Introduction
Gliomas are the most common malignant brain tumors
and are characterized by relentless growth and aggressive invasion
into the brain parenchyma (1).
Under the current standard of care, the life expectancy of patients
with glioma is ~14 months after diagnosis despite aggressive
surgery, radiation and chemotherapies (2). Therefore, a better understanding of
the mechanism through which glioma develops is necessary for
efficient and specific inhibition of the progression of this form
of cancer.
Potassium (K+) channels are the most
diverse ion channels in the plasma membrane (3). Over the last 10 years, accumulating
evidence has indicated that diverse types of K+
channels, including voltage-gated, calcium-activated, two-pore
domain and inward rectifier K+ channels, are
overexpressed in a number of tumor types, such as prostate, colon
and breast (4–6). Most previous studies have demonstrated
that, in addition to controlling physiological parameters,
K+ channels also play important roles in the onset,
proliferation and malignant progression of various types of cancer
(7). However, mammalian cells
constitutively express an array of various types of K+
channels. Although most previous studies have described the
expression of a particular type of K+ channel in a
cancer cell line and have correlated the expression and functional
activity of the channel with proliferation, it is not clear to what
degree these individual K+ channels contribute to
proliferation or whether a specific association exists between
particular K+ channel subtypes and proliferation.
Moreover, the mechanisms by which these K+ channels
facilitate cell proliferation remain obscure.
Increasing evidence has demonstrated that a variety
of K+ channels, such as voltage-gated K+
channels (KV), calcium-activated K+ channels
(KCa) and ATP-sensitive K+ channels
(KATP) (8–10), are overexpressed in glioma biopsies
and cultured cells. A previous study found a correlation between
the activity of KATP channels and proliferation of
glioma cells and xenografted tumors (8). However, it remains uncertain whether
other K+ channels are also proliferative molecules in
glioma cells. Identification of K+ channels that play a
role in glioma growth may provide novel therapeutic targets. In the
present study, we attempted to identify K+ channels that
affect the growth of human glioma U87-MG cells in vitro and
in vivo. Using a variety of K+ channel blockers
and openers, we found that KV and KATP
channels play roles in controlling cell proliferation and
tumorigenesis, whereas other K+ channel subtypes do not.
We also analyzed the mechanism of action of K+ channels
in cell proliferation by studying the relationship between
K+ channel activities and Ca2+ entry with the
use of a fluorescent cytosolic Ca2+ assay. Our results
demonstrated that KV and KATP channels may
affect cell proliferation by modulating Ca2+ influx.
Materials and methods
Chemicals and drug preparations
Tetraethylammonium (TEA), 4-aminopyridine (4-AP),
glibenclamide, phloretin,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
diazoxide, iberiotoxin, tetrodotoxin (TTX) and propidium iodide
(PI) were obtained from Sigma Chemical Co. (St. Louis, MO, USA).
RPMI-1640 medium and fetal bovine serum (FBS) were obtained from
Invitrogen Life Technologies (Carlsbad, CA, USA). The Annexin
V-fluorescein isothiocyanate/propidium iodide (Annexin V-FITC/PI)
apoptosis detection kit was procured from Antgene Biotech (Wuhan,
China). RNase A was purchased from Fermentas International Inc.
(Burlington, Ontario, Canada). All other chemicals were of standard
analytical grade.
Cell culture
The human glioma cell line U87-MG was purchased from
the American Type Culture Collection (ATCC, Manassas, VA, USA) and
grown in RPMI-1640 supplemented with 10% FBS and 100 units of
penicillin/streptomycin in 5% CO2 at 37°C. The cells
were passaged every 3 days and maintained in exponential growth to
~80% confluency for later experiments.
MTT proliferation assay
A modified MTT assay was used to examine the effect
on cell proliferation. Briefly, cells were seeded in a 96-well
plate at 5,000 cells/well and incubated overnight. After drug
treatment for 48 h, 20 μl of MTT solution (5 mg/ml) was added to
each well, and the samples were incubated for an additional 4 h.
Subsequently, the supernatant was removed, and the cells were
dissolved in 150 μl of DMSO. Finally, absorbance was measured at
570 nm using a 96-well microplate reader (Thermo Scientific,
USA).
Annexin V-FITC/PI apoptosis assay
Cells were double stained using an Annexin V-FITC/PI
apoptosis detection kit. Briefly, after exposure to different drugs
for 48 h, the cells were harvested, washed twice with cold PBS and
resuspended in Annexin V binding buffer. Then, 5 μl of FITC-labeled
Annexin V and 5 μl of PI were added. The cells were incubated for
15 min at room temperature in the dark with gentle oscillation.
After the addition of 200 μl of binding buffer to each tube, the
cells were analyzed within 1 h using a flow cytometer
(Becton-Dickinson, USA).
Cell cycle assay
Cells were detached by trypsinization, seeded at
5×105 cells/well in a 6-well plate and incubated
overnight. The cells were then treated with various drugs at
different concentrations. Subsequently, the cells were harvested
into cold PBS at different time points, fixed in ice-cold 70%
ethanol and stored at 4°C overnight for subsequent cell cycle
analysis. Fixed cells were washed twice with PBS and resuspended in
1 ml of the staining reagents (100 μg/ml RNase A and 50 μg/ml PI).
The samples were incubated in the dark for 30 min, and the
distribution of cells in the various phases of the cell cycle was
measured by flow cytometry.
[Ca2+]i
measurements
Cells were grown overnight, washed twice with Hank’s
balanced salt solution (HBSS) and loaded with 1 μmol/l Fluo3-AM for
30 min in the dark at room temperature. Then cells were washed
twice with Ca2+-free HBSS, re-suspended in
Ca2+-free HBSS and incubated at room temperature for 20
min in the dark. When appropriate, the cells were pretreated with
K+ channel blockers or openers for 5 min before the
initiation of Ca2+ influx. Ca2+ influx was
measured as changes in fluorescent signals, which were recorded
using a fluorescence microscope (Olympus, Japan) and analyzed using
Matlab software.
