Introduction
Endometrial cancer is the most common malignancy of
the female genital tract in developed countries, with an incidence
rate of 12,9/100.000 women/year and a mortality rate of 2,4/100.000
(1). More than 88,000 new cases a
year are reported in the European Union (2). The incidence has considerably
increased during the last three decades and it is currently the
fourth most frequent cancer among women, exceeded only by cancer of
the breast, lung, colon and rectum (3), representing the seventh cause of
cancer-related mortality among women in Western Europe (2). In industrialized countries, most cases
of adenocarcinoma of the endometrium are diagnosed at an early
stage with an overall survival rate of ~90% (2).
The cancer spread pathways are: direct expansion,
free transtubal implantation, blood and lymphatic invasion. The
lymphatic spread is the most frequent pathway occurring three times
more than the blood spread, and it allows malignant cells to reach
the parametrium, vagina, ovaries and retroperitoneal, pelvic and
para-aortic lymph nodes (4). In
general, this type of cancer primarily involves the pelvic lymph
nodes: external iliac, internal iliac, common iliac (medial and
lateral) and obturator (external and internal in relation with the
obturator nerve). Other patterns of lymphatic spread are the
presacral, inguinal and lombo-aortic lymph nodes (i.e. para-caval,
precaval, retro-caval, right lateral aortic). In relation to
grading G1–G3, myometrial invasion and International Federation of
Gynecology and Obstetrics (FIGO) staging, pelvic positive lymph
nodes are reported in 8–15% of patients in clinical stage I, 30% of
patients in clinical stage II and in 45% of patients in clinical
stage III (5).
Among the pelvic lymph nodes, the external iliac
nodes are the most frequently affected by metastasis both in tumors
limited to the uterine corpus and to the cervix. An earlier spread
to the common iliac lymph nodes is reported in the latter one.
Para-aortic lymph nodes (1–6% of cases) are rarely involved as the
primary station, indeed they are mainly associated with metastatic
pelvic lymph nodes (6). As
demonstrated by several authors, risk factors for the recurrence of
endometrial carcinoma can be divided into uterine and extrauterine
factors (4). Uterine factors
include histologic type, grade (7),
depth of myometrial invasion (8),
cervical involvement (4), vascular
invasion (9,10), presence of atypical endometrial
hyperplasia (11), hormone receptor
status and DNA ploidy (12).
Extrauterine factors include adnexal involvement,
intraperitoneal metastasis, positive peritoneal cytology (13,14)
and pelvic and para-aortic lymph node metastasis (7,15).
Patients with no evidence of extrauterine disease, no cervical
involvement and no evidence of vascular invasion are at low overall
risk of recurrence. Grade and depth of invasion are important
prognostic factors for these patients. Women with evidence of
extrauterine disease, cervical involvement or vascular invasion
constitute a high risk group. If one of these three factors is
positive, the frequency of recurrence is 20%, increasing to 43% for
two positive factors and 63% for three factors.
Furthermore, some clinical factors assume prognostic
value; these include, BMI, age, race, socioeconomic status and
previous use of tamoxifen as hormonal treatment after breast cancer
(16). It has also been
demonstrated that younger women have a more favorable prognosis due
to a significantly higher proportion of early stage disease and
less myometrial invasion (4).
In relation to the risk of recurrence in stage I,
this can be divided into three categories: low risk (stage IA G1 G2
with endometrioid type), intermediate risk (stage IA G3 with
endometrioid type, stage IB G1 and G2 with endometrioid type), high
risk (stage IB G3 with endometrioid type, all stages with
non-endometrioid type) (2). The
standard approach for endometrial cancer is surgery which consists
in total hysterectomy, bilateral salpingo-oophorectomy, colpectomy
of the superior third and peritoneal washing (17).
FIGO recommends the evaluation of pelvic and
para-aortic lymph nodes in every case, but a few studies have
challenged its utility. The ASTEC study in particular examined
lymphadenectomy in >1,400 patients from 85 centres in 4
different states and showed that lymphadenectomy does not provide
improvement in low risk cancer (stage IA, G1–G2) (18). Lymphadenectomy, by contrast, is
necessary in intermediate-high risk cancer to guide surgical
staging and therapeutic choices.
The approach for stage II endometrial cancer
consists in radical hysterectomy with bilateral
salpingo-oophorectomy and systematic pelvic lymphadenectomy, with
or without para-aortic lymphadenectomy (17). Maximal surgical debulking is
imperative in stage III or IV cancer.
The aim of the present study was to assess tumor
size in patients undergoing surgical staging for endometrial cancer
in order to verify a correlation between this and the most
important prognostic factors, and to identify a novel helpful
element in planning surgical approach.
Patients and methods
One hundred and forty-seven patients with
endometrial cancer treated at the Gynecologic-Obstetric Clinic of
the University of Parma from August 2000 to January 2012
participated in the present study. All the enrolled patients were
properly informed regarding the collection of data and they
provided a written consent form to the use of data respecting their
privacy (Italian law 675/96).
