Introduction
Multiple myeloma (MM) accounts for nearly 20% of
mortality due to total hematologic tumors (1). The pathologic features of MM involve
aberrant activation of metabolism and signal pathways, including
uncontrolled and unlimited angiogenesis and increased glucose
consumption. These changes play important roles in the clinical
course of MM, particularly MM invasion and metastasis (2–4).
The angiogenic switch is controlled by the
activation of pro-tumor genes such as hypoxia inducible factor-1
(HIF-1) and brain-derived neurotrophic factor (BDNF), which encode
pro-angiogenic factors, such as vascular endothelial growth factor
(VEGF) (5–7). Studies have shown that angiogenesis is
associated with upregulated HIF/VEGF pathways in ~40% MM patients
(6), suggesting that other pathways
could be involved in the regulation of VEGF besides the HIF/VEGF
pathway.
PGC-1α is an important co-activator that
participates in the regulation of gene transcriptional activity
(8). It is now well established
that PGC-1α contributes to several important functions, including
mitochondrial biogenesis and metabolism, by interacting with
transcription factors such as nuclear respiratory factors(NRFs) and
estrogen-related receptor (ERR)-α (9–11).
PGC-1α influences glucose consumption by regulating glucose
transporter-4 (GLUT-4), which is upregulated in MM and is
responsible for basal glucose consumption and maintenance of
myeloid cell leukemia-1 (Mcl-1) expression, growth and survival
(3). Studies have also found that
PGC-1α promotes angiogenesis by binding with ERR-α, thereby
increasing VEGF expression (12).
Thus, angiogenesis and metabolism may be linked via PGC-1α. However
it is not clear whether PGC-1α is involved in the regulation of
angiogenesis and glucose metabolism in MM. Therefore, in the
present study, we explored the role of PGC-1α in angiogenesis and
glucose metabolism in MM.
Materials and methods
Cells and culture
Chemicals were purchased from HyClone, Sigma and
Thermo Fisher Scientific, unless otherwise noted. The human MM cell
line RPMI-8226 was obtained from the American Type Culture
Collection (ATCC; Manassas, VA, USA) and was grown in RPMI-1640
medium (HyClone, Thermo Fisher Scientific, Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS) and 100 U/ml
penicillin/streptomycin. Human umbilical vein endothelial cells
(HUVECs) were cultured using the trypsin digestion method. All
cells were grown at 37°C in an atmosphere containing 5% (v/v)
CO2. Peripheral blood mononuclear cells from normal
healthy volunteers were separated and used as the normal
control.
Cell proliferation assay with CCK-8
reagent
Cell proliferation was assayed using Cell Counting
Kit-8 (CCK-8) according to the manufacturer’s protocol (Dojindo
Laboratories, Kumamoto, Japan). Cells were suspended at a
concentration of 5×104/ml in complete medium, seeded in
96-well plates at 0.1-ml suspension/well, and cultured at 37°C.
Then, 10 μl CCK-8 solution was added to each well after 24 and 48 h
of culture, respectively. After incubation at 37°C for 1 h, the
plate was examined with a microplate reader (Bio-Rad, La Jolla, CA,
USA) and the absorbance at 450 nm was recorded. Each experiment was
performed in triplicate.
siRNA transfection
siRNA duplexes for ERR-α and PGC-1α were designed
and produced by Shanghai GenePharma Co., Ltd. (Shanghai, China).
RPMI-8226 cells were transfected with Lipofectamine®
2000 reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s protocol. The sequence information for siRNA was:
siERR-α, 5′-GGCAGAAA CCUAUCUCAGGUU-3′ (sense) and 5′-CCUGAGAUAG
GUUUCUGCCUC-3′ (antisense); siPGC-1α, 5′-GCCAAA CCAACAACUUUAUUU-3′
(sense) and 5′-AUAAAGUU GUUGGUUUGGCUU-3′ (antisense).
Migration assay
Endothelial cell migration was assessed as
previously described (13).
Briefly, 1.5–2.0×104 of RPMI-8226 cells were loaded in
the lower chamber of Transwell, and siRNA targeting PGC-1α or ERR-α
was added. After 4–6 h of transfection, the culture medium was
replaced with fresh RPMI-1640 medium, and then cultured for 24 h.
Then, 1.5–2.0×104 of HUVECs were seeded in the upper
chamber (8-μm pore; Costar Corp., Cambridge, MA, USA). The plates
were then cultured at 37°C in 5% CO2 for 12 h. The cells
migrated across the membrane and adhered to the lower part of the
membrane, while those that did not migrate were removed with a
cotton swab. The former were stained with crystal violet and
examined under a microscope. The cell number before and after the
experiments was counted in order to quantify proliferation.
RT-PCR
Total RNA was extracted using the TRIzol-based
method (Sigma) from RPMI-8226 cells and the biopsy samples.
