Introduction
Gastric adenocarcinoma is the second leading cause
of cancer-related mortality worldwide (1), and its prognosis mainly depends on
early detection and the appropriate treatment (2). In spite of the use of endoscopy with
biopsy for diagnosis and health screening, due to the relatively
asymptomatic nature of the disease in early stages, the majority of
patients are diagnosed at an advanced stage (3). Although surgical intervention and
chemotherapy have significantly improved patient outcome, the
curative ratio for gastric carcinoma remains quite low (4). Therefore, the identification of
available biomarkers is an urgent goal for the early detection and
treatment of gastric cancer.
The myc proto-oncogene family mainly includes
c-myc, N-myc and L-myc, which are involved in
cell growth, cell cycle progression and genome instability through
many other genes (5,6). Although deregulation of c-myc
is known to be related to tumorigenesis of digestive system
neoplasms (5), the specific
mechanisms are still not fully understood. Mina53 is a recently
identified novel myc target gene, whose expression was found to be
directly induced by the oncogene c-myc, encoding a protein
localized in the nucleus with a molecular weight of 53 kDa
(7). Studies have shown that
mina53 expression levels are elevated in human colon cancer
(8), esophageal squamous cell
carcinoma (9), hepatocellular
carcinoma (10) and
cholangiocarcinoma (11). However,
quantitive investigation of mina53 expression has not been
carried out, and there are only a few studies concerning
mina53 expression in gastric carcinoma.
Laser-capture microdessection (LCM) is a newly
developed technology by which the heterogeneity of tissues is
satisfactorily resolved in situ (12) and has been widely used in gastric
carcinoma research (13–16). In the present study, we examined the
expression of mina53 in the gastric adenocarcinoma tissues
quantitively by LCM combined with real-time polymerase chain
reaction (real-time PCR) or western blot analysis. Lauren’s
classification is an important classification for scientific
research. Therefore, the expression of Mina53 during gastric
carcinogenesis and its correlation with clinicopathological factors
in intestinal gastric carcinoma (IGC) and diffuse gastric carcinoma
(DGC) was also immunohistochemically investigated. The correlation
between the expression of mina53 and patient survival time was also
investigated.
Materials and methods
Patients and samples
For real-time quantitative PCR detection, 12 pairs
of human gastric tumor tissues and their adjacent normal gastric
tissues were obtained within 30 min after surgical resection at
Renji Hospital, Shanghai Jiaotong University in 2011. Twelve of
these cases were chosen to extract proteins for western blot
analysis. These samples were frozen immediately in liquid nitrogen
for 20 sec and then stored at −80ºC until use. For
immunohistochemical examinations, formalin-fixed paraffin-embedded
(FFPE) samples were obtained from 64 patients who underwent surgery
for gastric carcinoma. The specimens consisting of 30 normal
gastric tissues, 44 chronic atrophic gastritis without intestinal
metaplasia, 34 intestinal metaplasia, 36 dysplasia were obtained by
biopsy during July 2009 and June 2010 at Renji Hospital. All of the
patients provided informed consent, and the study protocol was
approved by the Ethics Committee of Renji Hospital. An experienced
gastrointestinal pathologist (X.-Y. Chen) evaluated the tumor stage
and grade by microscopic examination of the samples following
laser-capture microdissection.
Fresh-frozen samples were embedded in optimal
cutting temperature compound (Sakura Finetek, Torrance, CA, USA)
and then serially cut into 8-μm sections in a cryostat at −20ºC.
The first section was mounted on ordinary glass slide and stained
with H&E for diagnosis, and the other was mounted on an
ethylene vinyl acetate (EVA) membrane, followed by incubation in
70% ethanol (1 min), ddH2O (10 sec), Mayer’s hematoxylin
(30 sec), twice ddH2O (20 sec), eosin (5 sec), 95%
ethanol (30 sec) and 100% ethanol (1 min). After air-drying, the
sections were subjected to laser-capture using an LMD system (Leica
Microsystems, Wetzlar, Germany), and the cells of interest were
selectively collected following the manufacturer’s recommendations
within 40 min. In order to harvest a sufficient amount of cells, 8
h was required for real-time PCR and 15 h for western blot
analysis, respectively. Each dissection was determined to be
>95% homogeneous by review of the histologic sections. The
captured malignant and normal cells were preserved in 0.5-ml
microcentrifuge tubes containing 200 μl TRIzol reagent for
isolating total RNA or containing nothing for extracting protein
and then stored at −80ºC until use.