In vivo therapy experiments
To further verify the antiproliferative efficacies
of K+ channel blockers, an in vivo experiment was
performed using 6- to 8-week old nude mice. The nude mice were
randomly divided into control and treated groups. The treated
groups were subcutaneously injected with a suspension of
5×106 U87-MG cells with 4-AP (5 mmol/l), TEA (20 mmol/l)
or glibenclamide (200 μmol/l), and mice in the control group were
injected with a suspension of 5×106 cells with 0.01
mol/l PBS (8). Tumor size was
measured every 3 days and tumor volume was determined by
calculating (length × width)2/2. At the end of the
experiment, tumors were excised and weighed. A tumor growth curve
was plotted according to the tumor volume, and the inhibitory rates
of tumor growth were calculated according to the tumor weight. All
procedures were performed in accordance with the National
Institutes of Health Guide for the Care and Use of Laboratory
Animals.
Electrophysiological assay
Membrane currents were recorded using a whole-cell
voltage clamp and borosilicate glass pipettes (outer diameter, 1.5
mm; direct current resistance, 3–6 MΩ) constructed using a two-step
puller (P-97; Sutter, USA). To investigate the KV
currents, the pipette solution consisted of (in mmol/l) KCl 140,
MgCl2 2.5, HEPES 10, EGTA 11 and ATP 5 and the pH was
adjusted to 7.2 (11). To
investigate the KATP currents, the micropipettes were
filled with (in mmol/l) KCl 140, MgCl2 1, ATP 0.01,
EGTA-KOH 5 and HEPES-KOH 5 and the pH was adjusted to 7.3 (12). The cells were bathed in an
extracellular solution containing (in mmol/l) NaCl 135, KCl 5.4,
MgCl2 1.0, NaH2PO4 0.33,
CaCl2 1.8, HEPES 10 and D-glucose 10 with 1 μmol/l TTX.
The osmolarity was adjusted to 330 mOsm/l with sucrose and the pH
was adjusted to 7.4. Whole-cell patch clamp recordings were
performed at room temperature using a patch clamp amplifier
(Axon-200B) (13). Adjustments of
capacitance compensation and series resistance compensation were
performed before the membrane currents were recorded. The membrane
currents were filtered at 10 kHz (−3 dB) and the data were
processed using Clampfit (Axon, USA).
Statistical analysis
