Introduction
Malignant tumor is one of the leading causes of
mortality. The mortality rates of liver cancer are the third
highest in the world (1).
Hepatocellular carcinoma (HCC) is a malignant tumor with a potent
ability to invade locally and metastasize distantly (2). Due to the lack of effective
chemoprevention or systematic treatment, the prognosis of HCC is
very poor (3). Therefore, it is of
utmost importance to explore the molecular mechanisms of HCC.
Prostaglandin E2 (PGE2), a
predominant metabolic product of cyclooxygenase-2 (COX-2), has been
shown to affect numerous tumorigenic progressions, such as HCC
(4), renal cell carcinoma (5) and prostate cancer (6). Endogenous and exogenous
PGE2 might promote carcinoma cell growth (7), invasion (8) and migration (6) via activation of a series of signal
transduction pathways. PGE2 exerts its biological
functions through binding with four types of E prostanoid receptors
on the cell surface membrane (9,10),
among which, the EP2 receptor is believed to be involved in cancer
cell proliferation and invasion (11–13).
Tumor invasion and metastasis are characterized by
epithelial-mesenchymal transition (EMT) (14). EMT is a critical process enabling
the tumor cells to migrate from the primary tumor and metastasize
to distant sites (15). Previous
studies suggested that Snail is a zinc-finger transcriptional
repressor governing EMT during tumor progression (16–18).
Snail has been found to play a major role in promoting tumor cell
migration and invasion in many cancer types (19–21),
and its expression predicts a poor outcome in patients with
metastatic cancer (16).
In HCC, the most abundant prostaglandin is
PGE2 (22); increased
COX-2 expression has been documented (23) and Snail expression was significantly
higher (24). Based on these
findings, our previous studies showed that PGE2 could
significantly enhance HCC cell invasion and migration through
upregulation of Snail expression level; however, the detailed
mechanisms through which PGE2 regulates Snail protein
expression remains to be further clarified. In the present study,
PGE2 was found to upregulate Snail expression level via
the EP2 receptor in Huh-7 cells. Src, EGFR, Akt and mTOR were all
involved in the EP2 receptor-mediated Snail protein expression.
These findings reveal that PGE2 could promote HCC cell
invasion through upregulating Snail expression level via the
EP2/Src/EGFR/Akt/mTOR pathway.
Materials and methods
Materials
The human HCC cell line Huh-7 was obtained from the
American Type Culture Collection (ATCC; Manassas, VA, USA).
Dulbecco’s modified Eagle’s medium (DMEM) was from Invitrogen
(Carlsbad, CA, USA). PGE2 and PI3K inhibitor LY294002
were from Cayman Chemical Co. (Ann Arbor, MI, USA). EP2 receptor
agonist butaprost, Src inhibitor PP2 and anti-β-actin antibody were
from Sigma-Aldrich (St. Louis, MO, USA). EGFR inhibitor AG1478 and
mTOR inhibitor PP242 were from Merck Millipore. Anti-phosphorylated
EGFR (Tyr1173) antibody was from SAB (Signalway Antibody, Nanjing,
China), anti-EGFR antibody, anti-phosphorylated Akt (Ser473)
antibody, anti-Akt antibody, anti-phosphorylated mTOR antibody,
anti-mTOR antibody, anti-Snail antibody were from Cell Signaling
Technology (Danvers, MA, USA). The protein assay was from Bio-Rad
Laboratories (Hercules, CA, USA). Electrochemiluminescence (ECL)
reagents were from Amersham Biosciences (Piscataway, NJ, USA). The
Transwell unit was from Costar Corning (Cambridge, MA, USA).
Matrigel was from BD Biosciences, (Discovery Labware, Bedford, MA,
USA).
Cell line and culture
HCC Huh-7 cells were cultured in DMEM, supplemented
with 10% fetal calf serum at 37°C in a humidified 5% CO2
incubator. The experiments were performed when cells reached 80%
confluence and were conducted in serum-free medium with serum
deprivation for 12 h before the experiments.
Cell migration assays
Cell migration assays were performed in 24-well
Transwell chambers Prior to experiment, the lower surfaces of the
membranes were coated with gelatin (1%) diluted in PBS. Cells
(5×104) were added to the upper Transwell chamber and
media with 10%FBS were added to the lower Transwell chamber. The
serum-free media plus pharmacological agents were added in the
upper Transwell chamber. After 12 h of incubation at 37°C, the
cells were fixed and stained by 0.1% crystal violet for 30 min at
room temperature. After washing the wells with PBS, the cells on
the upper surface of the filter were removed with a cotton swab.
The migrating cells on the lower surface of the filter were
solubilized with 10% acetic acid 10 min and quantified by measuring
the absorbance at 550 nm.
Cell invasion assays
Cell invasion assays were performed in
Matrigel-coated 24-well Transwell chambers. Cells
(5×104) were added to the upper Transwell chamber and
media with 10% FBS were added to the lower Transwell chamber. The
serum-free media plus pharmacological agents were added in the
upper Transwell chamber. After 24 h of incubation at 37°C, the
cells were fixed and stained by 0.1 % crystal violet for 30 min at
room temperature. After washing the wells with PBS, the cells on
the upper surface of the filter were removed with a cotton swab.
The invading cells on the lower surface of the membrane were
solubilized with 10% acetic acid 10 min and quantified by measuring
the absorbance at 550 nm.
Western blot analysis
Different pharmacological agents were used for the
treatment of HCC Huh-7 cells for various times. The cells were
collected into modified radioimmunoprecipitation assay (RIPA)
buffer (50 mM Tris-HCl pH 7.4, 1% NP-40, 0.25% Na-deoxycholate, 150
mM NaCl, 1 mM EDTA, 1 mM PMSF, protease inhibitor cocktail) and
placed on ice for 30 min. Lysates were sonicated on ice and
centrifuged at 15,000 × g/min for 30 min. Protein concentrations of
cells were measured by Bio-Rad protein assay kit. Equal amounts of
proteins (40–60 μg) were separated by SDS-PAGE and transferred onto
nitrocellulose membranes. Membranes were blocked with 5% non-fat
dry milk-PBST buffer for 1 h at room temperature and incubated with
the corresponding primary antibodies overnight at 4°C with gentle
shaking. Then, membranes were washed by PBST and incubated for 2 h
with the peroxidase-conjugated secondary anti-rabbit or anti-mouse
antibodies at room temperature. The signals were detected by
enhanced chemiluminescent reagent (ECL) and analyzed with the
ImageJ analysis software.
Statistical analysis
Data are expressed as the means ± SD. Student’s
t-test was used for evaluation of statistical significance and a
value of P<0.05 was considered to indicate a statistically
significant difference.