RNA extraction and RT-PCR
RNA was isolated from the captured cells using the
Optimum FFPE RNA Isolation kit (Ambion, Austin, TX, USA) following
the recommended manufacturer’s instructions. Chloroform (40 μl) was
added to each tube, and the tubes were shaken violently by hand for
15 sec before incubation at room temperature for 5 min. After
centrifugation at 12,000 rpm for 15 min at 4ºC, the aqueous layer
was transferred to a 1.5-ml Eppendorf tube. Glycogen (1 μl) (10
μg/μl) carrier and 100 μl isopropanol were added successively and
precipitation was carried out at room temperature for 10 min
followed by centrifugation at 12,000 rpm for 15 min at 4ºC. The RNA
pellet was washed in 500 ml 75% ethanol, dried and redissolved in
20 μl diethylpyrocarbonate-treated RNase-free water before storing
at −80ºC. RNA samples were treated with DNase (Roche Diagnostics
Corp., Indianapolis, IN, USA), and then reverse transcription was
performed using 4 μl of 5× PrimeScipt™, 1 μl of Oligo(dT) primer
(50 μM), 1 μl of random 6 mers (100 μM), 1 μl of PrimeScript™ RT
Enzyme Mix I, × μl (1 μg) of total RNA and (13-x) μl of RNase-free
H2O in a total volume of 20 μl, followed by incubation
at 37ºC for 15 min (first-strand synthesis) and at 85ºC for 5 sec
(inactivation of the reverse transcriptase) then the samples were
maintained at 4ºC until use.
Real-time quantitative PCR
Real-time quantitative PCR was performed using the
ABI PRISM 7300 sequence detection system (ABI-7300H; Applied
Biosystems, Foster City, CA, USA) in a 10-μl reaction system
including 5 μl of SYBR® Premix Ex Taq™ II (2X) (Takara
Corp.) 0.4 μl of PCR forward primer (10 μM), 0.4 μl of PCR reverse
primer (10 μM), 0.2 μl of ROX Reference Dye (50X), 1 μl of
mina53 cDNA or c-myc cDNA and 3 μl of
dH2O. All the reactions were performed in triplicate.
The sequence of primer pairs were: sense, GGG ACA CAA CAT TGG GTA
TCA TCA and antisense, AAC ATG GGC AAT TCA GGC AGA for
mina53; sense, CCA CAG CAA ACC TCC TCA CAG and antisense,
GCA GGA TAG TCC TTC CGA GTG for c-myc; and sense, GTG AAG
GTC GGA GTC AAC G and antisense, TGA GGT CAA TGA AGG GGT C for
GAPDH (reference gene). The reactions were carried out at
95ºC for 30 sec initially followed by 40 cycles of 95ºC for 5 sec,
60ºC for 31 sec and 72ºC for 30 sec. Reaction products were
identified by agarose gel electrophoresis and GoldView staining.
The threshold cycle (Ct) of every target gene for each sample was
assessed using the 2−ΔΔCt method [ΔΔCt = (tumor gene Ct
- GAPDH Ct) − (normal epithelial gene Ct - GAPDH Ct)]. A
fold-change ≥2 was determined to be high expression.
Western blot analysis
Cells from 12 pairs of samples were lysed in a mixed
buffer (9.5 mol/l urea, 65 mmol/l DTT, 4% CHAPS, 0.2% IPG buffer)
for 1 h in an ice-bath and then centrifuged at 12,000 rpm for 1 h
at 4ºC. Sample proteins (20 μg) were subjected to 8% sodium
dodecylsulfate-polyacrylamide gel electrophoresis followed by
electrotransfer onto a polyvinylidene difluoride microporous
membrane (Millipore, Bedford, MA, USA) and then blocked with 5%
skim milk/0.01% Tween for 2 h at room temperature. After treatment
with rabbit anti-Mina53 polyclonal antibody at a dilution of
1:2,500 (Abcam Biotechnology), rabbit anti-c-Myc monoclonal
antibody (Bioget Technology) at a dilution of 1:1,000 or
HRP-conjugated rabbit anti-GAPDH monoclonal antibody (Shanghai
Kangcheng Biotechnology Co., Ltd., Shanghai, China) at a dilution
of 1:3,000 at 4ºC overnight and incubated with HRP-conjugated
secondary antibodies except for GAPDH at room temperature for 1 h,
blots were then detected with enhanced chemiluminescence for 1 min
(Pierce Biotechnology).
Immunohistochemical staining for
anti-Mina53
Routinely processed FFPE serial sections (4 μm) were
mounted on coated slides and deparaffinized in xylene and graded
alcohol. After pretreatment with 3% H2O2,
deparaffinized 4-μm tissue sections were autoclaved for Mina53.
After treatment with 5% non-immune goat serum, the sections were
incubated overnight at 4ºC with the anti-Mina53 polyclonal antibody
at a dilution of 1:500 (Abcam Biotechnology), followed by
peroxidase-labeled goat anti-mouse or goat anti-rabbit IgG Fab’.
Color was developed with 3,3-diaminobenzidine before the light
counterstaining with hematoxylin. The sections were then air-dried,
coverslipped and observed with an Olympus BX51 microscope (Olympus
Optical Co., Ltd., Tokyo, Japan).
