Introduction
The accuracy of radiation treatment has improved
with recent advances in the capacity for information processing.
Neurosurgeon Lars Leksell developed the first stereotactic
technique for brain lesions; he described the use of directed
narrow beams of radiant energy to achieve local destruction of
undesirable brain tumor tissue (1).
This therapy differs from conventional radiotherapy, which involves
exposing large areas of intracranial tissue to relatively broad
fields of radiation over a number of sessions, and is a minimally
invasive technique for the accurate delivery of highly focused
ionizing radiation with a minimal effect on normal surrounding
structures over a short treatment period.
This stereotactic technique was applied to
extracranial lesions in the 1990s. Blomgren et al (2) first reported the successful use of
hypofractionated, stereotactic, high-dose radiation therapy for the
treatment of extracranial malignancies such as solitary tumors of
the liver, lung or retroperitoneal space. Stereotactic body
radiotherapy (SBRT) is increasingly indicated for various types of
tumors, including primary or metastatic peripheral lung cancer,
hepatocellular carcinoma, metastatic liver tumors, recurrent
abdominal and pelvic tumors and bone metastases (3–5).
SBRT has already been reported to be safe and
feasible for the curative treatment of patients with operable stage
I non-small cell lung cancer (NSCLC). Local control and the overall
survival rate in 5 years after SBRT were potentially comparable to
surgery (6). Local control rates in
pulmonary oligometastases range from 67 to 96% at 2 years (7). Habermehl et al (8) reported that local control rates in
liver metastases, including colorectal adenocarcinoma, breast
cancer, pancreatic adenocarcinoma and ovarian cancer are 87, 69 and
59% after 6, 12, and 18 months, respectively.
Studies have reported that complete resection of
pulmonary and liver metastases derived from CRC is associated with
relatively long-term survival (9–11);
thus, sequential resection is warranted in a select group of
patients. However, surgical treatment may be difficult for various
reasons, such as repeated surgery, poor general condition of the
patient and refusal of surgery. SBRT offers a minimally invasive
and precise alternative for such cases. We report the successful
treatment of three patients with four CRC metastases using SBRT.
SBRT is a safe and alternative technique with which to resect
pulmonary and liver metastases.
Materials and methods
At our institute, the indications for SBRT treatment
of colorectal oligometastases using a liniac system (Siemens
Industry Inc., ONCOR Impression Plus: Osaka University; Varian
Inc., Trilogy: Saito Yukoukai Hospital) are histologically
confirmed colorectal adenocarcinoma, radical resection of the
primary tumor, inoperable tumors as assessed by a trained surgeon,
tumors not amenable to another local treatment or patient refusal
to undergo surgery, progression or stable disease after
chemotherapy for recurrence, the presence of one to three lesions
confined to one organ as determined by CT or PET/CT, and the
maximum diameter of the largest lesion is 5 cm on CT.
Gross tumor volume (GTV) was identified and
contoured on each axial CT image. GTV was considered to be equal to
the clinical target volume (CTV). Next, we determined the internal
target volume (ITV) by summing the CTVs of all CT scan images,
including both the CVT in the inspiratory phase and in the
expiratory phase. Planning target volume (PTV) was defined as the
ITV plus a setup margin of 5 mm in all directions. The maximum
moving distance of the tumor was determined in the x-, y- and
z-axis directions using four-dimensional CT. When the maximum value
was >1 cm, we often use respiratory gating. The tumor must be
within the PTV under the on-board image (OBI) before irradiation
and the beam must irradiate the target tumor in the expiratory
phase. Critical structures, such as the esophagus, spinal cord and
trachea, are avoided by contouring. The radiation dose varies
according to lesion site.