Results
PGE2 promotes HCC cell
migration and invasion
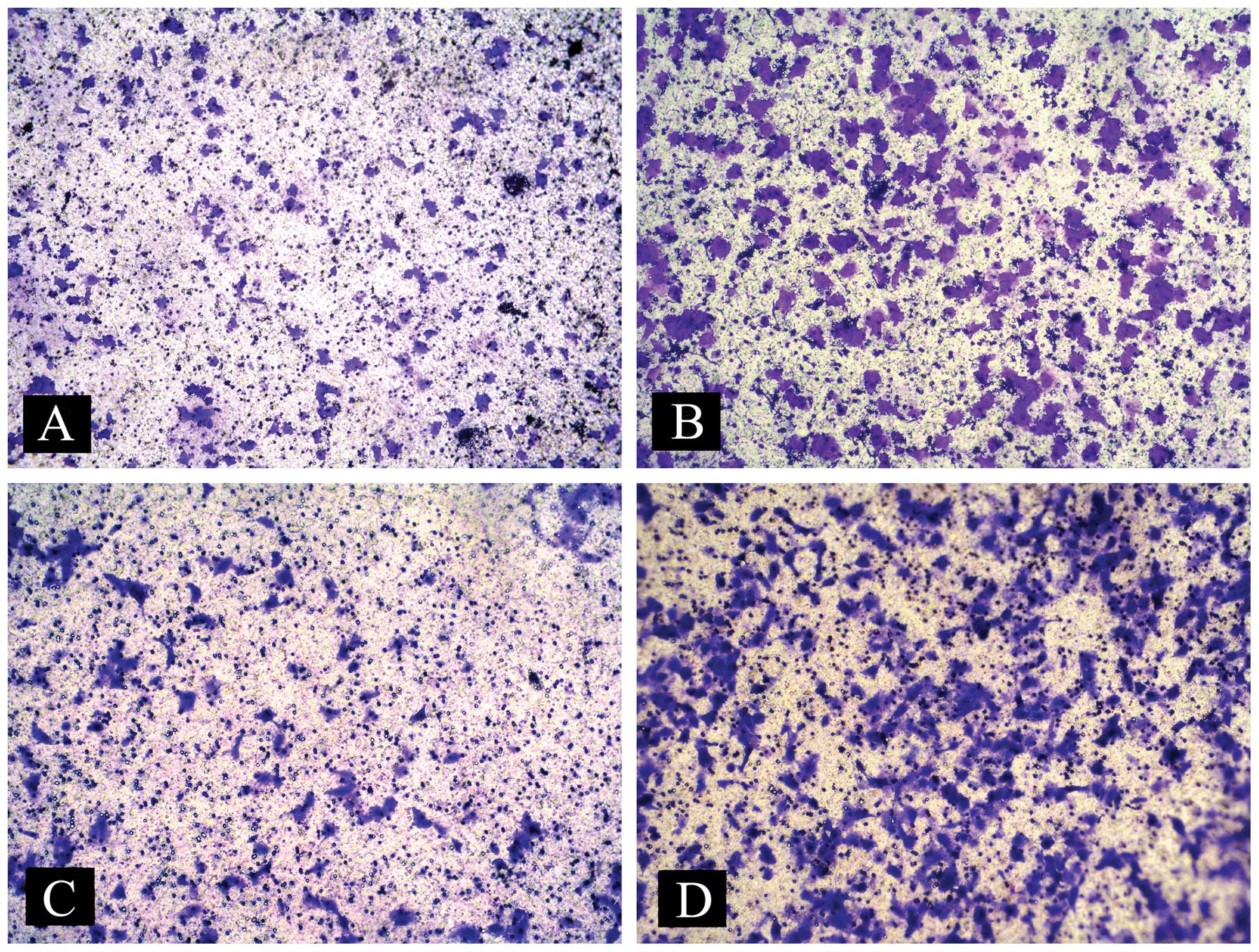
The cell invasion assays were utilized to analyze
the effects of PGE2 on HCC cell migration and invasion.
Huh-7 cells were treated with vehicle or exogenous 10 μM
PGE2 in the upper chamber and media plus 10% FBS were
added to the lower Transwell chamber. As shown in Fig. 1, in the Transwell assay, cell
migration was found to increase by 192% when the cells were treated
with PGE2 for 12 h. Cell invasion was found to increase
by 186% when the cells were treated with PGE2 for 24 h.
These results demonstrate that PGE2 significantly
promotes Huh-7 cell migration and invasion.
PGE2 induces Snail expression
in HCC cells
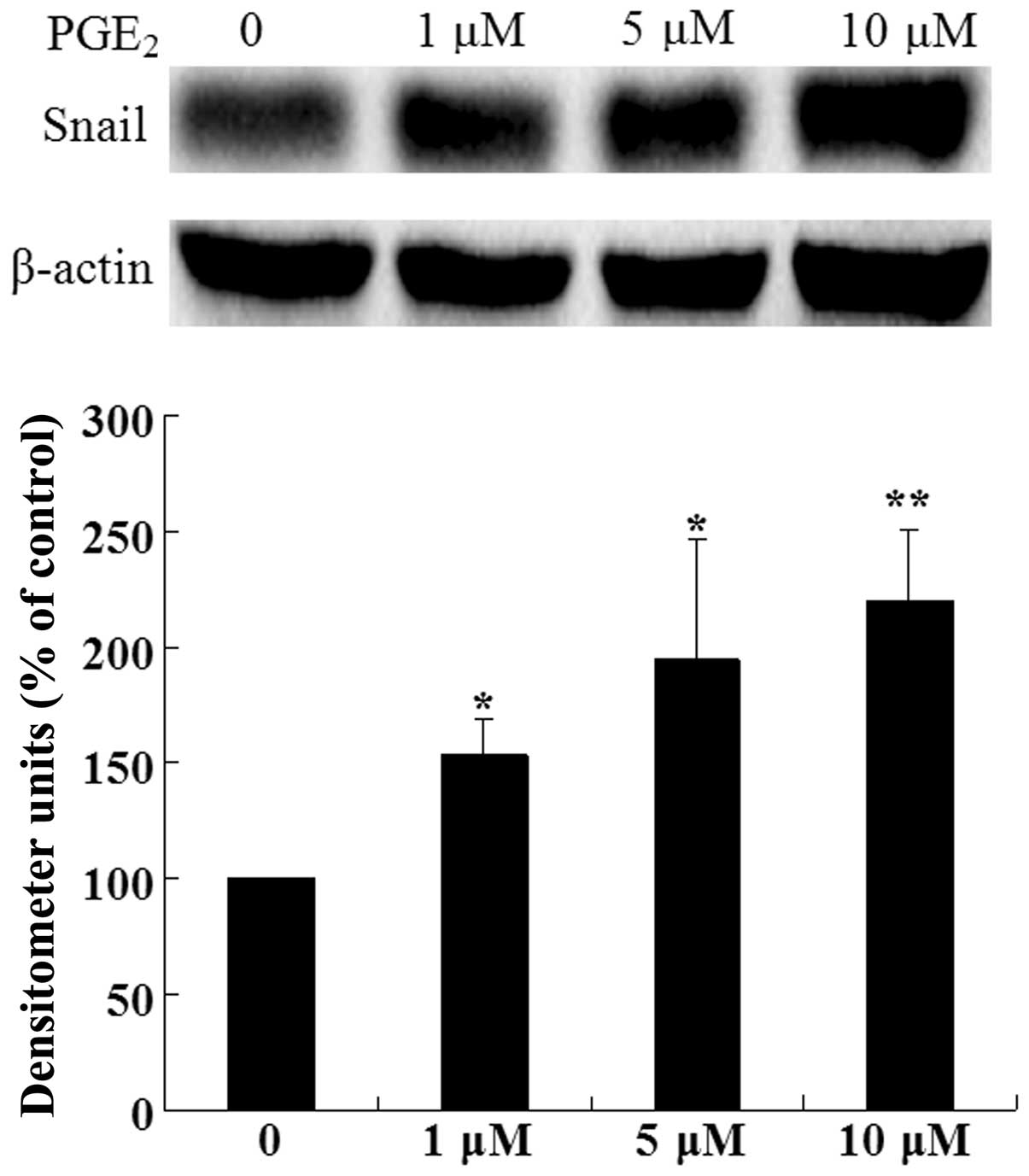
To identify the potential effects of PGE2
on Snail expression, Huh-7 cells were treated with various
concentrations of PGE2 for 24 h. As shown in Fig. 2, treatment of Huh-7 cells with
PGE2 significantly increased the expression level of
Snail protein compared with the control group. These data indicate
that PGE2 upregulates Snail expression in a
dose-dependent manner in Huh-7 cells.
