Hepatocellular carcinoma (HCC) is the third leading
cause of cancer-related mortality worldwide, owing to a very high
mortality to incidence ratio of 0.93 (1). Surgery, including liver
transplantation, remains the only curative modality for HCC.
However, despite resection with curative intent, the clinical
course is variable and the long term prognosis is poor with
reported 5-year survival rates ranging from 17 to 53% (2). There is an ongoing search for
predictive biomarkers of cancer prognosis, where, among others,
protein biomarkers, mRNA expression level, pathological parameters,
clinicopathologic parameters and genomic DNA abnormalities are
surveyed (3–5). Among all the biomarkers of HCC
prognosis, mRNA expression level of some genes is associated with
prognosis of patients with HCC, including tumor-suppressor and
promoter genes, genes involved in growth inhibition and apoptosis,
genes regulating DNA damage, cell cycle regulators, genes
responding to the cell invasion and metastasis, genes responsible
for cell-cell interaction (2).
Among other things, a large number of genes regulating cell cycle
have been proven to be associated with prognosis in HCC patients.
For instance, overexpression of cyclin A, D1, and E has been found
to correlate with the relapse of HCC and are independent predictive
markers for recurrence and prognosis (6,7). In
addition, Singhal et al (2)
reported that p15, p16, p18, p19, p21, p27 and p57, which were all
potent negative cell cycle regulators inhibiting the G1/S
transition, are associated with prognosis in patients with HCC.
E2F is a family of transcription factors that, in
coordination with DP and pocket proteins [retinoblastoma protein
(pRB), p107, p130], regulates the G1/S-phase transition of cell
cycle (8) and its activity is
regulated by the pRB, a tumor-suppressor that is functionally
inactivated in most human tumors (9–11). E2F
transcription factors have been proven to be associated with
prognosis in cancer. For example, high expression of E2F1 and E2F2
was associated with poor prognosis in breast cancer (12,13),
and the expression of E2F4 is associated with poor prognosis in HCC
(14). Although E2F transcription
factor 3 (E2F3), a member of E2F transcription factors, plays
important roles in the development of HCC and other types of
cancer, its role in prognosis in patients with HCC and other types
of cancer has not been reported. In the present study, on the basis
of comprehensive bioinformatics analyses, we illustrated that the
overexpression of E2F3 may be a biomarker for prognosis in patients
with HCC.
The target gene, E2F3, which is a member of the
genes involved in the regulation of cell cycle (KEGG map 04110)
according to the Kyoto Encyclopedia of Genes and Genomes (KEGG)
pathway maps (http://www.genome.jp/kegg/pathway.html) (15) was selected for bioinformatics
analysis.
E2F3 can specifically bind to pRB in a cell
cycle-dependent manner and has a central role in linking cell cycle
proteins, such as cyclin-dependent kinases (CDKs), cyclins and pRB,
to the expression of a variety of genes involved in cell cycle
progression and cellular proliferation (9,11). In
addition, as an oncogene with strong proliferative potential, E2F3
modulates different genes that play a crucial role in DNA
synthesis, transcription and signal transduction, and it is
regularly dysregulated or overexpressed in cancer (20). It has been proven that E2F3 is
involved in many processes during cancer development, such as cell
proliferation, DNA damage, apoptosis and drug resistance. Using
both in vitro and in vivo approaches, Martinez et
al (21) demonstrated that E2F3
functions as a master regulator of the DNA damage response and is
required for DNA damage-induced apoptosis. Amplification and
overexpression of the E2F3 gene in human bladder cancer is
associated with increased tumor stage, grade and proliferation
index and, in prostate cancer, E2F3 overexpression is linked to
tumor aggressiveness. For both bladder and prostate cancer,
overexpression of the E2F3 protein may cooperate with removal of
the E2F inhibitor tumor-suppressor pRB to drive cellular
proliferation. Thus, E2F3 is considered to have a critical role in
modifying cellular proliferation rate in human bladder and prostate
cancer (22). In addition,
downregulation of miR-200b may lead to E2F3 overexpression and in
turn contribute to decreasing the sensitivity of lung
adenocarcinoma cells to docetaxel (23).
Studies on E2F3 with HCC are limited. Only few
studies indicated that E2F3 may play important roles in the
development of liver cancer. miR-195 can block the G1/S
transition by suppressing Rb-E2F signaling through targeting
multiple molecules including E2F3, suggesting that E2F3 plays
important roles in cell cycle control and in the molecular etiology
of HCC (24). The inhibition of
expression of miRNAs miR-34a, miR-127, miR-200b and miR-16a is
involved in the regulation of apoptosis, cell proliferation,
cell-to-cell connection and epithelial-mesenchymal transition,
while the mechanistic link between these alterations in miRNA
expression and the development of HCC was mediated by the
corresponding changes in the levels of E2F3, NOTCH1, BCL6, ZFHX1B
and BCL2 proteins targeted by these miRNAs. These results indicate
that E2F3 may be associated with apoptosis, cell proliferation,
cell-to-cell connection and epithelial-mesenchymal transition in
HCC (25).
E2F3 is involved in many processes and plays key
roles in cancer, while its relationship with HCC is limited and its
associations with prognosis in cancer are rare, with only one study
indicating that E2F3 may be an independent factor predicting
overall survival and cause-specific survival in prostate cancer
(26). Thus, in the present study,
based on the comprehensive bioinformatics analysis, we elucidated
that the overexpression of E2F3 may be associated with prognosis in
patients with HCC.