Evaluation of immunostaining
The positive staining intensity of cells was scored
as follows: 0, no staining; 1, shallow brown; 2, brown; 3, dark
brown. The percentage of positive cells was divided into 4 levels:
0, ≤10% positive cells; 1, 11–50% positive cells; 2, 51–75%
positive cells; 3, >75% positive cells. The total score for the
staining of samples was calculate by multiplication of the
intensitiy and percentage scores: 0, (−); 1–2, (+); 3–4, (++); 5–9,
(+++). Each score ≥3–4 (++) was considered to be high
expression.
Statistical analysis
The comparison of the mRNA and proteins between the
malignant cells and the adjacent non-malignant cells was tested
using Wilcoxon signed rank test, and the relationship between the
mRNA or protein of mina53 and c-Myc was checked by Pearson
correlation analysis. The staining index ratio of Mina53 was
compared between different tissues using ANOVA analysis of
variance. The correlation between Mina53 expression and Lauren’s
type was examined using χ2 test, and the same method was
carried out between Mina53 expression and the various
clinicopathological factors in IGC and DGC, respectively. Crude
survival curves were calculated using the Kaplan-Meier method.
P-values <0.05 were assigned to indicate statistically
significant results. All statistical analyses were carried out
using SPSS 16.0 (SPSS, Inc., Chicago, IL, USA).
Results
Expression and correlation of mina53 and
c-Myc RNA
When compared with the adjacent normal epithelial
tissues, mina53 and c-myc mRNA expression in the
malignant tissue was highly increased (P<0.05 and P<0.01) as
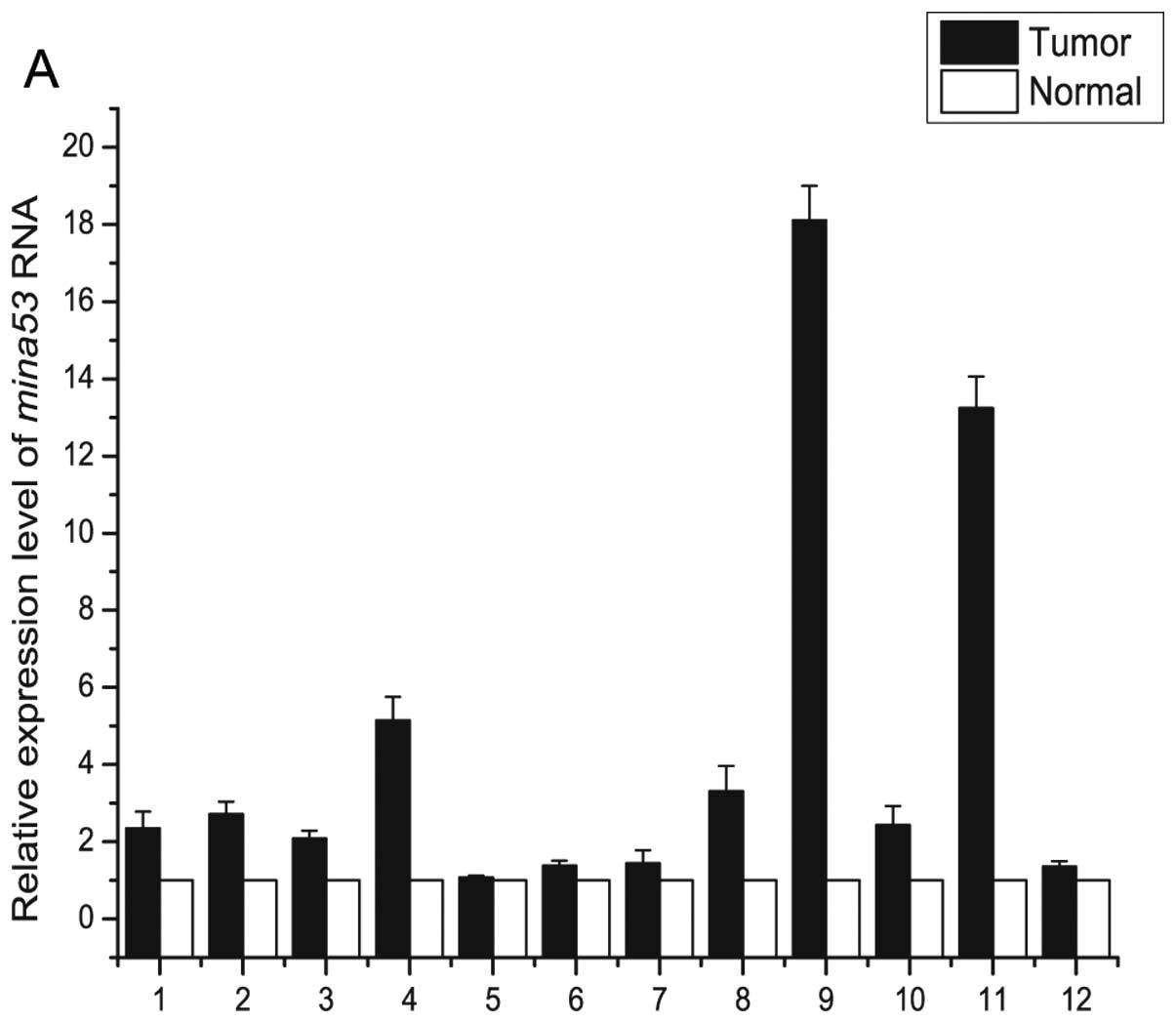
determined using real-time quantitative PCR. Mina53
expression was higher (fold-change ≥2) in 9 cases and c-Myc
expression was higher (fold-change ≥2) in 12 cases (Fig. 1). A significant positive correlation
between mina53 mRNA and c-myc mRNA in the gastric
carcinoma was noted (r=0.58, P<0.05).