The data are expressed as the means ± standard error
(± SE). Statistical significance was assessed using analysis of
variance (ANOVA). P-values of <0.05 were considered to indicate
statistically significant results.
Results
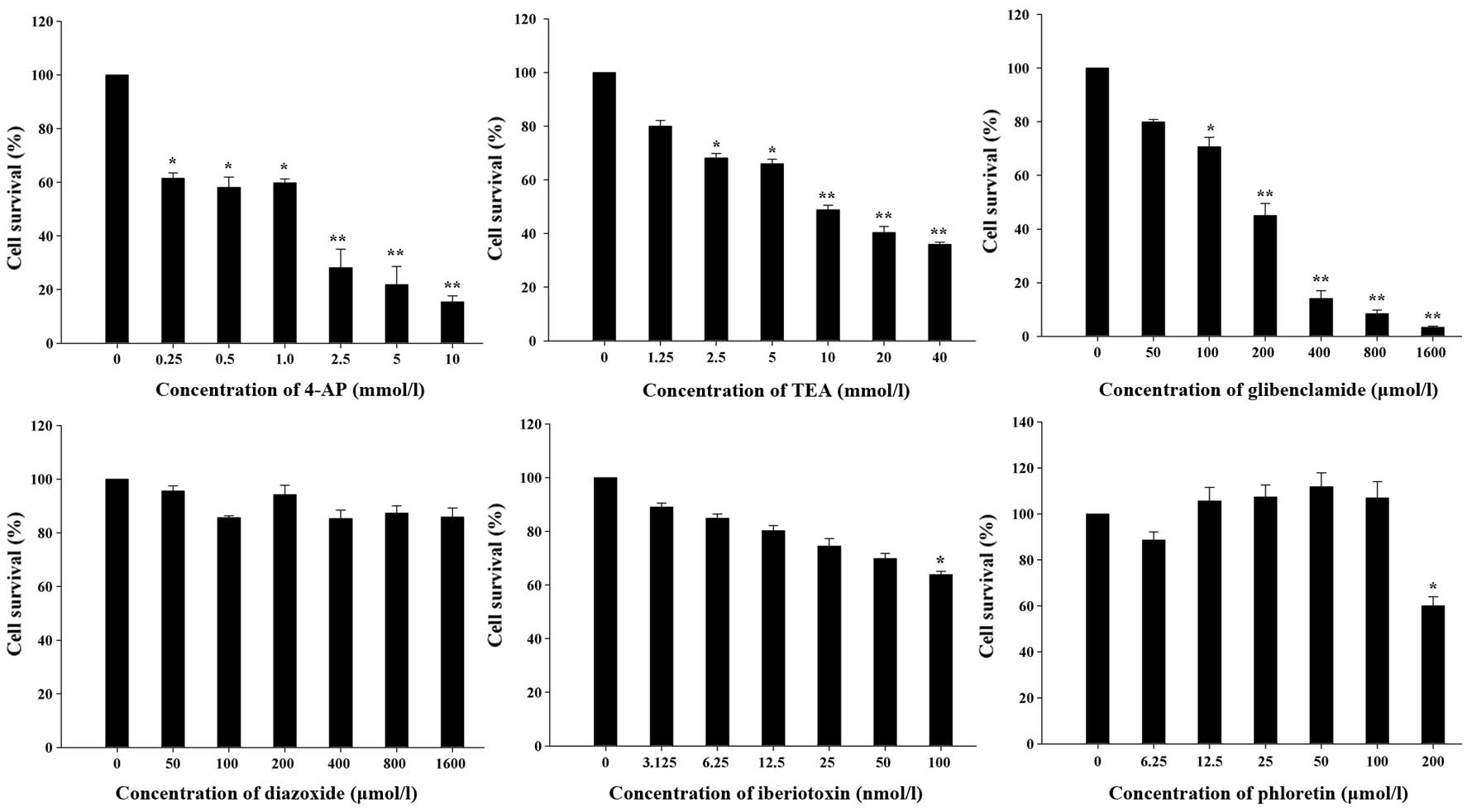
Effects of K+ channel blockers
and openers on cell proliferation
To identify the K+ channels that affect
U87-MG cell proliferation, we used a variety of K+
channel blockers and openers and an MTT assay was used to determine
the number of live cells remaining after the drug treatment. Among
the blockers, 4-AP and TEA are transient outward and delayed
rectifier KV channel blockers, respectively (14), whereas glibenclamide is a specific
blocker of KATP channels, and iberiotoxin is a specific
KCa channel blocker. As shown in Fig. 1, cell proliferation was
significantly inhibited by 4-AP and TEA in a dose-dependent manner
and glibenclamide also significantly decreased the U87-MG cell
number. However, 100 nmol/l iberiotoxin, a concentration that
completely blocks KCa channels (15), only moderately inhibited U87-MG cell
proliferation. Meanwhile, diazoxide, an opener of KATP
channels and phloretin, an opener of KCa channels, had
no significant effects on cell proliferation. Taken together, these
data reveal that KV and KATP channels but not
KCa channels have important roles in the proliferation
of U87-MG cells.
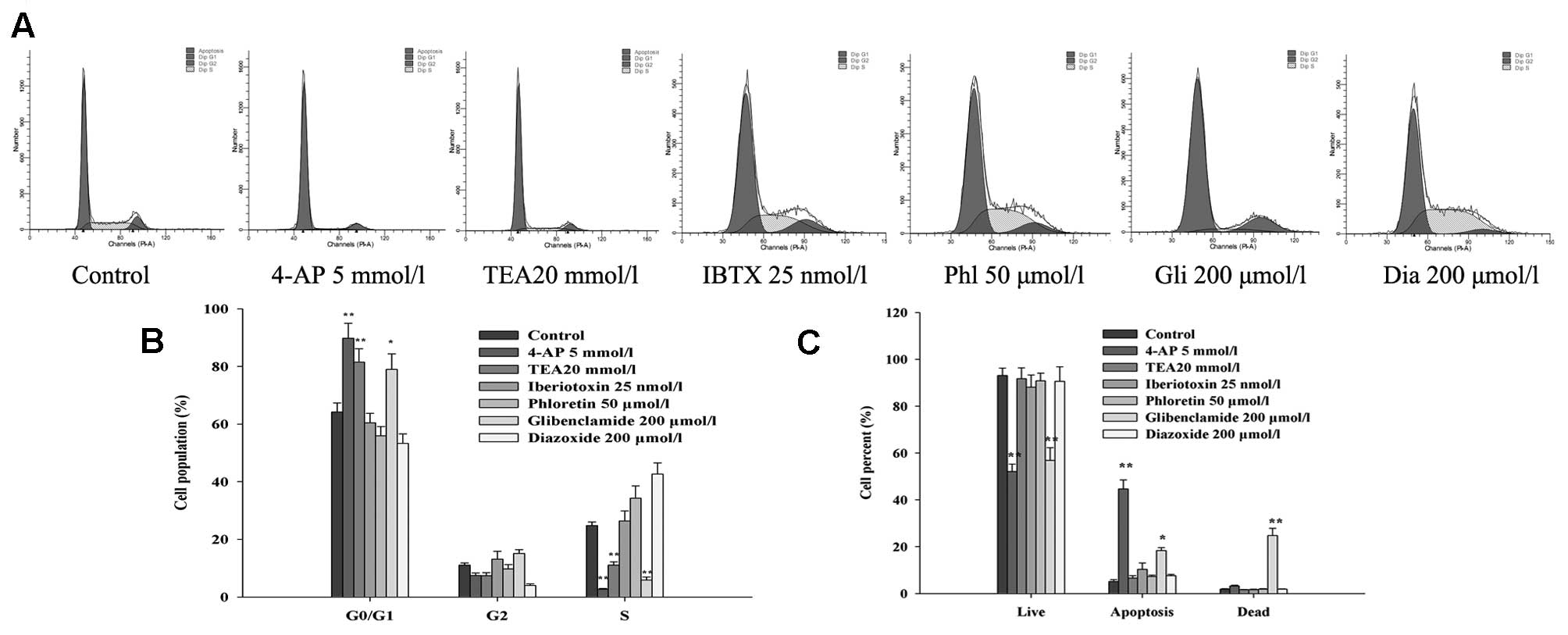
Effects of K+ channel blockers
and openers on the cell cycle distribution
To elucidate the mechanisms through which
K+ channels influence the proliferation of glioma cells,
flow cytometry was performed to investigate whether the U87-MG cell
cycle may be influenced by various K+ channel blockers
or openers. As shown in Fig. 2A and
B, the percentage of U87-MG cells in the
G0/G1 phase was significantly enhanced after
exposure to 4-AP, TEA and glibenclamide for 48 h, whereas the
percentage of cells in the S phase was markedly reduced.
Iberiotoxin had no obvious effect on the U87-MG cell cycle at a
concentration of 25 nmol/l. Meanwhile, activating KATP
channels with diazoxide and activating KCa channels with
phloretin increased the percentage of U87-MG cells in the S phase.