All patients underwent a surgical approach according
to current international guidelines; laparotomic hysterectomy with
bilateral salpingo-oophorectomy and systematic pelvic
lymphadenectomy in 129 (87.8%) patients and laparoscopic
hysterectomy with bilateral salpingo-oophorectomy and systematic
pelvic lymphadenectomy in 18 (12.2%). We studied both patients
undergoing follow-up and patients with no follow-up.
In the present retrospective study, the following
inclusion criteria were used: histological diagnosis of endometrial
endometrioid, primitive cancer, no important comorbidity, treatment
with surgical lymphadenectomy, measurement of the largest dimension
of the tumor by the pathologist. We collected data about cancer
prognostic factors: FIGO stage, grading, peritoneal washing,
positive lymph nodes. This information is kept in the Gynecologic
ward archive and in the central archive of our hospital.
We also gathered data regarding tumor markers CA 125
reported in the patients’ medical reports. We revised the patients’
staging in order to conform them to the 2009 FIGO staging review.
All tumors were measured by the expert pathologist and were
reported in the original pathology reports. According to other
authors and histological reports available, we considered the
largest diameter.
We studied the state of the lymph nodes considering
positive or negative nodes independently of their number or
percentage and we correlated primary diameters with other
prognostic factors such as stage, grading, peritoneal cytologic
results, lymph node metastasis tumor markers.
Statistical analysis was performed by SPSS (Chicago,
IL, USA) software for Windows version 19, using parametric and
non-parametric tests where appropriate. We performed the
Kolmogorov-Smirnov test for the normality of distribution.
Continuous data were tested with the t-test, and categorical
variables were tested with the χ2 test or Fisher’s exact
test where appropriate. The results obtained from the data
collection are expressed in absolute number and percentage for
discrete variables, in means ± standard deviation for continuous
variables. Statistical significance of differences was defined as
p<0.05.
Results
The distribution of the FIGO stage of the study
group showed 67 patients (45.6%) in stage IA, 49 (33.3%) in stage
IB, 6 (4.1%) in stage II, 9 (6.1%) in stage IIIA and 16 (10.9%) in
stage IIIC. There were no patients in stage IV. The grading
distribution indicated 64 cases (43.5%) G1, 66 (44.9%) G2 and 17
(11.6%) G3. Peritoneal washings for cytologic assessment were
