Introduction
Macrophages are important elements in the tumor
microenvironment (TME) that are not only affected by cytokines and
other cellular products but also produce many of their own
cytokines and products to affect survival, proliferation, behavior
and the function of tumors and other cells to further affect the
regulatory network of cytokines and the TME. It is generally
accepted that tumor-associated macrophages (TAM) in the TME are
important antitumor effector cells. Mantovani et al
(1), proposed the famous
‘macrophage balanced hypothesis’ in 1992, which suggested that TAMs
had dual functions in killing tumors and promoting tumor growth.
More and more studies in recent years (2,3) have
shown that TAMs are involved in processes associated with the
incidence, growth, invasion and migration of tumors, particularly
in processes closely related to vascularization and lymph vessel
formation in tumors.
Tumor cells can recruit macrophages in the TME, and
macrophages can affect tumor cells in return, thus forming a
vicious cycle. Measures can be taken to prevent the recruitment of
macrophages, attenuate the secretory functions of macrophages,
antagonize the cytokines secreted by macrophages, block signaling
pathways in tumor cells, in order to block this vicious cycle.
However, most of the previous studies have focused on the effects
of tumor cells on TAMs and the changes in the cytokines secreted by
TAMs. The changes in gene expression in tumor cells under the
effects of macrophages are still largely unknown. Therefore, the
present study was carried out to illustrate the changes in the gene
expression profile in lung cancer cells under the effects of a
macrophage-conditioned medium based on microarray data from the
Gene Expression Omnibus (GEO) database and to find the
transcription factors playing key roles to provide new clues for
blocking this vicious cycle.
Materials and methods
Microarray data
The gene expression profile data were derived from
the GEO database GSE9315 of the National Center for Biotechnology
Information (NCBI) (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE9315)
submitted by Lee CY et al. Human monocyte THP-1 and a highly
invasive human pulmonary adenocarcinoma cell line, CL1-5, were
utilized as the subjects for investigation. THP-1 cells were
pre-treated with phorbol myristate acetate (PMA) for 24 h to allow
for differentiation into macrophages, and the cells were further
incubated in serum-free culture media for another 24 h. After the
PMA was washed out, the supernatant of the culture solution was
used as the conditioned medium. The GSM234967 sample was derived
from CL1-5 cells that were not treated with the conditioned medium,
and the sample GSM234968 was derived from CL1-5 cells that were
treated with the conditioned medium.
The microarray utilized in the present study was
GPL5968, which is a human cDNA CHIP that contains 1,152 human EST
clones with putative gene names related to cell adhesion, motility,
angiogenesis, signal transduction, tumorigenesis and
metastasis.
Methods for microarray data analysis
The microarray analysis was carried out for
GSM234967 and GSM234968 samples. The CEL data compression package
for microarray was downloaded in the supplementary file of GEO and
was decompressed into another folder for further use. Additionally,
the original data for the sample were also downloaded in TXT
format. The downloaded data were standardized using Bioconductor
(version 2.10.1) (3). The RMA
algorithm was used to calculate the expression level, and the MAS
algorithm was used to calculate the detection call. Samples with no
less than 2 detection calls were retained as the P filter low
expression probe number, and human HGNC gene abbreviations were
used to unify the gene names. Afterwards, the VLOOKUP function was
used as a substitute for the probe number in the original data, and
the expression datasheet correlating the gene names and samples was
established. The LIMMA differential gene screening algorithm was
used to screen for the upregulated genes and the downregulated
genes in GSM234968 as compared to GSM234967. The fold-change,
P-value and FDR values were calculated.
Bioinformatic analysis of the
differentially expressed genes
DAVID software was used for Gene Ontology (GO) and
Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses.
The DAVID database (http://david.abcc.ncifcrf.gov/) was opened and the
gene cluster was submitted for further analysis. The corresponding
gene designator was selected, and the whole genome of the human
served as the background. Then, the ‘Functional Annotation Tool’
was used to obtain the results for the GO and KEGG enrichment
analysis (P-value was set to 0.05).
The protein-protein interaction (PPI) network was
obtained from the HPRD database (http://www.hprd.org/), which contained 36,874 lines
and 9,453 nodes. Forty differentially expressed genes were
projected into the PPI network, and the correlation pairs that
correlated with every differentially expressed gene were retained;
thus, a network composed of the nodes was directly connected with
the differentially expressed genes.
The GO terms or KEGG pathways of interest and their
corresponding genes were selected, and the genes with the same
functions were correlated by constructing a visualization network
using Cytoscape software (version 2.6.3).
Screening of the regulatory small
molecules
The connectivity map (CMap) database consists of
data from whole-genome transcription expression profiles of human
cells under the influence of active small molecules, including
7,256 expression profiles from 6,100 groups of small molecule
interference experiments (a small molecule interference group and
the normal control group). In total, 1,309 small molecules are
included in the 6,100 groups of small molecule interference
experiments.
According to the interference experiments on cancer
cells using the 1,309 small molecules documented in the CMap
database, we analyzed the differentially expressed genes from the
expression profile data derived from the pulmonary adenocarcinoma
samples (GSM234968 vs. GSM234967). The drugs and the gene sets
affected by the drugs (the differentially expressed genes) were
obtained. Then, the differences in gene expression for pulmonary
adenocarcinoma were analyzed and compared with the differentially
expressed genes induced by the interference of the molecules to
identify the small molecules that may be driving variations in gene
expression between normal and diseased cells.
The genes showing differential expression in the
diseased cells in comparison to the normal cells were categorized
into downregulated genes and upregulated genes, and then these
genes were used to make a probe set under the HG-U133A platform.
This probe set was used to compare the differentially expressed
genes after treatment with the small molecules in the CMap
database, and finally an enrichment value representing similarity
was obtained.
Results
Results of the analysis of the
differentially expressed genes
After the analysis of the microarray data, 1,041
genes were analyzed, among which 70 genes showed either an
expression increase of at least 2-fold or an expression decrease of
at least 50%. The 70 differentially expressed genes were uploaded
for functional annotation, and 40 genes were annotated (as shown in
Table I): ANXA4, CD1D, CDK2, COMT,
GJA1, GNG11, HMGB2, IL12A, IL18, JUNB, STMN1, MMP7, MYBL2, NUBP1,
NFKB2, PLAU, RBBP4, RGS4, SMN2, STC1, YWHAH, EIF2B4, EIF2B5, NFS1,
AURKB, PTTG1, CIAO1, MRC2, TUBB4B, NID2, RNF167, RPS27L, DPH5,
SNIP1, COL21A1, PLA2G12A, RNF135, C1orf87, PCNA-AS1 and
SEPT5-GP1BB.
 | Table IDifferentially expressed genes in the
CL1-5 cells following treatment with the macrophage-conditioned
medium. |
Table I
Differentially expressed genes in the
CL1-5 cells following treatment with the macrophage-conditioned
medium.
| Gene symbol | Entrez ID | Description | Fold-change
value |
|---|
| 1 | ANXA4 | 307 | Annexin A4 | 2.024670261 |
| 2 | CD1D | 912 | CD1d molecule | 2.309465826 |
| 3 | CDK2 | 1017 | Cyclin-dependent
kinase 2 | 2.464015819 |
| 4 | COMT | 1312 |
Catechol-O-methyltransferase | 2.967239056 |
| 5 | GJA1 | 2697 | Gap junction
protein, α 1 | 2.284841855 |
| 6 | GNG11 | 2791 | Guanine nucleotide
binding protein (G protein), γ 11 | 2.593119266 |
| 7 | HMGB2 | 3148 | High mobility group
box 2 | 2.421030043 |
| 8 | IL12A | 3592 | Interleukin 12A
(natural killer cell stimulatory factor 1, cytotoxic lymphocyte
maturation factor 1, p35) | 2.266825069 |
| 9 | IL18 | 3606 | Interleukin 18
(interferon-γ-inducing factor) | 2.115577065 |
| 10 | JUNB | 3726 | Jun B
proto-oncogene | 0.497792634 |
| 11 | STMN1 | 3925 | Stathmin 1 | 2.409299142 |
| 12 | MMP7 | 4316 | Matrix
metallopeptidase 7 (matrilysin, uterine) | 2.166427269 |
| 13 | MYBL2 | 4605 | V-myb
myeloblastosis viral oncogene homolog (avian)-like 2 | 2.181687983 |
| 14 | NUBP1 | 4682 | Nucleotide binding
protein 1 | 2.162368172 |
| 15 | NFKB2 | 4791 | Nuclear factor of κ
light polypeptide gene enhancer in B-cells 2 (p49/p100) | 0.450935487 |
| 16 | PLAU | 5328 | Plasminogen
activator, urokinase | 0.33083469 |
| 17 | RBBP4 | 5928 | Retinoblastoma
binding protein 4 | 2.300745137 |
| 18 | RGS4 | 5999 | Regulator of
G-protein signaling 4 | 2.030545158 |
| 19 | SMN2 | 6607 | Survival of motor
neuron 2, centromeric | 2.05350515 |
| 20 | STC1 | 6781 | Stanniocalcin
1 | 0.308078625 |
| 21 | YWHAH | 7533 | Tyrosine
3-monooxygenase/tryptophan 5-monooxygenase activation protein, η
polypeptide | 2.018610231 |
| 22 | EIF2B4 | 8890 | Eukaryotic
translation initiation factor 2B, subunit 4 δ, 67 kDa | 2.024384748 |
| 23 | EIF2B5 | 8893 | Eukaryotic
translation initiation factor 2B, subunit 5 ɛ, 82 kDa | 2.051791918 |
| 24 | NFS1 | 9054 | NFS1 nitrogen
fixation 1 homolog (S. cerevisiae) | 2.078554339 |
| 25 | AURKB | 9212 | Aurora kinase
B | 2.143919742 |
| 26 | PTTG1 | 9232 | Pituitary
tumor-transforming 1 | 2.323462232 |
| 27 | CIAO1 | 9391 | Cytosolic
iron-sulfur protein assembly 1 | 0.462791283 |
| 28 | MRC2 | 9902 | Mannose receptor, C
type 2 | 2.106472697 |
| 29 | TUBB4B | 10383 | Tubulin, β 4B class
IVb | 2.09408162 |
| 30 | NID2 | 22795 | Nidogen 2 | 0.379487576 |
| 31 | RNF167 | 26001 | Ring finger protein
167 | 2.075793535 |
| 32 | RPS27L | 51065 | Ribosomal protein
S27-like | 2.319500882 |
| 33 | DPH5 | 51611 | DPH5 homolog (S.