Approximately 2 μg of total RNA was reverse-transcribed into the
first-strand complementary DNA (cDNA) pool using a First-Strand
cDNA Synthesis kit and real-time RT-PCR was carried out using
SYBR-Green (Toyobo Co., Ltd., Osaka, Japan) with the Applied
Biosystems 7500 System (Life Technologies, Carlsbad, CA, USA)
according to the manufacturer’s instructions. Data analysis was
carried out using the comparative Ct method. The following
human-specific primers were used: β-actin,
5′-TTCCAGCCTTCCTTCCTGG-3′ (forward) and 5′-TTGCGCTCAGGAGCAAT-3′
(reverse); PGC-1α, 5′-TGGTGCCACCACCATCAAAGA-3′ (forward) and 5′-TC
ACCAAACAGCCGCAGACTG-3′ (reverse); ERR-α, 5′-GTG
GATGGAGGTGCTGGTGCT-3′ (forward) and 5′-AGCCT CGGCATCTTCGATGTG-3′
(reverse); MEF2C, 5′-GCCCT GAGTCTGAGGACAAG-3′ (forward) and
5′-AGTGAG CTGACAGGGTTGCT-3′ (reverse); GLUT-4, 5′-GGCTTC
TTCATCTTCACCTTCT-3′ (forward) and 5′-CTCAGTTC TGTGCTGGGTTTC-3′
(reverse); VEGF, 5′-GAAGTGGTG AAGTTCATGGATGTC-3′ (forward) and
5′-CGATCGTTC TGTATCAGTCTTTCC-3′ (reverse).
Western blot analysis
Total cell proteins were prepared, fractionated and
electroblotted on sodium dodecyl sulfate gels, and western blot
analysis was performed as previously described (12).
Statistical analysis
To measure overall differences, particularly those
between different treatments and control, SPSS 17.0 (SPSS Inc.,
Chicago, IL, USA) was used. Analysis of variance and post-hoc tests
(two-sided Dunnett’s t test) were applied to analyze the average
values of replicate results obtained by independent experiments. A
P-value of <0.05 was considered to indicate a statistically
significant difference.
Results
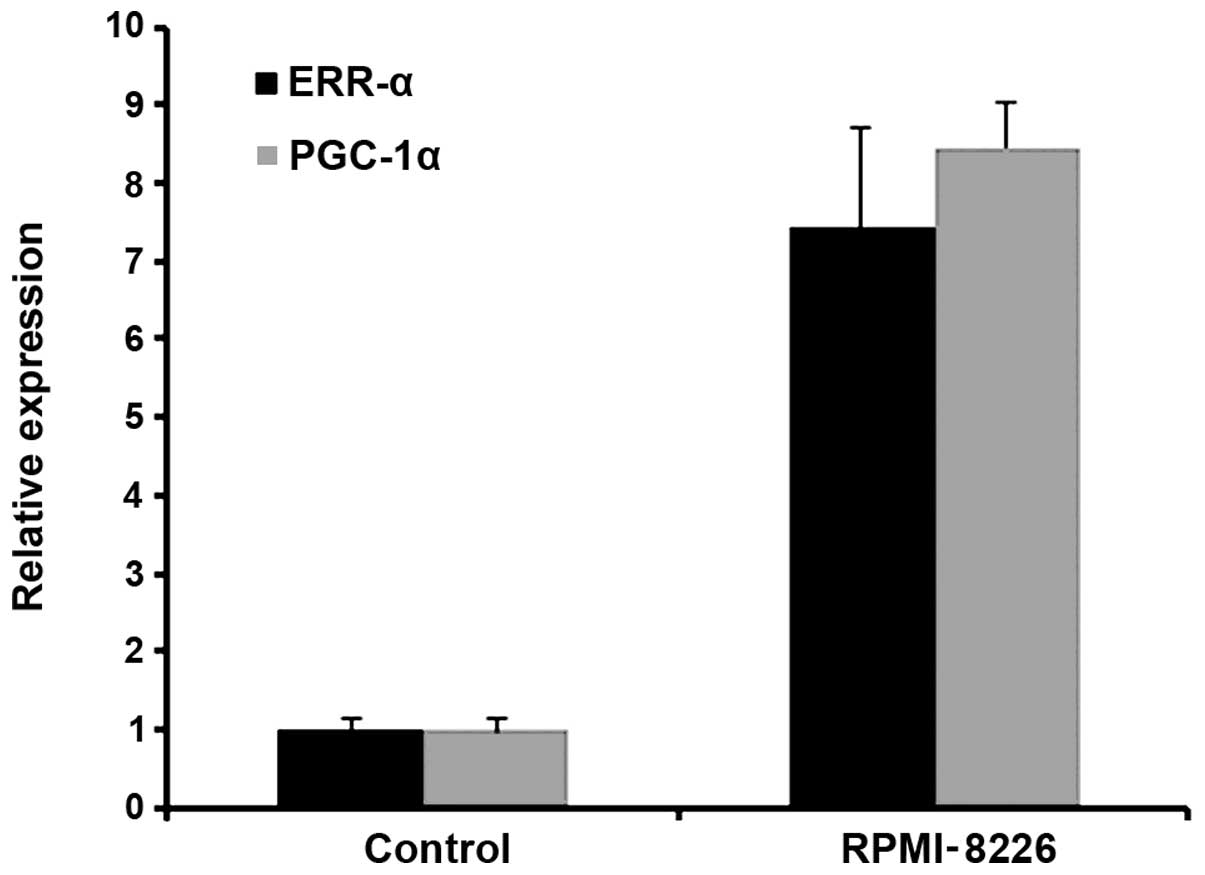
PGC-1α is upregulated in MM
We found that the level of PGC-1α was upregulated in
the MM RPMI-8226 cells. The level of ERR-α, which is a PGC-1α
related co-activated factor, was also increased. The relative
expression of PGC-1α and ERR-α in RPMI-8226 cells was >7-fold
higher than that in the control (Fig
1).