available from 122 patients. Peritoneal cytologic results were
negative in 118 (96.7%) patients and positive in 4 (3.3%).
All patients underwent pelvic lymphadenectomy, the
median of the lymph nodes removed was 16; in 131 (89.1%) cases
nodes were negative, in 16 (10.9%) cases they were positive
(Table I). CEA was available for 25
patients, CA 125 for 83, CA 19.9 for 58 and CA 15.3 for 57.
 | Table IDetailed data regarding surgical and
pathological features of patients. |
Table I
Detailed data regarding surgical and
pathological features of patients.
| Total, n (%) |
|---|
| Patients | 147 (100) |
| Stage | 147 (100) |
| IA | 67 (45.6) |
| IB | 49 (33.3) |
| II | 6 (4.1) |
| IIIA | 9 (6.1) |
| IIIB | 0 (0.0) |
| IIIC | 16 (10.9) |
| IVA | 0 (0.0) |
| IVB | 0 (0.0) |
| Grading | 147 (100) |
| 1 | 64 (43.5) |
| 2 | 66 (44.9) |
| 3 | 17 (11.6) |
| Peritoneal cytologic
results | 122 (83) |
| Positive | 4 (3.3) |
| Negative | 118 (96.7) |
| Lymphadenectomy | 147 (100) |
| Positive | 16 (10.9) |
| Negative | 131 (89.1) |
We correlated tumor size with all these variables
(Table II). The mean tumor largest
diameter was 4.26 (range 0.4–13 cm). Tumor size was significantly
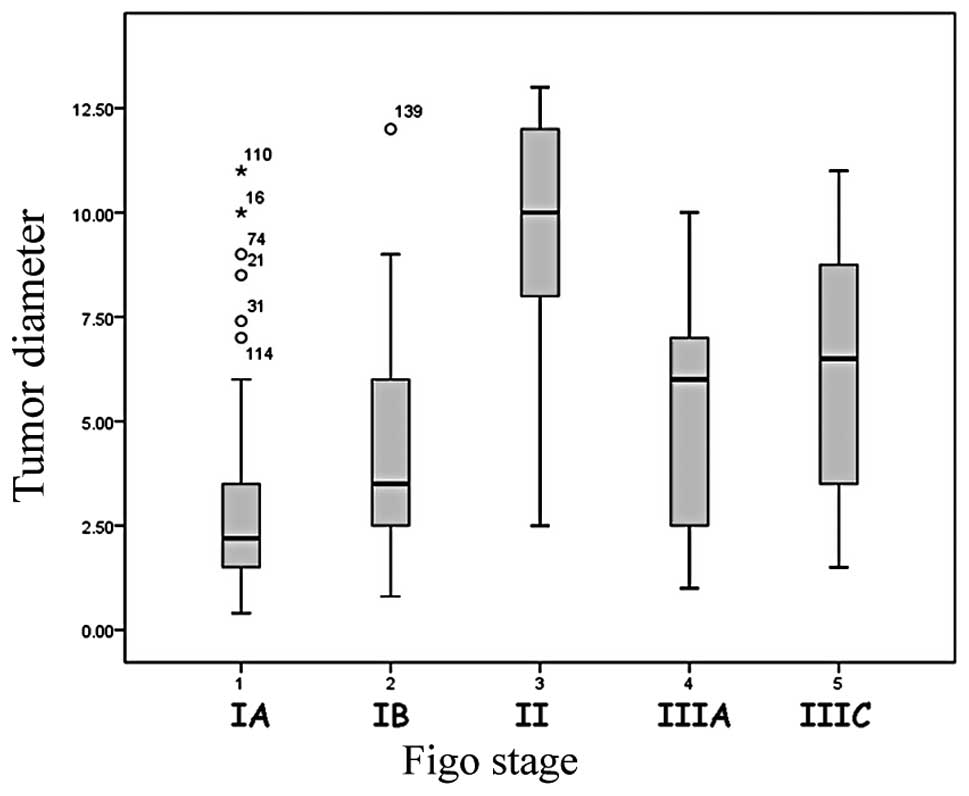
related to FIGO stage (p<0.01) (Fig.
1). The average tumor dimension in stage IA was 2.9 cm (±2.3;
median, 2.2 cm), 4.4 cm (±2.5) in stage IB (median, 3.5 cm), 9.6 cm
(±3.9) in stage II (median, 10 cm), 5.4 cm (±3) in stage IIIA
(median, 6 cm), and 6.3 cm (±3.1) in stage IIIC (median, 6.5
cm).
 | Table IICorrelation between primary tumor
diameter and stage, grading, peritoneal cytology and lymph node
status. |
Table II
Correlation between primary tumor
diameter and stage, grading, peritoneal cytology and lymph node
status.
| Variables | Tumor diameter mean
(± SD) | P-value |
|---|
| Stage |
| IA | 2.9 (2.3) | 0.01 |
| IB | 4.4 (2.5) | 0.01 |
| II | 9.3 (3.9) | 0.01 |
| IIIA | 5.4 (3) | 0.01 |
| IIIC | 6.3 (3.1) | 0.01 |
| Grading |
| 1 | 3.3 (2.6) | 0.01 |
| 2 | 4.8 (3.2) | 0.01 |
| 3 | 5.8 (2.5) | 0.01 |
| Peritoneal
cytologic results |
| Positive | 7.5 (2.6) | <0.05 |
| Negative | 4.1 (2.8) | <0.05 |
| Lymph node
status |
| Positive | 6.3 (3.1) | 0.01 |
| Negative | 4 (2.8) | 0.01 |
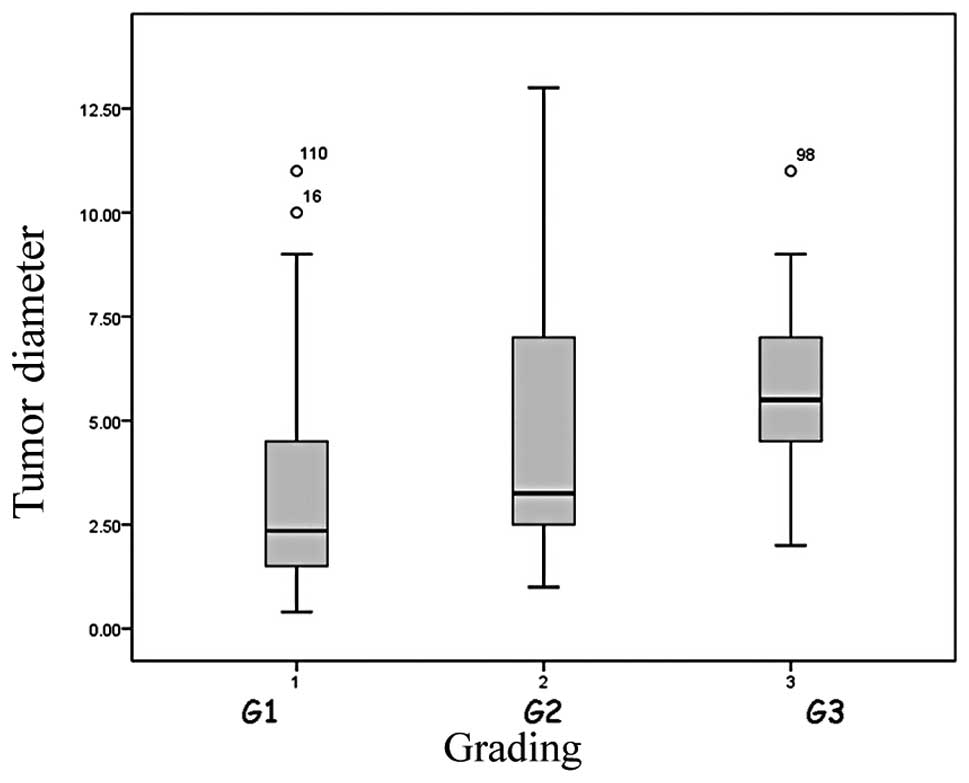
Histological grading also correlated with the tumor
largest diameter (p<0.01). When the tumor grade was 1 the mean
size was 3.3 cm (±2.6; median, 2.5 cm); when it was 2, the average
diameter was 4.8 cm (±3.2; median, 3.2 cm), and when the tumors
were undifferentiated, grade 3, the mean dimension was 5.8 cm
(±2.5; median, 5.5 cm) (Fig.
