Introduction
Cancer cells are recognized by the immune system as
foreign objects, and are destroyed by immune cells (1). However, cancer cells are known to
evade the antitumor immune response by multiple immunosuppressive
mechanisms, such as i) losing major histocompatibility complex
(MHC) molecules so as to avoid recognition by immune cells
(2), ii) producing
immunosuppressive cytokines (3),
and iii) inducing immunosuppressive cells including myeloid derived
suppressor cells (MDSCs) (4) and
regulatory T cells (Tregs) (5) to
interfere with attack by other immune cells. In particular, MDSCs
and Tregs have been focused on and studied recently as targets of
cancer therapy.
Regulatory T cells are one of immune suppressor
cells and play an important role in maintaining immunological
tolerance. However, this mechanism is one of the main obstacles to
overcome when attempting to improve antitumor immunity. In fact,
the accumulation of Tregs in tumor tissues (6) and peripheral blood (7) is correlated with poor prognosis in
patients with cancer. Recently, several studies have also reported
the importance of a balance between immune effective cells and
immune suppressor cells. For example, the CD8+ T
cell/Treg ratio was found to be associated with overall survival
time in patients with colorectal cancer (8). These studies, thus, show that Tregs
are the major player in suppressing antitumor effects. Various
methods to deplete Tregs have been evaluated in clinical trials
(9–11). Previous studies have demonstrated
that naïve T cells differentiate into Tregs in the presence of
TGF-β in the periphery (12,13).
Therefore, TGF-β plays a critical role in the induction and
maintenance of Tregs.
PSK is a protein-bound polysaccharide purified from
the mycelium of Corious versicolor, and has been used in
Japan as an adjuvant therapy for gastric and colorectal cancer in
combination with chemotherapy, and as an adjuvant treatment for
small cell lung cancer in combination with chemoradiotherapy.
Various actions of protein-bound polysaccharide-K (PSK) have been
reported, such as direct suppression of tumor growth (14) and induction of cytokine production
(15,16). PSK has also been reported to improve
the immunosuppressive state of tumor-bearing hosts by suppressing
TGF-β activity in vitro(17)
and in vivo(18).
We, therefore, hypothesized that PSK reduces Tregs
through suppression of TGF-β resulting in augmentation of antitumor
immunity. In fact, a clinical trial previously reported that PSK
reduces the proportion of Tregs in the peripheral blood of gastric
cancer patients (19). However, the
relationship between PSK and Tregs as well as the mechanism by
which PSK reduces the proportion of Tregs remain unclear. In the
present study, we investigated whether PSK affects Tregs in
vitro and in vivo, and found that reduction of TGF-β by
PSK decreases the proportion of Tregs in the spleen and augments
antitumor immunity.
Materials and methods
Mice
C57BL/6 and BALB/c female mice were purchased from
Charles River Laboratories Japan, Inc. (Yokohama, Japan). All
animals were maintained under a specific pathogen-free condition.
BALB/c mice were used in experiments at 6 weeks of age. C57BL/6
mice were used from 6 weeks to 12 weeks of age. All animal
experiments were performed under the Institutional Guidelines for
Care and Use of Laboratory Animals (Kureha Corp., Tokyo, Japan).
The experimental protocol was approved by the Ethics Committee on
Animal Experiments of the Biomedical Research Laboratories of
Kureha Corp., and the mice were handled in accordance with the
guidelines of the committee.
Reagents
PSK (Kureha Corp.) was dissolved in sterilized
physiological saline, and then diluted to appropriate
concentrations for use in the in vivo and in vitro
experiments. The anti-CD3e and anti-CD28 antibodies (BD
Biosciences, Franklin Lakes, NJ, USA) were used in the in
vitro assay. The anti-CD25 antibody produced by hybridoma PC61
(ATCC) was a rat IgG1 antibody. For in vivo administration,
the anti-CD25 antibody (PC61) was used after purification from
hybridoma ascites produced in SCID mice. The concentration of IgG
was determined by the Rat IgG1 ELISA Quantitation kit (Bethyl
Laboratories, Montgomery, TX, USA). The FITC-anti-CD4 antibody
(clone RM4-5; eBioscience, San Diego, CA, USA), APC-anti-CD25
antibody (clone PC61; eBioscience), PE-anti-Foxp3 antibody (clone
FJK-16; eBioscience), and PerCP-anti-CD8a antibody (clone 53-6.7;
BioLegend, San Diego, CA, USA) were used in the flow cytometry.
Regulatory T cell conversion assay
Spleens from C57BL/6 mice were gently minced into
single-cell suspensions in complete RPMI-1640 containing 10% fetal
bovine serum (FBS) (BioWest, Nuaillé, France), and
CD4+CD25− T cells were purified using MACS
beads (Miltenyi Biotec, Bergisch Gladbach, Germany). The purity of
the CD4+CD25− T cells was confirmed to be
>90% by flow cytometry. These cells were suspended in RPMI-1640
supplemented with 10% FBS, 1 mmol/l sodium pyruvate, 2 mmol/l
L-glutamine, non-essential amino acids, 25 mmol/l HEPES buffer and
50 μmol/l 2-mercaptoethanol. The cell suspension was adjusted to a
density of 1×106 cells/ml and used in the conversion
assays.
For conversion to Tregs,
CD4+CD25− cells were cultured with 2 μg/ml of
the anti-CD3 antibody and 2 μg/ml of the anti-CD28 antibody in the
presence of 20 U/ml recombinant human IL-2 (PeproTech Inc., Rocky
Hill, NJ, USA) and 2 ng/ml recombinant TGF-β (R&D Systems,
Minneapolis, MN, USA) for 4 days in a 96-well plate. After
incubation, the proportions of Tregs were analyzed by a flow
cytometer.