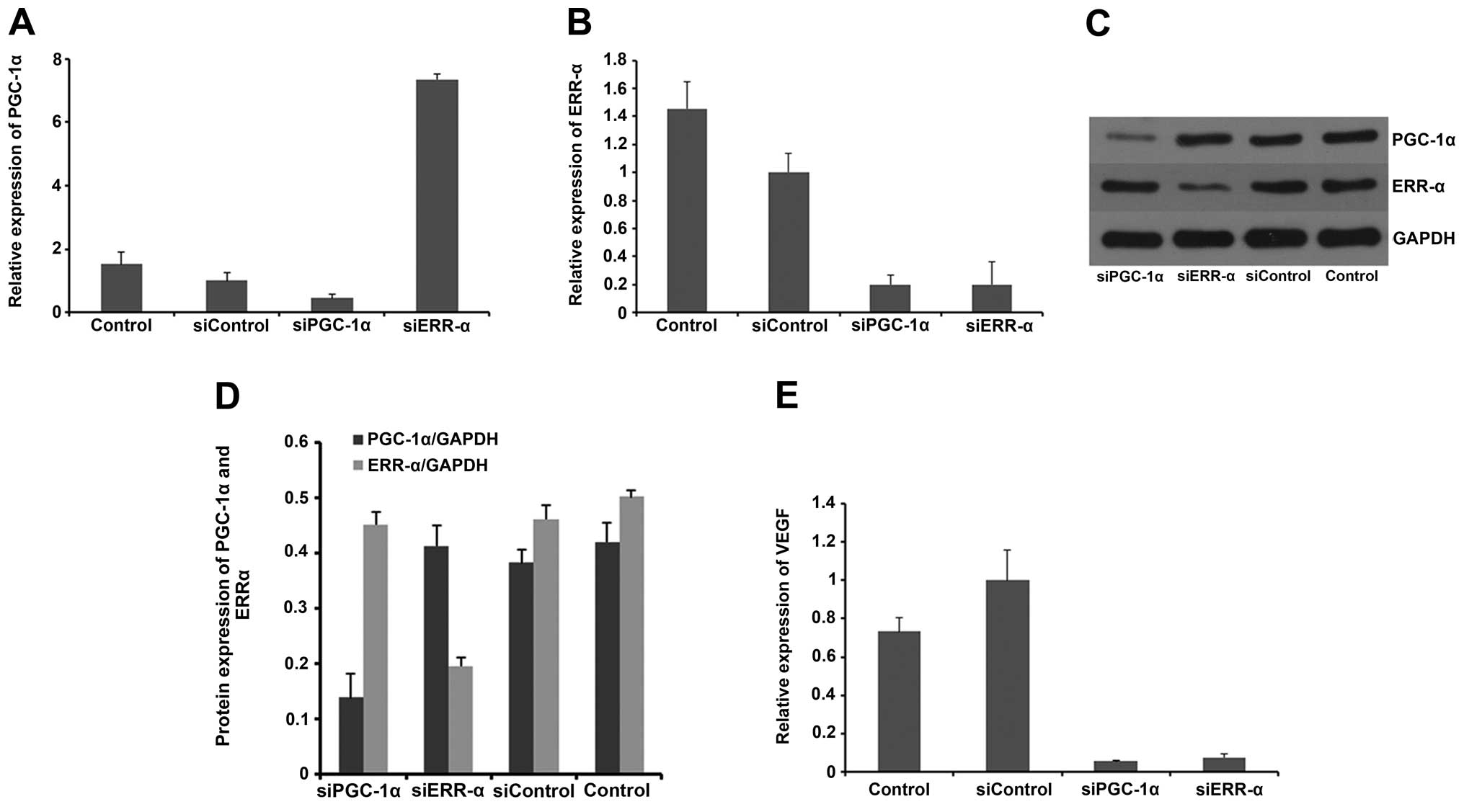
PGC-1α regulates the expression of VEGF
in vitro
The effect of PGC-1α and ERR-α on the expression of
VEGF in the MM cell line RPMI-8226 was examined. PGC-1α was
suppressed by siRNA in the RPMI-8226 cells, and the VEGF mRNA
expression was then measured by RT-PCR. As shown in Fig. 2, our results showed that VEGF mRNA
was significantly lower in the siPGC-1α group than in the control.
Next, we treated the RPMI-8226 cells with siRNA targeting ERR-α and
found that mRNA level of VEGF was also markedly reduced. These data
indicated that both PGC-1α and ERR-α were required for the
regulation of VEGF in RPMI-8226 cells, and these results are in
accordance with those of other studies (12).