2).
The most statistically significant difference was
between the mean tumor size of grade 1 and 2 cancer and between the
mean tumor size of grade 1 and 3 tumor (p=0.012) as calculated by
the post-hoc test. There were no notable differences between other
comparisons.
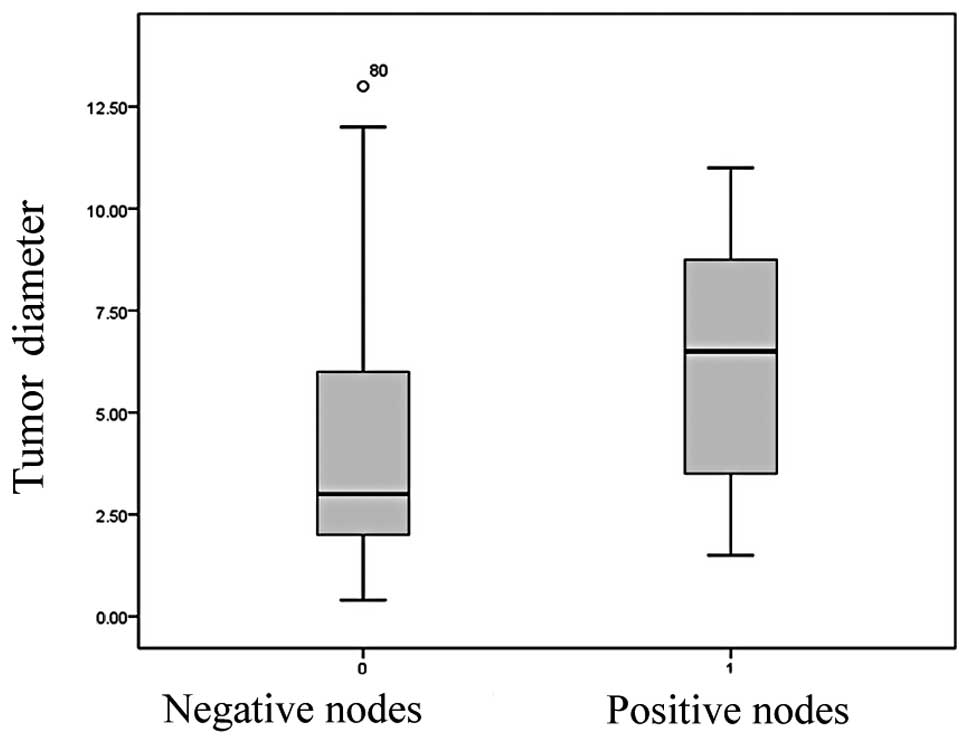
A marked correlation existed between tumor largest
diameter and nodal metastases (Fig.
3). The mean tumor size was 4 cm (±2.8) and the median was 3 cm
when lymph nodes resulted negative. The average dimension of tumor
with nodal metastases was 6.3 cm (±3.1) and the median was 6.5 cm.
The correlation coefficient here was 0.003 (p<0.01).