Depletion of Tregs
BALB/c mice were inoculated subcutaneously with
1×106 methylcholanthrene-induced fibrosarcoma (Meth A)
cells on day 0. These mice were injected intraperitoneally with 500
μg of the anti-CD25 antibody on days 1, 7, 14 and 21 to selectively
deplete CD25+ cells. Control mice were administered rat
IgG. Tumor size was measured using calipers twice a week.
Antitumor effect following administration
of PSK in vivo
BALB/c mice were inoculated subcutaneously with
1×106 Meth A cells on day 0. From day 1, the mice
received intraperitoneal injection of PSK (50 mg/kg) three times
per week. Control mice were administered saline intraperitoneally.
Tumor size was measured twice a week. After 4 weeks, all mice were
euthanized, and the tumor weights were measured. The proportion of
Tregs and the CD8+ cell-to-Treg cell
(CD8+/Treg) ratio in the spleen, plasma TGF-β
concentration and IFN-γ production by spleen cells were also
measured.
Flow cytometry
The proportions of regulatory T cells were measured
using a Mouse Regulatory T cell staining kit (eBioscience). In
brief, the cells from the in vitro Treg conversion assay
were stained with the FITC-anti-CD4 and APC-anti-CD25 antibodies,
and then suspended in Fix/Perm buffer and incubated. The cells were
washed with permeabilization buffer and stained with the anti-mouse
Foxp3 antibody. After washing with permeabilization buffer, the
cells were analyzed by a FACScalibur flow cytometer (BD
Biosciences).
For the in vivo study, spleens from mice were
minced into single-cell suspensions in complete RPMI-1640
containing 10% FBS, and monolayer cells were also stained with the
FITC-anti-CD4, APC-anti-CD25, PE-anti-Foxp3, and PerCP-anti-CD8a
antibodies following the above protocol.
The data were analyzed using FlowJo software (Tomy
Digital Biology Co., Ltd., Tokyo, Japan). Tregs were defined as
CD4+CD25+Foxp3+ cells. The
proportion of Tregs in the spleen was defined as the percentage of
CD25+Foxp3+ cells to CD4+ cells in
the spleen. The CD8+/Treg ratio in the spleen was
calculated as follows: Number of CD8+ cells divided by
number of Treg cells in the spleen.
Measurement of TGF-β in plasma
Blood from mice was collected into tubes containing
EDTA (BD Biosciences) and centrifuged at 1000 × g for 20 min at
4°C. The plasma samples were collected into fresh tubes and
centrifuged again at 10,000 × g for 10 min at 4°C. The supernatant
was used to determine the plasma TGF-β1 level by a
Canine/Mouse/Porcine/Rat TGF-β1 Quantikine ELISA kit (R&D
Systems).
Measurement of IFN-γ production by spleen
cells
Spleen cells (5×106) obtained from the
mice in the in vivo study were cultured with Meth A cells
(5×104) pretreated with 50 μg/ml mitomycin-C (MMC) for
45 min. After 2 days, the cell culture supernatants were collected,
and the levels of IFN-γ were measured using a Mouse IFN-gamma
Quantikine ELISA kit (R&D Systems).
Statistical analysis
Data are presented as means ± standard error of the
mean (SE). Statistical analyses were performed using Student’s
t-test and Mann-Whitney U test. P-values <0.05 were considered
to indicate statistically significant results.
Results
PSK reduces the proportion of
TGF-β-induced Tregs in vitro
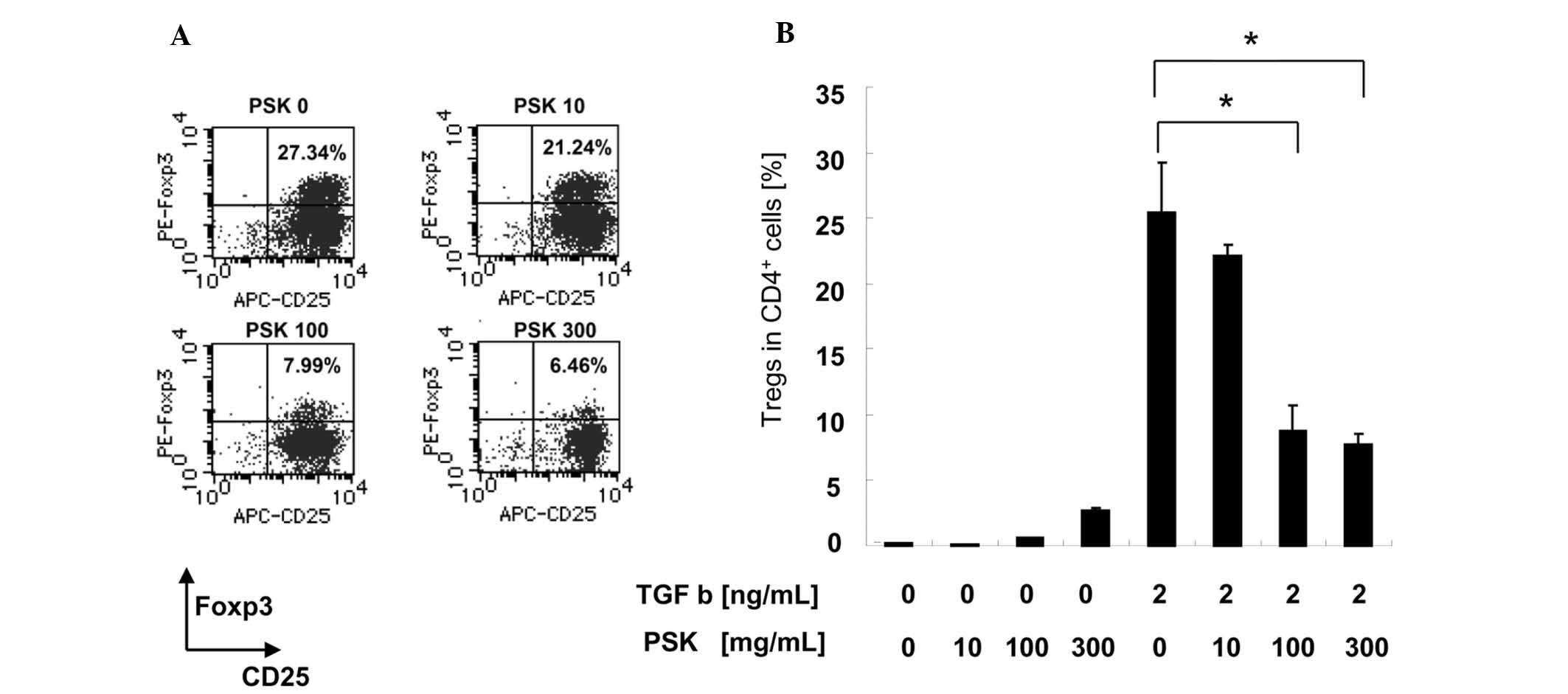
We first confirmed whether Tregs were induced by
TGF-β from CD4+CD25− T cells by an in
vitro conversion assay. After culturing
CD4+CD25− T cells for 4 days in the presence
of TGF-β, the proportion (mean ± SE) of
CD25+Foxp3+ cells to CD4+ cells
was 25.42±3.83% (Fig. 1B).