Expression of Mina53 and c-Myc
protein
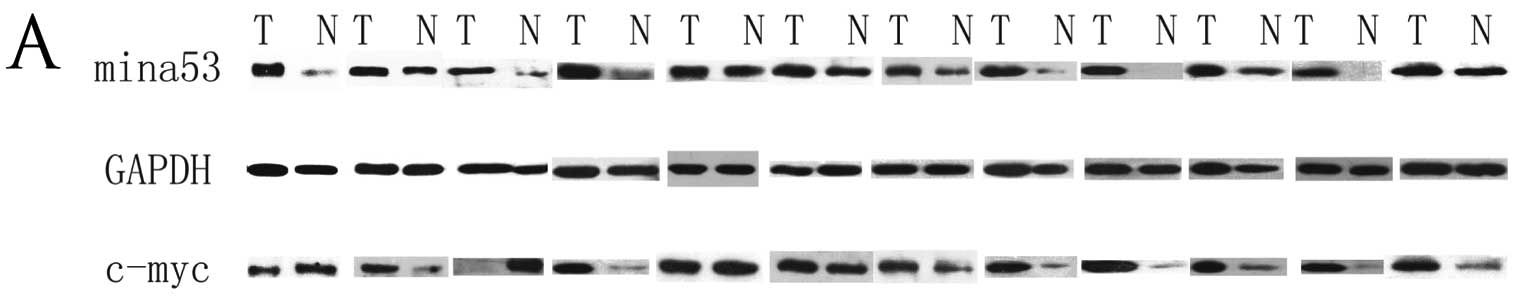
The intensity of the band for Mina53 in the
malignant tissue was increased when comparing to that in the
adjacent normal epithelial samples in all 12 cases (P<0.01)
(Fig. 2), and the expression level
of c-Myc was found to be higher in malignant cells when compared to
that in the adjacent normal epithelial tissues (P<0.05).
Nevertheless, the c-Myc expression level was observed to be lower
in the malignant than that in the matched non-malignant tissues in
3 (25%) cases. The Mina53 expression was found to be significantly
correlated with that of c-Myc (r=0.876, P<0.01).
Immunohistochemistry of Mina53
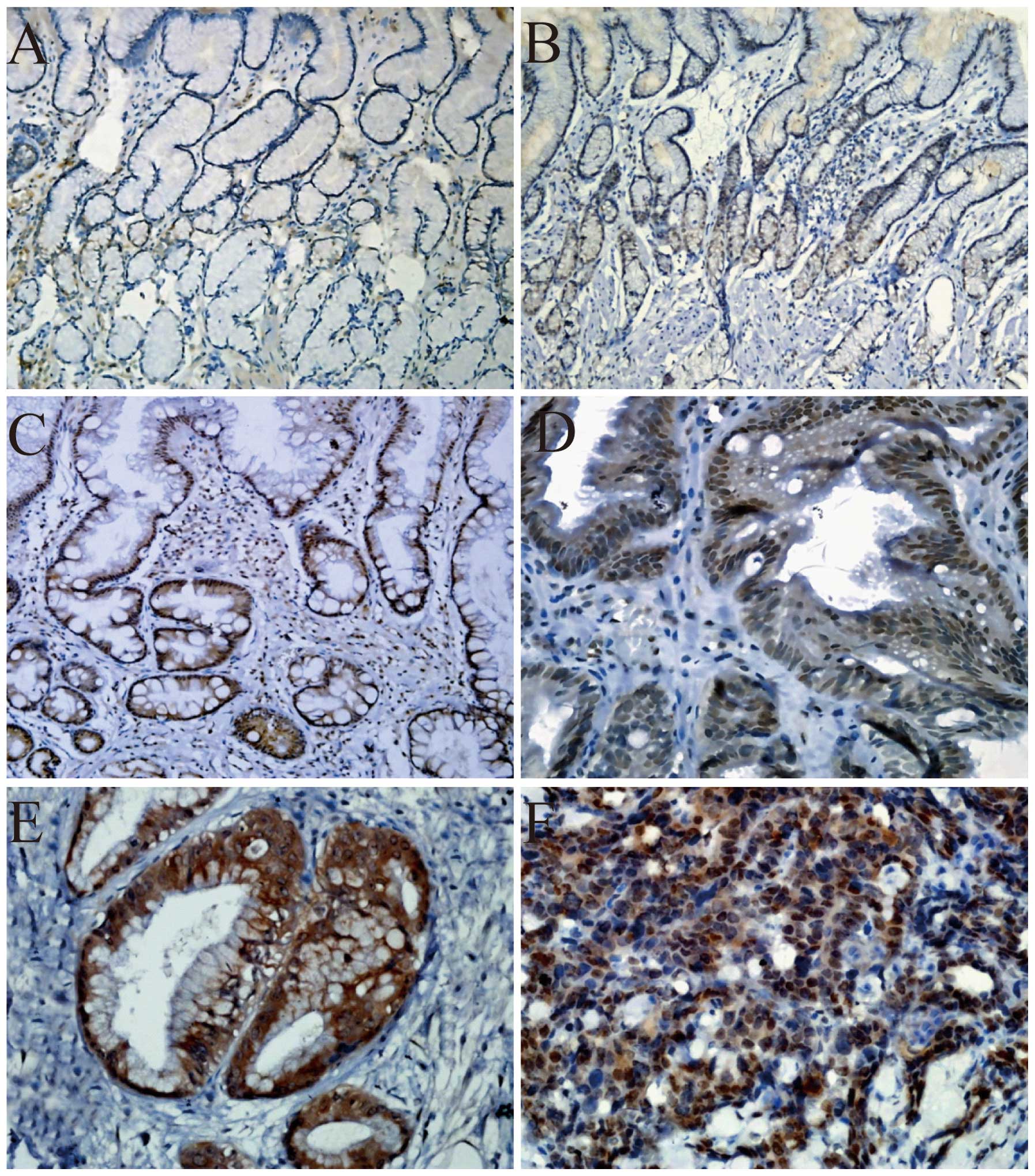
The percentage of cases with overexpression of
Mina53 was determined in normal gastric tissues (0%), chronic
atrophic gastritis without intestinal metaplasia (9.1%), intestinal
metaplasia (29.4%), dysplasia (41.7%) and IGC tissues (50%). The
frequency of the overexpression of Mina53 was progressively
increased (Table I), with a
statistically significant difference (P<0.01). Mina53 expression
was observed to be mainly localized in the nucleus and partly in
the cytoplasm. Only a few cells in the neck of the glands
eliminating mature foveola gastricae and proper gastric glands were
detected to express Mina53 with low intensity in normal gastric
tissues. The expression of Mina53 was increased in chronic atrophic
gastritis without intestinal metaplasia which was still mainly
located in the neck of the glands. In chronic atrophic gastritis
with intestinal metaplasia, the expression of Mina53 was observed