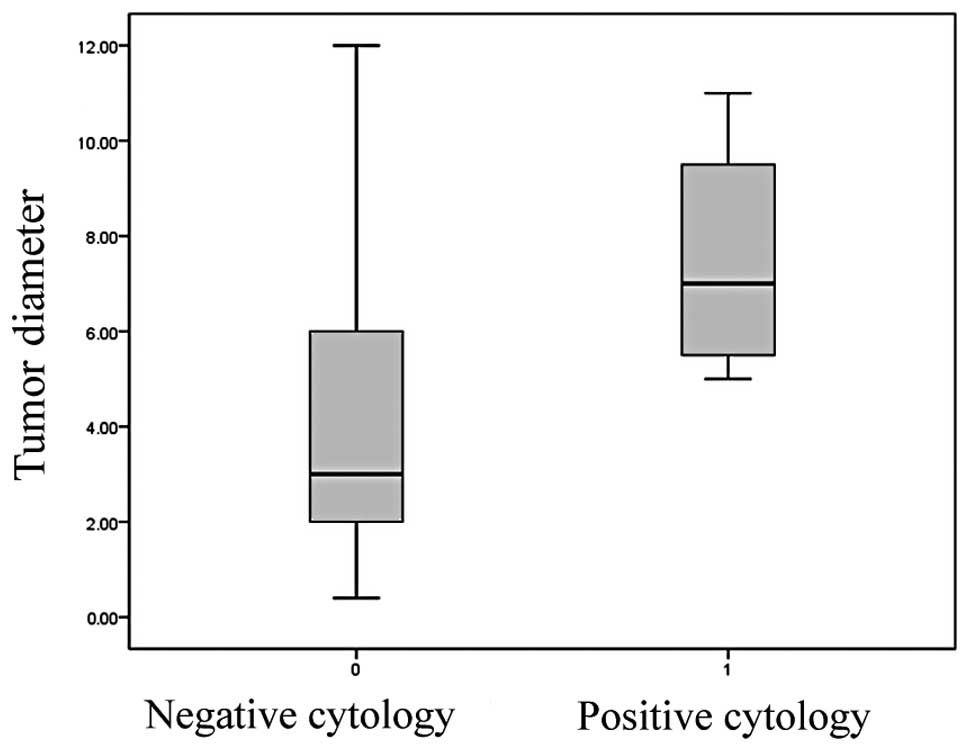
The t-test also demonstrated an association between
peritoneal cytologic results and tumor size (Fig. 4). Types of cancers with negative
peritoneal cytologic results were smaller (4.1±2.8 cm) than cancers
with negative results (7.5±2.6 cm) (p=0.020). A correlation
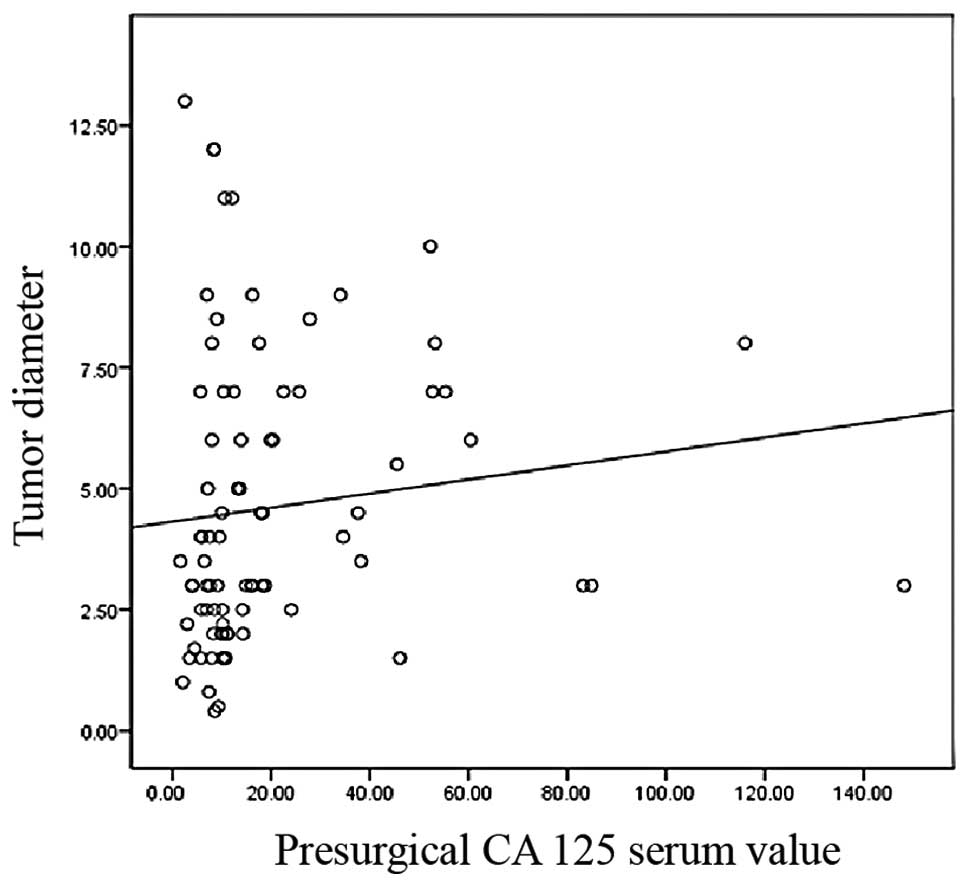
(p=0.009) existed between preoperative CA 125 values and tumor
largest diameter (Fig. 5).