Results
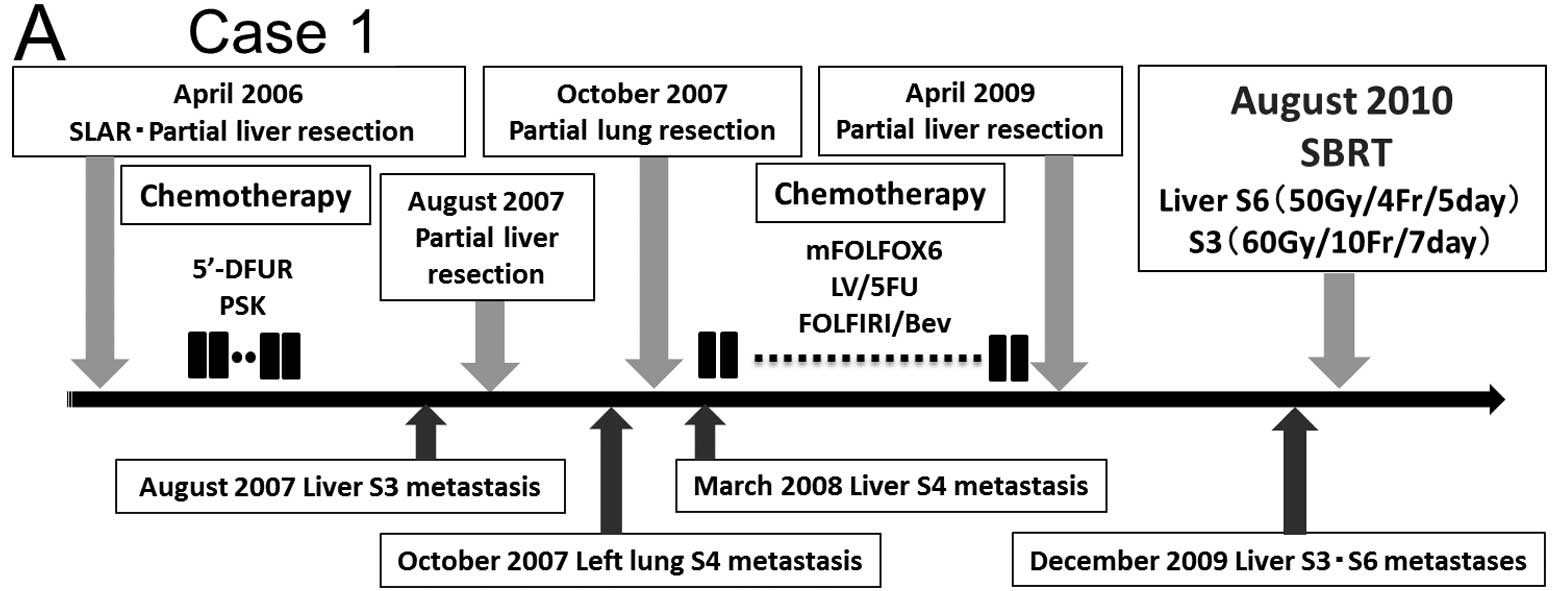
Case 1
A 70-year-old man with lower rectal cancer and liver
metastasis (stage IV) underwent super-low anterior resection (SLAR)
and partial liver resection for S6 in April 2006 (Fig. 1A). The tumor was a moderately
differentiated adenocarcinoma. Adjuvant chemotherapy with 5′-DFUR
and PSK was administered after surgery. During chemotherapy, the
recurrent tumor appeared on S3 in the liver, and the patient
underwent a second partial liver resection. Two months after
surgery, the metastatic tumor appeared on S4 in the left lung, and
the patient underwent a partial lung resection. Five months later,
the metastatic tumor appeared on S4 in the liver. Systemic
chemotherapy with mFOLFOX6, followed by LV/5FU and
FOLFIRI/bevacizumab, was administered. Because the tumor exhibited
progressive disease (PD), the patient underwent a third partial
liver resection. In December 2009, the patient had two liver
metastases on S3 (tumor diameter 1.8 cm and volume 3.0 ml) and S6
(tumor diameter 1.3 cm and volume 1.2 ml) (Fig. 1B). Due to repeated surgery and the
patient’s refusal to undergo surgery, SBRT (60 Gy/10 Fr and 50 Gy/4
Fr) was performed for the liver metastases in August 2010. No
adverse events were observed during the radiotherapy and the
patient completed the treatment. In May 2013, 33 months after the
therapy, a CT scan revealed CR of the liver metastases (Fig. 1B). As of June 2013, the patient has
not had a recurrent tumor.