EP2 receptor is involved in
PGE2-induced Snail expression
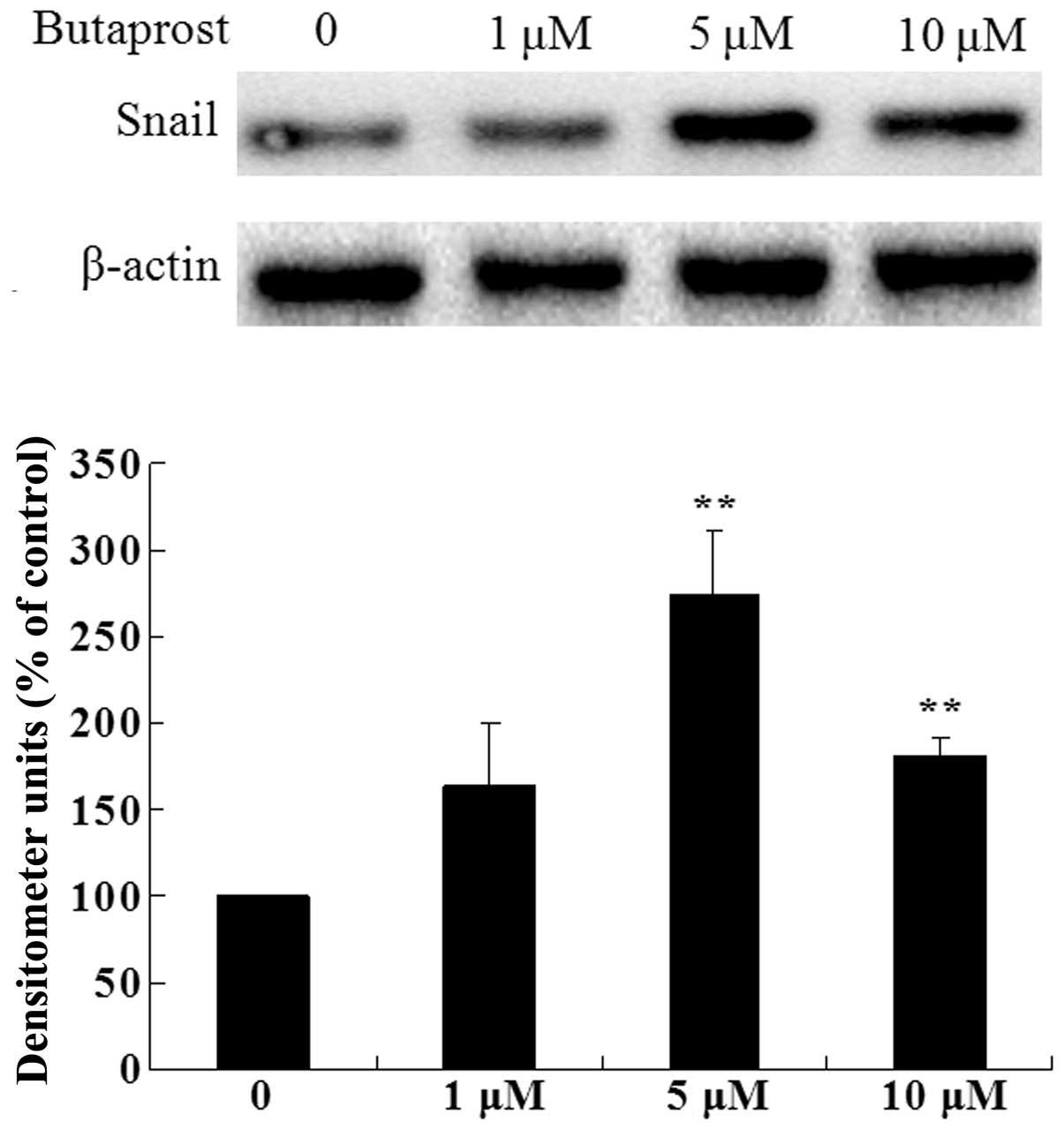
Based on our previous results, we know that
PGE2 promotes HCC cell proliferation and invasion via
EP2 receptor, and PGE2 also markedly increases the Snail
expression level. Thus, we postulated that PGE2 could
increase the expression level of Snail protein via EP2 receptor. To
evaluate this hypothesis, we treated Huh-7 cells with various
concentrations of EP2 agonist butaprost; as shown in Fig. 3, treatment of Huh-7 cells with
butaprost significantly increased the expression level of Snail
protein compared with the control group. These results indicate
that EP2 receptor plays an important role in
PGE2-induced Snail expression.