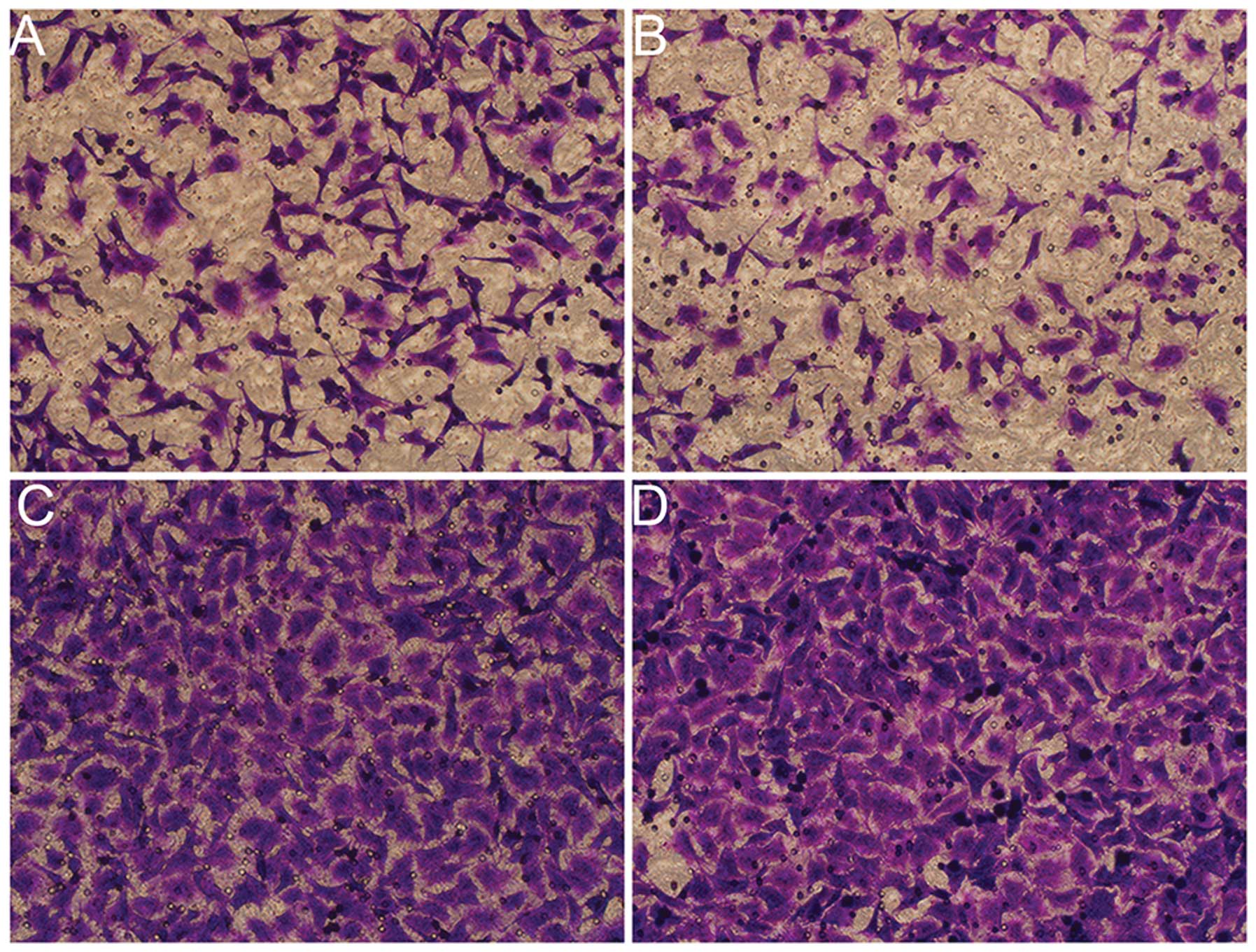
Suppression of PGC-1α inhibits in vitro
angiogenesis in RPMI-8226 cells
In the present study, we also determined whether
PGC-1α and ERR-α inhibition of MM cells affects the migration of
human vascular endothelial cells. As shown in Fig. 3, RPMI-8226 cells without any
treatment evidently promoted the migration of HUVECs, wherein a
large number of cells were observed to have crossed the membrane of
the Transwell chamber. RPMI-8226 cells transfected with siRNA
targeting PGC-1α or ERR-α inhibited HUVEC migration and the counts
of the migratory HUVECs reduced.
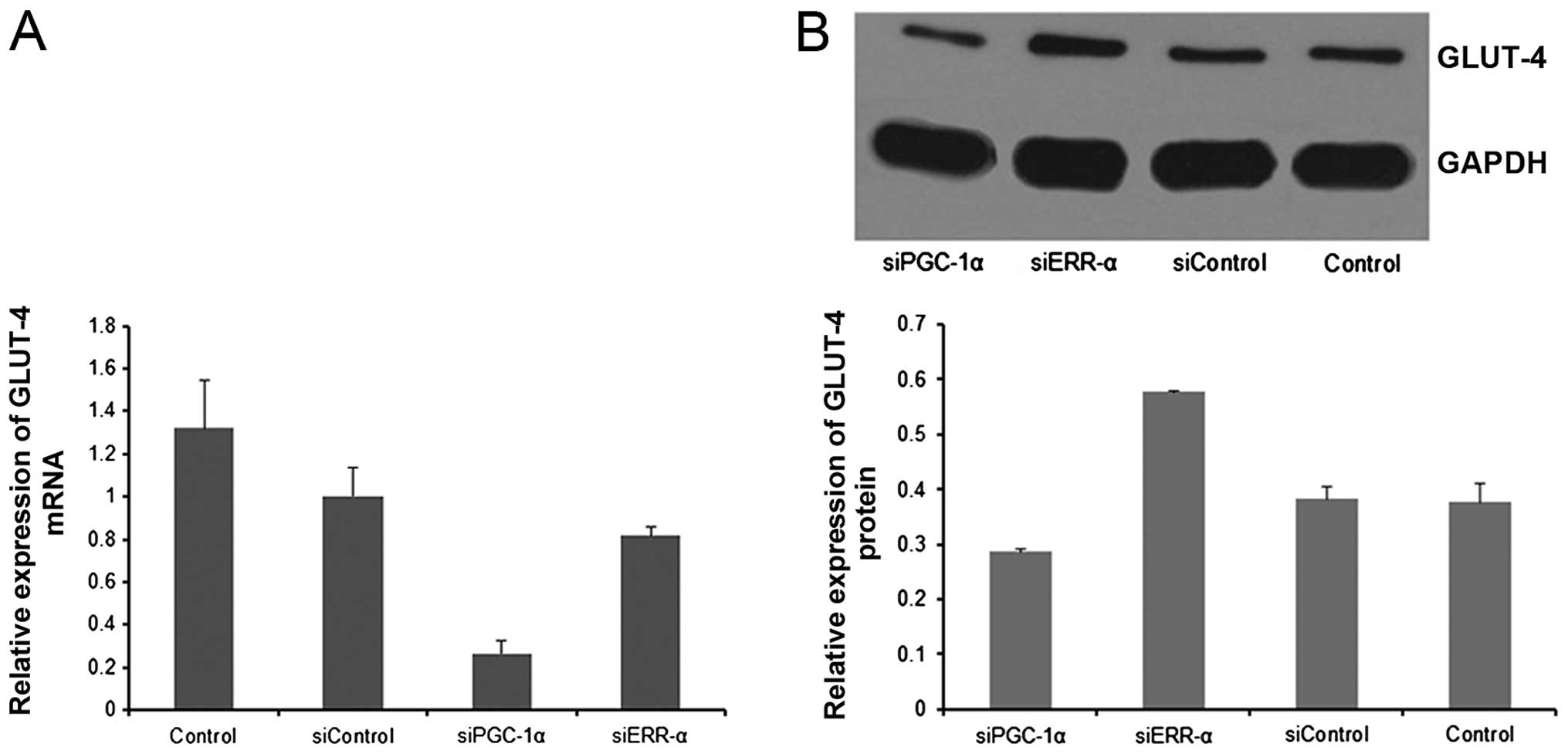
Suppression of PGC-1α reduces GLUT-4
expression in MM
Myeloma cells exhibit upregulated expression of
GLUT-4, which is necessary for glucose consumption, lactate
production, growth and viability (3). We sought to determine whether PGC-1α
plays a role in the regulation of GLUT-4 in MM. As shown in
Fig. 4, suppression of PGC-1α led
to decreased GLUT-4 expression in the RPMI-8226 cells.
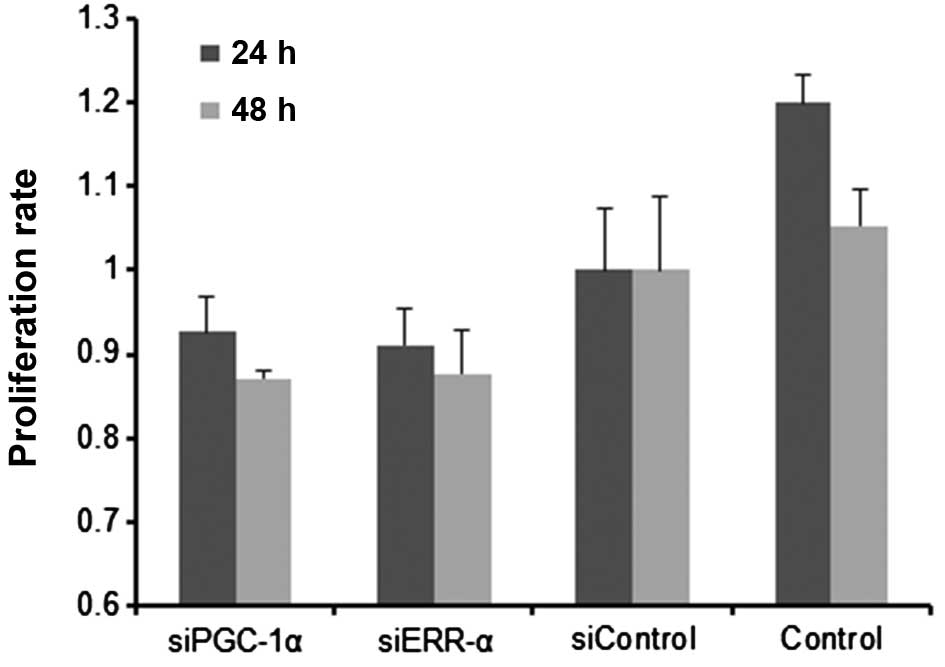
Inhibition of PGC-1α results in growth
and proliferation defects
Cells transfected with siPGC-1α or siERR-α were
found to grow more slowly than the control cells. Similarly, the
results of the CCK-8 assay also showed that proliferation of
RPMI-8226 cells was diminished by siPGC-1α or siERR-α (Fig. 5), indicating that PGC-1α or ERR-α
inhibition hampers the proliferation of myeloma cells.
Discussion
MM is a hematological malignancy characterized by
the aberrant expression of malignant plasma cells within the bone
marrow (14). A number of studies
have shown that increased microvessel density (MVD) correlates with
disease state, suggesting that increased bone marrow angiogenesis
is important in myeloma progression (15). Other studies indicate that a high
rate of glucose consumption beyond that necessary for ATP synthesis
is exhibited by transformed cells including MM cells (16). This phenomenon has been further
confirmed by F-18 fluorodeoxyglucose positron emission
tomography-computed tomography (F-18-FDG PET-CT) scanning. Studies
have shown that MM exhibits a high uptake rate of F-18 FDG and is
positively correlated with the percentage of CD38/CD138-expressing
myeloma cells in the bone marrow (17,18).
Moreover, Kaira et al (19)
showed that F-18 FDG uptake in cancers is determined by the
presence of glucose metabolism, angiogenesis and other factors,
suggesting that MM with high rates of F-18 FDG uptake is most
likely characterized by increased angiogenesis and glucose
metabolism.