in intestinal metaplasia excluding proper gastric glands (Fig. 3).
 | Table IMina53 protein expression in the
multistage tissues of gastric carcinogenesis. |
Table I
Mina53 protein expression in the
multistage tissues of gastric carcinogenesis.
| | Mina53 protein
expression | | |
|---|
| |
| | |
|---|
| Group | Total cases | − | + | ++ | +++ | High expression
(%) | P-value |
|---|
| Normal | 30 | 28 | 2 | 0 | 0 | 0 | |
| NCAG | 44 | 23 | 17 | 4 | 0 | 9.1 | |
| IM | 34 | 8 | 16 | 10 | 0 | 29.4 | 0 |
| DYS | 36 | 4 | 17 | 10 | 5 | 41.7 | |
| IGC | 30 | 3 | 12 | 9 | 6 | 50 | |
Correlation between Mina53 overexpression
and clinicopathological factors in intestinal-type gastric
carcinoma (IGC) and diffuse-type gastric carcinoma (DGC)
No relationship was observed between the percentage
of cases with Mina53 overexpression and patient gender and tumor
site in both types of cancers. High expression of Mina53 was found
to be correlated to age (P<0.05), tumor diameter (P<0.05),
depth of invasion (P<0.01), lymph node metastasis (P<0.05),
distant metastasis (P<0.05) and TNM staging (P<0.05) in IGC
cases, while high expression of Mina53 was found to be correlated
only with lymph node metastasis (P<0.01) in DGC cases. The
Mina53 expression level was higher in the groups with age ≤63
years, tumor diameter >5 cm, depth of invasion (T3+T4), lymph
node metastasis, distant metastasis and TNM stage (II, III, IV) in
the IGC cases (Table II).
 | Table IICorrelation between Mina53 expression
and clinicopathological factors in IGC and DGC. |
Table II
Correlation between Mina53 expression
and clinicopathological factors in IGC and DGC.