These results were consistent with previous reports (16) that K+ channels are
believed to facilitate progression through G1/S
checkpoint.
Effects of K+ channel blockers
and openers on cell apoptosis
To determine whether the reduction in cell viability
caused by K+ channel blockers was related to apoptotic
cell death, the effects of different K+ channel blockers
and openers on U87-MG cell apoptosis were studied using Annexin
V-FITC/PI staining. As illustrated in Fig. 2C, the percentage of Annexin
V-FITC-positive apoptotic cells was increased by adding 4-AP or
glibenclamide to the U87-MG cells (5.1±0.8% of control, 44.7±3.8%
of 5 mmol/l 4-AP and 18.3±1.32% of 200 μmol/l glibenclamide).
However, 4-AP only slightly increased necrosis, whereas
glibenclamide induced noticeable necrosis (1.8±0.26% of control,
3.2±0.26% of 5 mmol/l 4-AP and 24.8±3.08% of 200 μmol/l
glibenclamide). In contrast, at some of the tested concentrations,
treatment with TEA, iberiotoxin, phloretin or diazoxide for 48 h
had no apparent effect on cell apoptosis.
Effects of K+ channel blockers
and openers on Ca2+ influx
To further explore the mechanism of K+
channel involvement in U87-MG cell proliferation, we examined the
effects of different K+ channel blockers and openers on
Ca2+ influx. Ca2+ influx was induced by the
addition of 0.5 mmol/l CaCl2 to the bathing medium
(Ca2+-free HBSS), which caused a rapid increase in
cytosolic Ca2+ to a level that was sustained for several
minutes. As shown in Fig. 3, 4-AP
(5 mmol/l) and glibenclamide (200 μmol/l) nearly abolished the
increase in [Ca2+]i induced by exogenously
applied Ca2+ (P<0.01), and TEA (20 mmol/l) reduced
the peak Ca2+ response by 8.5±0.5% (P<0.05),
indicating that KV and KATP channels may
modulate Ca2+ influx into glioma cells and subsequently
modulate the proliferation and apoptosis of these cells.
Iberiotoxin (25 nmol/l) decreased the peak Ca2+ response
by 2.1±0.1%, which was not significant when compared with the
control cells (P>0.05).
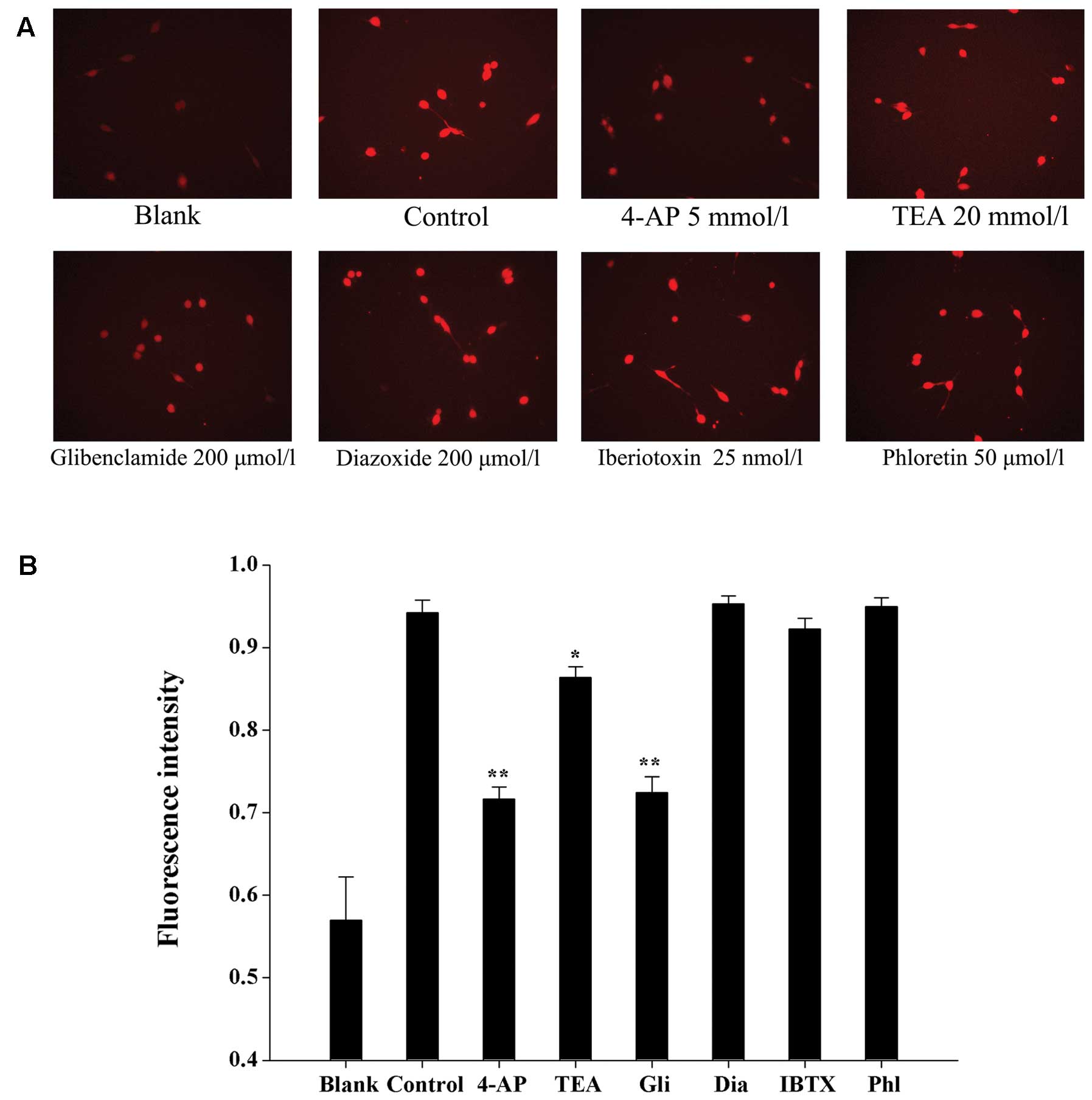 | Figure 3Effects of K+ channel
blockers and openers on the increase in
[Ca2+]i induced by exogenously applied
Ca2+. (A) Cells were placed in Ca2+-free HBSS
with or without blockers. Blank, cells were incubated with
Ca2+-free HBSS. Control, Ca2+ influx was
induced by adding 0.5 mmol/l CaCl2 to
Ca2+-free HBSS. (B) The results of Ca2+
influx were analyzed using Matlab software and [Ca2+]i
was determined based on the fluorescence intensity. 4-AP,
4-aminopyridine; TEA, tetraethylammonium; Gli, glibenclamide; Dia,
diazoxide; IBTX, iberiotoxin; Phl, phloretin. *P<0.05
and **P<0.01 when compared to the control.