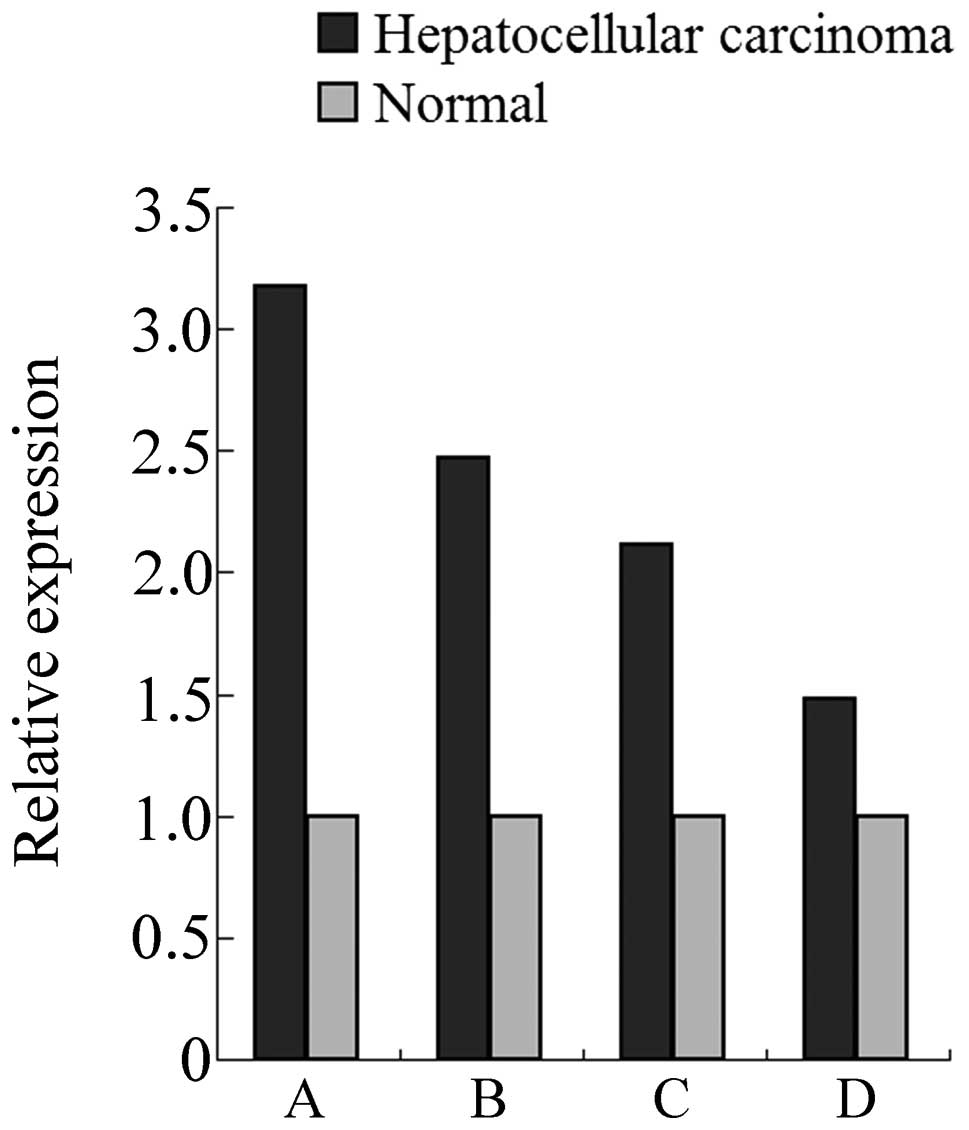
The relative expression of E2F3 in HCC and normal
livers was analyzed based on the array data retrieved from the
Oncomine online database. As shown in Fig. 1, on the basis of 4 independent
microarray data sets which covered 385 cases of HCC and 327 cases
of normal livers, we revealed that E2F3 was upregulated in HCC when
compared with normal controls, with at least 1.5-fold and on
average 2.3-fold changes. This result indicated that E2F3, an
oncogene, may play significant roles in the development of HCC and
its overexpression in tumors may be associated with prognosis.
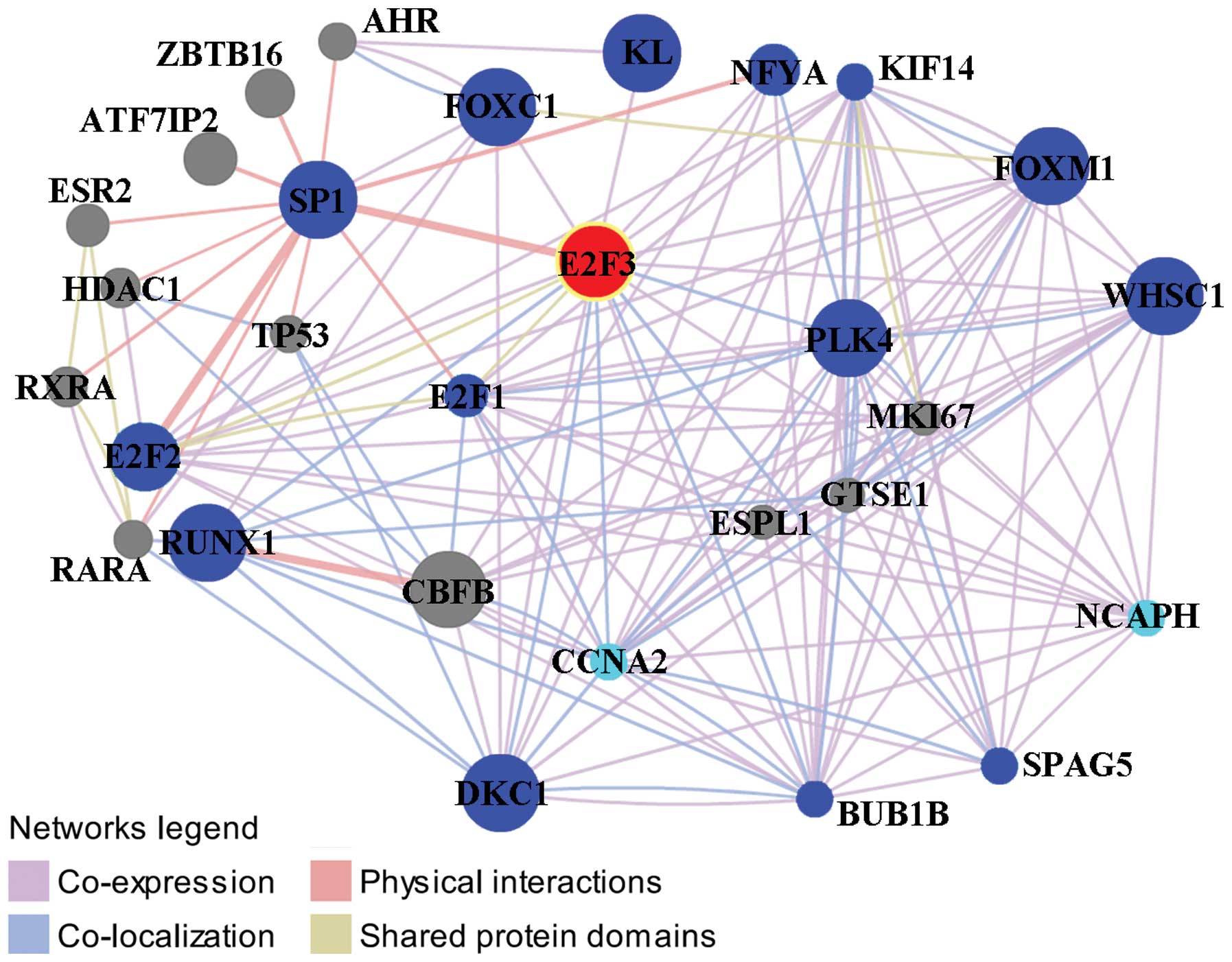
Protein-protein interactions of E2F3 with other
proteins were analyzed using GeneMANIA online tool. As shown in
Fig. 2, E2F3 had direct
interactions with a total of 16 proteins and, among them, E2F3
co-expressed with FOXC1, KL, NFYA, KIF14, FOXM1, WHSC1 and NCAPH,
co-expressed and co-localized with BUB1B and DKC1, co-localized
with PLK4, SPAG5, CCNA2 and RUNX1, co-expressed and shared protein
domains with E2F1 and E2F2 and had strong physical interactions
with SP1. With the exception of the NCAPH and CCNA2, these
proteins/genes are associated with prognosis in patients with HCC
and other types of cancer. It has been proven that high expression
of FOXC1 is associated with poor prognosis in several types of
solid tumors including non-small cell lung cancer (27), pancreatic ductal adenocarcinoma
(28) and basal-like breast cancer
(29). In HCC, patients with
positive FOXC1 expression have shorter overall survival times and
higher recurrence rates than those with negative FOXC1 expression,
indicating that high expression of FOXC1 is an independent,
significant risk factor for recurrence and survival (30). Klotho gene (KL) is identified as a
tumor-suppressor in several tumors, and its downregulation is
closely associated with poor prognosis in several types of tumors
such as gastric (31) and breast
cancer (32). In HCC, a significant
loss of KL mRNA and protein expression was observed in tumors,
which parallels the increased methylation in KL promoter DNA, and
both KL expression and methylation correlated with the poor
prognosis of HCC (33). KIF14 mRNA
and protein expression are respectively increased 5.5- and 4.2-fold
on average in glioma tissues when compared to non-neoplastic brain
tissues. Furthermore, both univariate and multivariate Cox
regression analyses determined that KIF14 overexpression
effectively predicted decreased overall survival in patients with
gliomas (34). In HCC, the
expression of KIF14 is significantly increased and has significant
associations with an unfavorable prognosis (35). Overexpression of FOXM1 can greatly
increase the death hazard [hazard ratio, 1.899; 95% confidence
interval (CI), 1.016–3.551] and thus closely associates with poor
prognosis of non-small cell lung cancer patients through promoting
tumor metastasis (36). Similarly,
FOXM1 expression is significantly associated with cisplatin-based
chemotherapy resistance and poor prognosis in advanced non-small
cell lung cancer patients (37). In
HCC, overexpression of FOXM1, which is regulated by the
TNF-α/reactive oxygen species/HIF-1 pathway (38), is associated with aggressive tumor
features and poor prognosis of patients (39). WHSC1 is also a poor prognostic