Next, the effect of PSK on Treg conversion was
examined. The proportion (mean ± SE) of
CD25+Foxp3+ cells to CD4+ cells
was 22.18±0.824% with 10 μg/ml of PSK, 8.74±1.98% with 100 μg/ml of
PSK and 7.75±0.763% with 300 μg/ml of PSK (Fig. 1B).
These data indicate that PSK reduces the proportion
of TGF-β-induced Tregs in a dose-dependent manner.
Depletion of Tregs suppresses tumor
growth in Meth A-bearing mice
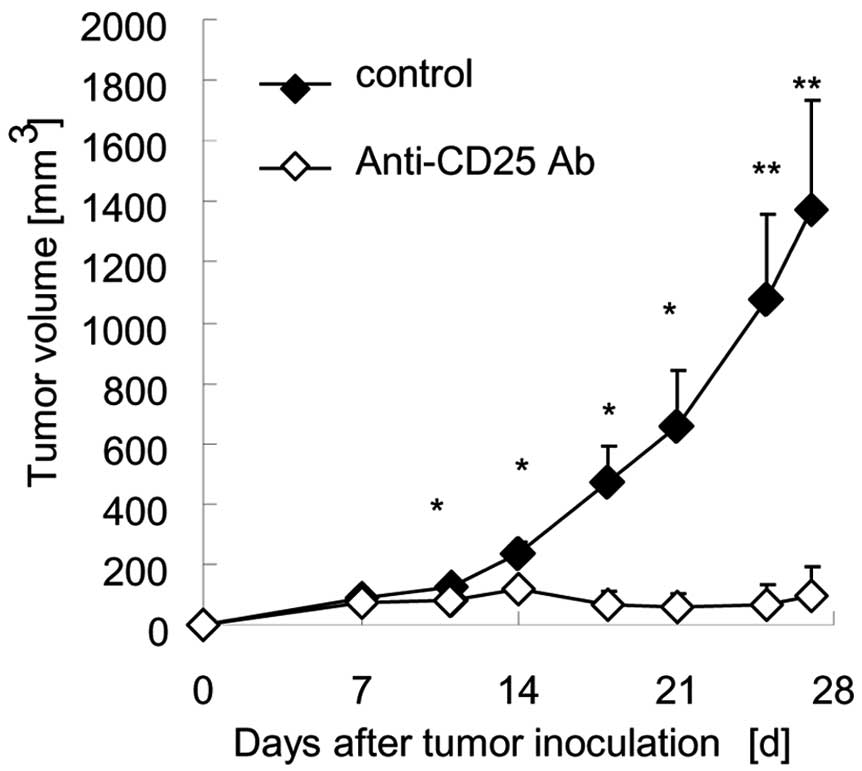
To exam whether tumor growth is related to Tregs,
BALB/c mice were inoculated subcutaneously with Meth A cells and
then received intraperitoneal injections of the anti-CD25 antibody
to deplete Tregs. In a preliminary study, when BALB/c mice were
administered the anti-CD25 antibody, the Tregs were depleted
immediately and recovered 7 days after injection (data not shown).
Therefore, we administered the antibody once a week. Tumor growth
was significantly suppressed by depletion of Tregs (Fig. 2).
The anti-CD25 antibody did not directly affect the
growth of Meth A cells in vitro. Therefore, the data of the
present study suggest that depletion of Tregs in mice augments the
activity of other immune cells and suppresses the growth of Meth A
tumor cells in vivo.