K+ channel, potassium channel; HBSS, Hank’s balanced
salt solution. |
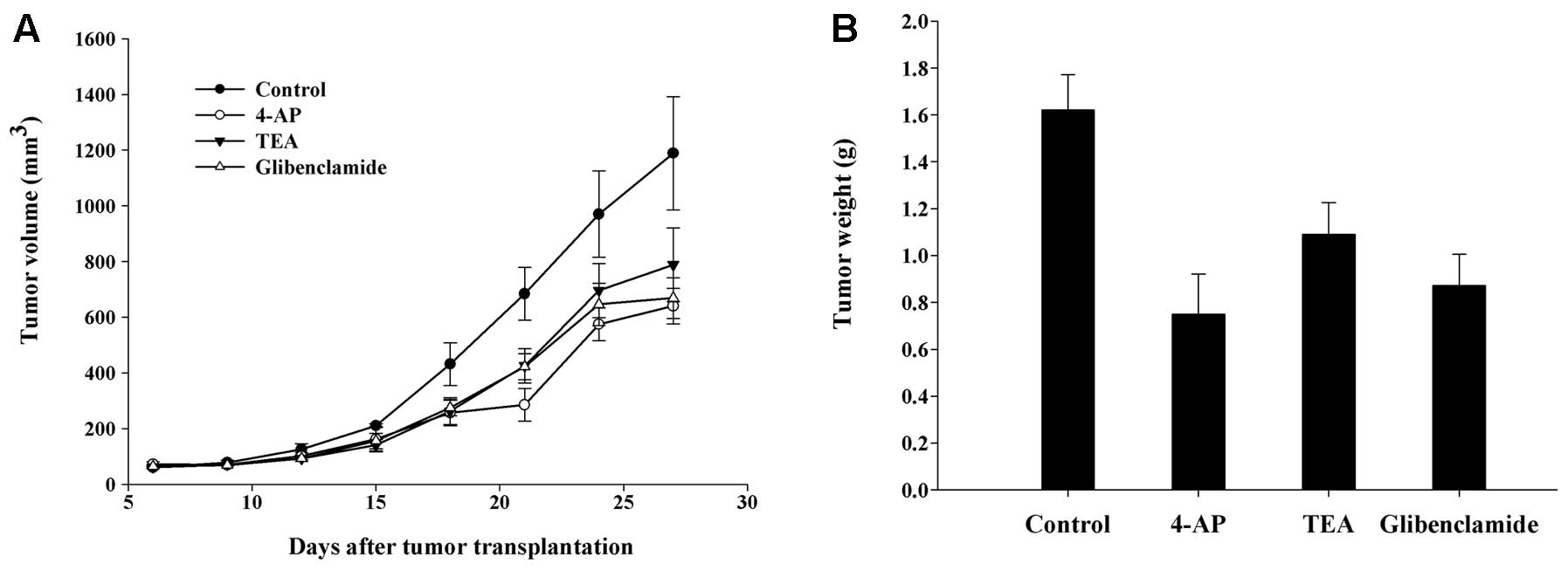
Therapeutic efficacy of K+
channel blockers
To provide direct evidence that KV or
KATP channels are responsible for tumor development, the
antitumor activities of KV or KATP channel
blockers were investigated in nude mice with established human
glioma U87-MG xenografts. As shown in Fig. 4A, U87 xenografts grew rapidly in
mice, and the tumor size in the control group reached 1189.2±203.3
mm3 over the duration of the experiment (27 days).
Statistically, there were significant differences in both tumor
volume and weight between the control and the treated groups. Mice
treated with 4-AP at 5 mmol/l and glibenclamide at 200 μmol/l
showed inhibition rates of 46.2 and 43.8%, respectively, which were
significant (P<0.01) when compared with the control. TEA at 20
mmol/l suppressed tumor growth by 33.7% (P<0.05). The actual
tumor weights at the time of sacrifice are shown in Fig. 4B. No obvious toxic effects were
observed in any of the groups during the experiment. These results
suggest that KV and KATP channels play
important roles in glioma development in vivo.