factor in cancer. A combined use of microarray technologies and
bioinformatics analyses revealed that a critical-gene model,
comprising FAM53B, KIF21B, WHSC1 and TMPO, predicted survival in
all data sets with follow-up information in multiple myeloma,
indicating that WHSC1 is a significant prognostic factor (40). Regarding BUB1B, unsupervised
clustering analysis strongly discriminated the malignant tumors,
suggesting that the combined expression of BUB1B and PINK1 is the
best predictor of overall survival (P<2×10−6)
(41). DKC1 is an essential
nucleolar protein involved in cell proliferation. DKC1 was shown to
be overexpressed in paraffin sections of 252 HCC cases when
compared with 80 non-cancerous liver tissues at the protein level
and was overexpressed in 80 HCC and 50 non-cancerous tissues at the
transcript level and the multivariate analysis suggested that
overexpression of DKC1 is an independent unfavorable prognosis
factor (hazard risk, 2.912; P=0.007) in HCC (42). Polo-like kinase 4 (PLK4), belonging
to the serine/threonine kinase family, is critical for centriole
replication and cell cycle progression and has been proposed as a
tumor-suppressor in HCC. Decreased expression of PLK4 is present in
72.4% (178/246) of HCC tissues and HCC patients with low PLK4
expression have shorter survival than those with high PLK4
expression (43). Patients with low
E2F1-expressing tumors are associated with favorable outcome
(hazard ratio=4.3; 95% CI=1.8–9.9; P=0.001) in breast cancer
(12), indicating that high
expression of E2F1 is associated with poor prognosis. In addition,
high expression of RUNX1 and SPAG5 are associated with poor
prognosis in acute myeloid leukemia (44), and estrogen receptor positive breast
cancer (45), respectively.
Nuclear factor YA (NFYA), E2F2 and SP1 are known to
be associated with unfavorable prognosis indirectly in cancer. For
example, high ALDH expression is associated with poor prognosis in
uterine endometrial adenocarcinoma, while the ALDH activity is
transcriptionally regulated by ALDH1A1 for which the promoter
region contained CCAAT and octamer binding motifs. Among
CCAAT-recognizing transcription factors, NFYA is involved in
ALDH1A1 transcription (46). This
result suggests that NFYA responds to poor prognosis via ALDH1A1
and ALDH in uterine endometrial adenocarcinoma. Overexpression of
the human epidermal growth factor receptor 2 (HER2) in breast
tumors is associated with poor prognosis, and the high expression
of E2F2, CCND1 and CCND3 can increase the levels of factors
upregulating HER2 (13). Regarding
the relationship of SP1 with cancer, a study revealed that the
promoter hypomethylation upregulates CD147 expression primarily
through increasing SP1 binding and is associated with poor
prognosis in HCC patients (47).
Similarly, SP1 binding site is important for mediating CADM1
promoter activity which is associated with poor prognosis in HCC
(48). These results indicate that
SP1 is closely related to poor prognosis in HCC patients through
mediating certain genes.
Among a total of 16 proteins/genes which had direct
interactions with E2F3, 14 of them were associated with poor
prognosis in several types of cancer, and among those 14
proteins/genes, 8 were associated with poor prognosis in patients
with HCC. These results strongly indicate that E2F3 may be
associated with poor prognosis in patients with HCC.