| Features | Mina53 expression
level | Mina53 over-
expression (%) | P-value |
|---|
|
|---|
| −/+ | ++/+++ |
|---|
| Intestinal type |
| Gender | | | | 0.5 |
| Male | 11 | 12 | 52.2 | |
| Female | 4 | 3 | 42.9 | |
| Age (years)a | | | | 0.03 |
| ≤63 | 5 | 11 | 68.8 | |
| >63 | 10 | 4 | 28.6 | |
| Site | | | | 0.617 |
| Antrum | 6 | 8 | 57.1 | |
| Corpus
ventriculi | 7 | 4 | 36.4 | |
| Cardia | 2 | 3 | 60 | |
| Tumor diameter
(cm)b | | | | 0.025 |
| ≤5 | 13 | 7 | 35 | |
| >5 | 2 | 8 | 80 | |
| Depth of
invasion | | | | 0 |
| T1+T2 | 10 | 3 | 23.1 | |
| T3+T4 | 5 | 12 | 70.1 | |
| Lymph node
metastasis | | | | 0.001 |
| Negative | 11 | 2 | 15.4 | |
| Positive | 4 | 13 | 76.5 | |
| Distant
metastasis | | | | 0.021 |
| Negative | 15 | 10 | 40 | |
| Positive | 0 | 5 | 100 | |
| Tumor stage | | | | 0.025 |
| I | 8 | 2 | 20 | |
| II+III+IV | 7 | 13 | 65 | |
| Diffuse type |
| Gender | | | | 0.565 |
| Male | 6 | 18 | 75 | |
| Female | 2 | 8 | 80 | |
| Age
(years)a | | | | 0.565 |
| ≤63 | 6 | 18 | 75 | |
| >63 | 2 | 8 | 80 | |
| Site | | | | 0.764 |
| Antrum | 5 | 11 | 68.8 | |
| Corpus
ventriculi | 2 | 11 | 84.6 | |
| Cardia | 1 | 4 | 80 | |
| Tumor diameter
(cm)b | | | | 0.352 |
| ≤5 | 4 | 9 | 69.2 | |
| >5 | 4 | 17 | 81 | |
| Depth of
invasion | | | | |
| T1+T2 | 2 | 3 | 60 | 0.334 |
| T3+T4 | 6 | 23 | 79.3 | |
| Lymph node
metastasis | | | | |
| Negative | 6 | 4 | 40 | 0.003 |
| Positive | 2 | 22 | 91.7 | |
| Distant
metastasis | | | | 0.402 |
| Negative | 6 | 16 | 72.7 | |
| Positive | 2 | 10 | 83.3 | |
| Tumor stage | | | | 0.126 |
| I | 3 | 3 | 50 | |
| II+III+IV | 5 | 23 | 82.1 | |
Mina53 expression in relation to survival
time
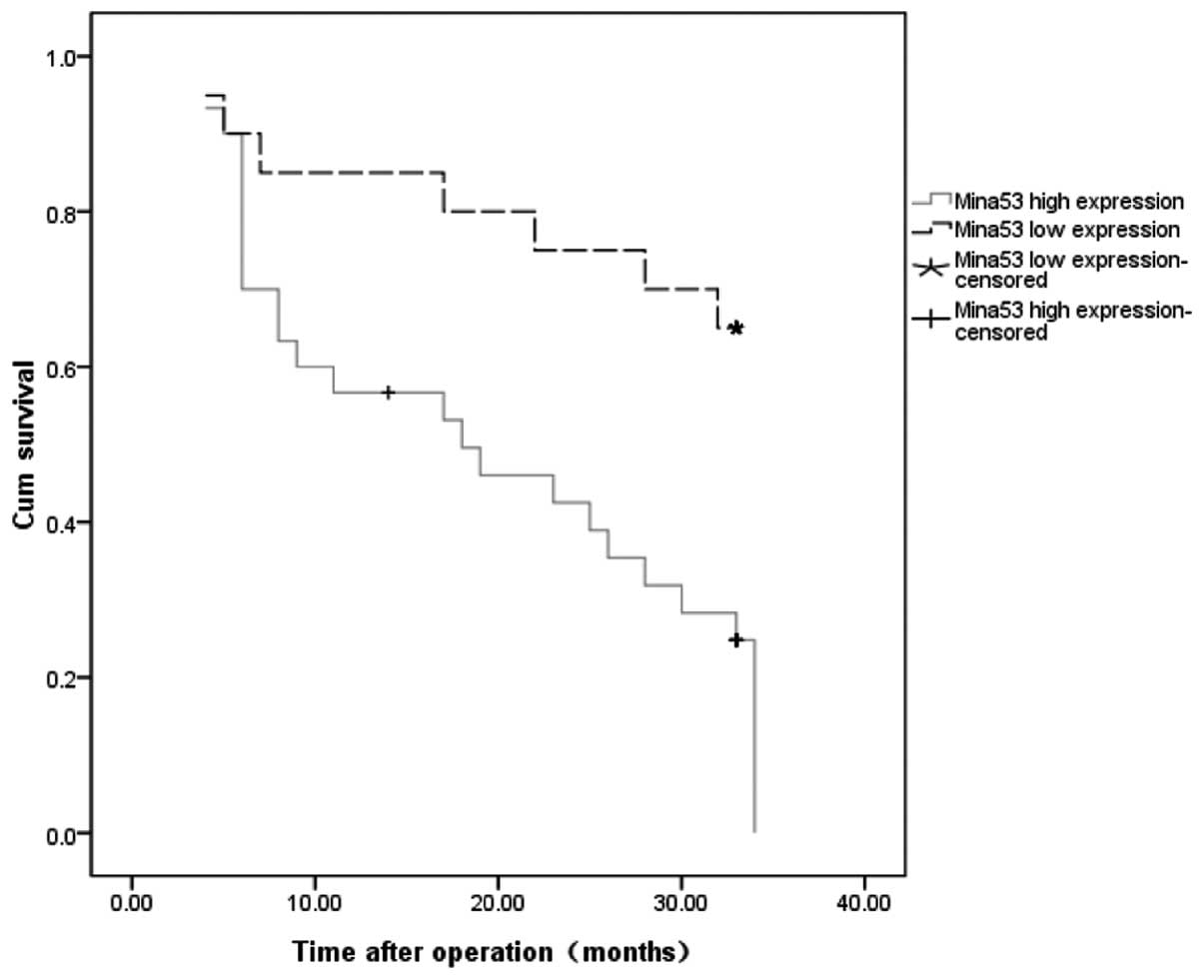
Fifty patients successfully followed up were divided
into two groups according to low or high Mina53 expression. Crude
survival curves were estimated for each group using the
Kaplan-Meier method. The survival rate was lower in patients with
tumors with high mina53 expression than in patients with tumors
with low mina53 expression (P=0.007; Fig. 4).
Discussion
Mina53 has been identified as a novel myc-induced
nuclear antigen. For quantitive analysis and to obtain ‘pure’ cells
of interest, we firstly combined laser-capture microdissection with
real-time quantitative PCR and western blot analysis to investigate
the mina53 and c-myc expression in gastric carcinoma
tissues and adjacent normal tissues without contamination.
A larger number of homogeneous cells were obtained
using laser-capture microdissection for mRNA and protein analysis,
and the levels of mina53 mRNA and protein expression were
found to be highly elevated in tumor cells. The c-Myc expression
was not always consistent with that of Mina53 by using western blot
analysis (Fig. 2). A similar result
was also reported in esophageal squamous cell carcinoma by Tsuneoka
et al (9). In 3 (25.0%)
cases, the expression of c-Myc in tumor cells was lower than that