Involvement of Src in
PGE2-induced Snail expression
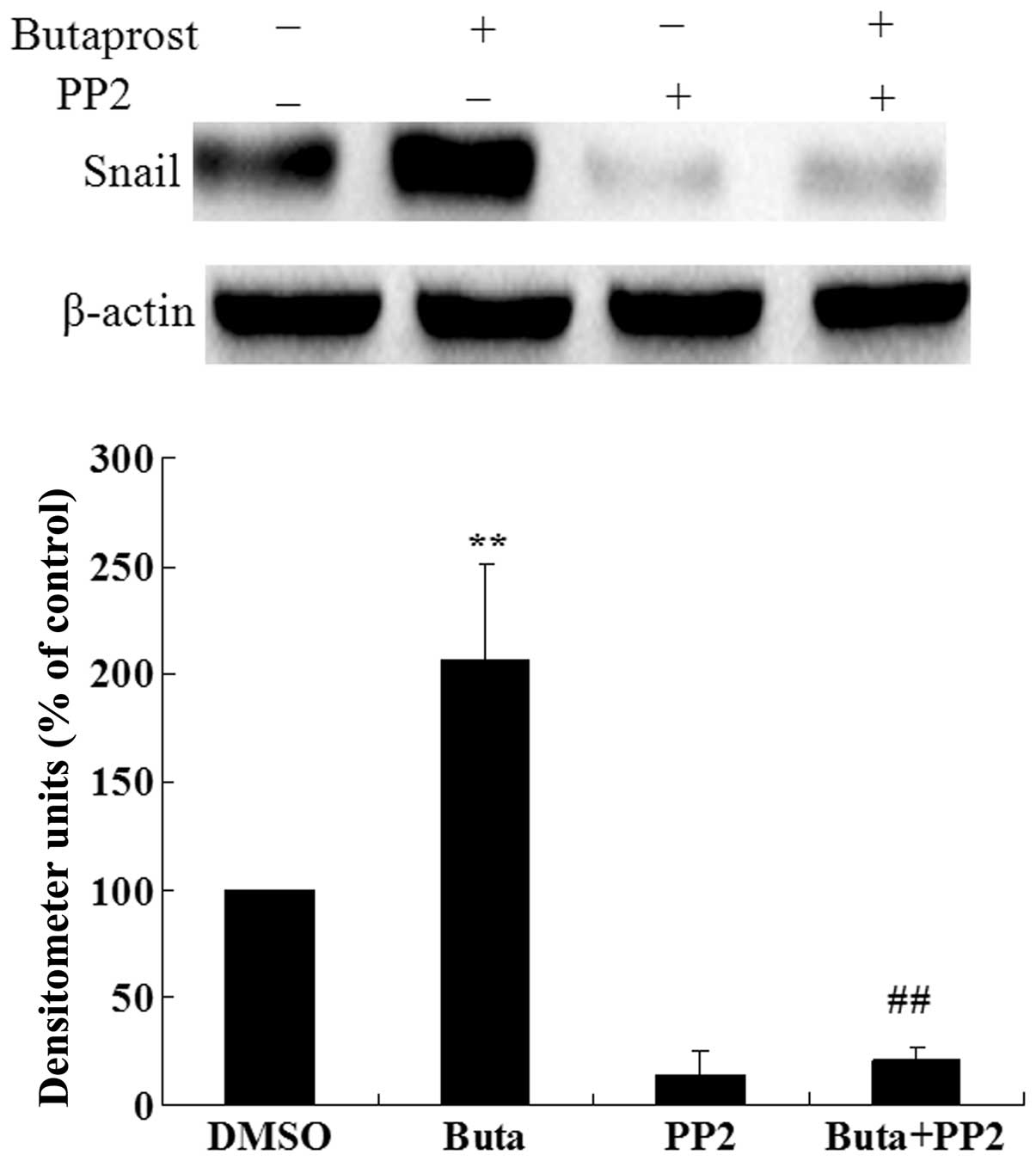
We further investigated whether Src is involved in
the Snail expression induced by butaprost. As shown in Fig. 4, pretreatment of Huh-7 cells with
Src inhibitor PP2 markedly suppressed the Snail expression induced
by butaprost. These observations indicate that Src kinase is
involved in EP2 receptor-mediated Snail expression.
EGFR/Akt is involved in EP2-mediated
Snail expression
Phosphatidylinositol 3-kinase (PI3K), the kinase
that modulates the phosphorylation of Akt, is one of the most
important downstream proteins in the EGFR signaling pathway. We
sought to clarify the EGFR and Akt effects on EP2 receptor
agonist-induced Snail expression. As PI3K modulates the
phosphorylation of Akt, Huh-7 cells were pre-treated with EGFR
inhibitor 5 μM AG1478 or PI3K inhibitor 10 μM LY294002 for 1 h
before 5 μM butaprost treatment for 24 h, and were subsequently
subjected to immunoblotting assay to assess the effect of these
inhibitors on butaprost-induced expression of Snail. The data in
Fig. 5A show that butaprost-induced
Snail expression was decreased ~67.92% by the EGFR inhibitor and
~56.63% by the PI3K inhibitor. To determine whether EGFR and Akt
were activated by EP2 receptor agonist stimulation, activation of
EGFR and Akt was measured by detecting the phosphorylation of EGFR
and Akt with western blot analysis. Huh-7 cells were exposed to 5
μM butaprost for different periods of time. EGFR and Akt activation
were detected at 5 min following butaprost treatment. As shown in
Fig. 5B and C, the phosphorylation
level of EGFR and Akt increased significantly after butaprost
treatment in Huh-7 cells, and the effect reached its maximum at 30
and 45 min, respectively. These data indicate that butaprost
induced EGFR and Akt phosphorylation in a time-dependent manner in
Huh-7 cells. Based on these findings, treatment with 5 μM butaprost
for 45 min was used for subsequent experiments. Huh-7 cells were
pre-treated with Src inhibitor 10 μM PP2 or EGFR inhibitor 5 μM
AG1478 for 1 h before 5 μM butaprost treatment for 45 min. The data
in Fig. 5D show that AG1478 and PP2
suppressed the butaprost-induced Akt phosphorylation, further
indicating that activated Src and EGFR are upstream of Akt. The
experiments in the above sections showed that EGFR/Akt is involved
in the EP2-mediated Snail expression in Huh-7 cells.