cerevisiae) | 2.095665817 |
| 34 | SNIP1 | 79753 | Smad nuclear
interacting protein 1 | 2.314891847 |
| 35 | COL21A1 | 81578 | Collagen, type XXI,
α 1 | 2.036490338 |
| 36 | PLA2G12A | 81579 | Phospholipase A2,
group XIIA | 2.430047715 |
| 37 | RNF135 | 84282 | Ring finger protein
135 | 2.033762661 |
| 38 | C1orf87 | 127795 | Chromosome 1 open
reading frame 87 | 0.367687812 |
| 39 | PCNA-AS1 | 100302739 | PCNA antisense RNA
1 | 3.150155333 |
| 40 | SEPT5-GP1BB | 100526833 | SEPT5-GP1BB read
through | 3.570031192 |
Results of the enrichment analysis of the
differentially expressed genes
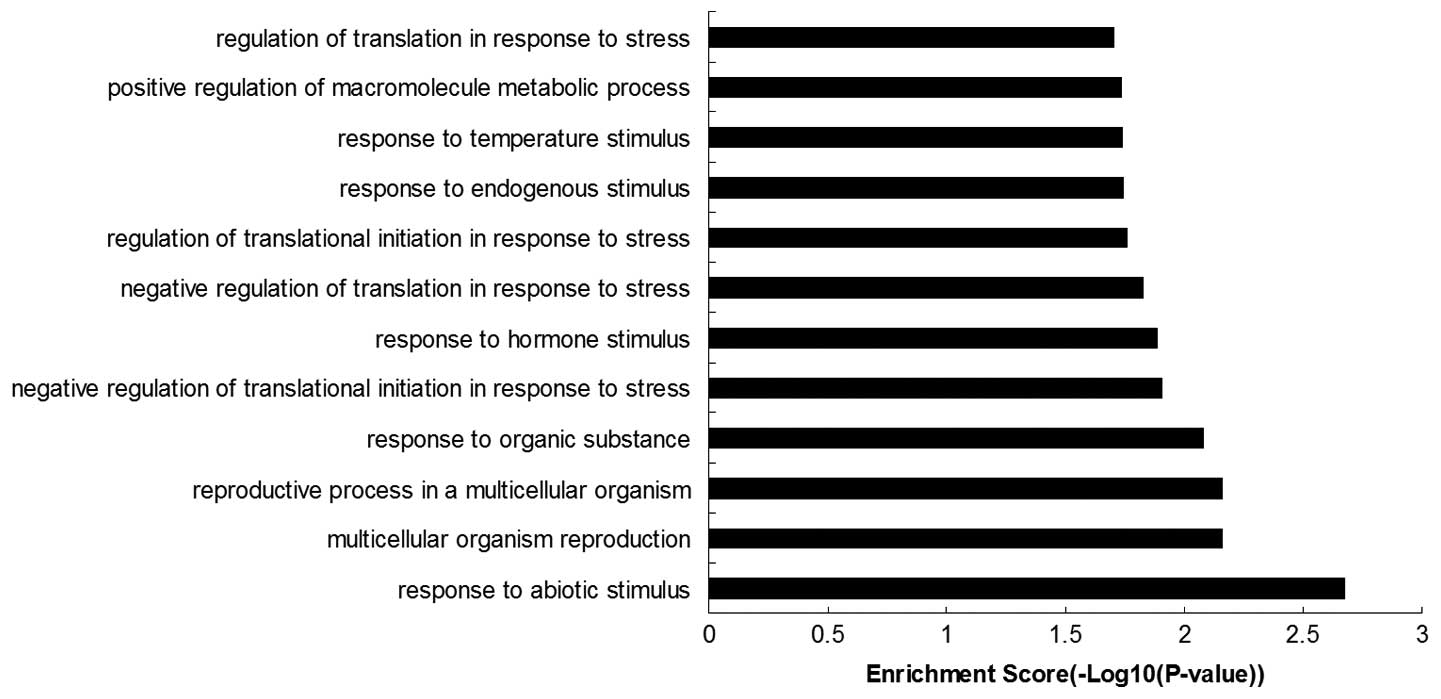
The 40 genes listed above were subjected to
functional enrichment analysis, and the top 15 GO terms were
selected (Fig. 1). Nine of these
genes were categorized as ‘response to abiotic stimulus’ (GO:
0009628), ‘response to organic substance’ (GO: 0010033), ‘negative
regulation of translational initiation in response to stress’ (GO:
0032057), ‘response to hormone stimulus’ (GO: 0009725), ‘negative
regulation of translation in response to stress’ (GO: 0032055),
‘regulation of translational initiation in response to stress’ (GO:
0043558), ‘response to endogenous stimulus’ (GO: 0009719),
‘response to temperature stimulus’ (GO: 0009266), ‘regulation of
translation in response to stress’ (GO: 0043555), or were
correlated with ‘stress’ and ‘responses’.
Among these 40 genes, 6 genes (RBBP4, IL12A, AURKB,
PTTG1, STMN1 and CDK2) were related to the ‘cell cycle’ (GO:
0007049, GO: 0051726, GO: 0000279, GO: 0022402, GO: 0000278, GO:
0022403); 4 genes (RBBP4, IL12A, GJA1 and COMT) were related to
‘cell proliferation’ (GO: 0008285); 3 genes (HMGB2, RBBP4 and CDK2)
were related to ‘DNA replication’ (GO: 0006260); 7 genes (HMGB2,
YWHAH, RGS4, SP1NI, RPS27L, GNG11 and STMN1) were related to an
‘intracellular signaling cascade’ (GO: 0007242) and 3 genes (IL18,
JUNB and PLAU) were related to ‘blood vessel morphogenesis’ (GO:
0048514).
An in-depth literature search revealed that TUBB4B,
STMN1 and NUBP1 were related to ‘the functions of microfilaments
and microtubules’; GJA1, MMP7, PLAU, NID2, MRC2 and COL21A1 were
related to ‘extracellular matrix’; SNIP1, MYBL2, PCNA-AS1, STC1 and
COMT were related to ‘lung cancer’; RGS4 and GNG11 were related to
‘breast cancer’; and GNG11 was related to ‘endometrial
carcinoma’.