in adjacent normal cells. Tsuneoka et al conjectured that
proteins in the Myc/Mad/Max network or some other factor or factors
which were able to control mina53 expression may be involved
in this issue (9). Yet, the precise
mechanisms remain unknown. This also demonstrated that it was a
complicated mechanism for gene expression from mRNA to protein.
Immunohistochemical staining showed that the Mina53
expression level was increased stepwisely during the process of
tumorigenesis in IGC. Teye et al (8) found that the expression level was
increased in colon adenoma. These results suggest that the increase
in Mina53 expression may be an early molecular event during gastric
carcinogenesis. Thus, Mina53 may be a potential marker for the
early diagnosis of gastric cancer.
Teye et al (8) also noted that the expression of Mina53
was increased in all pathological grades of colon cancer,
particularly in strongly invasive and metastatic cases. In the
present study, we observed that there was a higher percentage of
cases with high Mina53 expression in DGC (76.5%) when compared with
this percentage in IGC (50.0%). In addition, the correlation
between Mina53 overexpression and clinicopathological features also
differed in IGC and DGC. A high expression of Mina53 was associated
with age, the diameter of the tumor, depth of invasion, lymph node
metastasis, distant metastasis and TMN stage in IGC cases; however,
high expression of Mina53 was correlated with only lymph node
metastasis in DGC, exhibiting some differences from what was
previously described. These results confirm that IGC and DGC have
different histogenetic pathways (17) and suggest that mina53 may
participate in lymph node metastasis in DGC. In the present study,
we found that the staining index of Mina53 was associated with the
prognosis of patients with gastric carcinomas by a Kaplan-Meier
plot, and the prognosis of patients with elevated expression of
Mina53 was poor. A similar result was also found in esophageal
squamous cell carcinomas (9) and
advanced renal cell carcinomas (18). These findings suggest that Mina53
may serve as a potential marker for diagnosis and prognosis in a
subset of gastric cancer patients, particularly DGC. However, in a
study by Komiya et al (19),
patients with high Mina53 expression were found to have a more
favorable prognosis than those with low Mina53 expression. The
exact mechanism is still needed to be investigated.
In conclusion, high expression of Mina53 was
detected in gastric carcinoma and may be an early molecular events
in tumorigenesis. It was found to be closely correlated with many
dlinicopathological factors, and Mina53 may be a potential
molecular marker for diagnosis, molecular classification and
prognosis of gastric cancer. Elucidation of the mechanism of Mina53
in tumorigenesis and tumor progression may provide a novel
therapeutic target for gastric adenocarcinoma.