Research on the formation of new blood vessels and
VEGF in particular, is a major focus of MM investigations and has
led to the clinical approval of monoclonal anti-VEGF agents
(20). Although these agents show
significant preclinical and clinical anticancer activity, they
prolong overall survival of patients for months only, after which
the tumor continues to grow. Therefore, an understanding of tumor
angiogenesis is still needed (20).
The present study demonstrated that PGC-1α strongly
regulated VEGF expression and angiogenesis by coactivating the
orphan nuclear receptor ERR-α, suggesting that PGC-1α and ERR-α
control a novel angiogenic pathway that delivers the needed oxygen
and substrates (3). In addition, it
has been suggested that activation of the ERR-α/PGC-1α pathway
increases VEGF expression and angiogenesis (21), and that the ErbB2/Neu-induced
mammary tumor cells ectopically expressing PGC-1α exhibit increased
concentrations of the angiogenic factor VEGF compared with controls
(22). Our results showed that
PGC-1α and ERR-α are upregulated in MM and their expression was
associated with the VEGF level. They also showed that PGC-1α or
ERR-α inhibition can significantly suppress the expression of VEGF
in vitro. Taken together, the results suggest that PGC-1α
and ERRα, major regulators of mitochondrial function and cellular
energy metabolism, also play an important role in the regulation of
VEGF and angiogenesis, not only in normal cells and tissues but
also in MM. This also illustrates that only targeting the classical
pathways involved in angiogenesis could probably not achieve the
goal of anti-angiogenesis. Indeed, lenalidomide and other similar
agents show limited efficacy in the treatment of MM, and this
further supports the complexity of tumor angiogenesis. Targeting
PGC-1α and/or ERR-α may improve the anti-angiogenesis effect of
agents in clinical use.
Deregulation of glycolytic metabolism is another
feature of MM (23). This
observation and F-18-FDG PET results regarding MM suggest the
reliance of myeloma on increased glucose consumption and glycolysis
(24,25). Glucose is involved in generating
NADH and FADH, which contribute to maintaining the integrity of
mitochondria (26). Glucose also
prevents the release of cytochrome c by maintaining the
interaction between hexokinase II and mitochondria (27,28).
In addition to generating energy-related chemicals, such as ATP,
glucose also provides biosynthetic intermediates for lipid and
nucleotide synthesis and plays a role in the regulation of several
factors associated with cell death (i.e., Mcl-1, Bcl-2-associated
death promoter protein) (29–32).
The contribution of the glycolytic phenotype to increased
resistance to apoptosis-inducing agents (33,34)
supports the benefits of targeting glucose consumption.
Few studies have focused on determining the
biological and molecular role of GLUT activation in tumors,
knowledge that could facilitate the identification of potential
therapeutic targets. McBrayer et al (3) performed gene expression profiling
studies to identify deregulated GLUT family members in MM and
demonstrated that myeloma cells exhibit reliance on constitutively
cell surface-localized GLUT-4 for basal glucose consumption,
maintenance of Mcl-1 expression, growth and survival. It can, thus,
be concluded that GLUT-4 is highly important to the MM
proliferation and progression. GLUT-4 and its regulation controlled
by other factors are well studied in normal cells. However, the
pathway involved in regulating the expression of GLUT-4 is rarely
studied in cancer, especially in MM. Our investigation of the
interaction between MM cell proliferation and GLUT-4 activity
delineates a pathway linking GLUT-4 activity with the aberrant