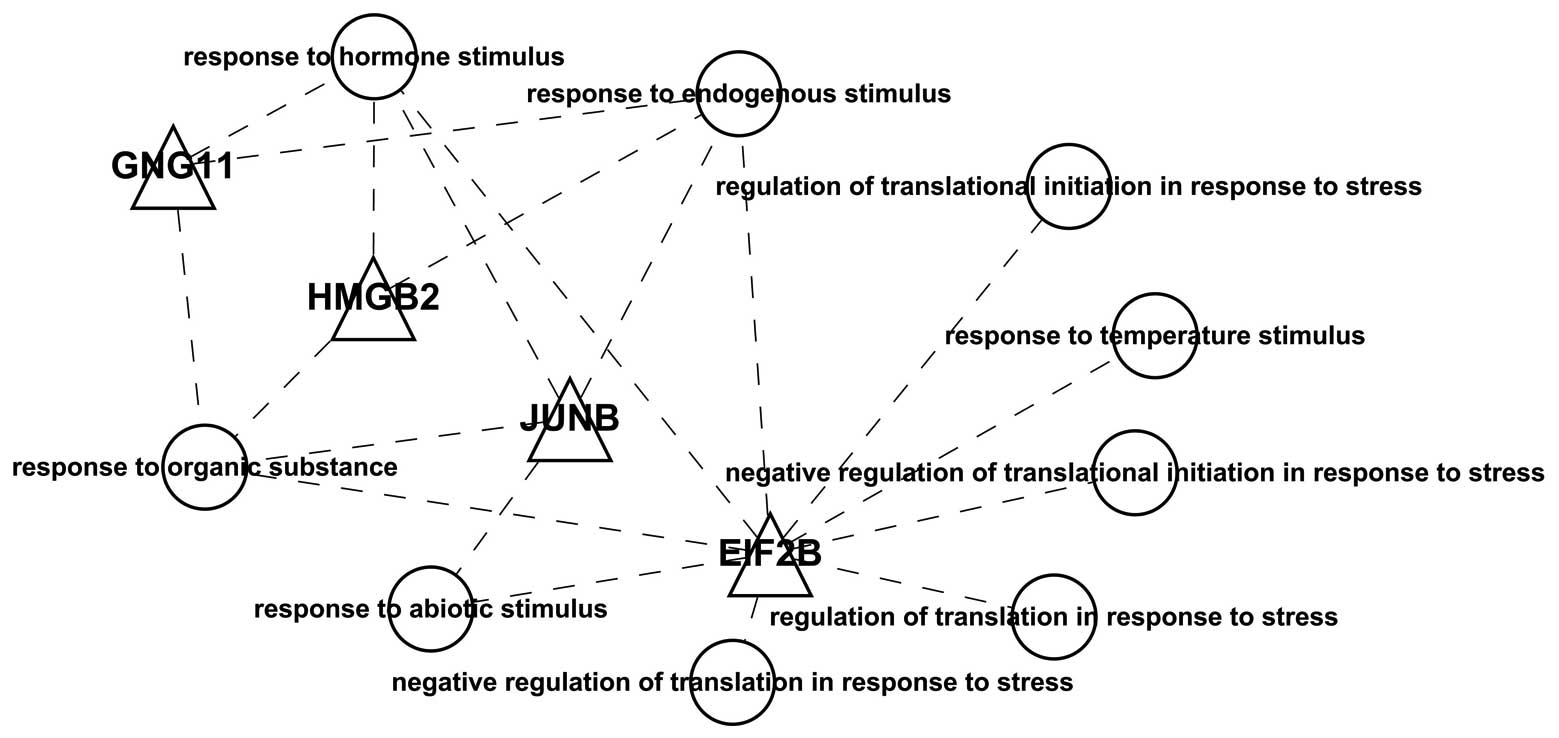
Notably, we found 5 differentially expressed
transcription factors: EIF2B4, EIF2B5, JUNB, GNG11 and HMGB2. Using
these transcription factors and the GO functional annotations, we
constructed a visualization graph (Fig.
2) and found that these transcription factors were all related
to ‘stress’ and ‘responses’.
Functional enrichment analysis and the
construction of the visualization network for transcription factors
and regulatory genes
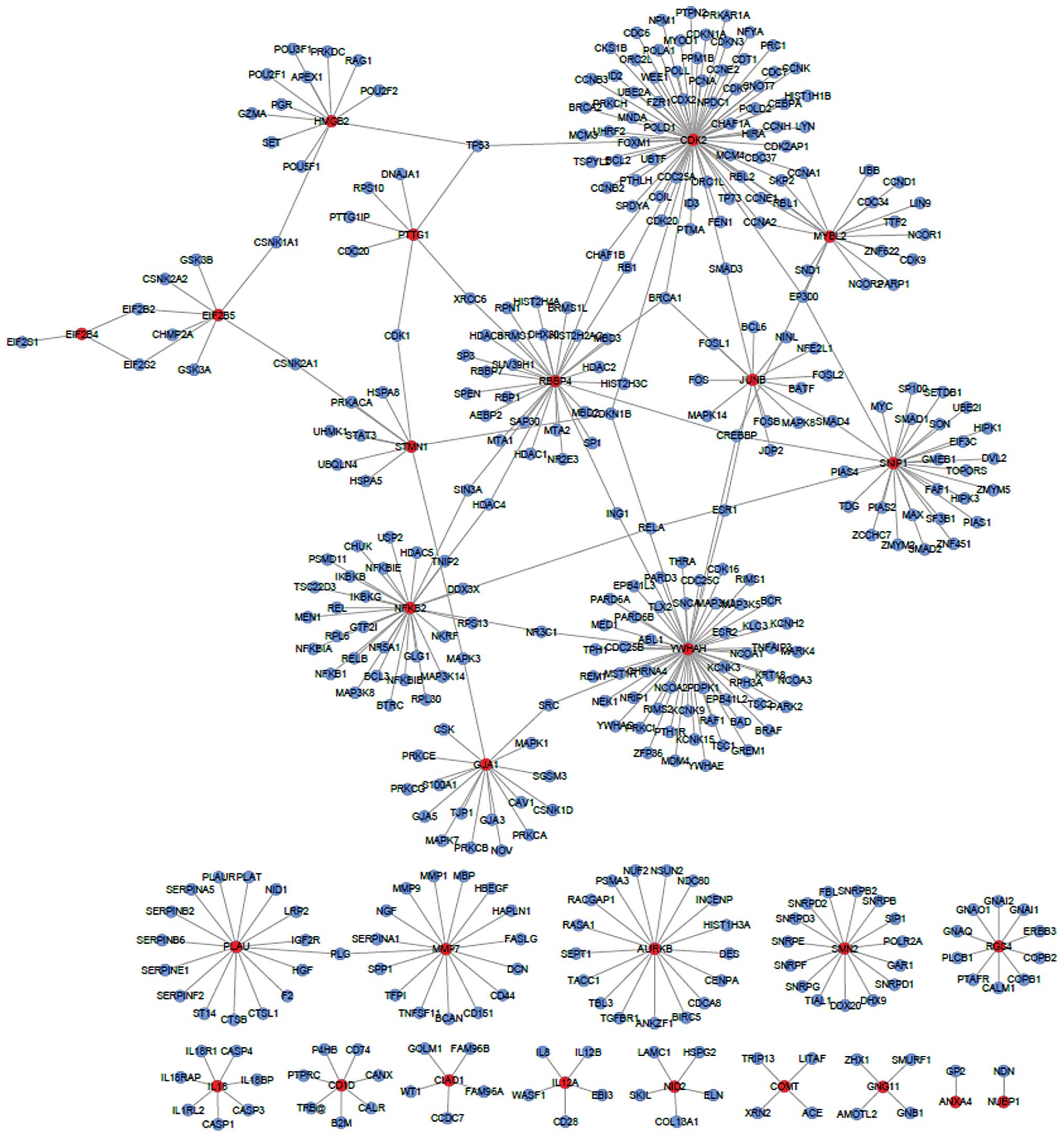
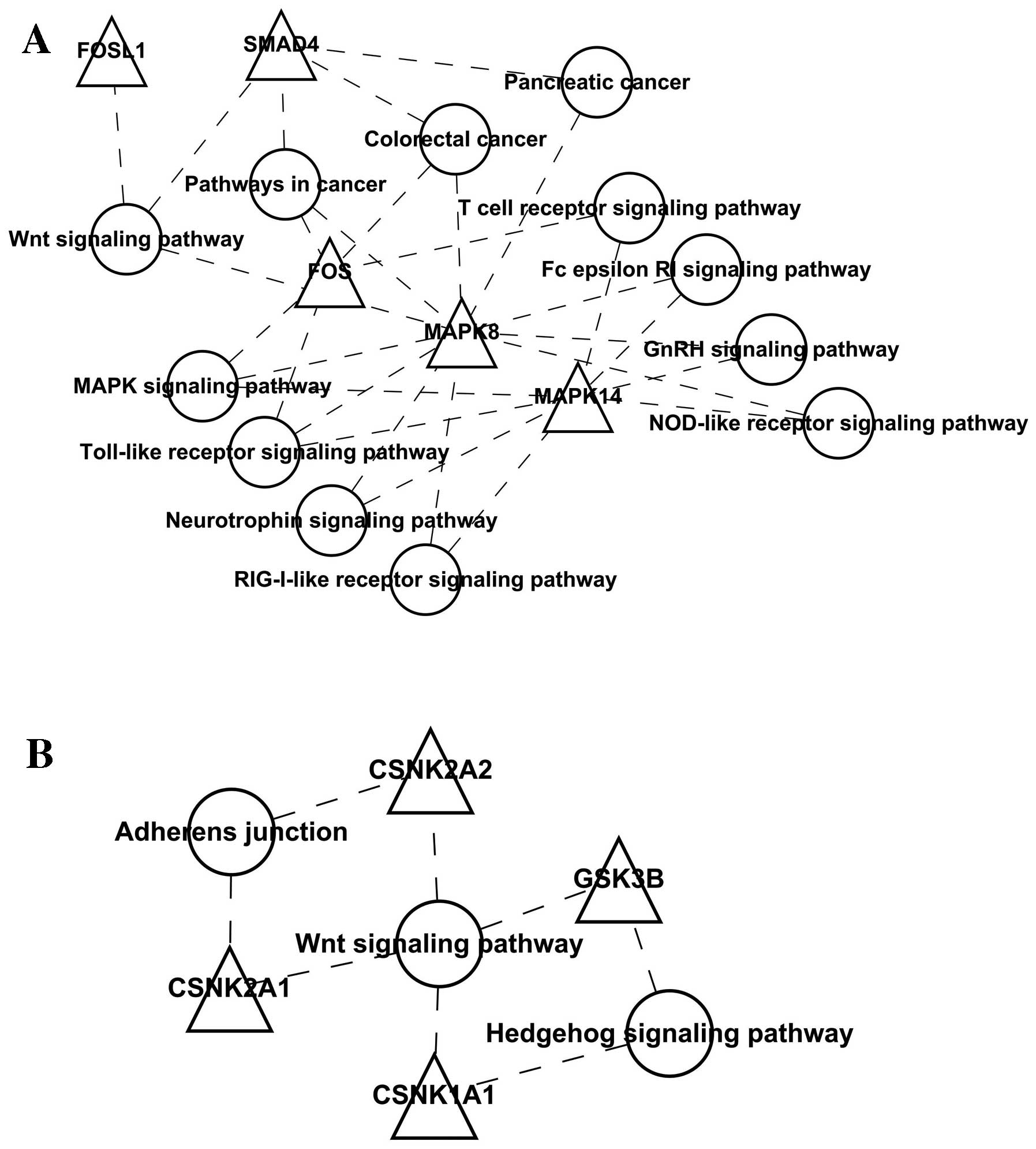
To better illustrate the functions of the
differentially expressed genes and their interactions, we
constructed a network composed of the nodes directly connecting the
differentially expressed genes (Fig.