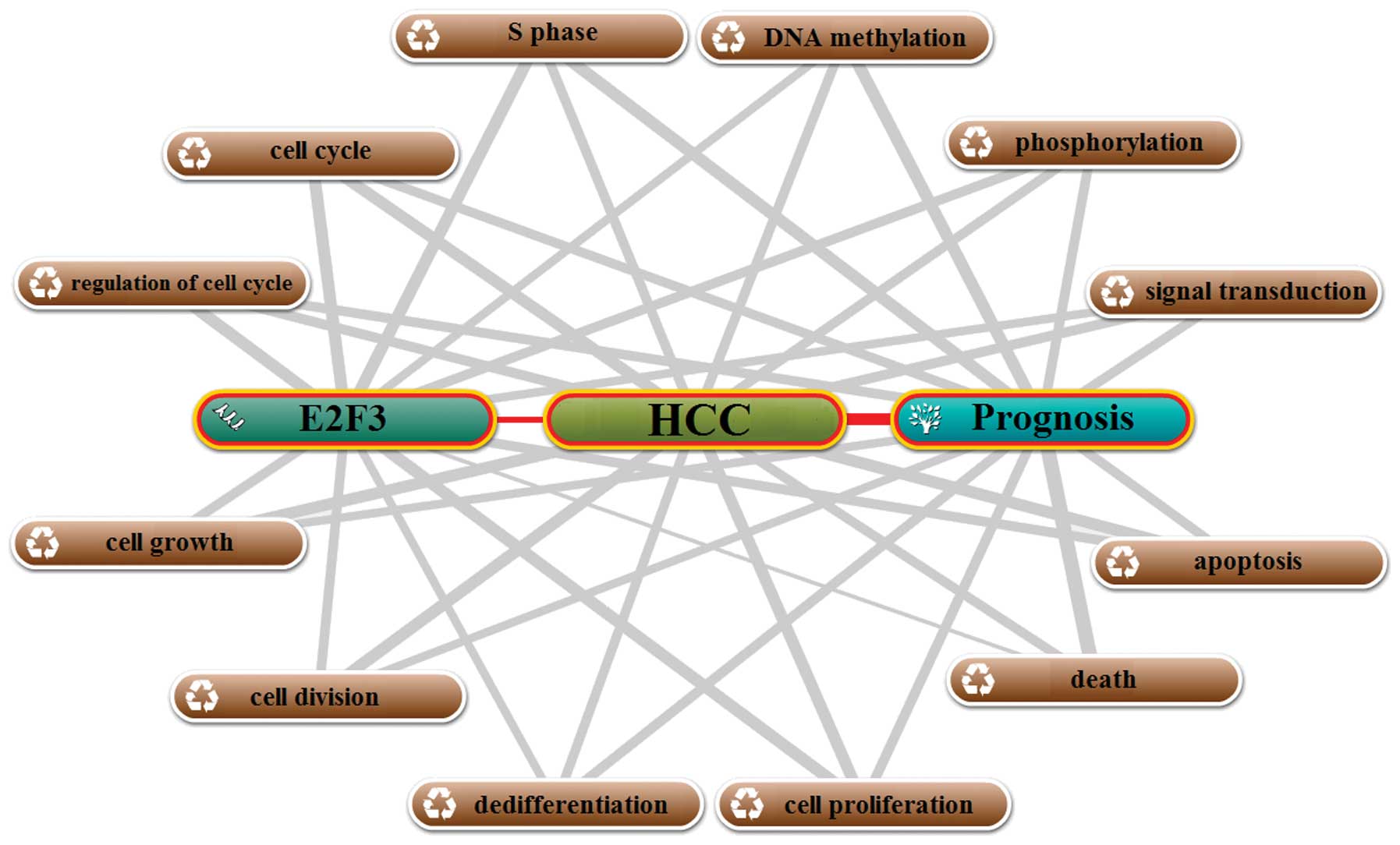
The annotation of biological processes of E2F3 with
HCC and prognosis were generated with the Coremine online tool. As
shown in Fig. 4, a total of 12
biological processes were closely associated with E2F3, HCC and
prognosis at the same time, suggesting that E2F3 may be involved in
the prognosis of HCC patients through these processes. Among the 12
processes, 3, including S phase, cell cycle and regulation of cell
cycle, were cell cycle-related, and 6, including cell growth, cell
division, dedifferentiation, cell proliferation, death and
apoptosis were ‘growth/death’-related. Previous studies proved that
E2F3 contributes to promote the G1/S-phase transition of
cell cycle and has a central role in linking cell cycle proteins to
the expression of a variety of genes involved in cell cycle
progression and cellular proliferation (9,11).
Furthermore, E2F3, an oncogene with strong proliferative potential
(20), plays important roles in
cell proliferation, DNA damage and apoptosis (21,22).
Thus, we conclude that E2F3 may be associated with prognosis in HCC
mainly through cell cycle, ‘growth/death’-related biological
processes.
HCC is a fatal liver malignancy with an
exceptionally high incidence in Asia and Africa. The number of new
cases in America and Europe is rapidly increasing, making HCC a
worldwide health problem (66).
Although the mortality of HCC has significantly decreased with the
development of surgical techniques, a considerable portion develop
tumor recurrence, which in many cases cannot be detected early,
making it the most important factor limiting the long-term survival
of HCC patients (67). Thus, it is
of utmost importance to find sensitive markers for monitoring
postoperative recurrence of HCC and to give adequate treatment for
HCC patients (68). A number of
factors and parameters would be used as biomarkers for prognosis in
patients with HCC; for example, Hao et al (5) revealed that 6 common clinicopathologic
parameters including tumor size, number of tumor nodules, tumor
stage, venous infiltration status and serum α-fetoprotein and total
albumin levels are significantly associated with the overall HCC
survival and disease-free survival. However, most studies focus on
circulating proteins, DNA, microRNAs (miRNAs), cancer cells and
regulatory T cells as serological prognostic biomarkers for
patients with HCC (66) and, among
these, the dys-expression of certain genes as promising prognosis
markers for patients with HCC.
Gene function prediction based on bioinformatics
analysis is a potential, feasible and valuable way for gene
function mining and many large-scale networks of molecular
interactions within the cell have made it possible to go beyond one
dimensional approaches to study protein function in the context of
a network (69). ONCOMINE is a
cancer microarray database and web-based data-mining platform aimed
at facilitating discovery from genome-wide expression analyses.
Differential expression analyses comparing most major types of
cancer with respective normal tissues as well as a variety of
cancer subtypes and clinical-based and pathology-based analyses are
available for exploration. Data can be queried and visualized for a
selected gene across all analyses or for multiple genes in a
selected analysis (16). ONCOMINE
contains 65 gene expression datasets comprising nearly 48 million
gene expression measurements from over 4,700 microarray experiments
by 2004 and the number of array data and genes grew quickly every
year. GeneMANIA is a web-based database and tool for prediction of
gene function on the basis of multiple networks derived from
different genomic or proteomic data/sources. It is fast enough to
predict gene function with great accuracy (17). Coremine Medical is a product of the
PubGene Company which grew out of Pubgene online tool. Pubgene is a
gene/protein database and web-based tool for literature mining. It
carries out automated extraction of experimental and theoretical
biomedical knowledge from publicly available gene and text
databases to create a gene-to-gene co-citation network for millions
of named human genes by automated analysis of titles and abstracts
in over 10 million MEDLINE records (70). Thus, the associations of E2F3 with
prognosis for patients with HCC through these online
databases/tools are potentially reliable.
miRNAs are a class of small (22 bp) endogenous
non-coding RNAs which regulate gene expression mainly by binding to
the 3′-UTR of the target mRNA and leading to mRNA cleavage,
destabilization or translational repression (71,72).
miRNA-mediated post-transcriptional gene regulation is considered a
significant regulator of many cellular processes, both
physiological and pathological (73,74).