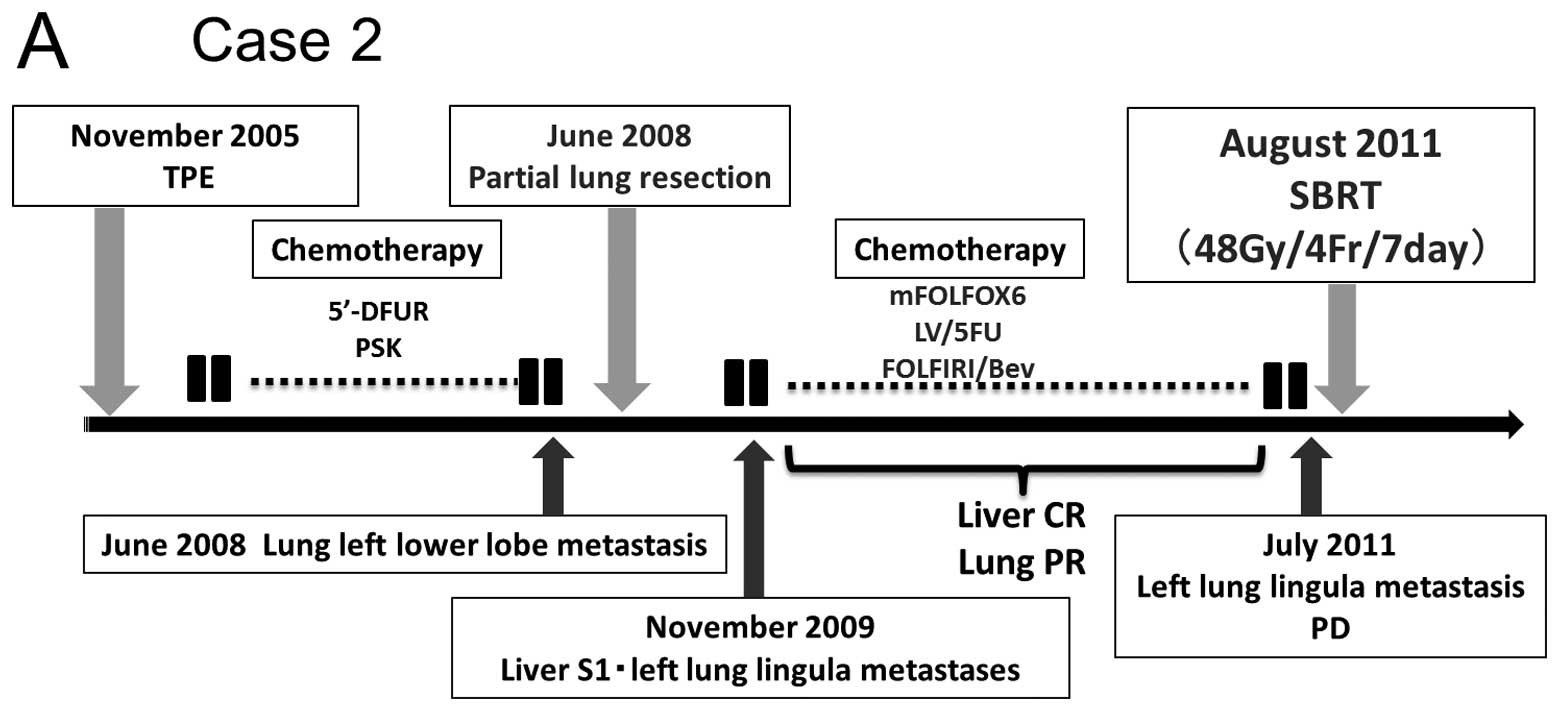
Case 2
In November 2005, a 65-year-old man with advanced
upper rectal cancer infiltrating the bladder and seminal vesicles
(stage IIIC) underwent total pelvic exenteration (TPE) with
complete resection (Cur A) (Fig.
2A). The tumor was a moderately differentiated adenocarcinoma.
Adjuvant chemotherapy with 5′-DFUR and PSK was administered after
surgery. Seven months after surgery, pulmonary metastasis appeared
on the left lower lobe and the patient underwent partial lung
resection. In November 2009, metastatic tumors appeared on S1 in
the liver and the lingula of the left lung. After systemic
chemotherapy with mFOLFOX6, followed by LV/5FU and
FOLFIRI/bevacizumab for approximately 12 months, the patient
experienced CR of the liver metastasis and PR of the lung
metastasis. However, in July 2011, CT showed that the left lung
metastasis volume had increased (tumor diameter 1.2 cm and volume
0.9 ml) (Fig. 2B). Due to repeated
surgery and PD in the metastatic pulmonary tumor after systemic
chemotherapy, SBRT (48 Gy/4 Fr) was performed for the lung
metastasis in August 2011. In May 2012, 9 months after the therapy,
CT showed CR of the lung tumor (Fig.