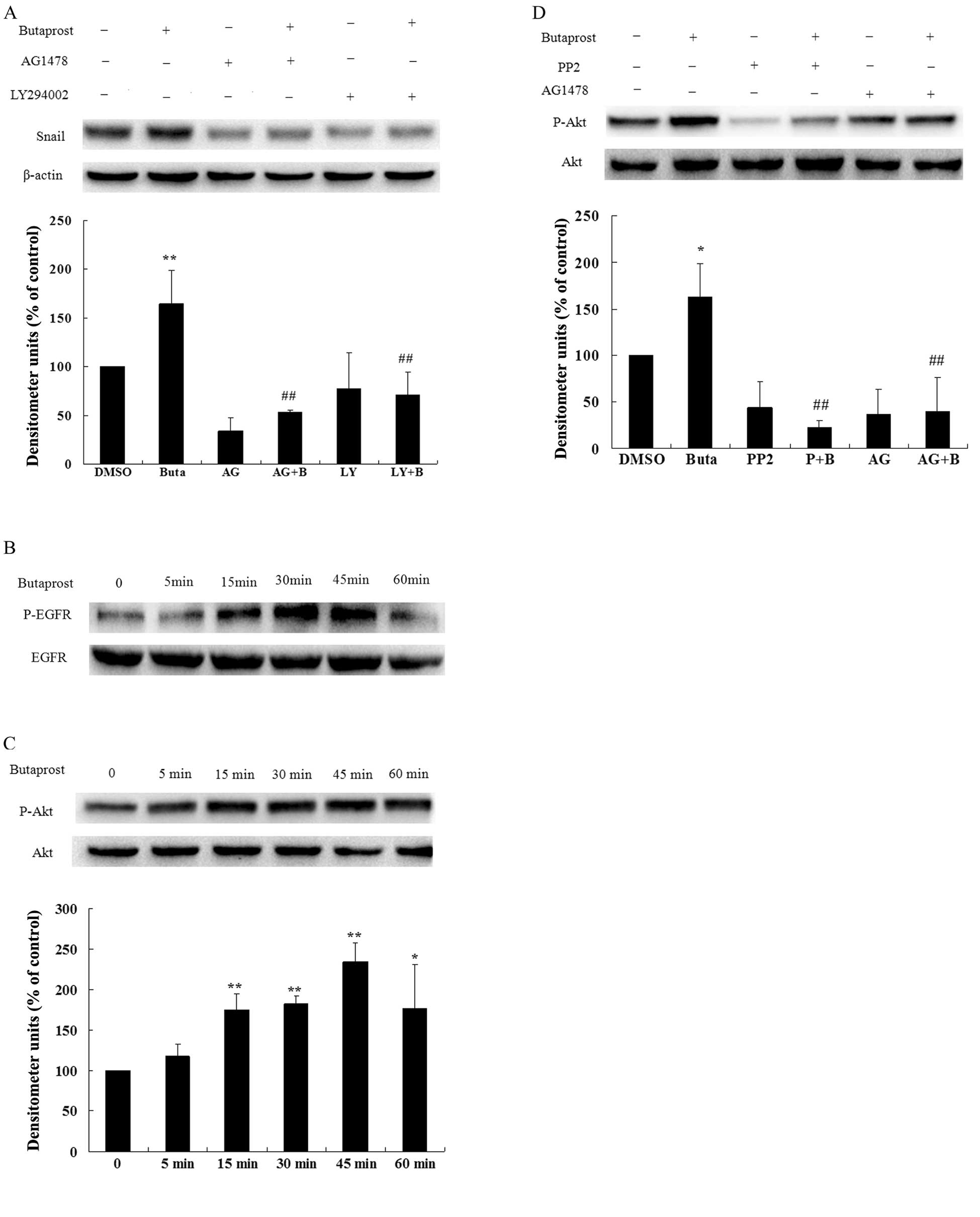 | Figure 5EGFR and Akt are involved in the
expression of Snail protein induced by PGE2 via EP2
receptor. (A) The effect of EGFR inhibitor AG1478 and PI3K
inhibitor LY294002 on Snail expression induced by the EP2 receptor.
Huh-7 cells cultured in serum-free medium were pretreated with 5 μM
AG1478 or 10 μM LY294002 for 1 h, and were then treated with 5 μM
butaprost for 24 h; 40 mM Licl and 10 μM MG132 were added 7 h
before lysis of the cells. Snail expression level was determined by
immunoblotting with anti-Snail antibody. β-actin as loading control
was determined by immunoblotting with anti-β-actin antibody.
Quantitative analysis of the Snail expression level was carried out
by calculating the ratio between Snail protein and β-actin
expression levels from three different experiments.
**P<0.01, compared with the control;
##P<0.01, compared with butaprost treatment. (B) The
effect of butaprost on EGFR phosphorylation. Huh-7 cells cultured
in serum-free medium were treated with butaprost at 5 μM for the
indicated times, and cell lysates were obtained. EGFR
phosphorylation level was determined by immunoblotting with
anti-phospho-EGFR antibody. Total EGFR expression level in cell
lysates was determined by reprobing the same blot with anti-EGFR
antibody. (C) The effect of butaprost on Akt phosphorylation. Huh-7
cells cultured in serum-free medium were treated with butaprost at
5 μM for the indicated times, and cell lysates were obtained. Akt
phosphorylation level was determined by immunoblotting with
anti-phospho-Akt antibody. Total Akt expression level in cell
lysates was determined by reprobing the same blot with anti-Akt
antibody. Quantitative analysis of the Akt phosphorylation level
was carried out by calculating the ratio between Akt protein and
phosphorylation level from three different experiments.
*P<0.05, **P<0.01 compared with the
control. (D) The Src and EGFR inhibitors suppressed
butaprost-induced phosphorylation of Akt. Huh-7 cells were
serum-starved for 12 h and then pre-treated with 10 μM PP2 or 5 μM
AG1478 for 1 h before 5 μM butaprost treatment for 45 min. Akt
phosphorylation level was determined by immunoblotting with
anti-phospho-Akt antibody. Total Akt expression level in cell
lysates was determined by reprobing the same blot with anti-Akt
antibody. Quantitative analysis of the Akt phosphorylation level
was carried out by calculating the ratio between Akt protein and
phosphorylation level from three different experiments.
*P<0.05, compared with the control;
##P<0.01 compared with butaprost treatment. Buta or
B, butaprost; P, PP2; AG, AG1478; LY, LY94002. |
mTOR plays a key role in EP2-mediated
Snail expression
Mammalian target of rapamycin (mTOR) is an important
downstream of PI3K/Akt signaling. To clarify the mTOR inhibitor 5
μM PP242 effects on EP2 agonist-induced Snail expression, Huh-7
cells were pre-treated with or without PP242 for 1 h prior to 5 μM
butaprost treatment for 24 h, and were subsequently subjected to
immunoblotting assay to assess the effect of these inhibitors on
butaprost-induced expression of Snail. As shown in Fig. 6A, butaprost-induced Snail expression
was almost completely suppressed by the mTOR inhibitor. Then, to
determine whether the mTOR was activated by EP2 receptor agonist
stimulation, activation of mTOR was measured by detecting the
phosphorylation of mTOR with western blot analysis. Huh-7 cells
were exposed to 5 μM butaprost for different periods of time. mTOR
activation was detected at 5 min following butaprost treatment. As
shown in Fig. 6B, the
phosphorylation level of mTOR increased significantly after
butaprost treatment, and the effect reached its maximum at 15 min.
The data indicate that butaprost induced mTOR phosphorylation in a
time-dependent manner in Huh-7 cells. The data presented in the
above sections indicate that mTOR is involved in the EP2-mediated
expression of Snail in Huh-7 cells.
Discussion
Cyclooxygenase-2 (COX-2) plays a significant role in
the progression of HCC; higher tumor cytosolic COX-2 level is
associated with poorer patient survival (25,26).