Since miRNAs perform their functions through the regulation on
their target genes, it has been well established that miRNAs
represent a class of genes with a great potential for use in
diagnostics, prognosis and therapy (75); therefore, we can predict the gene
function through the functions of miRNAs targeting the gene. In the
present study, miRWalk, which covered 9 miRNA-mRNA interaction
tools, was used to predict the miRNAs which targeted the E2F3.
MiRWalk is a comprehensive database on miRNAs, which collects
predicted and validated miRNA binding sites on all mRNAs,
mitochondrial genes and 10 kb upstream flanking regions of all
known genes of human, mouse and rat. More importantly, the miRWalk
is a potential real-time database, in which the ‘predicted target
module’ is updated every 6 months and ‘validated target module’ is
updated every month (18).
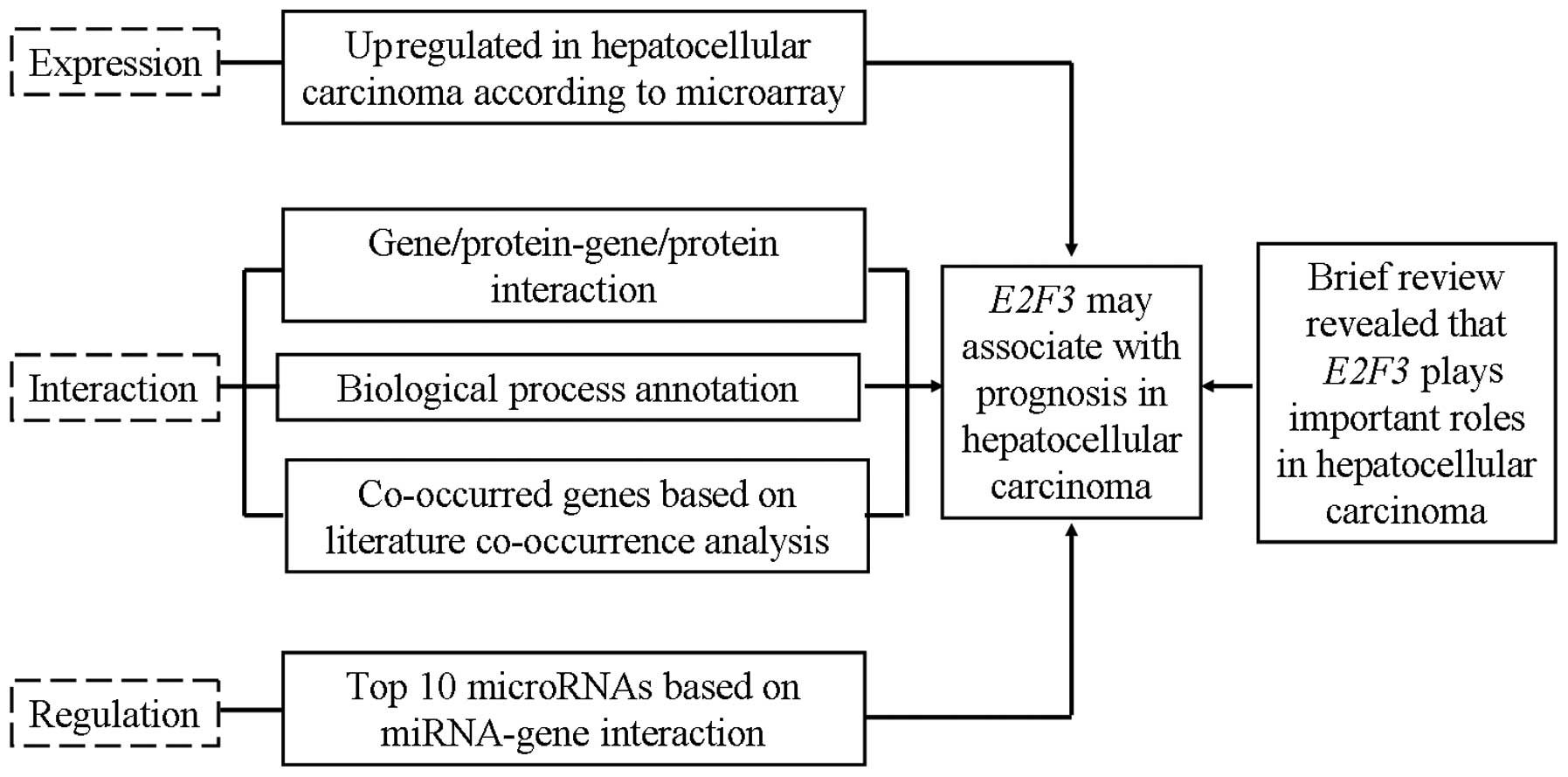
Collectively, on the basis of comprehensive
bioinformatics analyses including microarray data interpretation,
protein-protein interaction, biological processes annotation, gene
co-occurrence and miRNA-mRNA interaction (Fig. 5), we demonstrated that E2F3
interacted with tens of genes/proteins associated with poor
prognosis for patients with HCC and other types of cancer (Fig. 2) and gene co-occurrence indicated
that E2F3 co-occurred with 14 genes which were all associated with
unfavorable prognosis in HCC and other types of cancer (Fig. 3). Further analysis based on
annotation of biological processes suggested that E2F3 is closely
related to 12 biological processes which were closely associated
with HCC and prognosis. In addition, the top 10 miRNAs targeted to
the E2F3 are all associated with poor prognosis for patients with
cancer (Table I). These results
indicated that E2F3, which interacted with a large number of genes,
proteins, miRNAs and biological processes which were all associated
with poor prognosis, may also be associated with prognosis in HCC
and other types of cancer. Together with the notable upregulation
of E2F3 in HCC (Fig. 1), we
illustrated, for the first time, that the overexpression of E2F3 in
HCC may be associated with poor prognosis and it may be an
unfavorable biomarker for prognosis in HCC patients. The present
study provides important information for further investigation of
the prognosis-related functions of E2F3 in HCC.
The research was supported by the National Natural
Science Foundation of China (grant no. 81360448).
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singhal A, Jayaraman M, Dhanasekaran DN
and Kohli V: Molecular and serum markers in hepatocellular
carcinoma: predictive tools for prognosis and recurrence. Crit Rev
Oncol Hematol. 82:116–140. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Midorikawa Y, Makuuchi M, Tang W and
Aburatani H: Microarray-based analysis for hepatocellular
carcinoma: from gene expression profiling to new challenges. World
J Gastroenterol. 13:1487–1492. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chiriva-Internati M, Grizzi F, Wachtel MS,
Jenkins M, Ferrari R, Cobos E and Frezza EE: Biological treatment
for liver tumor and new potential biomarkers. Dig Dis Sci.