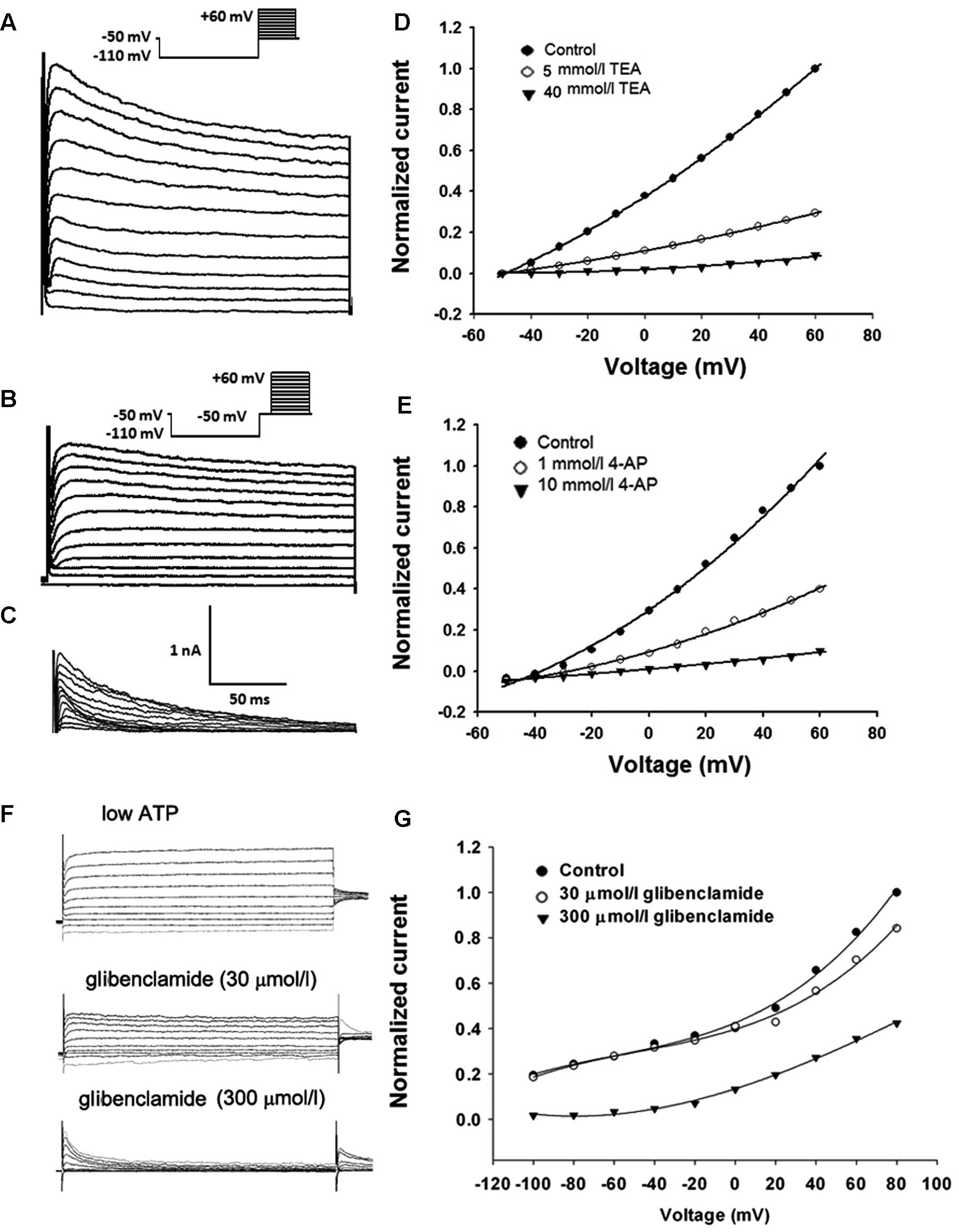
Effects of K+ channel blockers
on K+ currents
To determine whether K+ channel blockers
inhibits cell proliferation and tumorigenesis via blockage of
whole-cell K+ currents, we next studied the
dose-dependent effects of these three blockers on
KV/KATP currents in U87-MG cells.
Representative recordings of the KV currents (transient
and persistent K+ currents) were evoked with a step-up
depolarization protocol. Briefly, the membrane potential was
pre-hyperpolarized from −50 mV to −110 mV for 100 msec, depolarized
from −50 to +60 mV (10 mV increment/step, duration 200 msec) and
subsequently restored to the original depolarizing potential of −50
mV (Fig. 5A). Depolarizing the
voltage from −110 to −50 mV and maintaining the level of −50 mV for
100 msec inactivated transient K+ currents and a further
depolarization voltage step from −50 to +60 mV evoked persistent
K+ currents (Fig. 5B).
The transient component (Fig. 5C)
was then visualized in isolation using point-by-point subtraction
of the persistent component (as shown in Fig. 5B) from the total outward current (as
shown in Fig. 5A). As shown in
Fig. 5D, 5 mmol/l TEA remarkably
inhibited the persistent outward currents and the persistent
component was reduced completely after the application of TEA (40
mmol/l). Meanwhile, 4-AP (1 mmol/l) markedly suppressed the
transient outward current and 4-AP (10 mmol/l) completely and
reversibly blocked the transient components in each cell examined
(Fig. 5E).
To examine the effect of glibenclamide on
KATP currents, KATP currents were activated
by using standard whole cell recording with a pipette solution
containing only 10 mmol/l ATP (12). The membrane potential was normally
held at −40 mV and the currents were evoked by a series of 400 msec
depolarizing and hyperpolarizing current steps (−100 mV to +80 mV
in 20 mV steps, Fig. 5F). As shown
in Fig. 5G, the whole-cell
K+ currents observed with low intracellular ATP were
inhibited by extracellular glibenclamide (300 μmol/l). These data
suggest that these three K+ channel blockers have the
same working concentration range for inhibiting
KV/KATP currents, cell proliferation and
tumor growth, indicating that the effects of 4-AP, TEA and
glibenclamide are indeed mediated by blockage of K+
channels.
Discussion
As a crucial cellular function, cell growth is
strictly controlled by several independent mechanisms. Since the
pioneering study in lymphocytes by DeCoursey et al (17), accumulating evidence has indicated
that K+ channels are relevant players in the control of
this process. Diverse types of K+ channels have been
shown to be overexpressed in tumorous tissues and the roles of
K+ channels in tumor cell growth have been demonstrated
(9,15). However, the types of K+
channels involved in this process vary (18,19).