PGE2, the key product of COX-2, plays crucial roles in
the development of several human malignant tumors, including HCC
(27–30). However, the exact mechanisms through
which PGE2 promotes hepatocarcinogenesis are only
beginning to be resolved. Previous studies indicated that
PGE2 exerts its biological functions via interaction
with four types of G-protein-coupled receptors (GPCRs): EP1, EP2,
EP3 and EP4, on the cell surface membrane (31). The downstream signaling transduction
pathways of these EP receptors have been well characterized. The
EP1 receptor is coupled with Gαq protein and thus signals through
protein kinase C (PKC) and intracellular Ca2+; the EP3
receptor is coupled with Gαi protein, with inhibitory effects on
adenylyl cyclase (AC); the EP2 and EP4 receptors are both coupled
with Gαs protein, but they have different downstream signal
pathways; the EP4 receptor could activate the phosphatidylinositol
3-kinase (PI3K)-Akt pathway and the EP2 receptor could increase
intracellular cAMP level and thus activate protein kinase A (PKA)
pathway (10,32,33).
The epithelial-mesenchymal transition (EMT) is
regarded as a key step in epithelium-derived tumor invasion and
metastasis (34). During the EMT
process, epithelial cells lose cell polarity and adhesion, and
undergo transdifferentiation into a mesenchymal phenotype with
highly migratory abilities. Snail, as one of the zinc-finger
transcriptional factors, plays pivotal roles in a number of tumor
invasions and metastasis (15,35).
As one of the main transcription factors controlling the EMT
process, Snail could repress E-cadherin (one of the epithelial
marker proteins) transcription level by binding with E-box element
on the E-cadherin promoter, and increase some mesenchymal marker
proteins expression level, such as Vimentin (19,36).
Furthermore, recent studies have indicated that during the
progression of HCC, Snail expression level was markedly increased
and could enhance the cancer cell invasion ability through
upregulating the MMP gene family expression (37,38).
Meanwhile, in renal cell carcinoma, elevated Snail and MMP protein
expression level frequently indicated the poor prognosis of
patients with this malignant tumor (39).
It was reported that in non-small cell lung cancer
(NSCLC), knockdown of Snail protein interrupted the
PGE2-induced repression of E-cadherin expression level
(40). In the Huh-7 cell line, we
confirmed that PGE2 could promote HCC cell migration and
invasion. At the same time, we also found that PGE2
markedly increased Snail expression level. However, the mechanisms
underlying PGE2-upregulated Snail protein expression
level remain unclear. PGE2 could bind with four types of
EP receptors on the cell surface membrane and transmit the
different signal transduction pathways to show various
physiological and pathophysiological functions. Hence, which type
of EP receptor is mainly responsible for this phenomenon is of
particular interest to us. It is well-established that EP2 receptor
plays a crucial role in various types of carcinogenesis (11,41).
The present study showed that treatment of Huh-7 cells with
butaprost could significantly increase the Snail expression level,
which indicates that EP2 receptor plays a key role in
PGE2-induced Snail expression.
On the basis of previous research, we know that the
EP2 receptor is coupled with the Gαs protein to exert its
biological functions. The heterotrimeric G protein consists of α, β
and γ subunits, and α subunits could be divided into Gαs, Gαi and
Gαq. Different subtypes of Gα subunits could mediate specific
signaling pathways. When binding with PGE2, EP2 receptor
could activate the Gαs subunit, which increases intracellular cAMP
level, and thus enhances the PKA activity. Based on this canonic
pathway, treatment of Huh-7 cells with the AC inhibitor SQ22536 and
the PKA inhibitor H89 should block the effects of PGE2
or EP2 receptor agonist-induced-snail expression. However, we did
not observe these expected results in our experiments (data not
shown). These results indicate that the Gαs/AC/PKA pathway, the
canonic pathway of EP2 receptor may not be responsible for the EP2
receptor-mediated Snail protein expression.
Several studies have shown that GPCRs could also
modulate the activation of the EGFR (8,27,42,43).
In endometrial cancer, activation of EGFR resulted in
overexpression of Snail (44). In
human mesenchymal stem cells, PGE2 could promote cell
migration and proliferation, at least in part, via the EP2
receptor-dependent β-arrestin-1/JNK signaling pathways (45). In squamous cell carcinoma,
activation of the EP2 receptor could transactivate the EGFR via PKA
and c-Src kinases (46). In mouse
skin papilloma, the EP2 receptor could form a complex with
β-arrestin-1 and Src, which promoted the tumor formation (47). Therefore, we hypothesized that the
EP2 receptor might upregulate the Snail expression in
β-arrestin-1/Src/EGFR pathways. The observations that the inhibitor
of the Src and EGFR suppressed the expression of Snail induced by
EP2 receptor agonist support our hypothesis. These data suggest
that Src and EGFR are involved in butaprost-mediated Snail
expression.
The EGF receptor is a transmembrane tyrosine kinase
that belongs to the HER/ErbB protein family. EGFR controls a
variety of biological responses such as cell proliferation and
migration (48). These effects are
mediated via activation of downstream molecules, including the
PI3K/Akt pathway (43,49). PI3K is composed of the p85
regulatory subunit and the p110 catalytic subunit. When EGFR is
activated, which could recruit the p85 subunit, the p110 subunit is
activated, leading to PI3K activation. Activated PI3K can
phosphorylate PIP2 to form the second messenger PIP3. PIP3 could
activate 3′-phosphoinositide-dependent kinase (PDK) by binding to
the PH domain of PDK. Activated PDK activates Akt by
phosphorylating its Thr308 and Ser473. Early reports showed that,
in HCC cells and human liver cancer tissues, the level of COX-2
expression and Akt phosphorylation is correlated positively with
the cell proliferation (50).
Blocking of either COX-2 or the Akt pathway can inhibit the process
of EMT (51). In glioma C6 cells,
PGE2 induces HO-1 protein expression via EP2 receptor
through PKA and PI3K signaling pathways (52). These findings suggest that Akt may
play an important role in the EP2 receptor-mediated Snail
expression. Our data showed that when binding with PGE2,
the EP2 receptor could markedly enhance Akt activity.
Activation of Akt mainly goes through
phosphorylation of the forkhead family transcription factor FKHR
and inhibition of BAD phosphorylation activity to anti-apoptosis,
via mTOR to mediate cell proliferation, via GSK-3β, caspase-9 and
other downstream substrates to exert biological effects (53). One protein that is emerging as a
central downstream of Akt is mTOR, which regulates tumor cell
proliferation, growth and survival (54,55).
mTOR usually regulates biogenesis with two types of complexes,
mTORC1 and mTORC2, activating p70S6 kinase, which enhances the
translation of mRNAs, and inhibiting 4E-BP1, a translational
repressor of mRNAs, contributes to cell growth and proliferation
(56,57). Our data showed that EP2 receptor
agonist triggered the activation of mTOR. The results suggest that
the activation of mTOR may be involved in EP2-mediated Snail
expression.
