathways in human aortic smooth
muscle cells. Biochem Biophys Res Commun. 351:118–125. 2006.
|
|
36
|
Wang XF, Zhou QM, Du J, Zhang H, Lu YY and
Su SB: Baicalin suppresses migration, invasion and metastasis of
breast cancer via p38MAPK signaling pathway. Anticancer Agents Med
Chem. 13:923–931. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim WJ, Lee SJ, Choi YD and Moon SK:
Decursin inhibits growth of human bladder and colon cancer cells
via apoptosis, G1-phase cell cycle arrest and extracellular
signal-regulated kinase activation. Int J Mol Med. 25:635–641.
2010.PubMed/NCBI
|
|
38
|
Son SH, Park KK, Park SK, et al: Decursin
and decursinol from Angelica gigas inhibit the lung
metastasis of murine colon carcinoma. Phytother Res. 25:959–964.
2011.
|
|
39
|
Lee HS, Seo EY, Kang NE and Kim WK:
[6]-Gingerol inhibits metastasis of MDA-MB-231 human breast cancer
cells. J Nutr Biochem. 19:313–319. 2008.
|
|
40
|
Luqman S and Pezzuto JM: NFκB: a promising
target for natural products in cancer chemoprevention. Phytother
Res. 24:949–963. 2010.
|