53:836–843. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hao K, Luk JM, Lee NP, Mao M, Zhang C,
Ferguson MD, Lamb J, Dai H, Ng IO, Sham PC and Poon RT: Predicting
prognosis in hepatocellular carcinoma after curative surgery with
common clinicopathologic parameters. BMC Cancer. 9:3892009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chao Y, Shih YL, Chiu JH, Chau GY, Lui WY,
Yang WK, Lee SD and Huang TS: Overexpression of cyclin A but not
Skp 2 correlates with the tumor relapse of human hepatocellular
carcinoma. Cancer Res. 58:985–990. 1998.PubMed/NCBI
|
|
7
|
Ohashi R, Gao C, Miyazaki M, Hamazaki K,
Tsuji T, Inoue Y, Uemura T, Hirai R, Shimizu N and Namba M:
Enhanced expression of cyclin E and cyclin A in human
hepatocellular carcinomas. Anticancer Res. 21:657–662.
2001.PubMed/NCBI
|
|
8
|
Attwooll C, Lazzerini Denchi E and Helin
K: The E2F family: specific functions and overlapping interests.
EMBO J. 23:4709–4716. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leone G, DeGregori J, Yan Z, Jakoi L,
Ishida S, Williams RS and Nevins JR: E2F3 activity is regulated
during the cell cycle and is required for the induction of S phase.
Genes Dev. 12:2120–2130. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lees JA, Saito M, Vidal M, Valentine M,
Look T, Harlow E, Dyson N and Helin K: The retinoblastoma protein
binds to a family of E2F transcription factors. Mol Cell Biol.
13:7813–7825. 1993.PubMed/NCBI
|
|
11
|
Humbert PO, Verona R, Trimarchi JM, Rogers
C, Dandapani S and Lees JA: E2f3 is critical for normal
cellular proliferation. Genes Dev. 14:690–703. 2000.
|
|
12
|
Vuaroqueaux V, Urban P, Labuhn M,
Delorenzi M, Wirapati P, Benz CC, Flury R, Dieterich H, Spyratos F,
Eppenberger U and Eppenberger-Castori S: Low E2F1 transcript levels
are a strong determinant of favorable breast cancer outcome. Breast
Cancer Res. 9:R332007. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Justenhoven C, Pierl CB, Haas S, Fischer
HP, Hamann U, Baisch C, Harth V, Spickenheuer A, Rabstein S,
Vollmert C, Illig T, Pesch B, Brüning T, Dippon J, Ko YD and Brauch
H: Polymorphic loci of E2F2, CCND1 and CCND3 are associated with
HER2 status of breast tumors. Int J Cancer. 124:2077–2081. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Feo F, De Miglio MR, Simile MM, Muroni MR,
Calvisi DF, Frau M and Pascale RM: Hepatocellular carcinoma as a
complex polygenic disease. Interpretive analysis of recent
developments on genetic predisposition. Biochim Biophys Acta.
1765:126–147. 2006.PubMed/NCBI
|
|
15
|
Ogata H, Goto S, Sato K, Fujibuchi W, Bono
H and Kanehisa M: KEGG: Kyoto Encyclopedia of Genes and Genomes.
Nucleic Acids Res. 27:29–34. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rhodes DR, Yu J, Shanker K, Deshpande N,
Varambally R, Ghosh D, Barrette T, Pandey A and Chinnaiyan AM:
ONCOMINE: a cancer microarray database and integrated data-mining
platform. Neoplasia. 6:1–6. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mostafavi S, Ray D, Warde-Farley D,
Grouios C and Morris Q: GeneMANIA: a real-time multiple association
network integration algorithm for predicting gene function. Genome
Biol. 9(Suppl 1): S42008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dweep H, Sticht C, Pandey P and Gretz N:
miRWalk - database: prediction of possible miRNA binding sites by
‘walking’ the genes of three genomes. J Biomed Inform. 44:839–847.
2011.
|
|
19
|
de Leeuw N, Dijkhuizen T, Hehir-Kwa JY,
Carter NP, Feuk L, Firth HV, Kuhn RM, Ledbetter DH, Martin CL, van
Ravenswaaij-Arts CM, Scherer SW, Shams S, Van Vooren S, Sijmons R,
Swertz M and Hastings R: Diagnostic interpretation of array data
using public databases and internet sources. Hum Mutat. 33:930–940.
2012.PubMed/NCBI
|
|
20
|
Miles WO, Tschöp K, Herr A, Ji JY and
Dyson NJ: Pumilio facilitates miRNA regulation of the E2F3
oncogene. Genes Dev. 26:356–368. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Martinez LA, Goluszko E, Chen HZ, Leone G,
Post S, Lozano G, Chen Z and Chauchereau A: E2F3 is a mediator of
DNA damage-induced apoptosis. Mol Cell Biol. 30:524–536. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Olsson AY, Feber A, Edwards S, Te Poele R,
Giddings I, Merson S and Cooper CS: Role of E2F3 expression in
modulating cellular proliferation rate in human bladder and
prostate cancer cells. Oncogene. 26:1028–1037. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Feng B, Wang R, Song HZ and Chen LB:
MicroRNA-200b reverses chemoresistance of docetaxel-resistant human
lung adenocarcinoma cells by targeting E2F3. Cancer. 118:3365–3376.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu T, Zhu Y, Xiong Y, Ge YY, Yun JP and
Zhuang SM: MicroRNA-195 suppresses tumorigenicity and regulates
G1/S transition of human hepatocellular carcinoma cells.
Hepatology. 50:113–121. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tryndyak VP, Ross SA, Beland FA and
Pogribny IP: Down-regulation of the microRNAs miR-34a,
miR-127, and miR-200b in rat liver during
hepatocarcinogenesis induced by a methyl-deficient diet. Mol
Carcinog. 48:479–487. 2009.
|
|
26
|
Foster CS, Falconer A, Dodson AR, Norman
AR, Dennis N, Fletcher A, Southgate C, Dowe A, Dearnaley D, Jhavar
S, Eeles R, Feber A and Cooper CS: Transcription factor E2F3
overexpressed in prostate cancer independently predicts clinical
outcome. Oncogene. 23:5871–5879. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wei LX, Zhou RS, Xu HF, Wang JY and Yuan
MH: High expression of FOXC1 is associated with poor clinical
outcome in non-small cell lung cancer patients. Tumour Biol.
34:941–946. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang L, Gu F, Liu CY, Wang RJ, Li J and Xu
JY: High level of FOXC1 expression is associated with poor
prognosis in pancreatic ductal adenocarcinoma. Tumour Biol.