In conclusion, the results showed that the subtype 2
of PGE2 receptor upregulating Snail protein appears not
to be through the canonic G protein-dependent activation of PKA
pathway but through the Src-EGFR-Akt-mTOR pathway. The present
study provides further insight into the mechanisms by which
PGE2 promotes HCC invasion and migration. Targeting of
the PGE2/EP2/Snail pathway may be a novel therapeutic
strategy in the prevention and treatment of malignant diseases.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (30871015 and 81172003) and by
a Project Funded by the Priority Academic Program Development of
Jiangsu Higher Education Institutions (PAPD).
References
|
1
|
Wu T: Cyclooxygenase-2 in hepatocellular
carcinoma. Cancer Treat Rev. 32:28–44. 2006. View Article : Google Scholar
|
|
2
|
Pang RW, Joh JW, Johnson PJ, Monden M,
Pawlik TM and Poon RT: Biology of hepatocellular carcinoma. Ann
Surg Oncol. 15:962–971. 2008. View Article : Google Scholar
|
|
3
|
Uchino K, Tateishi R, Shiina S, et al:
Hepatocellular carcinoma with extrahepatic metastasis: clinical
features and prognostic factors. Cancer. 117:4475–4483. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bai XM, Zhang W, Liu NB, et al: Focal
adhesion kinase: Important to prostaglandin E2-mediated
adhesion, migration and invasion in hepatocellular carcinoma cells.
Oncol Rep. 21:129–136. 2009.PubMed/NCBI
|
|
5
|
Wu J, Zhang Y, Frilot N, Kim JI, Kim WJ
and Daaka Y: Prostaglandin E2 regulates renal cell
carcinoma invasion through the EP4 receptor-Rap GTPase signal
transduction pathway. J Biol Chem. 286:33954–33962. 2011.
|
|
6
|
Vo BT, Morton D Jr, Komaragiri S, Millena
AC, Leath C and Khan SA: TGF-β effects on prostate cancer cell
migration and invasion are mediated by PGE2 through
activation of PI3K/AKT/mTOR pathway. Endocrinology. 154:1768–1779.
2013.
|
|
7
|
Zhang L, Jiang L, Sun Q, et al:
Prostaglandin E2 enhances mitogen-activated protein kinase/Erk
pathway in human cholangiocarcinoma cells: involvement of EP1
receptor, calcium and EGF receptors signaling. Mol Cell Biochem.
305:19–26. 2007. View Article : Google Scholar
|
|
8
|
Han C, Michalopoulos GK and Wu T:
Prostaglandin E2 receptor EP1 transactivates EGFR/MET
receptor tyrosine kinases and enhances invasiveness in human
hepatocellular carcinoma cells. J Cell Physiol. 207:261–270.
2006.
|
|
9
|
Breyer RM, Bagdassarian CK, Myers SA and
Breyer MD: Prostanoid receptors: subtypes and signaling. Annu Rev
Pharmacol Toxicol. 41:661–690. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bos CL, Richel DJ, Ritsema T,
Peppelenbosch MP and Versteeg HH: Prostanoids and prostanoid
receptors in signal transduction. Int J Biochem Cell Biol.
36:1187–1205. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiang J and Dingledine R: Role of
prostaglandin receptor EP2 in the regulations of cancer cell
proliferation, invasion, and inflammation. J Pharmacol Exp Ther.
344:360–367. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pan MR, Hou MF, Chang HC and Hung WC:
Cyclooxygenase-2 up-regulates CCR7 via EP2/EP4 receptor signaling
pathways to enhance lymphatic invasion of breast cancer cells. J
Biol Chem. 283:11155–11163. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang X and Klein RD: Prostaglandin E2
induces vascular endothelial growth factor secretion in prostate
cancer cells through EP2 receptor-mediated cAMP pathway. Mol
Carcinog. 46:912–923. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guarino M, Rubino B and Ballabio G: The
role of epithelial-mesenchymal transition in cancer pathology.
Pathology. 39:305–318. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fan F, Samuel S, Evans KW, et al:
Overexpression of snail induces epithelial-mesenchymal transition
and a cancer stem cell-like phenotype in human colorectal cancer
cells. Cancer Med. 1:5–16. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Shi J, Chai K, Ying X and Zhou BP:
The role of Snail in EMT and tumorigenesis. Curr Cancer Drug
Targets. 13:963–972. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Emadi Baygi M, Soheili ZS, Schmitz I,
Sameie S and Schulz WA: Snail regulates cell survival and inhibits
cellular senescence in human metastatic prostate cancer cell lines.
Cell Biol Toxicol. 26:553–567. 2010.PubMed/NCBI
|
|
18
|
Nishioka R, Itoh S, Gui T, et al: SNAIL
induces epithelial-to-mesenchymal transition in a human pancreatic
cancer cell line (BxPC3) and promotes distant metastasis and
invasiveness in vivo. Exp Mol Pathol. 89:149–157. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Haslehurst AM, Koti M, Dharsee M, et al:
EMT transcription factors snail and slug directly contribute to
cisplatin resistance in ovarian cancer. BMC Cancer. 12:912012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang H, Wang HS, Zhou BH, et al:
Epithelial-mesenchymal transition (EMT) induced by TNF-α requires
AKT/GSK-3β-mediated stabilization of snail in colorectal cancer.
PLoS One. 8:e566642013.
|
|
21
|
He H, Chen W, Wang X, et al: Snail is an
independent prognostic predictor for progression and patient
survival of gastric cancer. Cancer Sci. 103:1296–1303. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Han C, Demetris AJ, Michalopoulos G,
Shelhamer JH and Wu T: 85-kDa cPLA2 plays a critical
role in PPAR-mediated gene transcription in human hepatoma cells.
Am J Physiol Gastrointest Liver Physiol. 282:G586–G597.
2002.PubMed/NCBI
|
|
23
|
Rahman MA, Dhar DK, Yamaguchi E, et al:
Coexpression of inducible nitric oxide synthase and COX-2 in
hepatocellular carcinoma and surrounding liver: possible
involvement of COX-2 in the angiogenesis of hepatitis C
virus-positive cases. Clin Cancer Res. 7:1325–1332. 2001.
|
|
24
|
Li T, Zhu Y, Ren W, et al: High
co-expression of vascular endothelial growth factor receptor-1 and
Snail is associated with poor prognosis after curative resection of
hepatocellular carcinoma. Med Oncol. 29:2750–2761. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang TC, Poon RT, Lau CP, Xie D and Fan
ST: Tumor cyclooxygenase-2 levels correlate with tumor invasiveness
in human hepatocellular carcinoma. World J Gastroenterol.
11:1896–1902. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Koga H: Hepatocellular carcinoma: is there
a potential for chemoprevention using cyclooxygenase-2 inhibitors?