For example, KCa channels were found to be involved in
prostate (5), gastric (20) and colorectal cancer (4). In other tumors, proliferation was
supported by KATP channels or two-pore (2P)-domain
channels (21,22). Nevertheless, in the majority of
cancer cells, KV channels were correlated with
proliferation (7,16,23).
It remains unknown why different types of K+ channels
induce proliferation in different cancer models.
In the present study, we showed that 4-AP, TEA and
glibenclamide significantly suppressed cell proliferation in
vitro. The percentage of U87-MG cells in the
G0/G1 phase was also significantly enhanced
after exposure to 4-AP, TEA or glibenclamide for 48 h. On the other
hand, diazoxide and phloretin increased the percentage of cells in
the S phase. These findings, together with previous reports
(8,24), demonstrate that K+
currents are the key determinant of progression through the
G1/S checkpoint of the cell cycle. In addition, we also
found that 4-AP and glibenclamide both significantly increased the
percentage of apoptotic cells. In the U87-MG xenograft model in
nude mice, 4-AP, TEA and glibenclamide markedly suppressed tumor
growth in vivo. Taken together, these results suggest that
KV and KATP channels are the critical
K+ channel subtypes for U87-MG glioma growth.
Given the important role of K+ channels
in tumors, it is necessary to clarify their role in proliferation.
One hypothesis is that K+ channels keep the resting
membrane potential sufficiently polarized to allow the influx of
Ca2+ via membrane ion channels (25), implying that blocking K+
channels will directly modulate Ca2+ entry in malignant
cells. This may be a partial reason for the induction of tumor
growth via the abnormal expression of K+ channel
subtypes since Ca2+ acts as an activator involved in
many cellular signal transduction pathways, including the cell
growth and mitosis pathways (26).
For example, early reports indicate that growth of the colorectal
adenocarcinoma cell line DLD-1 and the ovarian cancer cell line
A2780 was associated with the regulatory effect of KV
channels on Ca2+ influx (24,25).
Nevertheless, to date, no direct evidence has ever been presented
to support the relationship between K+ channel activity
and Ca2+ ion entry in glioma cells. Here, we found that
4-AP and glibenclamide nearly abolished the increase in
[Ca2+]i induced by exogenously added
Ca2+. Sine the change in [Ca2+]i
may be due to a change in Ca2+ influx from the
extracellular medium or an alteration in Ca2+ release
from an internal store, we further examined the potential effects
of K+ channels on the release of Ca2+ from
intracellular stores, and we found that
[Ca2+]i in U87-MG cells was not affected by
K+ channel blockers or openers (data not shown). Through
these experiments, we established a link between KV and
KATP channel activities and Ca2+ influx,
indicating that modulation of Ca2+ influx may influence
the proliferation of U87-MG cells.
The expression of KCa channels is
correlated with glioma malignancy, with higher levels of
KCa protein observed in more malignant glioma biopsy
samples (27). However, the
relationship between KCa channels and glioma cells is
controversial. Some recent publications have proposed the idea that
KCa channels may have antiproliferative and
antitumorigenic properties (28).
Other results implicating KCa channels in the control of
glioma cell growth have been collected using specific cell growth
conditions, such as an elevated extracellular K+
concentration (29) or serum
deprivation (30). Our data showed
that the inhibitory effect of iberiotoxin was only observable at
concentrations greatly exceeding those necessary for complete
channel blockage (15), and
increases in apoptotic cells and cells in the
G0/G1 phase were not clearly observed,
suggesting that KCa channels may not play a role in the
growth of U87-MG glioma cells in vitro.
Although all the blockers of KV and
KATP channels tested in this study suppressed glioma
cell proliferation and tumor development, it remains unclear
whether their effects occur at the concentrations required to block
K+ channel activities. Electrophysiological results
showed that 4-AP, TEA and glibenclamide inhibited cell
proliferation and tumor growth in the concentration range required
to block KV/KATP channel currents, indicating
that these K+ channel blockers suppress glioma cell
proliferation by blocking K+ channel activities and
providing further support for the roles of
KV/KATP channels in mediating cell
proliferation and tumor growth.
In summary, the present findings provide strong
evidence that KV and KATP channel blockers
inhibit proliferation and tumorigenesis of U87-MG glioma cells. It
is likely that K+ channel activities modulate
Ca2+ influx into U87-MG cells and therefore affect the
proliferation, cell cycle progression and apoptosis of these cells.
Further study is needed to define the precise mechanisms
responsible for the antiproliferative effects of pharmacological
blockers of KV and KATP channels. In any
case, it is worthwhile to further explore the possibility of using
KV and KATP channels as new anti-glioma
targets in the coming years.
Acknowledgements
The present study was supported by the Wuhan
Science and Technology Foundation (201250499145-31) and Natural
Science Foundation of China (81302203).
References
|
1
|
Ru Q, Shang BY, Miao QF, Li L, Wu SY, Gao
RJ and Zhen YS: A cell penetrating peptide-integrated and
enediyne-energized fusion protein shows potent antitumor activity.
Eur J Pharm Sci. 47:781–789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Van Meir EG, Hadjipanayis CG, Norden AD,
Shu HK, Wen PY and Olson JJ: Exciting new advances in
neuro-oncology: the avenue to a cure for malignant glioma. CA
Cancer J Clin. 60:166–193. 2010.PubMed/NCBI
|
|
3
|
Fiske JL, Fomin VP, Brown ML, Duncan RL
and Sikes RA: Voltage-sensitive ion channels and cancer. Cancer
Metastasis Rev. 25:493–500. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Spitzner M, Ousingsawat J, Scheidt K,
Kunzelmann K and Schreiber R: Voltage-gated K+ channels
support proliferation of colonic carcinoma cells. FASEB J.