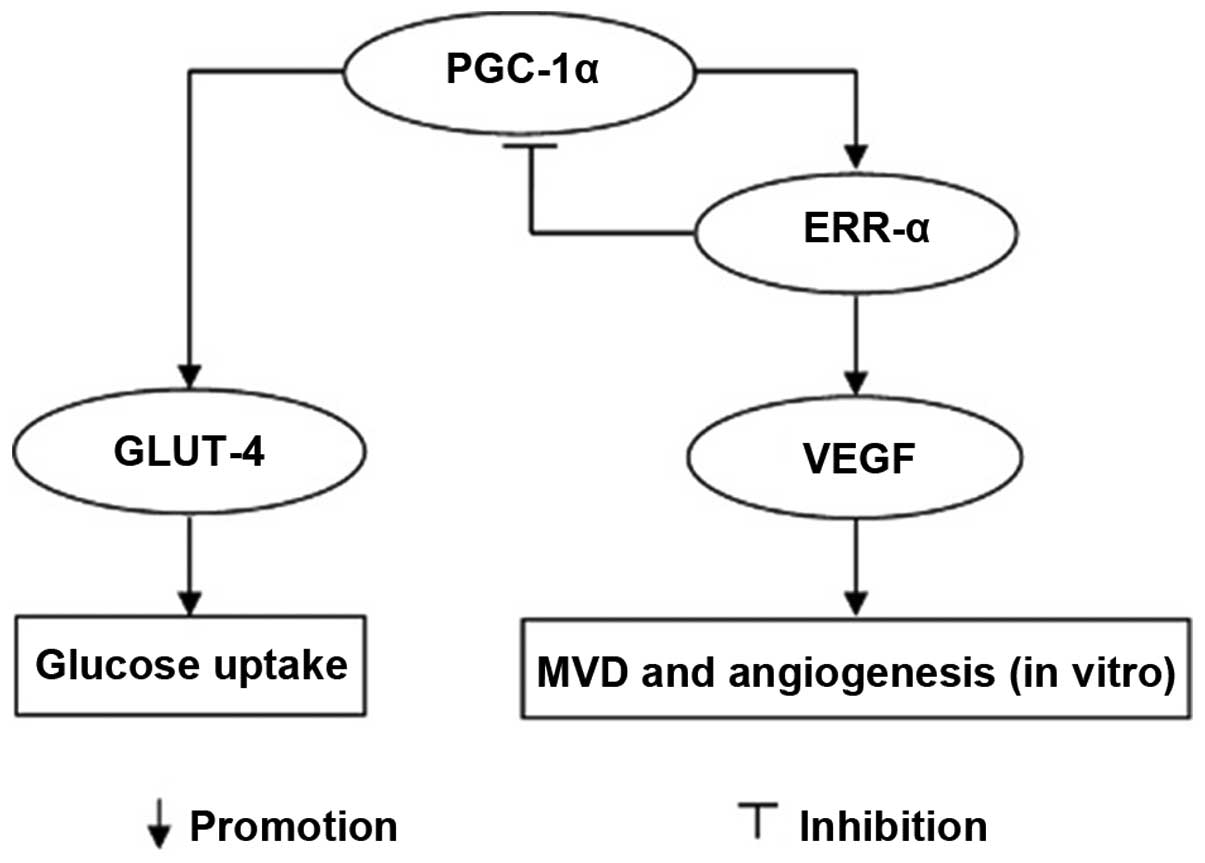
expression of PGC-1α. PGC-1α inhibition can decrease GLUT-4
expression and results in hampered proliferation of RPMI-8226
cells. However, by silencing ERR-α expression, PGC-1α expression is
as much as 7-fold increased and GLUT-4 expression is also
increased, suggesting that ERR-α is a repressor of PGC-1α and that
the latter is associated with GLUT-4 regulation, probably by
enhancing transcription of the GLUT-4 gene (Fig. 6).
ERR-α is an orphan member of the nuclear receptor
superfamily of transcription factors whose activity is regulated by
the expression level and/or activity of its obligate co-regulator,
PGC-1α. Under normal physiological conditions, and in response to
different environmental stimuli, the ERR-α/PGC-1α complex is
involved in regulating metabolic homeostasis under conditions of
high energy demand in brown adipocytes, proliferating T cells and
muscle. Notably, increased expression and activity of the
ERR-α/PGC-1α axis have also been shown to correlate with
unfavorable clinical outcomes in both breast and ovarian tumors
(35). However, little is known
about the role of ERR-α/PGC-1α in hematological malignances,
particularly in MM.
In conclusion, our results demonstrate that PGC-1α
and ERR-α are both upregulated in MM and human myeloma RPMI-8226
cells. Furthermore, this upregulation affects the in vitro
expression of GLUT-4 and the angiogenesis in MM by increasing VEGF
expression. Suppression of PGC-1α impairs the proliferation of
RPMI-8226 cells, although not to a very great extent. Targeting
PGC-1α may provide another effective and probably more potent way
to curb the increased needs of glucose and angiogenesis in MM.
References
|
1
|
Munshi NC: Plasma cell disorders: an
historical perspective. Hematology Am Soc Hematol Educ Program.
297:2008.PubMed/NCBI
|
|
2
|
Ria R, Reale A, De Luisi A, Ferrucci A,
Moschetta M and Vacca A: Bone marrow angiogenesis and progression
in multiple myeloma. Am J Blood Res. 1:76–89. 2011.PubMed/NCBI
|
|
3
|
McBrayer SK, Cheng JC, Singhal S, Krett
NL, Rosen ST and Shanmugam M: Multiple myeloma exhibits novel
dependence on GLUT4, GLUT8, and GLUT11: implications for glucose
transporter-directed therapy. Blood. 119:4686–4697. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stocks T, Rapp K, Bjorge T, et al: Blood
glucose and risk of incident and fatal cancer in the metabolic
syndrome and cancer project (me-can): analysis of six prospective
cohorts. PLoS Med. 6:e10002012009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vacca A, Ribatti D, Roccaro AM, Frigeri A
and Dammacco F: Bone marrow angiogenesis in patients with active
multiple myeloma. Semin Oncol. 28:543–550. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Giatromanolaki A, Bai M, Margaritis D, et
al: Hypoxia and activated VEGF/receptor pathway in multiple
myeloma. Anticancer Res. 30:2831–2836. 2010.PubMed/NCBI
|
|
7
|
Sun CY, Hu Y, Huang J, et al:
Brain-derived neurotrophic factor induces proliferation, migration,
and VEGF secretion in human multiple myeloma cells via activation
of MEK-ERK and PI3K/AKT signaling. Tumour Biol. 31:121–128. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Puigserver P, Wu Z, Park CW, Graves R,
Wright M and Spiegelman BM: A cold-inducible coactivator of nuclear
receptors linked to adaptive thermogenesis. Cell. 92:829–839. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Handschin C and Spiegelman BM: Peroxisome
proliferator-activated receptor γ coactivator 1 coactivators,
energy homeostasis, and metabolism. Endocr Rev. 27:728–735.
2006.
|
|
10
|
Puigserver P and Spiegelman BM: Peroxisome
proliferator-activated receptor-γ coactivator 1 α (PGC-1 α):
transcriptional coactivator and metabolic regulator. Endocr Rev.