PSK suppresses tumor growth in Meth
A-bearing mice
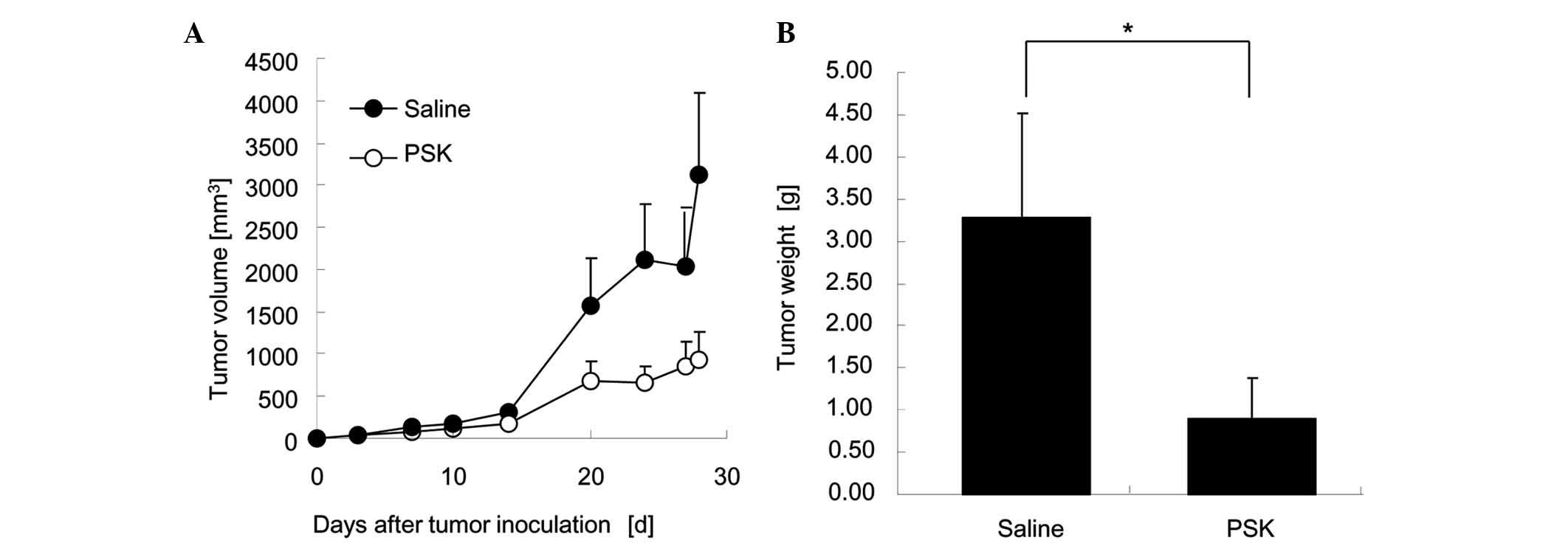
We examined the effect of PSK on the growth of Meth
A tumors. Tumor growth in mice treated with 50 mg/kg PSK was
significantly suppressed compared to the saline control group
(Fig. 3).
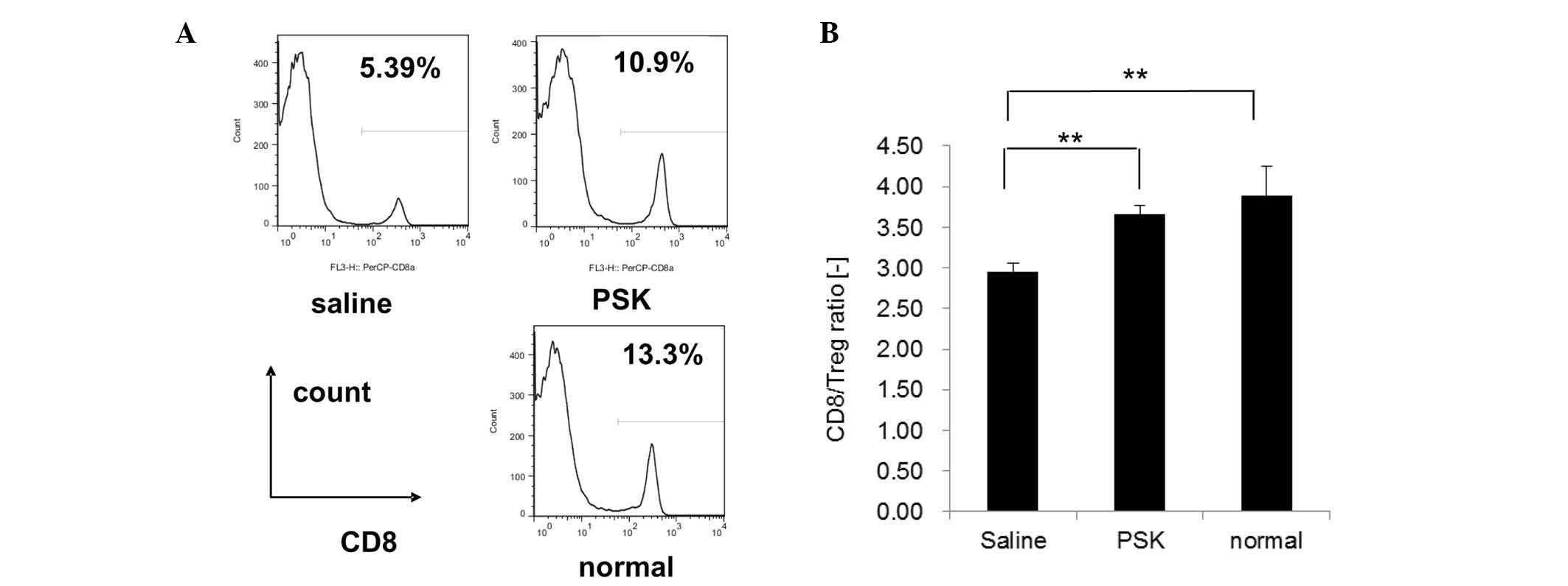
PSK reduces the proportion of Tregs and
increases the CD8/Treg ratio in Meth A-bearing mice
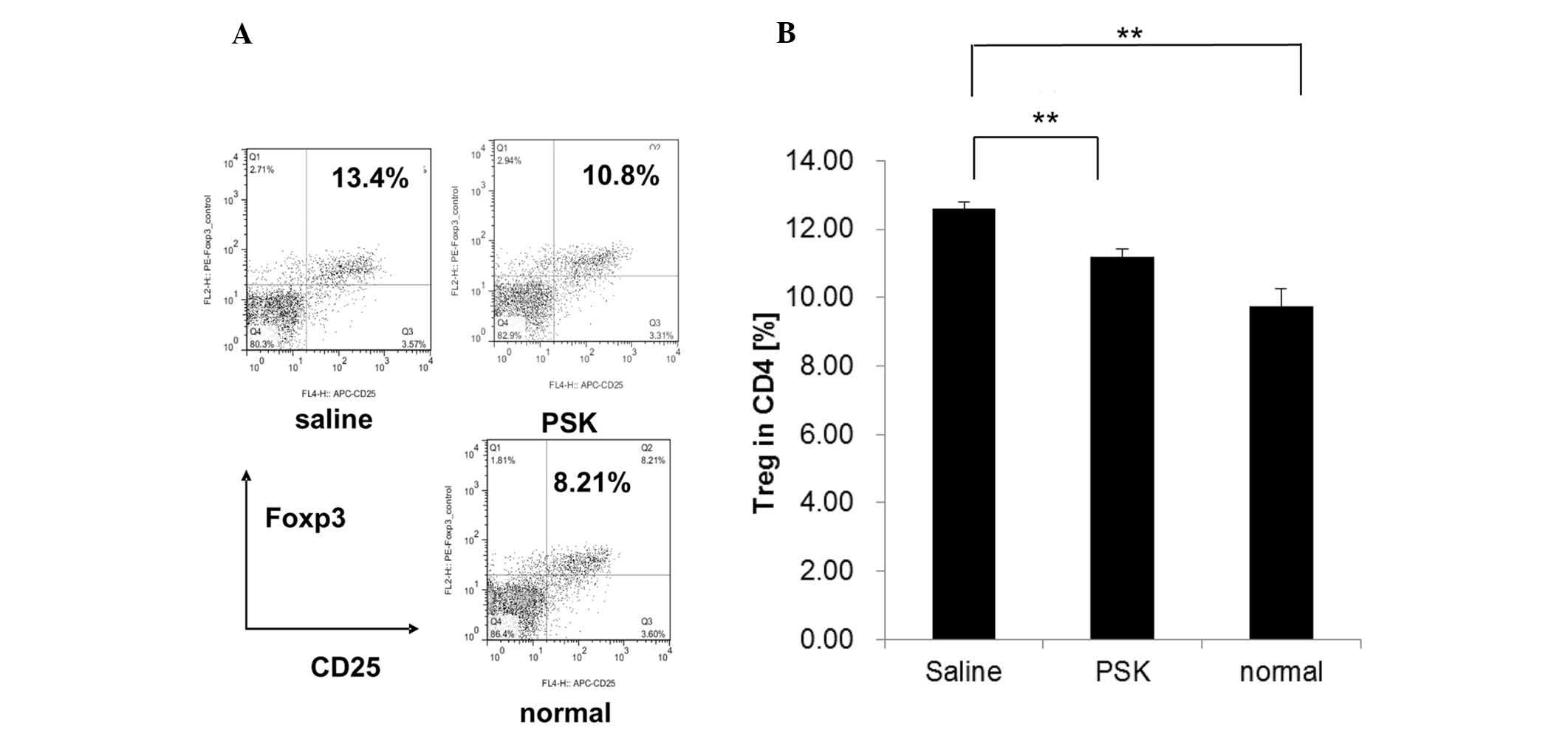
We next attempted to examine the underlying
mechanism by which PSK inhibits tumor growth. Since the growth of
Meth A cells in vivo was related to Tregs, we investigated
the proportion of Tregs and the CD8+/Treg ratio in the
spleen of Meth A-bearing mice with or without PSK treatment. Our
results showed that the proportion of Tregs in the saline control