34:853–858. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ray PS, Bagaria SP, Wang J, Shamonki JM,
Ye X, Sim MS, Steen S, Qu Y, Cui X and Giuliano AE: Basal-like
breast cancer defined by FOXC1 expression offers superior
prognostic value: a retrospective immunohistochemical study. Ann
Surg Oncol. 18:3839–3847. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xia L, Huang W, Tian D, Zhu H, Qi X, Chen
Z, Zhang Y, Hu H, Fan D, Nie Y and Wu K: Overexpression of forkhead
box C1 promotes tumor metastasis and indicates poor prognosis in
hepatocellular carcinoma. Hepatology. 57:610–624. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang L, Wang X, Jie P, Lu H, Zhang S, Lin
X, Lam EK, Cui Y, Yu J and Jin H: Klotho is silenced through
promoter hypermethylation in gastric cancer. Am J Cancer Res.
1:111–119. 2011.PubMed/NCBI
|
|
32
|
Jeschke J, Van Neste L, Glöckner SC, Dhir
M, Calmon MF, Deregowski V, Van Criekinge W, Vlassenbroeck I, Koch
A, Chan TA, Cope L, Hooker CM, Schuebel KE, Gabrielson E,
Winterpacht A, Baylin SB, Herman JG and Ahuja N: Biomarkers for
detection and prognosis of breast cancer identified by a functional
hypermethylome screen. Epigenetics. 7:701–709. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xie B, Zhou J, Yuan L, Ren F, Liu DC, Li Q
and Shu G: Epigenetic silencing of Klotho expression correlates
with poor prognosis of human hepatocellular carcinoma. Hum Pathol.
44:795–801. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Q, Wang L, Li D, Deng J, Zhao Z, He
S, Zhang Y and Tu Y: Kinesin family member 14 is a candidate
prognostic marker for outcome of glioma patients. Cancer Epidemiol.
37:79–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim TM, Yim SH, Shin SH, Xu HD, Jung YC,
Park CK, Choi JY, Park WS, Kwon MS, Fiegler H, Carter NP, Rhyu MG
and Chung YJ: Clinical implication of recurrent copy number
alterations in hepatocellular carcinoma and putative oncogenes in
recurrent gains on 1q. Int J Cancer. 123:2808–2815. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu N, Jia D, Chen W, Wang H, Liu F, Ge H,
Zhu X, Song Y, Zhang X, Zhang D, Ge D and Bai C: FoxM1 is
associated with poor prognosis of non-small cell lung cancer
patients through promoting tumor metastasis. PLoS One.
8:e594122013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Y, Wen L, Zhao SH, Ai ZH, Guo JZ and
Liu WC: FoxM1 expression is significantly associated with
cisplatin-based chemotherapy resistance and poor prognosis in
advanced non-small cell lung cancer patients. Lung Cancer.
79:173–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xia L, Mo P, Huang W, Zhang L, Wang Y, Zhu
H, Tian D, Liu J, Chen Z, Zhang Y, Hu H, Fan D, Nie Y and Wu K: The
TNF-α/ROS/HIF-1-induced upregulation of FoxMI expression promotes
HCC proliferation and resistance to apoptosis. Carcinogenesis.
33:2250–2259. 2012.
|
|
39
|
Sun HC, Li M, Lu JL, Yan DW, Zhou CZ, Fan
JW, Qin XB, Tang HM and Peng ZH: Overexpression of Forkhead box M1
protein associates with aggressive tumor features and poor
prognosis of hepatocellular carcinoma. Oncol Rep. 25:1533–1539.
2011.PubMed/NCBI
|
|
40
|
Agnelli L, Forcato M, Ferrari F, Tuana G,
Todoerti K, Walker BA, Morgan GJ, Lombardi L, Bicciato S and Neri
A: The reconstruction of transcriptional networks reveals critical
genes with implications for clinical outcome of multiple myeloma.
Clin Cancer Res. 17:7402–7412. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
de Reyniès A, Assié G, Rickman DS, Tissier
F, Groussin L, René-Corail F, Dousset B, Bertagna X, Clauser E and
Bertherat J: Gene expression profiling reveals a new classification
of adrenocortical tumors and identifies molecular predictors of
malignancy and survival. J Clin Oncol. 27:1108–1115.
2009.PubMed/NCBI
|
|
42
|
Liu B, Zhang J, Huang C and Liu H:
Dyskerin overexpression in human hepatocellular carcinoma is
associated with advanced clinical stage and poor patient prognosis.
PLoS One. 7:e431472012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu L, Zhang CZ, Cai M, Fu J, Chen GG and
Yun J: Downregulation of polo-like kinase 4 in hepatocellular
carcinoma associates with poor prognosis. PLoS One. 7:e412932012.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mendler JH, Maharry K, Radmacher MD,
Mrózek K, Becker H, Metzeler KH, Schwind S, Whitman SP, Khalife J,
Kohlschmidt J, Nicolet D, Powell BL, Carter TH, Wetzler M, Moore
JO, Kolitz JE, Baer MR, Carroll AJ, Larson RA, Caligiuri MA,
Marcucci G and Bloomfield CD: RUNX1 mutations are associated
with poor outcome in younger and older patients with
cytogenetically normal acute myeloid leukemia and with distinct
gene and microRNA expression signatures. J Clin Oncol.
30:3109–3118. 2012. View Article : Google Scholar
|
|
45
|
Buechler S: Low expression of a few genes
indicates good prognosis in estrogen receptor positive breast
cancer. BMC Cancer. 9:2432009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mamat S, Ikeda J, Tian T, Wang Y, Luo W,
Aozasa K and Morii E: Transcriptional regulation of aldehyde
dehydrogenase 1A1 gene by alternative spliced forms of nuclear
factor Y in tumorigenic population of endometrial adenocarcinoma.
Genes Cancer. 2:979–984. 2011. View Article : Google Scholar
|
|
47
|
Kong LM, Liao CG, Chen L, Yang HS, Zhang
SH, Zhang Z, Bian HJ, Xing JL and Chen ZN: Promoter hypomethylation
up-regulates CD147 expression through increasing Sp1 binding and
associates with poor prognosis in human hepatocellular carcinoma. J
Cell Mol Med. 15:1415–1428. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang W, Zhou L, Ding SM, Xie HY, Xu X, Wu
J, Chen QX, Zhang F, Wei BJ, Eldin AT and Zheng SS: Aberrant
methylation of the CADM1 promoter is associated with poor prognosis
in hepatocellular carcinoma treated with liver transplantation.