Cancer. 98:661–667. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bai XM, Jiang H, Ding JX, et al:
Prostaglandin E2 upregulates survivin expression via the
EP1 receptor in hepatocellular carcinoma cells. Life Sci.
86:214–223. 2009.
|
|
28
|
Ma J, Chen M, Xia SK, et al: Prostaglandin
E2 promotes liver cancer cell growth by the upregulation
of FUSE-binding protein 1 expression. Int J Oncol. 42:1093–1104.
2013.
|
|
29
|
Mayoral R, Fernandez-Martinez A, Bosca L
and Martin-Sanz P: Prostaglandin E2 promotes migration
and adhesion in hepatocellular carcinoma cells. Carcinogenesis.
26:753–761. 2005.PubMed/NCBI
|
|
30
|
Sheng H, Shao J, Washington MK and DuBois
RN: Prostaglandin E2 increases growth and motility of
colorectal carcinoma cells. J Biol Chem. 276:18075–18081.
2001.PubMed/NCBI
|
|
31
|
Breyer MD and Breyer RM: G protein-coupled
prostanoid receptors and the kidney. Annu Rev Physiol. 63:579–605.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Boie Y, Stocco R, Sawyer N, et al:
Molecular cloning and characterization of the four rat
prostaglandin E2 prostanoid receptor subtypes. Eur J
Pharmacol. 340:227–241. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Regan JW: EP2 and
EP4 prostanoid receptor signaling. Life Sci. 74:143–153.
2003.
|
|
34
|
Sanchez-Tillo E, Liu Y, de Barrios O, et
al: EMT-activating transcription factors in cancer: beyond EMT and
tumor invasiveness. Cell Mol Life Sci. 69:3429–3456. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Blechschmidt K, Kremmer E, Hollweck R, et
al: The E-cadherin repressor snail plays a role in tumor
progression of endometrioid adenocarcinomas. Diagn Mol Pathol.
16:222–228. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guaita S, Puig I, Franci C, et al: Snail
induction of epithelial to mesenchymal transition in tumor cells is
accompanied by MUC1 repression and ZEB1 expression. J Biol Chem.
277:39209–39216. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miyoshi A, Kitajima Y, Sumi K, et al:
Snail and SIP1 increase cancer invasion by upregulating MMP family
in hepatocellular carcinoma cells. Br J Cancer. 90:1265–1273. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Miyoshi A, Kitajima Y, Kido S, et al:
Snail accelerates cancer invasion by upregulating MMP expression
and is associated with poor prognosis of hepatocellular carcinoma.
Br J Cancer. 92:252–258. 2005.PubMed/NCBI
|
|
39
|
Mikami S, Katsube K, Oya M, et al:
Expression of Snail and Slug in renal cell carcinoma: E-cadherin
repressor Snail is associated with cancer invasion and prognosis.
Lab Invest. 91:1443–1458. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dohadwala M, Yang SC, Luo J, et al:
Cyclooxygenase-2-dependent regulation of E-cadherin: prostaglandin
E2 induces transcriptional repressors ZEB1 and snail in
non-small cell lung cancer. Cancer Res. 66:5338–5345. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kuo KT, Wang HW, Chou TY, et al:
Prognostic role of PGE2 receptor EP2 in esophageal squamous cell
carcinoma. Ann Surg Oncol. 16:352–360. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Buchanan FG and DuBois RN: Emerging roles
of β-arrestins. Cell Cycle. 5:2060–2063. 2006.
|
|
43
|
Fischer OM, Hart S, Gschwind A and Ullrich
A: EGFR signal transactivation in cancer cells. Biochem Soc Trans.
31:1203–1208. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hipp S, Walch A, Schuster T, et al:
Activation of epidermal growth factor receptor results in snail
protein but not mRNA overexpression in endometrial cancer. J Cell
Mol Med. 13:3858–3867. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yun SP, Ryu JM, Jang MW and Han HJ:
Interaction of profilin-1 and F-actin via a β-arrestin-1/JNK
signaling pathway involved in prostaglandin E2-induced
human mesenchymal stem cells migration and proliferation. J Cell
Physiol. 226:559–571. 2010.
|
|
46
|
Donnini S, Finetti F, Solito R, et al: EP2
prostanoid receptor promotes squamous cell carcinoma growth through
epidermal growth factor receptor transactivation and iNOS and
ERK1/2 pathways. FASEB J. 21:2418–2430. 2007. View Article : Google Scholar
|
|
47
|
Chun KS, Lao HC, Trempus CS, Okada M and
Langenbach R: The prostaglandin receptor EP2 activates multiple
signaling pathways and β-arrestin1 complex formation during mouse
skin papilloma development. Carcinogenesis. 30:1620–1627. 2009.
|
|
48
|
Ayuso-Sacido A, Moliterno JA, Kratovac S,
et al: Activated EGFR signaling increases proliferation, survival,
and migration and blocks neuronal differentiation in post-natal
neural stem cells. J Neurooncol. 97:323–337. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Glaysher S, Bolton LM, Johnson P, et al:
Targeting EGFR and PI3K pathways in ovarian cancer. Br J Cancer.
109:1786–1794. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Leng J, Han C, Demetris AJ, Michalopoulos
GK and Wu T: Cyclooxygenase-2 promotes hepatocellular carcinoma
cell growth through Akt activation: evidence for Akt inhibition in
celecoxib-induced apoptosis. Hepatology. 38:756–768. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ogunwobi OO, Wang T, Zhang L and Liu C:
Cyclooxygenase-2 and Akt mediate multiple growth-factor-induced
epithelial-mesenchymal transition in human hepatocellular
carcinoma. J Gastroenterol Hepatol. 27:566–578. 2011. View Article : Google Scholar
|
|
52
|
Park MK, Kang YJ, Ha YM, et al: EP2
receptor activation by prostaglandin E2 leads to
induction of HO-1 via PKA and PI3K pathways in C6 cells. Biochem
Biophys Res Commun. 379:1043–1047. 2009.PubMed/NCBI
|
|
53
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and Gonzalez-Baron M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004.PubMed/NCBI
|
|
54
|
Lau MT and Leung PC: The PI3K/Akt/mTOR
signaling pathway mediates insulin-like growth factor 1-induced
E-cadherin down-regulation and cell proliferation in ovarian cancer
cells. Cancer Lett. 326:191–198. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hung CM, Garcia-Haro L, Sparks CA and
Guertin DA: mTOR-dependent cell survival mechanisms. Cold Spring
Harb Perspect Biol. 4:a0087712012.PubMed/NCBI
|
|
56
|
Garcia-Maceira P and Mateo J: Silibinin
inhibits hypoxia-inducible factor-1α and mTOR/p70S6K/4E-BP1
signalling pathway in human cervical and hepatoma cancer cells:
implications for anticancer therapy. Oncogene. 28:313–324.
2009.
|
|
57
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|