group was significantly higher when compared to the proportion in
the normal group, and PSK treatment decreased the proportion of
Tregs significantly compared to the saline group (Fig. 4).
In addition, the CD8+/Treg ratio in the
saline group was significantly lower when compared to this ratio in
the normal group, and PSK treatment increased the proportion
significantly when compared to the saline group (Fig. 5).
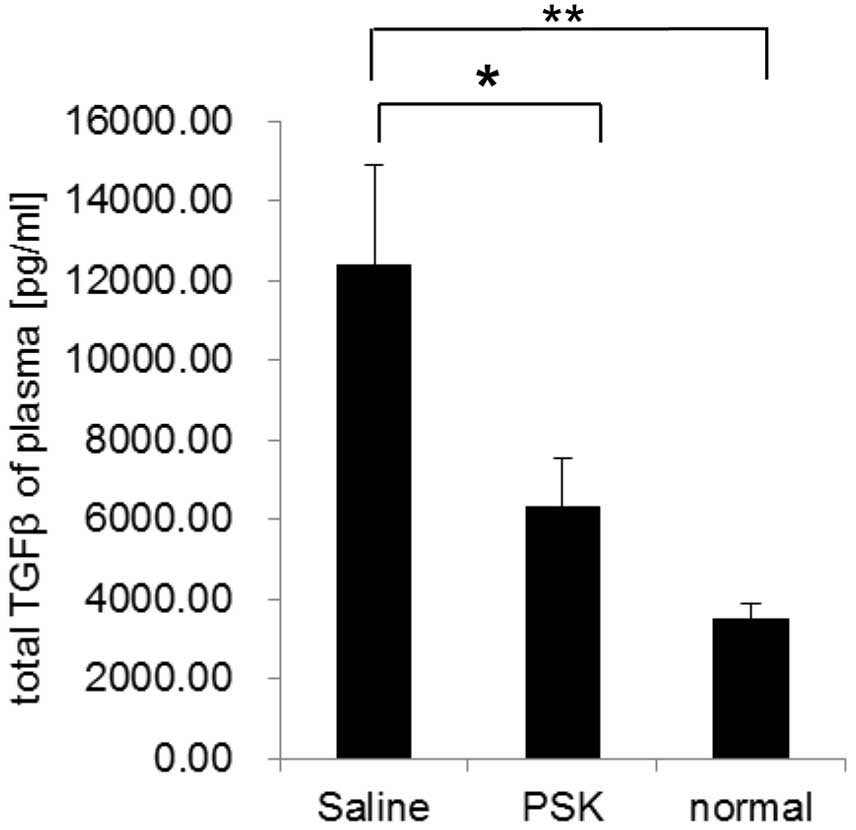
PSK decreases the plasma TGF-β levels in
the tumor-bearing mice
We also measured the plasma TGF-β levels in Meth
A-bearing mice by ELISA. The concentration of TGF-β was 12.4 ng/ml
in the saline group, 6.33 ng/ml in the PSK group and 3.52 ng/ml in
the normal group. PSK treatment reduced the plasma TGF-β level
significantly when compared to the level in the saline group
(Fig. 6).