Oncol Rep. 25:1053–1062. 2011.PubMed/NCBI
|
|
49
|
Song MA, Tiirikainen M, Kwee S, Okimoto G,
Yu H and Wong LL: Elucidating the landscape of aberrant DNA
methylation in hepatocellular carcinoma. PLoS One. 8:e557612013.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang YJ, Chen SY, Chen CJ and Santella
RM: Polymorphisms in cyclin D1 gene and hepatocellular carcinoma.
Mol Carcinog. 33:125–129. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Villanueva A and Hoshida Y: Depicting the
role of TP53 in hepatocellular carcinoma
progression. J Hepatol. 55:724–725. 2011.
|
|
52
|
Stroescu C, Dragnea A, Ivanov B, Pechianu
C, Herlea V, Sgarbura O, Popescu A and Popescu I: Expression of
p53, Bcl-2, VEGF, Ki67 and PCNA and prognostic significance in
hepatocellular carcinoma. J Gastrointestin Liver Dis. 17:411–417.
2008.PubMed/NCBI
|
|
53
|
Laurent-Puig P and Zucman-Rossi J:
Genetics of hepatocellular tumors. Oncogene. 25:3778–3786. 2006.
View Article : Google Scholar
|
|
54
|
Frau M, Tomasi ML, Simile MM, Demartis MI,
Salis F, Latte G, Calvisi DF, Seddaiu MA, Daino L, Feo CF,
Brozzetti S, Solinas G, Yamashita S, Ushijima T, Feo F and Pascale
RM: Role of transcriptional and posttranscriptional regulation of
methionine adenosyltransferases in liver cancer progression.
Hepatology. 56:165–175. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Huang YH, Lin KH, Chen HC, Chang ML, Hsu
CW, Lai MW, Chen TC, Lee WC, Tseng YH and Yeh CT: Identification of
postoperative prognostic microRNA predictors in hepatocellular
carcinoma. PLoS One. 7:e371882012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Cheng H, Zhang L, Cogdell DE, Zheng H,
Schetter AJ, Nykter M, Harris CC, Chen K, Hamilton SR and Zhang W:
Circulating plasma MiR-141 is a novel biomarker for metastatic
colon cancer and predicts poor prognosis. PLoS One. 6:e177452011.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liu XG, Zhu WY, Huang YY, Ma LN, Zhou SQ,
Wang YK, Zeng F, Zhou JH and Zhang YK: High expression of serum
miR-21 and tumor miR-200c associated with poor prognosis in
patients with lung cancer. Med Oncol. 29:618–626. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Chen HY, Lin YM, Chung HC, Lang YD, Lin
CJ, Huang J, Wang WC, Lin FM, Chen Z, Huang HD, Shyy JY, Liang JT
and Chen RH: miR-103/107 promote metastasis of colorectal cancer by
targeting the metastasis suppressors DAPK and KLF4. Cancer Res.
72:3631–3641. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Brenner B, Hoshen MB, Purim O, David MB,
Ashkenazi K, Marshak G, Kundel Y, Brenner R, Morgenstern S, Halpern
M, Rosenfeld N, Chajut A, Niv Y and Kushnir M: MicroRNAs as a
potential prognostic factor in gastric cancer. World J
Gastroenterol. 17:3976–3985. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Jamieson NB, Morran DC, Morton JP, Ali A,
Dickson EJ, Carter CR, Sansom OJ, Evans TR, McKay CJ and Oien KA:
MicroRNA molecular profiles associated with diagnosis,
clinicopathologic criteria, and overall survival in patients with
resectable pancreatic ductal adenocarcinoma. Clin Cancer Res.
18:534–545. 2012. View Article : Google Scholar
|
|
61
|
Hu X, Macdonald DM, Huettner PC, Feng Z,
El Naqa IM, Schwarz JK, Mutch DG, Grigsby PW, Powell SN and Wang X:
A miR-200 microRNA cluster as prognostic marker in advanced ovarian
cancer. Gynecol Oncol. 114:457–464. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hiroki E, Akahira J, Suzuki F, Nagase S,
Ito K, Suzuki T, Sasano H and Yaegashi N: Changes in microRNA
expression levels correlate with clinicopathological features and
prognoses in endometrial serous adenocarcinomas. Cancer Sci.
101:241–249. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wang X, Wang J, Ma H, Zhang J and Zhou X:
Downregulation of miR-195 correlates with lymph node metastasis and
poor prognosis in colorectal cancer. Med Oncol. 29:919–927. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Karakatsanis A, Papaconstantinou I,
Gazouli M, Lyberopoulou A, Polymeneas G and Voros D: Expression of
microRNAs, miR-21, miR-31, miR-122, miR-145, miR-146a, miR-200c,
miR-221, miR-222, and miR-223 in patients with hepatocellular
carcinoma or intrahepatic cholangiocarcinoma and its prognostic
significance. Mol Carcinog. 52:297–303. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yu J, Ohuchida K, Mizumoto K, Sato N,
Kayashima T, Fujita H, Nakata K and Tanaka M: MicroRNA,
hsa-miR-200c, is an independent prognostic factor in pancreatic
cancer and its upregulation inhibits pancreatic cancer invasion but
increases cell proliferation. Mol Cancer. 9:1692010. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wong KF, Xu Z, Chen J, Lee NP and Luk JM:
Circulating markers for prognosis of hepatocellular carcinoma.
Expert Opin Med Diagn. 7:319–329. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Anzola M: Hepatocellular carcinoma: role
of hepatitis B and hepatitis C viruses proteins in
hepatocarcinogenesis. J Viral Hepat. 11:383–393. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Yao DF, Dong ZZ and Yao M: Specific
molecular markers in hepatocellular carcinoma. Hepatobiliary
Pancreat Dis Int. 6:241–247. 2007.PubMed/NCBI
|
|
69
|
Sharan R, Ulitsky I and Shamir R:
Network-based prediction of protein function. Mol Syst Biol.
3:882007. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Jenssen TK, Laegreid A, Komorowski J and
Hovig E: A literature network of human genes for high-throughput
analysis of gene expression. Nat Genet. 28:21–28. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Behm-Ansmant I, Rehwinkel J and Izaurralde
E: MicroRNAs silence gene expression by repressing protein
expression and/or by promoting mRNA decay. Cold Spring Harb Symp
Quant Biol. 71:523–530. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kloosterman WP and Plasterk RH: The
diverse functions of microRNAs in animal development and disease.
Dev Cell. 11:441–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Croce CM and Calin GA: miRNAs, cancer, and
stem cell division. Cell. 122:6–7. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Tili E, Michaille JJ, Gandhi V, Plunkett
W, Sampath D and Calin GA: miRNAs and their potential for use
against cancer and other diseases. Future Oncol. 3:521–537. 2007.
View Article : Google Scholar : PubMed/NCBI
|