21:35–44. 2007.
|
|
5
|
Abdul M and Hoosein N: Expression and
activity of potassium ion channels in human prostate cancer. Cancer
Lett. 186:99–105. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang L, Zou W, Zhou SS and Chen DD:
Potassium channels and proliferation and migration of breast cancer
cells. Sheng Li Xue Bao. 61:15–20. 2009.PubMed/NCBI
|
|
7
|
Le Guennec JY, Ouadid-Ahidouch H, Soriani
O, Besson P, Ahidouch A and Vandier C: Voltage-gated ion channels,
new targets in anti-cancer research. Recent Pat Anticancer Drug
Discov. 2:189–202. 2007.PubMed/NCBI
|
|
8
|
Huang L, Li B, Li W, Guo H and Zou F:
ATP-sensitive potassium channels control glioma cell proliferation
by regulating ERK activity. Carcinogenesis. 30:737–744. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abdullaev IF, Rudkouskaya A, Mongin AA and
Kuo YH: Calcium-activated potassium channels BK and IK1 are
functionally expressed in human gliomas but do not regulate cell
proliferation. PLoS One. 5:e123042010. View Article : Google Scholar
|
|
10
|
Lefranc F, Pouleau HB, Rynkowski M and De
Witte O: Voltage-dependent K+ channels as oncotargets in
malignant gliomas. Oncotarget. 3:516–517. 2012.
|
|
11
|
Akamine T, Nishimura Y, Ito K, Uji Y and
Yamamoto T: Effects of haloperidol on K+ currents in
acutely isolated rat retinal ganglion cells. Invest Ophthalmol Vis
Sci. 43:1257–1261. 2002.PubMed/NCBI
|
|
12
|
Du Q, Jovanović S, Sukhodub A, Barratt E,
Drew E, Whalley KM, Kay V, McLaughlin M, Telfer EE, Barratt CL and
Jovanović A: Human oocytes express ATP-sensitive K+
channels. Hum Reprod. 25:2774–2782. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yi CL, Liu YW, Xiong KM, Stewart RR,
Peoples RW, Tian X, Zhou L, Ai YX, Li ZW, Wang QW and Li CY:
Conserved extracellular cysteines differentially regulate the
inhibitory effect of ethanol in rat P2X4 receptors.
Biochem Biophys Res Commun. 381:102–106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schaarschmidt G, Wegner F, Schwarz SC,
Schmidt H and Schwarz J: Characterization of voltage-gated
potassium channels in human neural progenitor cells. PLoS One.
4:e61682009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Weaver AK, Liu X and Sontheimer H: Role
for calcium-activated potassium channels (BK) in growth control of
human malignant glioma cells. J Neurosci Res. 78:224–234. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pardo LA: Voltage-gated potassium channels
in cell proliferation. Physiology. 19:285–292. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
DeCoursey TE, Chandy KG, Gupta S and
Cahalan MD: Voltage-gated K+ channels in human T
lymphocytes: a role in mitogenesis? Nature. 307:465–468. 1984.
|
|
18
|
Pardo LA, Contreras-Jurado C, Zientkowska
M, Alves F and Stühmer W: Role of voltage-gated potassium channels
in cancer. J Membr Biol. 205:115–124. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Z: Roles of K+ channels in
regulating tumour cell proliferation and apoptosis. Pflugers Arch.
448:274–286. 2004.
|
|
20
|
Elso CM, Lu X, Culiat CT, Rutledge JC,
Cacheiro NL, Generoso WM and Stubbs LJ: Heightened susceptibility
to chronic gastritis, hyperplasia and metaplasia in Kcnq1
mutant mice. Hum Mol Genet. 13:2813–2821. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Patel AJ and Lazdunski M: The 2P-domain
K+ channels: role in apoptosis and tumorigenesis.
Pflugers Arch. 448:261–273. 2004.
|
|
22
|
Klimatcheva E and Wonderlin WF: An
ATP-sensitive K+ current that regulates progression
through early G1 phase of the cell cycle in MCF-7 human breast
cancer cells. J Membr Biol. 171:35–46. 1999.
|
|
23
|
Asher V, Warren A, Shaw R, Sowter H, Bali
A and Khan R: The role of Eag and HERG channels in cell
proliferation and apoptotic cell death in SK-OV-3 ovarian cancer
cell line. Cancer Cell Int. 11:62011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhanping W, Xiaoyu P, Na C, Shenglan W and
Bo W: Voltage-gated K+ channels are associated with cell
proliferation and cell cycle of ovarian cancer cell. Gynecol Oncol.
104:455–460. 2007.
|
|
25
|
Yao X and Kwan HY: Activity of
voltage-gated K+ channels is associated with cell
proliferation and Ca2+ influx in carcinoma cells of
colon cancer. Life Sci. 65:55–62. 1999.
|
|
26
|
Shen Z, Yang Q and You Q: Research toward
potassium channels on tumor progressions. Curr Top Med Chem.
9:322–329. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jones RD, Kerr DJ, Harnett AN, Rankin EM,
Ray S and Kaye SB: A pilot study of quinidine and epirubicin in the
treatment of advanced breast cancer. Br J Cancer. 62:133–135. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fishman MC and Spector I: Potassium
current suppression by quinidine reveals additional calcium
currents in neuroblastoma cells. Proc Natl Acad Sci USA.
78:5245–5249. 1981. View Article : Google Scholar
|
|
29
|
Basrai D, Kraft R, Bollensdorff C,
Liebmann L, Benndorf K and Patt S: BK channel blockers inhibit
potassium-induced proliferation of human astrocytoma cells.
Neuroreport. 13:403–407. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Weiger TM, Colombatto S, Kainz V,
Heidegger W, Grillo MA and Hermann A: Potassium channel blockers
quinidine and caesium halt cell proliferation in C6 glioma cells
via a polyamine-dependent mechanism. Biochem Soc Trans. 35:391–395.
2007. View Article : Google Scholar : PubMed/NCBI
|