These results suggest that the reduction in the
blood TGF-β concentration by PSK reduces the proportion of Tregs in
the spleen and enhances antitumor immunity.
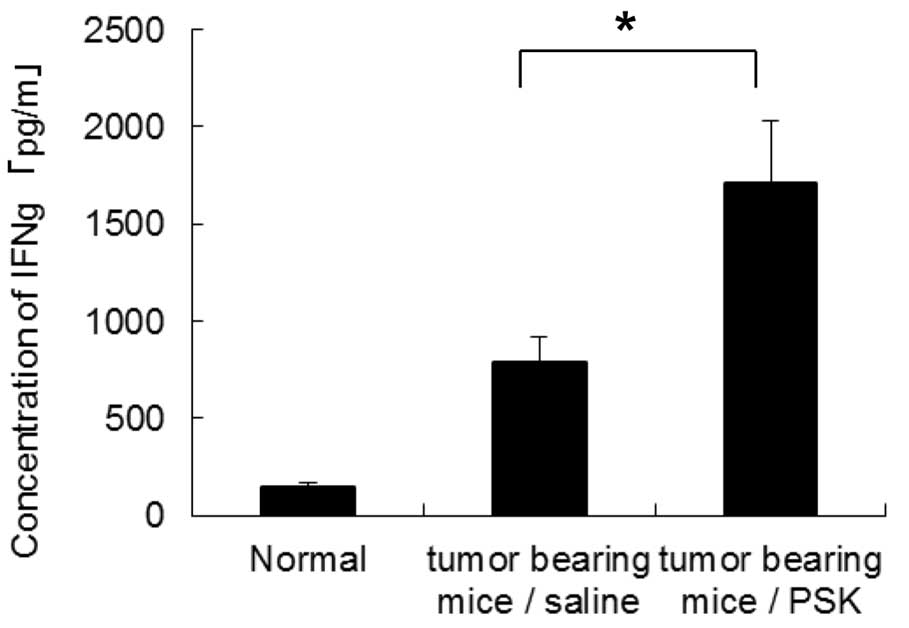
PSK increases IFN-γ production by spleen
cells in tumor-bearing mice
We also examined whether PSK increases
tumor-reactive cells in tumor-bearing mice. Spleen cells collected
from mice in the in vivo study were cultured with
MMC-treated Meth A in vitro, and the concentrations of IFN-γ
in the supernatants were measured by ELISA. Spleen cells from
PSK-administered mice showed significantly higher IFN-γ production
when compared to the production in the spleen cells from
saline-injected mice (Fig. 7).
These results suggest that PSK effectively increases the activity
of tumor-reactive cells in tumor-bearing mice.
Discussion
Clinical studies have demonstrated that PSK reduces
the proportion of Tregs in the peripheral blood of gastric cancer
(19) and colorectal cancer
patients (20). Therefore, we
attempted to clarify the relationship between PSK and Tregs, and
the mechanism by which PSK reduces Tregs, by conducting in
vitro and in vivo studies. In the present study, we
demonstrated that PSK suppressed the proportion of Tregs in
vitro and in vivo. Moreover, we showed the possibility
that PSK reduces Tregs through reducing the level of TGF-β.
There are various clinical approaches by which to
abolish immunosuppression induced by Tregs (9,10,21),
such as depletion of Tregs with anti-CD25 antibodies. Daclizumab,
the humanized anti-CD25 antibody, caused long-lasting depletion of
Tregs and enhancement of anti-peptide immune responses in a peptide
vaccination trial for breast cancer (11). In the present study, growth of Meth
A tumor cells in mice was also suppressed by depletion of Tregs
with the anti-CD25 antibody. This finding shows that Tregs are
absolutely essential for tumor growth. Many reports have shown that
the anti-CD25 antibody is useful if administered before grafting of
a tumor (21). However, in the
present study, the anti-CD25 antibody administered after
subcutaneous inoculation of Meth A tumor cells was also effective.
Therefore, the Meth A tumor-bearing mouse model may be sensitive to
the depletion of Tregs. While therapy targeting Tregs is effective
as mentioned above, the suppression of Tregs also produces adverse
effects (9) and the anti-CD25
antibody depletes not only Tregs but also effector cells (10). Therefore, the timing of antibody
administration and the dose have to be considered carefully when
Tregs are directly suppressed. On the other hand, since the present
study suggests that PSK reduces Tregs probably not by direct action
on Tregs but by indirect control via the reduction of TGF-β, PSK
may offer an advantage over other direct depletion methods in terms
of fewer adverse effects.