24:78–90. 2003.
|
|
11
|
Finck BN and Kelly DP: PGC-1 coactivators:
inducible regulators of energy metabolism in health and disease. J
Clin Invest. 116:615–622. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arany Z, Foo SY, Ma Y, et al:
HIF-independent regulation of VEGF and angiogenesis by the
transcriptional coactivator PGC-1α. Nature. 451:1008–1012.
2008.PubMed/NCBI
|
|
13
|
Lu J, Zhang K, Chen S and Wen W: Grape
seed extract inhibits VEGF expression via reducing HIF-1α protein
expression. Carcinogenesis. 30:636–644. 2009.PubMed/NCBI
|
|
14
|
Otjacques E, Binsfeld M, Noel A, Beguin Y,
Cataldo D and Caers J: Biological aspects of angiogenesis in
multiple myeloma. Int J Hematol. 94:505–518. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nikhil C and Kenneth C: Advances in
Biology and Therapy of Multiple Myeloma. 1. Basic Science.
Springer; New York, NY: 2012
|
|
16
|
Adekola K, Rosen ST and Shanmugam M:
Glucose transporters in cancer metabolism. Curr Opin Oncol.
24:650–654. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ak I and Gulbas Z: F-18 FDG uptake of bone
marrow on PET/CT scan: its correlation with CD38/CD138 expressing
myeloma cells in bone marrow of patients with multiple myeloma. Ann
Hematol. 90:81–87. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bartel TB, Haessler J, Brown TL, et al:
F18-fluorodeoxyglucose positron emission tomography in the context
of other imaging techniques and prognostic factors in multiple
myeloma. Blood. 114:2068–2076. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kaira K, Endo M, Abe M, et al: Biologic
correlation of 2-[18F]-fluoro-2-deoxy-D-glucose uptake
on positron emission tomography in thymic epithelial tumors. J Clin
Oncol. 28:3746–3753. 2010.
|
|
20
|
Podar K and Anderson KC: Emerging
therapies targeting tumor vasculature in multiple myeloma and other
hematologic and solid malignancies. Curr Cancer Drug Targets.
11:1005–1024. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Klimcakova E, Chenard V, McGuirk S, et al:
PGC-1α promotes the growth of ErbB2/Neu-induced mammary tumors by
regulating nutrient supply. Cancer Res. 72:1538–1546. 2012.
|
|
22
|
Altenberg B and Greulich KO: Genes of
glycolysis are ubiquitously overexpressed in 24 cancer classes.
Genomics. 84:1014–1020. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Durie BG, Waxman AD, D’Agnolo A and
Williams CM: Whole-body 18F-FDG PET identifies high-risk
myeloma. J Nucl Med. 43:1457–1463. 2002.PubMed/NCBI
|
|
24
|
Zhang K, Lu J, Mori T, et al: Baicalin
increases VEGF expression and angiogenesis by activating the
ERRα/PGC-1α pathway. Cardiovasc Res. 89:426–435. 2011.PubMed/NCBI
|
|
25
|
Bredella MA, Steinbach L, Caputo G, Segall
G and Hawkins R: Value of FDG PET in the assessment of patients
with multiple myeloma. AJR Am J Roentgenol. 184:1199–1204. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gendron MC, Schrantz N, Metivier D, et al:
Oxidation of pyridine nucleotides during Fas- and ceramide-induced
apoptosis in Jurkat cells: correlation with changes in
mitochondria, glutathione depletion, intracellular acidification
and caspase 3 activation. Biochem J. 353:357–367. 2001. View Article : Google Scholar
|
|
27
|
Mathupala SP, Ko YH and Pedersen PL:
Hexokinase II: cancer’s double-edged sword acting as both
facilitator and gatekeeper of malignancy when bound to
mitochondria. Oncogene. 25:4777–4786. 2006.
|
|
28
|
Gottlob K, Majewski N, Kennedy S, Kandel
E, Robey RB and Hay N: Inhibition of early apoptotic events by
Akt/PKB is dependent on the first committed step of glycolysis and
mitochondrial hexokinase. Genes Dev. 15:1406–1418. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
DeBerardinis RJ, Mancuso A, Daikhin E, et
al: Beyond aerobic glycolysis: transformed cells can engage in
glutamine metabolism that exceeds the requirement for protein and
nucleotide synthesis. Proc Natl Acad Sci USA. 104:19345–19350.
2007. View Article : Google Scholar
|
|
30
|
Zhao Y, Altman BJ, Coloff JL, et al:
Glycogen synthase kinase 3α and 3β mediate a glucose-sensitive
antiapoptotic signaling pathway to stabilize Mcl-1. Mol Cell Biol.
27:4328–4339. 2007.
|
|
31
|
Danial NN, Gramm CF, Scorrano L, et al:
BAD and glucokinase reside in a mitochondrial complex that
integrates glycolysis and apoptosis. Nature. 424:952–956. 2003.
View Article : Google Scholar
|
|
32
|
Rathmell JC, Fox CJ, Plas DR, Hammerman
PS, Cinalli RM and Thompson CB: Akt-directed glucose metabolism can
prevent Bax conformation change and promote growth
factor-independent survival. Mol Cell Biol. 23:7315–7328. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gatenby RA and Gillies RJ: Why do cancers
have high aerobic glycolysis? Nat Rev Cancer. 4:891–899. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Plas DR and Thompson CB: Cell metabolism
in the regulation of programmed cell death. Trends Endocrinol
Metab. 13:75–78. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chang CY and McDonnell DP: Molecular
pathways: the metabolic regulator estrogen-related receptor α as a
therapeutic target in cancer. Clin Cancer Res. 18:6089–6095.
2012.
|