2B). As of July 2013, the patient has not had a recurrent
tumor.
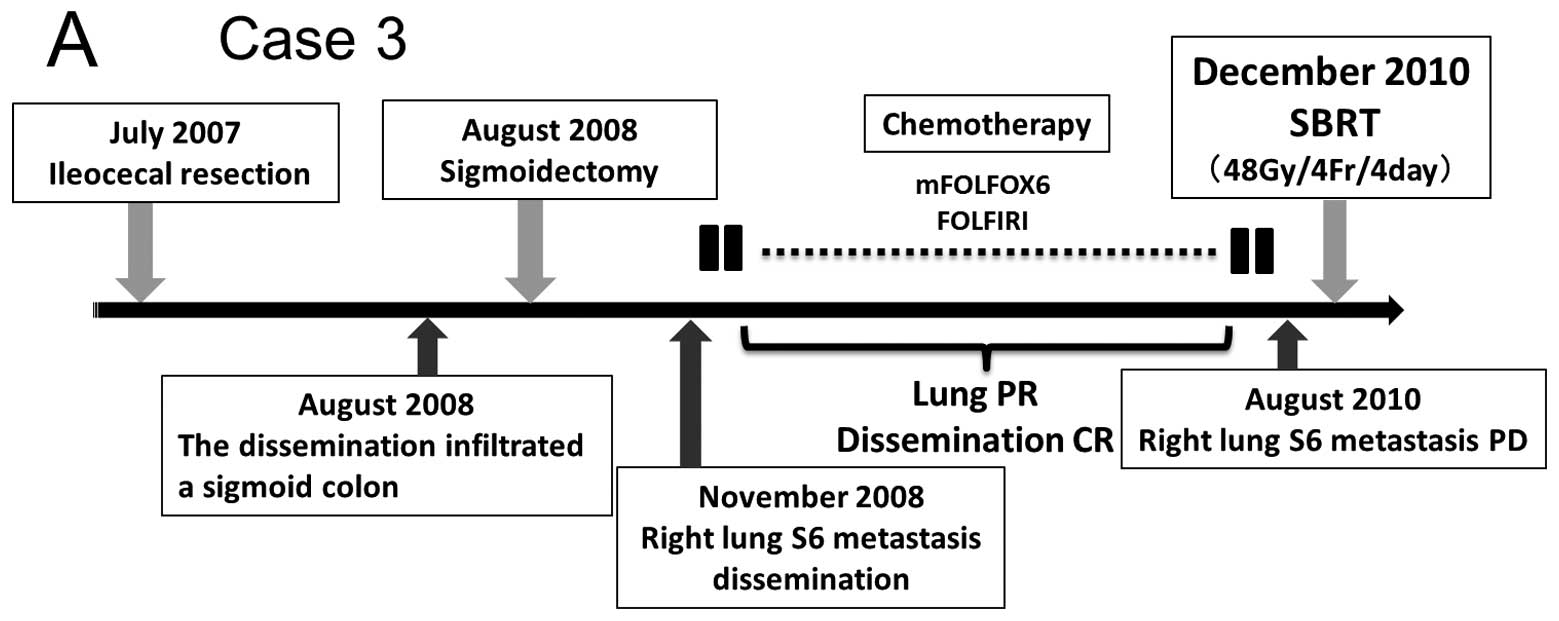
Case 3
In July 2007, a 70-year-old man with advanced cecum
cancer underwent ileocecal resection (Fig. 3A). The tumor was a moderately
differentiated adenocarcinoma (stage II). In August 2008, a
dissemination lesion that infiltrated the sigmoid colon developed
and the patient underwent sigmoidectomy. Seven months after
surgery, recurrent dissemination and right lung metastasis appeared
on S6. After systemic chemotherapy with mFOLFOX6, followed by
FOLFIRI, the patient experienced CR of the dissemination and PR of
the lung metastasis. However, in August 2010, CT showed that the
volume of the right lung metastasis increased (tumor diameter 0.8
cm and volume 0.27 ml) (Fig. 3B).
Due to the patient’s refusal of surgery and PD in the metastatic
lung tumor after systemic chemotherapy, SBRT (48 Gy/4 Fr) was
performed for the lung metastasis in December 2010. A PET/CT scan
15 months after therapy revealed CR of the lung tumor (Fig. 3B). The patient died of CRC in
December 2012.