TGF-β is known as an immunosuppressive cytokine and
has been shown recently to play an important role in inducing Tregs
and suppressing the functions of other immune cells via Tregs
(22). Plasma TGF-β levels and the
proportion of Tregs in the spleen were increased in tumor-bearing
mice than that in normal mice in the present study. On the other
hand, plasma TGF-β levels and the proportion of Tregs in
tumor-bearing mice administered PSK were not lower than those in
normal mice. This result implies that the reduction in TGF-β
concentration by PSK may reduce the population of TGF-β-induced
Tregs in tumor-bearing host and enhance antitumor immunity.
Moreover, PSK treatment not only decreased the
proportion of Tregs, but also increased the proportion of
CD8+ T cells and IFN-γ production by spleen cells. These
results indicate that PSK improves the immunosuppressive condition
in tumor-bearing hosts. Lu et al(23) also reported an increase in the
number of IFNγ-secreting tumor-specific T cells in PSK-treated
tumor-bearing mice. However, it remains unclear which immune cells
participated in the enhanced antitumor immunity in the present
study. We are planning to identify the effector cells using
depletion experiments by administering specific antibody to each
immune cell type.
Recently, new anticancer immunotherapies have been
evaluated in clinical studies (24,25).
Cancer vaccines are one of these novel approaches, but their
clinical efficacy is not yet sufficient (26). One major reason is that Treg-induced
immunosuppressive conditions interfere with cancer vaccine-induced
immune responses to cancer. Therefore, it is important to control
Tregs in cancer immunotherapies. In a murine osteosarcoma model,
administration of the anti-TGF-β antibody combined with cancer
vaccine therapy suppressed tumor volume and inhibited the
accumulation of Tregs, inducing CD8 T cell infiltration in tumor
tissues and Treg accumulation in the spleen (27). Therefore, TGF-β blockade when
combined with cancer vaccine is effective for the reduction of
Tregs and is one of the most effective strategies in cancer
immunotherapy.
In conclusion, the present study demonstrated that
PSK suppressed Tregs in vitro and in vivo. In the
future, identifying the effects of PSK on other immune cells would
not only elucidate the mechanism of action of PSK, but also confirm
the benefit of using PSK in combination with cancer vaccine
therapies.
Acknowledgements
The present study was conducted at Tokyo Women’s
Medical University, Tokyo, Japan; Ochanomizu University, Tokyo,
Japan; and Kureha Corp., Tokyo, Japan.
References
|
1
|
Dunn GP, Old LJ and Schreiber RD: The
immunobiology of cancer immunosurveillance and immunoediting.
Immunity. 21:137–148. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Algarra I, Cabrera T and Garrido F: The
HLA crossroad in tumor immunology. Hum Immunol. 61:65–73. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sharma S, Stolina M, Lin Y, Gardner B,
Miller PW, Kronenberg M and Dubinett SM: T cell-derived IL-10
promotes lung cancer growth by suppressing both T cell and APC
function. J Immunol. 163:5020–5028. 1999.PubMed/NCBI
|
|
4
|
Lesokhin AM, Hohl TM, Kitano S, Cortez C,
Hirschhorn-Cymerman D, Avogadri F, Rizzuto GA, Lazarus JJ, Pamer
EG, Houghton AN, Merghoub T and Wolchok JD: Monocytic
CCR2+ myeloid-derived suppressor cells promote immune
escape by limiting activated CD8 T-cell infiltration into the tumor
microenvironment. Cancer Res. 72:876–886. 2012.
|
|
5
|
Yamaguchi T and Sakaguchi S: Regulatory T
cells in immune surveillance and treatment of cancer. Semin Cancer
Biol. 16:115–123. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Curiel TJ, Coukos G, Zou L, Alvarez X,
Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L,
Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L,
Lackner A, Disis ML, Knutson KL, Chen L and Zou W: Specific
recruitment of regulatory T cells in ovarian carcinoma fosters
immune privilege and predicts reduced survival. Nat Med.
10:942–949. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Woo EY, Yeh H, Chu CS, Schlienger K,
Carroll RG, Riley JL, Kaiser LR and June CH: Cutting edge:
regulatory T cells from lung cancer patients directly inhibit
autologous T cell proliferation. J Immunol. 168:4272–4276. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suzuki H, Chikazawa N, Tasaka T, Wada J,
Yamasaki A, Kitaura Y, Sozaki M, Tanaka M, Onishi H, Morisaki T and
Katano M: Intratumoral CD8+ T/FOXP3 + cell
ratio is a predictive marker for survival in patients with
colorectal cancer. Cancer Immunol Immunother. 59:653–661. 2010.
|
|
9
|
Maker AV, Attia P and Rosenberg SA:
Analysis of the cellular mechanism of antitumor responses and
autoimmunity in patients treated with CTLA-4 blockade. J Immunol.
175:7746–7754. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jacobs JF, Punt CJ, Lesterhuis WJ,
Sutmuller RP, Brouwer HM, Scharenborg NM, Klasen IS, Hilbrands LB,
Figdor CG, de Vries IJ and Adema GJ: Dendritic cell vaccination in
combination with anti-CD25 monoclonal antibody treatment: a phase
I/II study in metastatic melanoma patients. Clin Cancer Res.