Discussion
Endometrial cancer, different from non-epithelial
cancer of corpus uteri (19,20),
is a gynecological malignancy with an elevated prevalence and a low
mortality rate in the initial stages, especially in developed
countries. These data are a result of the greater number of
diagnostic instruments, increased average life expectancy and
diagnosis in older patients.
According to recent literature (2), patients can be divided into two groups
based on the biological character of the malignancy. These two
groups, high risk (undifferentiated cancer of every stage or deeply
infiltrating cancer besides the stage) and low-risk (initially
infiltrating cancer, G1–G2) undergo different approaches; complete
intensive surgical staging in high-risk cancers while there is a
less invasive approach in low-risk cancers.
Due to this, the scientific community has been
interested in detecting new instruments to identify these two
groups of patients before surgery. Grading is already a suitable
indicator of the necessity of lymphadenectomy. Other parameters
such as myometrial invasion or cervix involvement have been studied
with ultrasound or magnetic resonance. It is generally agreed that
in the assessment of the depth of myometrial invasion by
endometrial carcinoma, TV-ultrasound has a high diagnostic accuracy
that is equivalent to that of magnetic resonance imaging (MRI).
MRI, in addition, can give more information about cervical or
parametrial involvement (21,22).
Tumor sizes are easily assessable by the use of current diagnostic
techniques such as hysteroscopy and ultrasound imaging.
In this retrospective study, analyzing the cancer
macroscopic dimensions, we found that tumor stage and grading
increase as tumor size increases. Nodal metastases and positive
peritoneal cytologic results risk increase too, therefore the risk
of extrauterine disease increases in the largest lesions. Indeed,
an association between tumor largest diameter and FIGO stage was
demonstrated. This correlation was not perfectly linear since the
mean size of the tumor in stage II (9.6±3.9 cm) was higher than
that of the tumor in stage IIIA (5.4±3 cm). We suppose that this
data has been distorted by the number of patients in the present
study; the patients in stage II were significantly fewer than
patients in other stages, and among the six patients in stage II,
one of them developed a large cancer.
An important difference between the size of cancer
in stage IA (2.9±2.3 cm) and IB (4.4±2.5 cm) was reported in the
present study. The difference between these two stages consists in
the invasion of more or less than 50% of the myometrium; therefore,
the present study, according to previous literature (23,24),
demonstrates that the probability of myometrial invasion increases
with the tumor size.
According to our data, grading and tumor dimension
are related. In particular, we noted a maximum correlation between
well differentiated and highly undifferentiated cancer in relation
to tumor size.
In contrast to Mariani et al (24), we correlated peritoneal cytologic
results with tumor size. In our study, 88% of patients underwent
peritoneal washing and only 3.3% had positive peritoneal cytologic
results. The present study shall be continued, in order to verify
the underlying significance of this correlation.
There are no studies that relate tumor size with
tumor markers. The present study showed an association between
tumor diameter and CA 125. CA 125 correlates with FIGO stage which
in turn is associated with tumor size in this study (25). A correlation between CA 125 and
tumor size derives from these data and further confirms our
findings. Different from CA 125, that often results increased in a
non-oncologic disease such as endometriosis (26), HE4 was recently proposed as a
diagnostic and prognostic marker in patients affected by
endometrial cancer. Despite HE4 showing better sensitivity and
specificity (albeit in early stages) than CA 125, it requires
further validation and estimation concerning the best cut-off
value, particularly in the case of coexisting chronic disease as
renal impairment (27).
Most of the literature focuses on the association
between tumor size and nodal metastases. In 1960, Gusberg et
al demonstrated a poorer prognosis when the tumor was >10 cm
(28). In 1979, Johnsson determined
an increase of extrauterine disease frequency in tumors larger than
a third of the uterine cavity (29). Shink et al (30) and Lurain et al (31) showed a decrease in the risk of lymph
node metastases and an increase of survival in tumors <2 cm.
The present study, according to these data,
demonstrated an important association between tumor size and the
risk of lymph node metastases. Among our 147 patients, 89.1% had no
nodal metastases; the mean tumor size in this group of patients was
4.1 cm (±2.8) and the median was 3 cm. This value comes
significantly close to the cut-off of 2 cm defined first by Shink
et al (23,30), and then demonstrated in other
studies (24,31,32).
Considering these data and particularly the
demonstrated association between tumor size and myometrial
invasion, grading, nodal metastases, the present study is in
accordance with Mariani et al (24) and Milam et al (32) and proposes the consideration of
endometrioid adenocarcinoma of the endometrium, grade 1 or 2, with
myometrial invasion <50% and tumor largest diameter ≤3 cm as
low-risk of nodal metastases. In accordance with these authors, we
maintain that it is reasonably safe to treat with lymphadenectomy
patients who are, according to these criteria, at high-risk of
metastases. According to Mariani et al (24), patients with positive peritoneal
cytologic results should undergo lymphadenectomy, but the 2009 FIGO
staging review does not include peritoneal cytologic results among
staging parameters, therefore we consider it the less important
parameter in the identification of patient risk.