3); we found that the differentially expressed genes had no
direct interaction, but an interaction network may be constructed
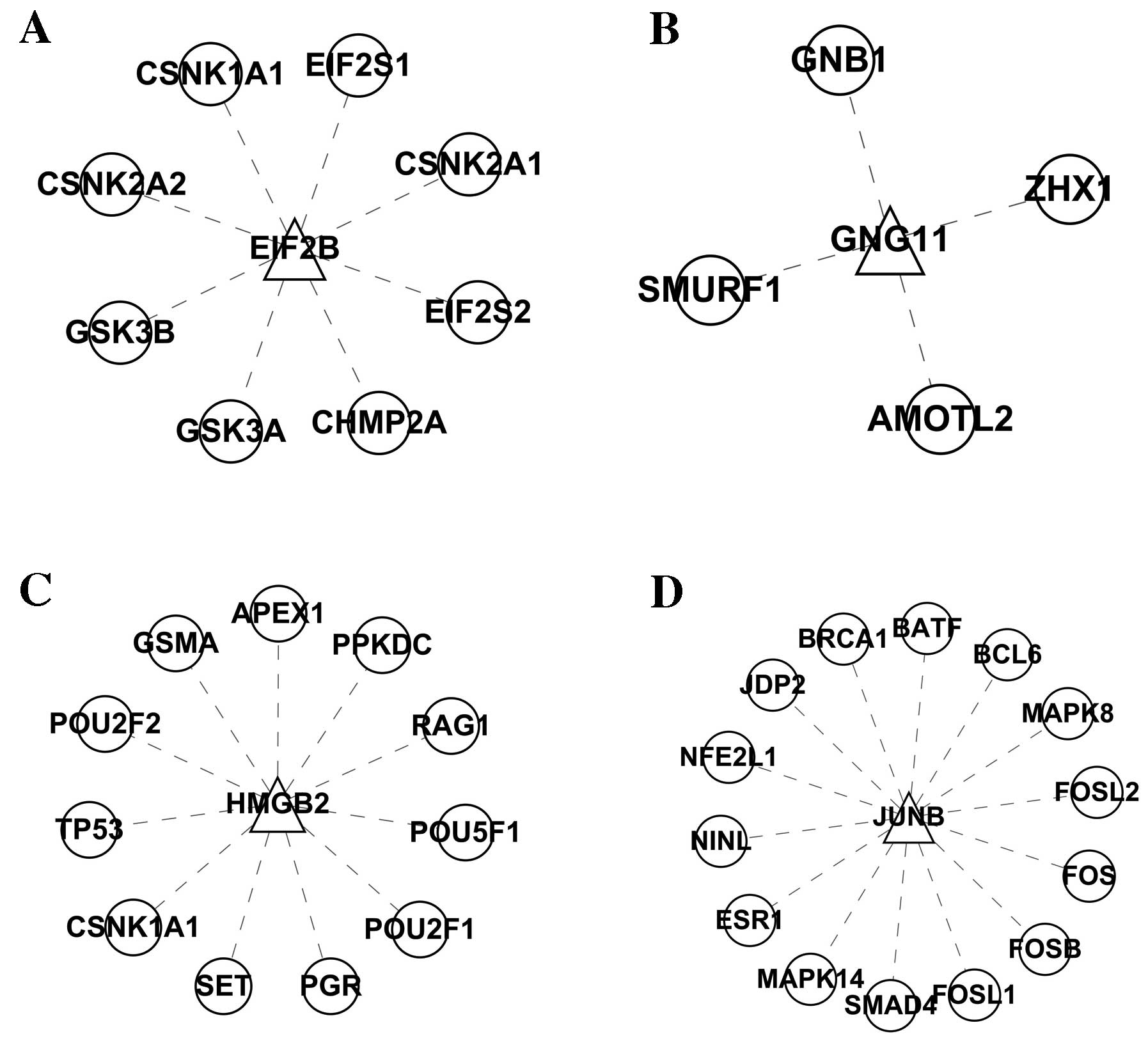
by using the expanded nodes. EIF2B, JUNB, GNG11 and HMGB2 and their
interacting genes were selected, and the visualization graph is
shown in Fig. 4. The KEGG
functional enrichment analysis for each gene cluster did not show
enrichment for GNG11 and HMGB2, but it did show enrichment for JUNB
and EIF2B, as shown in Figs. 5 and
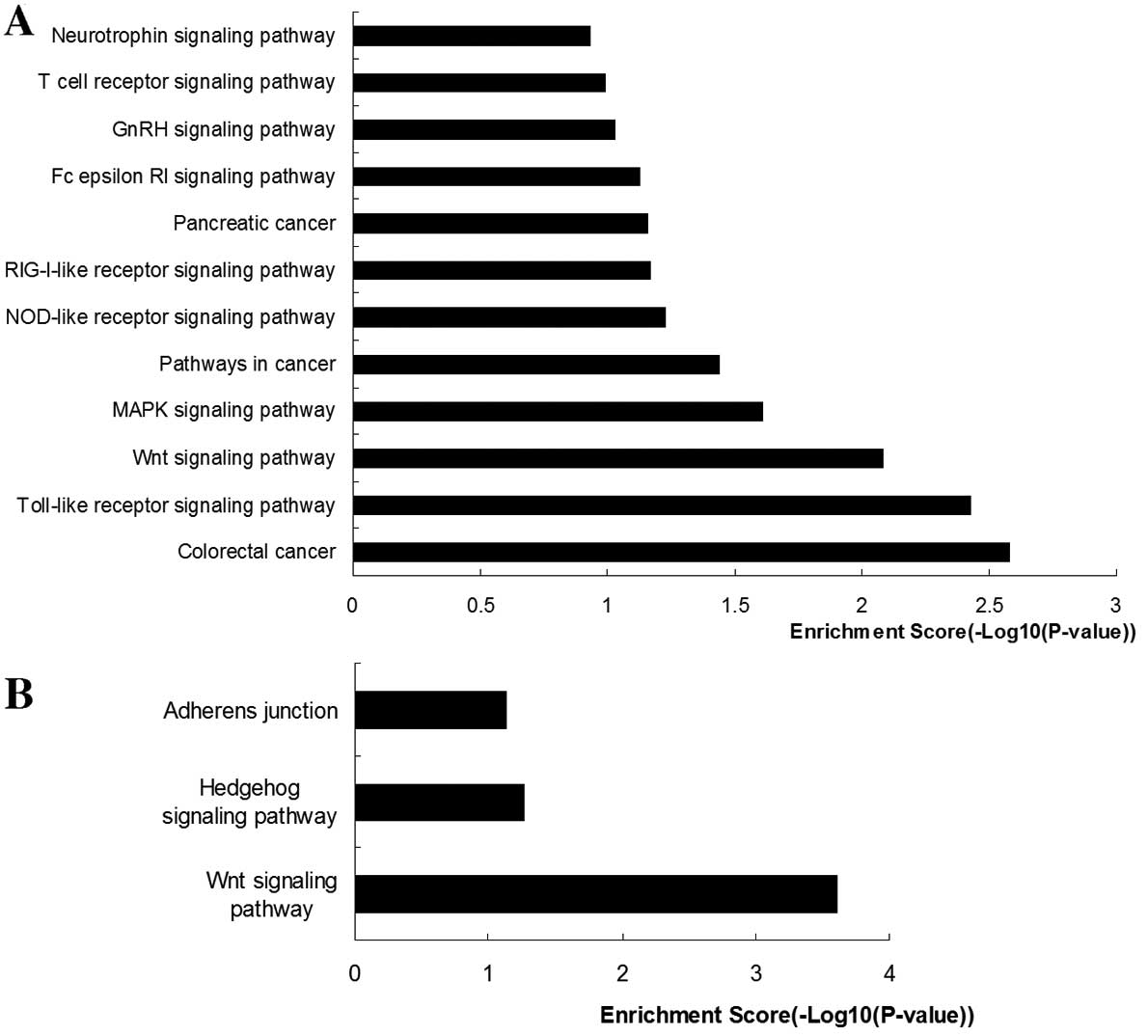
6. The functions of the JUNB
transcription factor were mainly enriched for the ‘colorectal
cancer pathway’, ‘pathways in cancer’, ‘pancreatic cancer pathway’,
‘Toll-like receptor signaling pathway’, ‘Wnt signaling pathway’,
‘MAPK signaling pathway’, ‘NOD-like receptor signaling pathway’,
‘RIG-I-like receptor signaling pathway’, ‘Fc epsilon RI signaling
pathway’, ‘GnRH signaling pathway’, ‘T cell receptor signaling
pathway’ and ‘neurotrophin signaling pathway’. The functions of the
EIF2B-related gene cluster were mainly enriched in ‘Wnt signaling
pathway’ and ‘Hedgehog signaling pathway’.