Discussion
SBRT is a minimally invasive radiation technology
that can provide a large dose of highly focused ionizing radiation
to the target tumor and reduce normal tissue toxicity. The therapy
for CRC oligometastasis was performed using fractionated
irradiation of 50 Gy/4 Fr and 60 Gy/10 Fr for Case 1, 48 Gy/4 Fr
for Case 2, and 48 Gy/4 Fr for Case 3. SBRT was performed in three
patients with distant metastases after surgical resection for
primary CRC, one liver metastasis and two pulmonary metastases. All
patients had CR during a follow-up period of 15 to 33 months as
evidenced by CT. No adverse events were detected during the
therapy.
Oligometastasis is described as a distant extension
of a primary cancer at an isolated site or less than five sites of
metastasis (12). Hellman et
al (13) reported that the
control of both the primary tumor and the oligometastatic lesion
leads to long-term survival in various types of cancers. Thus, the
local control of metastatic focus is particularly important.
To date, surgical resection has been the standard
therapy for liver and lung oligometastases. As for CRC, Salah et
al (14) reported that the
5-year survival rate is 52% for patients who have one
metastasectomy for pulmonary metastasis and 57.9% for patients who
have a second metastasectomy. In addition, Kobayashi et al
(9) reported that patients with
metastases derived from CRC who undergo both pulmonary and hepatic
resection have a 3-year survival rate of 36±8%, a 5-year survival
rate of 31±8% and an 8-year survival rate of 23±9%. A significant
difference was found in the cumulative survival of patients with a
solitary pulmonary metastasis compared to patients with multiple
pulmonary metastases. Thus, the resection of CRC oligometastases is
associated with long-term survival.
However, surgical resection may be difficult for
various reasons, such as repeated surgery, the poor general
condition of the patient and refusal of surgery. According to the
National Comprehensive Cancer Network (NCCN) guidelines, SBRT
should not be used in place of surgical resection for CRC (15). However, SBRT was recently reported
to be useful for CRC oligometastases and to offer a new treatment
alternative for cases of repeated surgery, systemic complications
and refusal to undergo surgery (16–18).
According to the Japanese Society for Therapeutic Radiology and
Oncology (JASTRO), the adaptation of SBRT in metastatic liver or
pulmonary tumors requires a diameter <5 cm and fewer than three
sites for other lesions (19). Our
three cases of CRC oligometastasis adapted well to SBRT.
Bae et al (16) retrospectively compared the local
control rate and overall survival of patients with distant CRC
metastases who were treated with SBRT and reported that only a
cumulative GTV <17 ml is a significantly favorable prognostic
factor for the local control rate, and that no significant factor
affects overall survival. A phase II study of SBRT in CRC
metastases was conducted by Hoyer et al (17), who showed that actuarial local
control was 86 and 63% at 2 years in a tumor- and patient-based
analysis, respectively, and that overall survival was 67, 38, 22,
13 and 13% after 1, 2, 3, 4 and 5 years, respectively. The largest
metastasis being <35 mm was significantly related to better
overall survival. However, patients with a tumor diameter <35 mm
did not have a significantly increased risk of local recurrence
compared to patients with smaller metastases. Kang et al
(18) retrospectively evaluated the
feasibility and efficacy of SBRT for CRC oligometastases and
reported that the 5-year overall survival and local control rates
were 29 and 19%, respectively, and that a cumulative GTV <23 ml
was a significantly favorable prognostic factor for the local
control rate and overall survival. Thus, SBRT provides therapeutic
benefits to select patients.
In our cases, the oligometastatic volume in the
liver and lung was much smaller than 17 ml and the diameter was
less than 35 mm. The eligibility criteria of SBRT for distant CRC
metastases must be defined by large-scale clinical trials.
In conclusion, SBRT is a promising treatment
associated with a more favorable prognosis for patients with
distant CRC metastases that are unresectable due to repeated
surgery, poor general condition of the patient, or refusal to
undergo surgery. This therapy may be one of the feasible treatments
for CRC with distant metastases.
Abbreviations:
|
SBRT
|
stereotactic body radiotherapy
|
|
CRC
|
colorectal cancer
|
|
NSCLC
|
non-small cell lung cancer
|
|
CR
|
complete response
|
|
S3
|
ventrolateral segment of left hepatic
lobe
|
|
S6
|
posteroinferior segment of right
hepatic lobe
|
|
CT
|
computed tomography
|
|
PET
|
positron emission tomography
|
|
GTV
|
gross tumor volume
|
|
CTV
|
clinical target volume
|
|
ITV
|
internal target volume
|
|
PTV
|
planning target volume
|
|
SLAR
|
super-low anterior resection
|
|
PD
|
progressive disease
|
|
PR
|
partial response
|
|
TPE
|
total pelvic exenteration
|
References
|
1
|
Leksell L: The stereotaxic method and
radiosurgery of the brain. Acta Chir Scand. 102:316–319.