16:5067–5078. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rech AJ, Mick R, Martin S, Recio A, Aqui
NA, Powell DJ Jr, Colligon TA, Trosko JA, Leinbach LI, Pletcher CH,
Tweed CK, DeMichele A, Fox KR, Domchek SM, Riley JL and Vonderheide
RH: CD25 blockade depletes and selectively reprograms regulatory T
cells in concert with immunotherapy in cancer patients. Sci Transl
Med. 4:134ra622012.
|
|
12
|
Chen W, Jin W, Hardegen N, Lei KJ, Li L,
Marinos N, McGrady G and Wahl SM: Conversion of peripheral
CD4+CD25− naive T cells to
CD4+CD25+ regulatory T cells by TGF-β
induction of transcription factor Foxp3. J Exp Med. 198:1875–1886.
2003.
|
|
13
|
Huber S, Stahl FR, Schrader J, Lüth S,
Presser K, Carambia A, Flavell RA, Werner S, Blessing M, Herkel J
and Schramm C: Activin a promotes the TGF-β-induced conversion of
CD4+CD25− T cells into Foxp3+
induced regulatory T cells. J Immunol. 182:4633–4640. 2009.
|
|
14
|
Hirahara N, Edamatsu T, Fujieda A, Fujioka
M, Wada T and Tajima Y: Protein-bound polysaccharide-K (PSK)
induces apoptosis via p38 mitogen-activated protein kinase pathway
in promyelomonocytic leukemia HL-60 cells. Anticancer Res.
32:2631–2638. 2012.
|
|
15
|
Sakagami H, Sugaya K, Utsumi A, Fujinaga
S, Sato T and Takeda M: Stimulation by PSK of interleukin-1
production by human peripheral blood mononuclear cells. Anticancer
Res. 13:671–675. 1993.PubMed/NCBI
|
|
16
|
Kato M, Hirose K, Hakozaki M, Ohno M,
Saito Y, Izutani R, Noguchi J, Hori Y, Okumoto S, Kuroda D, et al:
Induction of gene expression for immunomodulating cytokines in
peripheral blood mononuclear cells in response to orally
administered PSK, an immunomodulating protein-bound polysaccharide.
Cancer Immunol Immunother. 40:152–156. 1995. View Article : Google Scholar
|
|
17
|
Matsunaga K, Hosokawa A, Oohara M, Sugita
N, Harada M and Nomoto K: Direct action of a protein-bound
polysaccharide, PSK, on transforming growth factor-beta.
Immunopharmacology. 40:219–230. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Harada M, Matsunaga K, Oguchi Y, Iijima H,
Tamada K, Abe K, Takenoyama M, Ito O, Kimura G and Nomoto K: Oral
administration of PSK can improve the impaired anti-tumor
CD4+ T-cell response in gut-associated lymphoid tissue
(GALT) of specific-pathogen-free mice. Int J Cancer. 70:362–372.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yoshikawa K, Shimada M, Kurita N, Sato H,
Iwata T, Nishioka M, Morimoto S, Miyatani T, Komatsu M and And RN:
The effect of polysaccharide k with s-1 based chemotherapy in
advanced gastric cancer. Hepatogastroenterology. 60:1387–1390.
2013.PubMed/NCBI
|
|
20
|
Yoshino S, Yoshimura K, Suzuki N, Iida M,
Yoshida S, Maeda Y, Maeda K, Hazama S and Oka M: Immunoregulatory
effects of PSK on the Th1/Th2 balance and regulatory T-cells in
patients with colorectal cancer. Gan To Kagaku Ryoho. 37:2234–2236.
2010.(In Japanese).
|
|
21
|
Onizuka S, Tawara I, Shimizu J, Sakaguchi
S, Fujita T and Nakayama E: Tumor rejection by in vivo
administration of anti-CD25 (interleukin-2 receptor α) monoclonal
antibody. Cancer Res. 59:3128–3133. 1999.PubMed/NCBI
|
|
22
|
Shen E, Zhao K, Wu C and Yang B: The
suppressive effect of CD25+ Treg cells on Th1
differentiation requires cell-cell contact partially via TGF-β
production. Cell Biol Int. 35:705–712. 2011.
|
|
23
|
Lu H, Yang Y, Gad E, Wenner CA, Chang A,
Larson ER, Dang Y, Martzen M, Standish LJ and Disis ML:
Polysaccharide krestin is a novel TLR2 agonist that mediates
inhibition of tumor growth via stimulation of CD8 T cells and NK
cells. Clin Cancer Res. 17:67–76. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Aarntzen EH, De Vries IJ, Lesterhuis WJ,
Schuurhuis D, Jacobs JF, Bol K, Schreibelt G, Mus R, De Wilt JH,
Haanen JB, Schadendorf D, Croockewit A, Blokx WA, Van Rossum MM,
Kwok WW, Adema GJ, Punt CJ and Figdor CG: Targeting CD4+
T-helper cells improves the induction of antitumor responses in
dendritic cell-based vaccination. Cancer Res. 73:19–29.
2013.PubMed/NCBI
|
|
25
|
Sawada Y, Yoshikawa T, Nobuoka D,
Shirakawa H, Kuronuma T, Motomura Y, Mizuno S, Ishii H, Nakachi K,
Konishi M, Nakagohri T, Takahashi S, Gotohda N, Takayama T, Yamao
K, Uesaka K, Furuse J, Kinoshita T and Nakatsura T: Phase I trial
of a glypican-3-derived peptide vaccine for advanced hepatocellular
carcinoma: immunologic evidence and potential for improving overall
survival. Clin Cancer Res. 18:3686–3696. 2012. View Article : Google Scholar
|
|
26
|
Rosenberg SA, Yang JC and Restifo NP:
Cancer immunotherapy: moving beyond current vaccines. Nat Med.
10:909–915. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kawano M, Itonaga I, Iwasaki T, Tsuchiya H
and Tsumura H: Anti-TGF-β antibody combined with dendritic cells
produce antitumor effects in osteosarcoma. Clin Orthop Relat Res.
470:2288–2294. 2012.
|