Results for the screening for small
molecules regulating transcription factors
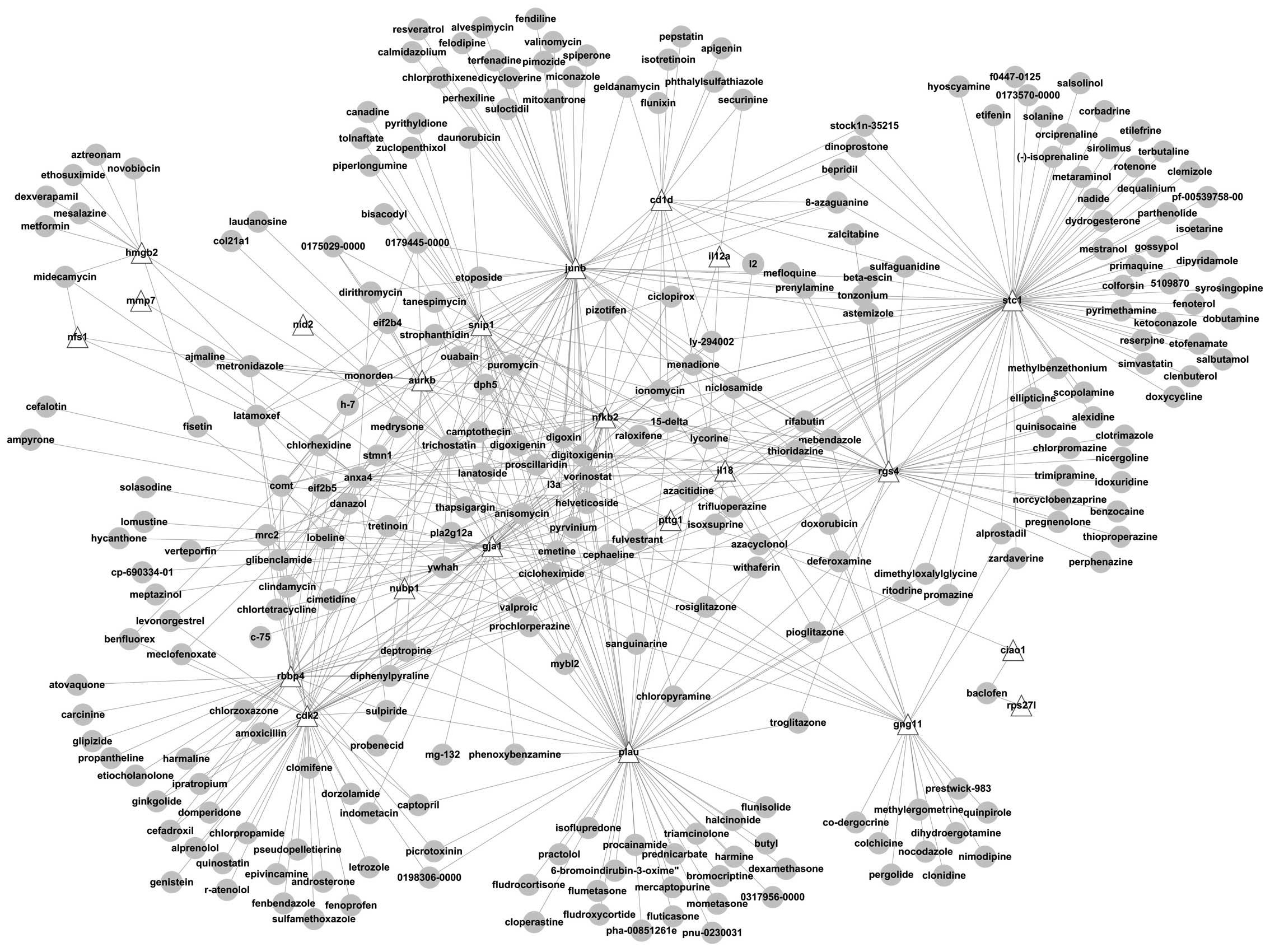
Using the 40 differentially expressed genes, a
comparison (map) was generated for the genes and the differentially
expressed genes interacting with the small molecules and the
correlations between each gene and the small molecules were
obtained (Fig. 7). The correlations
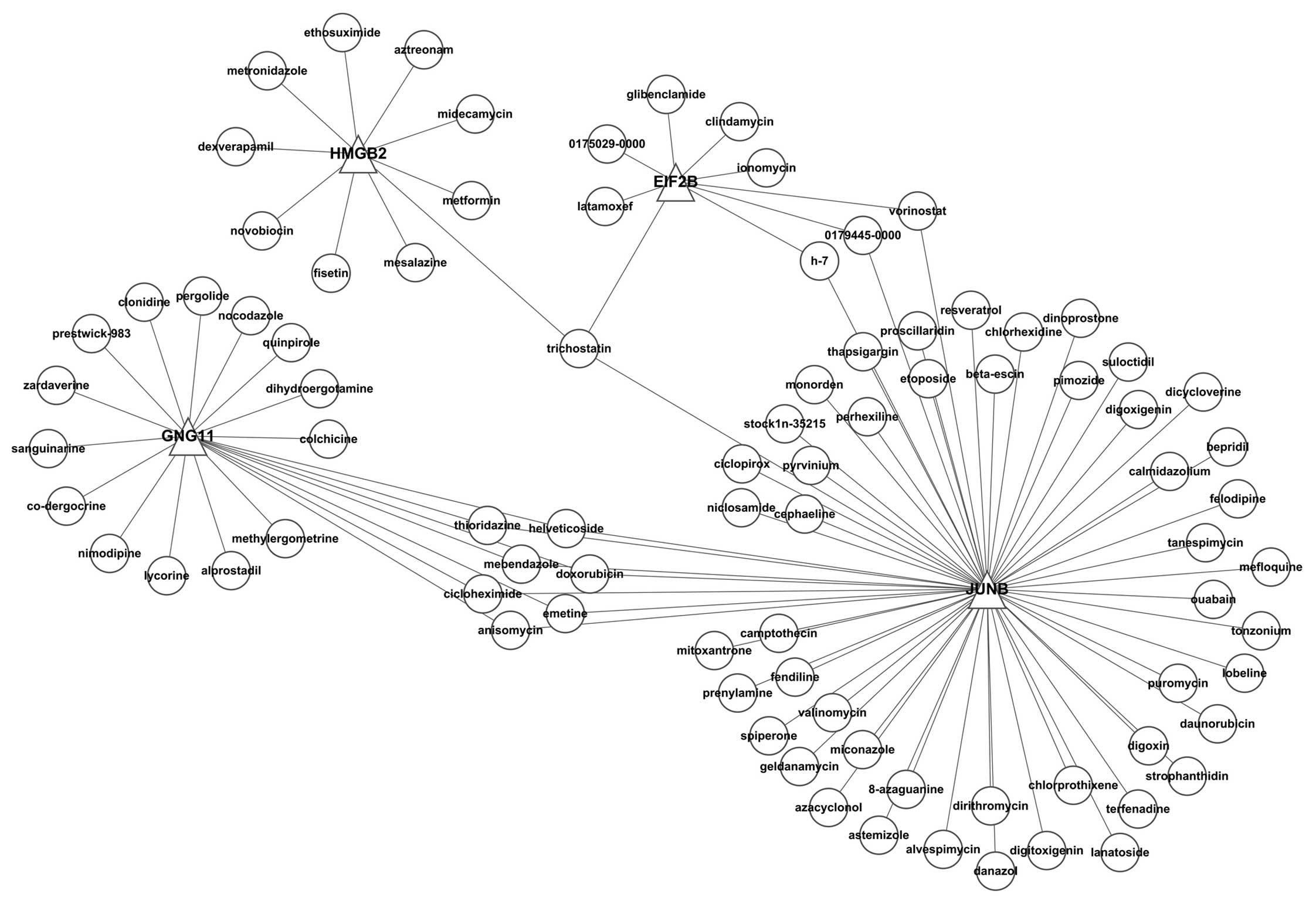
between the transcription factors and the small molecule are shown
in Fig. 8.