According to the recent literature, we concluded
that tumor size is correlated with stage and grade, which are
important prognostic factors to determine therapeutic approach. We
also found a correlation between tumor size and nodal metastasis,
positive peritoneal cytology and CA 125 values. Patients who have
grade 1 or 2 endometrioid corpus cancer, myometrial invasion
<50% and largest tumor diameter ≤3 cm can be treated only with
hysterectomy. Tumor largest diameter should be evaluated as a
preoperative parameter that indicates patients who do not require
lymphadenectomy.
The preoperative assessment of tumor size using
imaging techniques such as hysteroscopy (33), ultrasonography or magnetic
resonance, should be included in the diagnosis of patients with
suspected endometrial cancer in order to guide the surgeon to
determine the most appropriate surgical strategy for each
patient.
Acknowledgements
The authors acknowledge all the staff of the OB/GYN
Unit of Pharma University for their help in collecting the
data.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Colombo N, Preti E, Landoni F, Carinelli
S, Colombo A, Marini C and Sessa C; ESMO Guidelines Working Group.
Endometrial cancer: ESMO Clinical Practice Guidelines for
diagnosis, treatment and follow-up. Ann Oncol. 22(Suppl 6): 35–39.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Amant F, Moerman P, Neven P, Timmerman D,
Van Limbergen E and Vergote I: Endometrial cancer. Lancet.
366:491–505. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Patrelli TS, Berretta R, Rolla M, Vandi F,
Capobianco G, Gramellini D, Bacchi Modena A and Nardelli GB: Pelvic
lymphadenectomy in endometrial cancer: our current experience. Eur
J Gynaecol Oncol. 30:536–538. 2009.PubMed/NCBI
|
|
5
|
Zhang C, Wang C and Feng W:
Clinicopathological risk factors for pelvic lymph node metastasis
in clinical early-stage endometrioid endometrial adenocarcinoma.
Int J Gynecol Cancer. 22:1373–1377. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mariani A, Webb MJ, Keeney GL and Podratz
KC: Routes of lymphatic spread: a study of 112 consecutive patients
with endometrial cancer. Gynecol Oncol. 81:100–104. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morrow CP, Bundy BN, Kurman RJ, Creasman
WT, Heller P, Homesley HD and Graham JE: Relationship between
surgical-pathological risk factors and outcome in clinical stage I
and II carcinoma of the endometrium: a Gynecologic Oncology Group
study. Gynecol Oncol. 40:55–65. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Berretta R, Merisio C, Piantelli G, Rolla
M, Giordano G, Melpignano M and Nardelli GB: Preoperative
transvaginal ultrasonography and intraoperative gross examination
for assessing myometrial invasion by endometrial cancer. J
Ultrasound Med. 27:349–355. 2008.
|
|
9
|
Al Kushi A, Lim P, Aquino-Parsons C and
Gilks CB: Markers of proliferative activity are predictors of
patient outcome for low-grade endometrioid adenocarcinoma but not
papillary serous carcinoma of endometrium. Mod Pathol. 15:365–371.
2002.PubMed/NCBI
|
|
10
|
Nofech-Mozes S, Ackerman I, Ghorab Z,
Ismiil N, Thomas G, Covens A and Khalifa MA: Lymphovascular
invasion is a significant predictor for distant recurrence in
patients with early-stage endometrial endometrioid adenocarcinoma.
Am J Clin Pathol. 129:912–917. 2008. View Article : Google Scholar
|
|
11
|
Merisio C, Berretta R, De Ioris A,
Pultrone DC, Rolla M, Giordano G, Tateo S and Melpignano M:
Endometrial cancer in patients with preoperative diagnosis of
atypical endometrial hyperplasia. Eur J Obstet Gynecol Reprod Biol.
122:107–111. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Di Cristofano A and Ellenson LH:
Endometrial carcinoma. Annu Rev Pathol. 2:57–85. 2007.
|
|
13
|
Gu M, Shi W, Barakat RR, Thaler HT and
Saigo PE: Peritoneal washings in endometrial carcinoma. A study of
298 patients with histopathologic correlation. Acta Cytol.
44:783–789. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Turner DA, Gershenson DM, Atkinson N,
Sneige N and Wharton AT: The prognostic significance of peritoneal
cytology for stage I endometrial cancer. Obstet Gynecol.