1951.PubMed/NCBI
|
|
2
|
Blomgren H, Lax I, Naslund I and Svanstrom
R: Stereotactic high dose fraction radiation therapy of
extracranial tumors using an accelerator. Clinical experience of
the first thirty-one patients. Acta Oncol. 34:861–870. 1995.
View Article : Google Scholar
|
|
3
|
Wulf J, Hadinger U, Oppitz U, Olshausen B
and Flentje M: Stereotactic radiotherapy of extracranial targets:
CT-simulation and accuracy of treatment in the stereotactic body
frame. Radiother Oncol. 57:225–236. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Herfarth KK, Debus J, Lohr F, et al:
Stereotactic single-dose radiation therapy of liver tumors: results
of a phase I/II trial. J Clin Oncol. 19:164–170. 2001.PubMed/NCBI
|
|
5
|
Uematsu M, Shioda A, Suda A, et al:
Computed tomography-guided frameless stereotactic radiotherapy for
stage I non-small cell lung cancer: a 5-year experience. Int J
Radiat Oncol Biol Phys. 51:666–670. 2001.PubMed/NCBI
|
|
6
|
Onishi H, Shirato H, Nagata Y, et al:
Stereotactic body radiotherapy (SBRT) for operable stage I
non-small-cell lung cancer: can SBRT be comparable to surgery? Int
J Radiat Oncol Biol Phys. 81:1352–1358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Siva S, MacManus M and Ball D:
Stereotactic radiotherapy for pulmonary oligometastases: a
systematic review. J Thorac Oncol. 5:1091–1099. 2010.PubMed/NCBI
|
|
8
|
Habermehl D, Herfarth KK, Bermejo JL, et
al: Single-dose radiosurgical treatment for hepatic metastases -
therapeutic outcome of 138 treated lesions from a single
institution. Radiat Oncol. 8:1752013. View Article : Google Scholar
|
|
9
|
Kobayashi K, Kawamura M and Ishihara T:
Surgical treatment for both pulmonary and hepatic metastases from
colorectal cancer. J Thorac Cardiovasc Surg. 118:1090–1096. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lehnert T, Knaebel HP, Duck M, Bulzebruck
H and Herfarth C: Sequential hepatic and pulmonary resections for
metastatic colorectal cancer. Br J Surg. 86:241–243. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hamy A, Baron O, Bennouna J, Roussel JC,
Paineau J and Douillard JY: Resection of hepatic and pulmonary
metastases in patients with colorectal cancer. Am J Clin Oncol.
24:607–609. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Milano MT, Katz AW, Zhang H and Okunieff
P: Oligometastases treated with stereotactic body radiotherapy:
long-term follow-up of prospective study. Int J Radiat Oncol Biol
Phys. 83:878–886. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hellman S and Weichselbaum RR:
Oligometastases. J Clin Oncol. 13:8–10. 1995.
|
|
14
|
Salah S, Watanabe K, Park JS, et al:
Repeated resection of colorectal cancer pulmonary oligometastases:
pooled analysis and prognostic assessment. Ann Surg Oncol.
20:1955–1961. 2013. View Article : Google Scholar
|
|
15
|
Clinical Practice Guidelines in Oncology
(version 3.2013) provided by the National Comprehensive Cancer
Network (NCCN). http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf.
|
|
16
|
Bae SH, Kim MS, Cho CK, et al: High dose
stereotactic body radiotherapy using three fractions for colorectal
oligometastases. J Surg Oncol. 106:138–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hoyer M, Roed H, Traberg Hansen A, et al:
Phase II study on stereotactic body radiotherapy of colorectal
metastases. Acta Oncol. 45:823–830. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kang JK, Kim MS, Kim JH, et al:
Oligometastases confined one organ from colorectal cancer treated
by SBRT. Clin Exp Metastasis. 27:273–278. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
SBRT guidline provided by Japanese Society
for Therapeutic Radiology and Oncology (JASTRO). http://www.jastro.or.jp/guideline/.
|