After the upregulated and downregulated genes for
the disease were used to make the probe set under the HG-U133A
platform, the CMap database was used to search for disease-related
small molecules. We used the small molecules showing the highest
correlation between small molecule treatments and transcription
factors (the P-value is the smallest) as the examples. These small
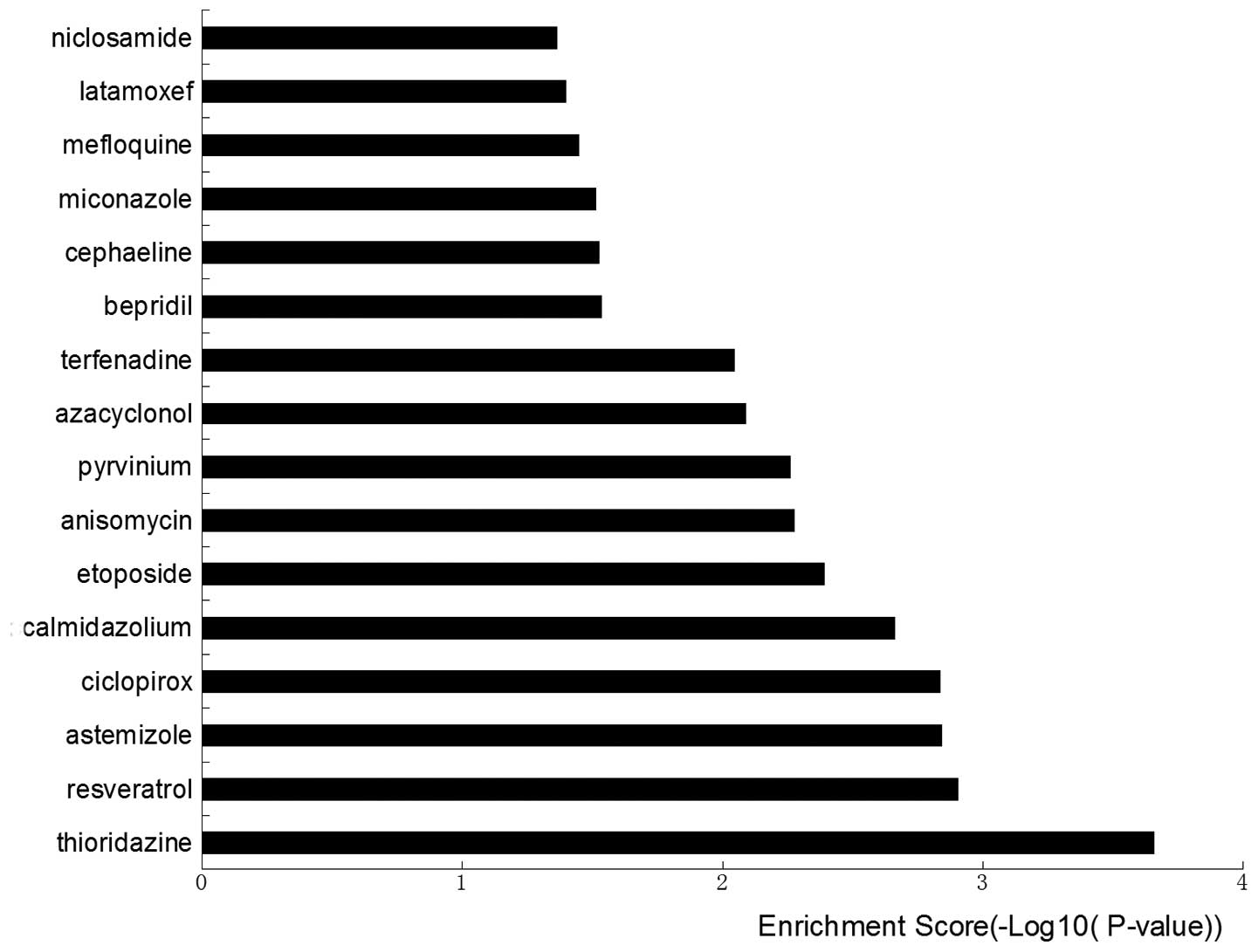
molecules are listed in Fig. 9.
Thioridazine, resveratrol, astemizole, ciclopirox, calmidazolium,
etoposide, anisomycin, pyrvinium, azacyclonol and terfenadine were
negatively correlated, which indicates that these small molecules
may be useful for the treatment of disease.
Discussion
The TME is composed of a series of resident cells,
such as fibroblasts and migrating cells, such as hematopoietic
cells. Macrophages are an important element in this complex
environment. Monocytes produced by the bone marrow enter the
bloodstream and select different tissues for nesting under the
effects of corresponding factors. Furthermore, these macrophages
display different functions under the effects of the
microenvironment after they enter the tissues. For example,
alveolar macrophages express lipoxidase at high levels; peritoneal
macrophages and hepatic Kupffer cells have relatively potent
phagocytotic functions and play antitumor roles (5); and animal tests have shown that
knockout of Kupffer cells can improve the metastatic rate of tumors
(6). When monocytes migrate to
tumor tissues, they differentiate into TAMs in the TME. TAMs may
have dual functions in tumor tissues and act as a ‘double-edged
sword’ (7). Macrophages have
plasticity and multi-directional differentiation capability
(8) and may have different
activation types and play different roles. TAMs can be divided into
2 types of macrophages: M1 and M2 types. Both of these 2 types of
macrophages can be found in tumors; however, the macrophages in
tumors are mainly M1 type in the early stages of tumorigenesis and
progression, and these macrophages play roles in immune
surveillance and antitumor processes. In contrast, the macrophages
in advanced-stage tumors are mainly M2 type, which function in
promoting tumor growth, invasion and metastasis.
Previous subjects of investigations have mainly been
TAMs and their secreted cytokines. Studies on tumor cells have
mainly focused on their growth, metastasis and vascularization,
while studies concerning the changes in gene expression and other
aspects in tumor cells under the effects of macrophages are scarce.
The present study identified 40 differentially expressed genes
after microarray data analysis and a comprehensive bioinformatic
analysis of these genes.
Through the functional enrichment analysis of the
differentially expressed genes, the present study found that among
the top 15 GO terms, 9 were related to ‘stress’ and ‘response’,
indicating that tumor cells were stimulated by various factors that
exist in the macrophage-conditioned medium and underwent stress
responses. Further investigation is warranted to determine whether
these responses are beneficial or detrimental.
The analysis showed that 5 of the differentially
expressed genes were related to lung cancer, 2 genes were related
to breast cancer, and 1 gene was related to endometrial carcinoma,
indicating that these genes have cancer-promoting functions.
After the construction and analysis of a
visualization network, we found that 6 differentially expressed
genes were related to the ‘cell cycle’, 4 genes were related to
‘cell proliferation’, 3 genes were related to ‘DNA replication’, 7
genes were related to ‘intracellular signaling cascades’ and 3
genes were related to ‘blood vessel morphogenesis’. All of these GO
terms were related to tumorigenesis and progression, indicating
that the tumor cells underwent changes beneficial for the
progression of tumors under the effects of the macrophage-
conditioned medium.
Many studies have reported on the functions of TAMs
in promoting tumor growth, and several studies have shown that TAMs
can express several types of cytokines, such as EGF, PDGF, TGF-β1,
HGF, EGFR and bFGF, that stimulate the proliferation and survival
of tumor cells (9,10). It has been confirmed by in
vitro co-incubation of tumor cells and macrophages that the
substances secreted by macrophages can stimulate the proliferation
of tumor cells (11). In
vivo tests have shown, through the depletion of macrophages,
that TAMs are essential for the growth of different types of tumors
(12). GO terms such as ‘cell
cycle’, ‘cell proliferation’ and ‘DNA replication’ identified in
the present study also support this opinion.