74:775–780. 1989.PubMed/NCBI
|
|
15
|
Hanson MB, van Nagell JR Jr, Powell DE,
Donaldson ES, Gallion H, Merhige M and Pavlik EJ: The prognostic
significance of lymph-vascular space invasion in stage I
endometrial cancer. Cancer. 55:1753–1757. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gizzo S, Di Gangi S, Bertocco A, Noventa
M, Fagherazzi S, Ancona E, Saccardi C, Patrelli TS, D’Antona D and
Nardelli GB: Levonorgestrel intrauterine system in adjuvant
tamoxifen treatment: balance of breast risks and endometrial
benefits systematic review of literature. Reprod Sci. Sep
23–2013.(Epub ahead of print).
|
|
17
|
Wright JD, Barrena Medel NI, Sehouli J,
Fujiwara K and Herzog TJ: Contemporary management of endometrial
cancer. Lancet. 379:1352–1360. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
ASTEC study group. Kitchener H, Swart AM,
Qian Q, Amos C and Parmar MK: Efficacy of systematic pelvic
lymphadenectomy in endometrial cancer (MRC ASTEC trial): a
randomised study. Lancet. 373:125–136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Patrelli TS, Silini EM, Gizzo S, Berretta
R, Franchi L, Thai E, Lukanovic A, Nardelli GB and Modena AB:
Extragenital Müllerian adenosarcoma with pouch of Douglas location.
BMC Cancer. 11:1712011.
|
|
20
|
Patrelli TS, Gizzo S, Di Gangi S, Guidi G,
Rondinelli M and Nardelli GB: Cervical Mullerian adenosarcoma with
heterologous sarcomatous overgrowth: a fourth case and review of
literature. BMC Cancer. 11:2362011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Savelli L, Testa AC, Mabrouk M, Zannoni L,
Ludovisi M, Seracchioli R, Scambia G and De Iaco P: A prospective
blinded comparison of the accuracy of transvaginal sonography and
frozen section in the assessment of myometrial invasion in
endometrial cancer. Gynecol Oncol. 124:549–552. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Savelli L, Ceccarini M, Ludovisi M,
Fruscella E, De Iaco PA, Salizzoni E, Mabrouk M, Manfredi R, Testa
AC and Ferrandina G: Preoperative local staging of endometrial
cancer: transvaginal sonography vs. magnetic resonance imaging.
Ultrasound Obstet Gynecol. 31:560–566. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schink JC, Rademaker AW, Miller DS and
Lurain JR: Tumor size in endometrial cancer. Cancer. 67:2791–2794.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mariani A, Webb MJ, Keeney GL, Haddock MG,
Calori G and Podratz KC: Low-risk corpus cancer: is lymphadenectomy
or radiotherapy necessary? Am J Obstet Gynecol. 182:1506–1519.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sorosky JI: Endometrial cancer. Obstet
Gynecol. 111:436–447. 2008. View Article : Google Scholar
|
|
26
|
Patrelli TS, Berretta R, Gizzo S, Pezzuto
A, Franchi L, Lukanovic A, Nardelli GB and Modena AB: CA 125 serum
values in surgically treated endometriosis patients and its
relationships with anatomic sites of endometriosis and pregnancy
rate. Fertil Steril. 95:393–396. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gizzo S, Ancona E, Saccardi C, D’Antona D,
Nardelli GB and Plebani M: Could kidney glomerular filtration
impairment represent the “Achilles heel” of HE4 serum marker? A
possible further implication. Clin Chem Lab Med. 52:e45–e46.
2014.
|
|
28
|
Gusberg SB, Jones HC Jr and Tovell HM:
Selection of treatment for corpus cancer. Am J Obstet Gynecol.
80:374–380. 1960.PubMed/NCBI
|
|
29
|
Johnsson JE: Recurrences and metastases in
carcinoma of the uterine body correlated to the size and
localization of the primary tumor. Acta Obstet Gynecol Scand.
58:405–408. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schink JC, Lurain JR, Wallemark CB and
Chmiel JS: Tumor size in endometrial cancer: a prognostic factor
for lymph node metastasis. Obstet Gynecol. 70:216–219.
1987.PubMed/NCBI
|
|
31
|
Lurain JR, Rice BL, Rademaker AW,
Poggensee LE, Schink JC and Miller DS: Prognostic factors
associated with recurrence in clinical stage I adenocarcinoma of
the endometrium. Obstet Gynecol. 78:63–69. 1991.PubMed/NCBI
|
|
32
|
Milam MR, Java J, Walker JL, Metzinger DS,
Parker LP and Coleman RL; Gynecologic Oncology Group. Nodal
metastasis risk in endometrioid endometrial cancer. Obstet Gynecol.
119:286–292. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Saccardi C, Gizzo S, Patrelli TS, Ancona
E, Anis O, Di Gangi S, Vacilotto A, D’Antona D and Nardelli GB:
Endometrial surveillance in tamoxifen users: role, timing and
accuracy of hysteroscopic investigation: observational longitudinal
cohort study. Endocr Relat Cancer. 20:455–462. 2013. View Article : Google Scholar
|