Acknowledgements
We thank Mr. Yan-Shen Peng for preparing serial
sections of the tissues. The authors are indebted to all patients
who participated in the study as well as the surgeons who aided in
the treatment of these patients. The present study was supported by
grants from the National key Basic Research and Development Program
(973) of China (no. 2010CB529304).
Abbreviations:
|
IGC
|
intestinal-type gastric carcinoma
|
|
DGC
|
diffuse-type gastric carcinoma
|
|
LCM
|
laser-capture microdissection
|
|
RT-real-time PCR
|
reverse transcription real-time
polymerase chain reaction
|
References
|
1
|
Huang CY, Chen YM, Zhao JJ, et al:
Decreased expression of transcription elongation factor A-like 7 is
associated with gastric adenocarcinoma prognosis. PLoS One.
8:e546712013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang L, Huang X, Chen Y, Jin X, Li Q and
Yi TN: Prognostic value of TP/PD-ECGF and thrombocytosis in gastric
carcinoma. Eur J Surg Oncol. 38:568–573. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pourhoseingholi MA, Moghimi-Dehkordi B,
Safaee A, Hajizadeh E, Solhpour A and Zali MR: Prognostic factors
in gastric cancer using log-normal censored regression model.
Indian J Med Res. 129:262–267. 2009.PubMed/NCBI
|
|
4
|
Henning GT, Schild SE, Stafford SL, et al:
Results of irradiation or chemo irradiation following resection of
gastric adenocarcinoma. Int J Radiat Oncol Biol Phys. 46:589–598.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gostissa M, Ranganath S, Bianco JM and Alt
FW: Chromosomal location targets different MYC family gene members
for oncogenic translocations. Proc Natl Acad Sci USA.
106:2265–2270. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ruggero D: The role of Myc-induced protein
synthesis in cancer. Cancer Res. 69:8839–8843. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsuneoka M, Koda Y, Soejima M, Teye K and
Kimura H: A novel myc target gene, mina53, that is involved
in cell proliferation. J Biol Chem. 277:35450–35459. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Teye K, Tsuneoka M, Arima N, et al:
Increased expression of a Myc target gene Mina53 in human
colon cancer. Am J Pathol. 164:205–216. 2004. View Article : Google Scholar
|
|
9
|
Tsuneoka M, Fujita H, Arima N, et al:
Mina53 as a potential prognostic factor for esophageal squamous
cell carcinoma. Clin Cancer Res. 10:7347–7356. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ogasawara S, Komuta M, Nakashima O, Akiba
J, Tsuneoka M and Yano H: Accelerated expression of a Myc target
gene Mina53 in aggressive hepatocellular carcinoma. Hepatol
Res. 40:330–336. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tan XP, Zhang Q, Dong WG, Lei XW and Yang
ZR: Upregulated expression of Mina53 in cholangiocarcinoma and its
clinical significance. Oncol Lett. 3:1037–1041. 2012.PubMed/NCBI
|
|
12
|
Wang Y, Antonopoulos DA, Zhu X, et al:
Laser captured microdissection and metagenomic analysis of intact
mucosa-associated microbial communities of human colon. Appl
Microbiol Biotechnol. 88:1333–1342. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang L, Zheng L, Wang SY, Zhu TF and Zhu
HG: Clonal analysis of gastric carcinoma and precancerous lesions
and its relation to Ki-67 protein expression. Neoplasma. 56:48–55.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee HJ, Nam KT, Park HS, et al: Gene
expression profiling of metaplastic lineages identifies CDH17 as a
prognostic marker in early stage gastric cancer. Gastroenterology.
139:213–225. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Z, Li M, Zhang G, Fang P, Yao H,
Xiao Z and Chen Z: Identification of human gastric carcinoma
biomarkers by differential protein expression analysis using
18O labeling and nanoLC-MS/MS coupled with laser capture
microdissection. Med Oncol. 27:296–303. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gutierrez-Gonzalez L, Graham TA,
Rodriguez-Justo M, et al: The clonal origins of dysplasia from
intestinal metaplasia in the human stomach. Gastroenterology.
140:1251–1260. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gürbüz Y, Kahlke V and Klöppel G: How do
gastric carcinoma classification systems relate to mucin expression
patterns? An immunohistochemical analysis in a series of advanced
gastric carcinomas. Virchows Arch. 440:505–511. 2002.
|
|
18
|
Ishizaki H, Yano H, Tsuneoka M, et al:
Overexpression of the myc target gene Mina53 in advanced renal cell
carcinoma. Pathol Int. 57:672–680. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Komiya K, Sueoka-Aragane N, Sato A, et al:
Expression of Mina53, a novel c-Myc target gene, is a favorable
prognostic marker in early stage lung cancer. Lung Cancer.
69:232–238. 2010. View Article : Google Scholar : PubMed/NCBI
|