Vascularization is an important event during tumor
growth and metastasis. White et al (13) found that the medium for
co-incubation of human peripheral blood monocytes and A549 cells
can increase the chemotaxis of vascular endothelial cells. Lin
et al (14) found in their
studies of transgenic Csfop/Csfop animals that TAMs were involved
in the metastasis of tumors and were ‘the switch for
vascularization’. In a PyMT-induced breast cancer model in mice,
the knockout of macrophages was found to significantly decrease the
pulmonary metastasis of tumors, indicating that TAMs are important
for establishing metastatic foci in tumors (15).
It was found that 6 differentially expressed genes
were related to the ‘extracellular matrix’. In vitro tests
have shown that the co-incubation of macrophages and tumor cells
can improve the TNF-α and MMP (matrix metalloproteinase)-dependent
invasive characteristics of tumor cells (16). MMPs are a group of zinc-dependent
matrix degrading enzymes, and MMP expression can damage tissue
structures and the basilar membrane, which is beneficial for the
growth, metastasis and spreading of tumor cells.
The above analysis demonstrated that a number of
genes in lung cancer cells under the functions of macrophages
underwent changes in their expression levels; consequently the
incidence and progression of tumors were promoted. To carry out
this analysis, we bioinformatically screened out 5 differentially
expressed transcription factors: EIF2B4, EIF2B5, JUNB, GNG11 and
HMGB2. Furthermore, the GO annotations for these transcription
factors were all related to ‘stress’ and ‘response’, indicating
that these factors may be important regulatory factors for the
changes in tumor cells and they may be potential targets for
regulating the growth of tumor cells.
Transcription factors are regulatory factors
required for gene expression in all organisms. They are considered
to have switching functions and can trigger gene expression by
binding to DNA or silencing genes by not binding to DNA. Data
mining showed that JUNB and EIF2B were differentially expressed in
tumors.
JUNB is a negative growth factor for cell
proliferation. In terms of cell cycle regulation, JUNB can promote
the growth of osteoblasts and chondroblasts by directly activating
the transcription of cyclin A (17). When JUNB is depleted, anoxia-induced
VEGF expression is inhibited. Studies in teratomas have also shown
that the neovascular rates and degrees are both significantly
decreased (18), which indicates
that JUNB molecules may be new targets for inhibiting
vascularization in tumors. It has been shown that the depletion of
JUNB may lead to the upregulation of RAE-ɛ on the surface of target
cells, which would activate the killing functions of NK cells on
target cells (19). This result
indicates that the high expression level of JUNB may function in
the immunological escape of tumor cells during incidence and tumor
progression.
Eukaryotic initiation factors (EIFs) are a group of
proteins that play important roles in the initiation of translation
in eukaryotic cells. EIF2B is a large complex composed of 5
subunits, and many human diseases have been shown to be directly or
indirectly related to EIF2B. In regards to tumors, the functions of
EIF2B are related to the GSK-3 protein. Many models for metastasis
and the incidence of cancer have shown that the intracellular
signaling pathways regulating EIF2B are different from those in
normal cells.
To illustrate the functions of these transcription
factors, we carried out bioinformatic analysis. Generally, a
transcription factor can regulate many genes, and a gene may be
regulated by many transcription factors, thus creating complex
regulatory networks. We constructed a visualization network for
transcription factors and their regulatory genes to investigate the
functions of the gene cluster regulated by the transcription factor
and further illustrate the functions of these transcription
factors.
In the KEGG functional enrichment analysis for the
regulatory gene assemblies of the transcription factors, the gene
assemblies regulated by GNG11 and HMGB2 did not show functional
enrichment, while the gene assemblies regulated by EIF2B and JUNB
obtained enrichment results.
The ‘Wnt signaling pathway’ showed enrichments in
the two gene assemblies. The ‘Wnt signaling pathway’ is a
well-known carcinogenic signaling pathway. Wnt protein, encoded by
the Wnt gene, can trigger the intracellular signaling transduction
pathway, which is involved in cell proliferation, differentiation
and migration. It was found that a number of genes in the
Wnt/β-catenin signaling pathway, such as MMP7, CIM4, VEGF, and
E-cadherin, are related to invasion and metastasis (20–22).
Previous studies have confirmed that over-activity of Wnt signaling
may lead to the hyperplasia of stem cells and malignant
transformation into tumor stem cells (23,24).
It has been reported that the ‘Wnt signaling pathway’ can be used
in the research and development of antitumor drugs and as a
therapeutic target for antitumor therapy. The enrichment of the
‘Wnt signaling pathway’ in the gene clusters regulated by EIF2B and
JUNB indicates that the cells underwent changes beneficial for
tumor growth under the functions of these 2 transcription
factors.
The ‘MAPK signaling pathway’ was also enriched among
the gene assemblies regulated by JUNB. The ‘MAPK signaling pathway’
is the common pathway for extracellular signals to induce nuclear
reactions (DNA transduction, cell proliferation, apoptosis). The
MAPK family is involved in cell growth, development, division,
synchronization of intercellular functions and other physiological
processes closely related to tumors. The intensive studies on
tumors in recent years have shown that the signaling transduction
pathway does not function independently, and a large amount of
crosstalk can be found among the signaling pathways. Many results
have shown that substantial crosstalk can be found during formation
and development of tumors between the ‘Wnt signaling pathway’ and
the ‘MAPK signaling pathway’. When the ‘Wnt signaling pathway’ or
the ‘MAPK signaling pathway’ becomes disordered, abnormal embryonic
development, defects or even early premature death may occur, and
this may lead to loss of control in cell proliferation and
differentiation, and further induce tumors (25–27).
The ‘Toll-like receptor (TLR) signaling pathway’,
‘NOD-like receptor (NLR) signaling pathway’ and ‘RIG-I-like
receptor (RLR) signaling pathway’ were also found to be enriched in
the JUNB gene cluster. These signaling pathways are all involved in
pathways expressing various types of pattern recognition receptors
(PRRs). Recently, many studies have confirmed that glioma, breast,
prostate, lung, ovarian and esophageal cancer cells are all related
to the ‘Toll-like receptor signaling pathway’ (28–31).
These studies used microorganism-derived TLR ligand to indicate
that the TLR expressed in the tumor cells had functional activities
(32). A recent study revealed that
several types of endogenous ligands of TLR exist in the TME
(33) and these endogenous ligands
affect the progression of tumors through the ‘Toll-like receptor
signaling pathway’. Ikebe et al (34) found that lipopolysaccharide can
promote the invasion and progression of pancreatic cancer through
the TLR4 signaling pathway. Other pathways, such as the ‘Fc ɛ RI
signaling pathway’ and the ‘T cell receptor signaling pathway’ also
play important roles in the immunological features of tumors
(35–37).
The functional enrichment analysis showed that the
gene assemblies regulated by JUNB enriched many tumor-related
functions, such as ‘colorectal cancer’, ‘pathways in cancer’ and
‘pancreatic cancer’, indicating that JUNB plays important roles
during the malignant progression of tumor cells under the
stimulation of macrophages. These results suggest that extensive
research must be conducted to investigate JUNB as a target for
blocking this vicious cycle.
Various genes have been correlated with previously
known drugs through biological network technique and network
pharmacology. These methods can be utilized to explore non-patent
‘targeting’ drugs and realize ‘new utilization for old drugs’, thus
saving research funds and significantly shortening the cycles for
research and development. For example, König et al (38) carried out an RNA interference
screening based on the whole genome. They utilized an integrated
systematic method and carried out analysis of gene subsets. The
results showed that 10 genes were related to the replication of the
influenza virus, and thus it was deduced that vATPase, CAMK2B and
other small molecule inhibitors may antagonize virus replication
after experimental confirmation. Chen et al (39) identified the inhibitor for the heat
shock protein HSP90, NVP-AUY922, using this method, and this
inhibitor is currently used to treat cholangiocarcinoma. Wei et
al (40) identified the mTOR
inhibitor, rapamycin, using this method and it is used for the
treatment of acute lymphocytic leukemia. The present study screened
thioridazine, resveratrol, astemizole, ciclopirox, calmidazolium,
etoposide, anisomycin, pyrvinium, azacyclonol and terfenadine based
on their interactions with transcription factors. These
interactions may inhibit the progression of pulmonary cancer cells
under the effect of macrophage-conditioned medium by inhibiting
JUNB and other transcription factors. The results of the present
study may provide new clues for further antitumor therapy and
research.
Acknowledgements
This research was supported by the Research
Fundation of the Education Department of Heilongjiang, China (no.
12531230).
References
|
1
|
Mantovani A, Bottazzi B, Colotta F,
Sozzani S and Ruco L: The origin and function of tumor-associated
macrophages. Immunol Today. 13:265–270. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hildenbrand R and Schaaf A: The
urokinase-system in tumor tissue stroma of the breast and breast
cancer cell invasion. Int J Oncol. 34:15–23. 2009.PubMed/NCBI
|
|
3
|
Lewis CE and Pollard JW: Distinct role of
macrophages in different tumor microenvironments. Cancer Res.
66:605–612. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gentleman RC, Carey VJ, Bates DM, et al:
Bioconductor: open software development for computational biology
and bioinformatics. Genome Biol. 5:R802004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Purohit V, Rapaka R, Kwon OS and Song BJ:
Roles of alcohol and tobacco exposure in the development of
hepatocellular carcinoma. Life Sci. 92:3–9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heuff G, Oldenburg HS, Boutkan H, et al:
Enhanced tumor growth in the rat liver after selective elimination
of Kupffer cells. Cancer Immunol Immunother. 37:125–130. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen JJ, Lin YC, Yao PL, et al:
Tumor-associated macrophages: the double-edged sword in cancer
progression. J Clin Oncol. 23:953–964. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goetze K, Walenta S, Ksiazkiewicz M,
Kunz-Schughart LA and Mueller-Klieser W: Lactate enhances motility
of tumor cells and inhibits monocyte migration and cytokine
release. Int J Oncol. 39:453–463. 2011.PubMed/NCBI
|
|
9
|
De Palma M and Lewis CE: Macrophage
regulation of tumor responses to anticancer therapies. Cancer Cell.
23:277–286. 2013.PubMed/NCBI
|
|
10
|
Horton LW, Yu Y, Zaja-Milatovic S,
Strieter RM and Richmond A: Opposing roles of murine duffy antigen
receptor for chemokine and murine CXC chemokine receptor-2
receptors in murine melanoma tumor growth. Cancer Res.
67:9791–9799. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Smith HA and Kang Y: The
metastasis-promoting roles of tumor-associated immune cells. J Mol
Med. 91:411–429. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sawant A, Hensel JA, Chanda D, et al:
Depletion of plasmacytoid dendritic cells inhibits tumor growth and
prevents bone metastasis of breast cancer cells. J Immunol.
189:4258–4265. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
White ES, Strom SR, Wys NL and Arenberg
DA: Non-small cell lung cancer cells induce monocytes to increase
expression of angiogenic activity. J Immunol. 166:7549–7555. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin EY, Li JF, Gnatovskiy L, et al:
Macrophages regulate the angiogenic switch in a mouse model of
breast cancer. Cancer Res. 66:11238–11246. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin EY, Nguyen AV, Russell RG and Pollard
JW: Colony-stimulating factor 1 promotes progression of mammary
tumors to malignancy. J Exp Med. 193:727–740. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hagemann T, Robinson SC, Schulz M, et al:
Enhanced invasiveness of breast cancer cell lines upon
co-cultivation with macrophages is due to TNF-α dependent
up-regulation of matrix metalloproteases. Carcinogenesis.
25:1543–1549. 2004.PubMed/NCBI
|
|
17
|
Andrecht S, Kolbus A, Hartenstein B, Angel
P and Schorpp-Kistner M: Cell cycle promoting activity of JunB
through cyclin A activation. J Biol Chem. 277:35961–35968. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schmidt D, Textor B, Pein OT, et al:
Critical role for NF-κB-induced JunB in VEGF regulation and tumor
angiogenesis. EMBO J. 26:710–719. 2007.
|
|
19
|
Nausch N, Florin L, Hartenstein B, et al:
Cutting edge: the AP-1 subunit JunB determines NK cell-mediated
target cell killing by regulation of the NKG2D-ligand RAE-1ɛ. J
Immunol. 176:7–11. 2006.PubMed/NCBI
|
|
20
|
Hussaini IM, Trotter C, Zhao Y,
Abdel-Fattah R, et al: Matrix metalloproteinase-9 is differentially
expressed in nonfunctioning invasive and noninvasive pituitary
adenomas and increases invasion in human pituitary adenoma cell
line. Am J Pathol. 170:356–365. 2007. View Article : Google Scholar
|
|
21
|
Pulukuri SM and Rao JS: Matrix
metalloproteinase-1 promotes prostate tumor growth and metastasis.
Int J Oncol. 32:757–765. 2008.PubMed/NCBI
|
|
22
|
Xu D, McKee CM, Cao Y, et al: Matrix
metalloproteinase-9 regulates tumor cell invasion through cleavage
of protease nexin-1. Cancer Res. 70:6988–6998. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kawaguchi-Ihara N, Murohashi I, Nara N and
Tohda S: Promotion of the self-renewal capacity of human acute
leukemia cells by Wnt3A. Anticancer Res. 28:2701–2704.
2008.PubMed/NCBI
|
|
24
|
Hu J, Dong A, Fernandez-Ruiz V, et al:
Blockade of Wnt signaling inhibits angiogenesis and tumor growth in
hepatocellular carcinoma. Cancer Res. 69:6951–6959. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim D, Rath O, Kolch W and Cho KH: A
hidden oncogenic positive feedback loop caused by crosstalk between
Wnt and ERK pathways. Oncogene. 26:4571–4579. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jeong AY, Lee MY, Lee SH, Park JH and Han
HJ: PPARδ agonist-mediated ROS stimulates mouse embryonic stem cell
proliferation through cooperation of p38 MAPK and Wnt/β-catenin.
Cell Cycle. 8:611–619. 2009.
|
|
27
|
Gazitt Y, Kolaparthi V, Moncada K, Thomas
C and Freeman J: Targeted therapy of human osteosarcoma with 17AAG
or rapamycin: Characterization of induced apoptosis and inhibition
of mTOR and Akt/MAPK/Wnt pathways. Int J Oncol. 34:551–561.
2009.PubMed/NCBI
|
|
28
|
Samara KD, Antoniou KM, Karagiannis K, et
al: Expression profiles of Toll-like receptors in non-small cell
lung cancer and idiopathic pulmonary fibrosis. Int J Oncol.
40:1397–1404. 2012.PubMed/NCBI
|
|
29
|
Tanaka J, Sugimoto K, Shiraki K, et al:
Functional cell surface expression of toll-like receptor 9 promotes
cell proliferation and survival in human hepatocellular carcinomas.
Int J Oncol. 37:805–814. 2010.PubMed/NCBI
|
|
30
|
Takala H, Kauppila JH, Soini Y, et al:
Toll-like receptor 9 is a novel biomarker for esophageal squamous
cell dysplasia and squamous cell carcinoma progression. J Innate
Immun. 3:631–638. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qiu J, Shao S, Yang G, Shen Z and Zhang Y:
Association of Toll like receptor 9 expression with lymph node
metastasis in human breast cancer. Neoplasma. 58:251–255. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lotze MT, Zeh HJ, Rubartelli A, et al: The
grateful dead: damage-associated molecular pattern molecules and
reduction/oxidation regulate immunity. Immunol Rev. 220:60–81.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sato Y, Goto Y, Narita N and Hoon DS:
Cancer cells expressing Toll-like receptors and the tumor
microenvironment. Cancer Microenviron. 2(Suppl 1): 205–214. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ikebe M, Kitaura Y, Nakamura M, et al:
Lipopolysaccharide (LPS) increases the invasive ability of
pancreatic cancer cells through the TLR4/MyD88 signaling pathway. J
Surg Oncol. 100:725–731. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Agha-Mohammadi S and Lotze MT:
Immunomodulation of cancer: potential use of selectively
replicating agents. J Clin Invest. 105:1173–1176. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tsai JP, Chen HW, Cheng ML, et al:
Analysis of host versus tumor interaction in cancer patients:
opposing role of transforming growth factor-β1 and interleukin-6 in
the development of in situ tumor immunity. Immunobiology.
210:661–671. 2005.PubMed/NCBI
|
|
37
|
Barbieri G, Rimini E and Costa MA: Effects
of human leukocyte antigen (HLA)-DR engagement on melanoma cells.
Int J Oncol. 38:1589–1595. 2011.PubMed/NCBI
|
|
38
|
König R, Stertz S, Zhou Y, et al: Human
host factors required for influenza virus replication. Nature.
463:813–817. 2010.PubMed/NCBI
|
|
39
|
Chen MH, Lin KJ, Yang WL, et al: Gene
expression-based chemical genomics identifies heat-shock protein 90
inhibitors as potential therapeutic drugs in cholangiocarcinoma.
Cancer. 119:293–303. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wei G, Twomey D, Lamb J, et al: Gene
expression-based chemical genomics identifies rapamycin as a
modulator of MCL1 and glucocorticoid resistance. Cancer Cell.
10:331–342. 2006. View Article : Google Scholar : PubMed/NCBI
|