Introduction
Lung cancer is the deadliest cancer in the world.
Its aggressive biology, resistance to conventional and targeted
therapeutic agents, and the absence of effective early detection
methods lead to a 5-year survival rate of only 14% (1–3).
According to the pathological and histological features, non-small
cell lung cancer (NSCLC) is the most prevalent type of lung cancer
(4). Despite modest improvements in
diagnosis and multimodality therapeutic methods, patients with lung
cancer still face a poor prognosis. It is critical to identify
molecular events that could lead to advances in lung cancer
therapeutics.
Lung tumorigenesis is associated with gene
alterations, such as gene dysregulation, mutations or loss of
heterozygosity (5,6). Ribosomal proteins (RPs), a major
component of ribosomes, are abundant RNA-binding proteins found in
every cell (7). Perturbations in
ribosomal biogenesis and translation associated with specific
genetic defects or syndromes may play a critical role in molecular
tumorigenesis (7). An excess of
free RPs can lead to cell-cycle arrest and apoptosis and regulate
extraribosomal functions, such as ribosomal protein S3 (RPS3) in
lymphocytic cells (8), RPL15 in
esophageal cancer (9), RPL19 in
breast tumors (10), RPL7A in
osteosarcoma (11) and RPL41 in
breast cancer (12). Another study
showed that RPL22 inactivation enhances the transformation
potential of lymphoblastic leukemia by inducing the stemness
factor, Lin28B (13) suggesting a
mechanistic basis by which RPL22 dysfunction could increase cancer
risk (14). Thus, RPs may be
particularly important regulators of cancer cell function.
In our previous study, we found that RPL22
transcript and protein expression was significantly downregulated
in NSCLC and that RPL22 may be involved in carcinogenesis (15); however, RPL22 has not been
implicated in any specific lung cancer mechanism.
As the elementary constituents of protein complexes
and regulatory pathways, protein-protein interactions are key
determinants of function (16,17).
Many free RPs including RPL5, L11, L23 and S7 interface with the
p53-MdM2 system, leading to cell-cycle arrest or apoptosis and
regulation of extraribosomal functions (18–21).
Thus, the characterization of protein-protein interactions is
critical to understand protein function and cell biology. Tandem
affinity purification (TAP)-tagging methods combined with LC-MS/MS
can be used to study protein interactions in eukaryotic cells
(16,17,22).
In the present study, we characterized RPL22-protein complexes in
NSCLC cells. By using a combination of TAP methods and GST
pull-down experiments, we demonstrated that RPL22 and casein kinase
2α (CK2α) interact in lung cancer cells in vitro and in
vivo. We also confirmed that RPL22 directly inhibits CK2α
substrate phosphorylation through this interaction. To the best of
our knowledge, this is the first report of this relationship in
lung cancer.
Materials and methods
Cell cultures
Cell lines from HBE, the NSCLC line LTEP-a-2 and
control cell line 293T (preserved in our department) were routinely
grown in RPMI-1640 medium (Life Technologies) supplemented with 10%
fetal bovine serum (FBS) and 1% penicillin/streptomycin in a moist
tissue culture incubator at 37°C in a 5% CO2 atmosphere.
Cells were seeded (1×108) and cultured in serum-free
medium for 24 h and then in complete medium for 48 h prior to RNA
and protein extraction.
Plasmid construction
cDNAs containing full-length wild-type RPL22 and
CK2α were obtained by RT-PCR from human bronchial epithelial (HBE)
cells. The fidelity of the amplified sequences was confirmed by DNA
sequencing. pCeMM-NTAP(GS) was kindly provided by the Austrian
Academy of Sciences Center for Molecular Medicine (CeMM). pGEX-6P1
was purchased from GE Healthcare and pcDNA3.1(+)was purchased from
Invitrogen. NotI and XhoI were used for molecular
cloning of the RPL22 sequence into pCeMM-NTAP(GS);
BamHI and XhoI were used to clone RPL22 into
pGEX-6P1 and pcDNA3.1(+); BamHI and EcoRI were used
to clone CK2α into pcDNA3.1(+).
Tandem affinity purification
pCeMM-NTAP(GS)-RPL22 was transfected into LTEP-a-2
cells, and GFP-positive cells were selected twice by flow
cytometry. RPL22-protein complexes were isolated by the TAP
procedure with non-transduced LTEP-a-2 cells as a negative control
as previously described (17).
Briefly, 4×108 GFP-positive cells were harvested, and
total protein lysates were extracted. Lysates (5 ml) were incubated
with 200 μl IgG-Sepharose beads (Sigma) for 2 h at 4°C and then
washed 4 times with 10 ml lysate buffer. The beads were incubated
in 500 μl TEV cleavage buffer with 100 U TEV protease (Promega) for
1 h at 16°C. The supernatant was collected by centrifugation at 500
× g; 200 μl streptavidin agarose beads was added and incubated for
4 h at 4°C. The beads were washed 5 times with 10 ml TEV cleavage
buffer. Proteins were heated and detached from the beads with 300
μl 1X Laemmli sample buffer containing 1% β-mercaptoethanol for 5
min at 95°C. Eluted proteins were separated by 4–15% (Bio-Rad
Laboratories) SDS-PAGE and visualized by silver staining.
Differentially expressed protein bands were excised for in
situ tryptic digestion, MALDI-TOF and LC-MS/MS. Mass spectra
were analyzed with Flex Analysis software (version 2.4; Bruker
Daltonik GmbH, Bremen, Germany) and searched against the NCBInr
database by an in-house Mascot (version 2.1; Matrix Science) search
engine.
GST pull-down assay
A 50-μl sample of 50% glutathione Sepharose 4B beads
(GE Healthcare) was equilibrated in 1X PBS buffer (pH 7.4). The
slurry was mixed with 15 μl of 0.4 mg/ml GST-fusion protein
(RPL22/CK2α, expressed by pGEX-6P1-RPL22/pGEX-6P1-CK2α) and
incubated for 30 min at 4°C in a rotating incubator to immobilize
the fusion protein on the glutathione Sepharose beads. The beads
were washed 4 times with 10 ml phosphate-buffered saline (PBS) and
then incubated with 1 ml of 2 mg/ml LTEP-a-2 cell lysates for 2 h
at 4°C. The beads were washed 5 times with 10 ml PBS. Laemmli
sample buffer (200 μl of 1X) containing 1% β-mercaptoethanol was
added and heated at 95°C for 5 min. Proteins (30 μl) were resolved
on precast 4–15% (Bio-Rad Laboratories) SDS-PAGE and visualized by
Coomassie blue staining or immunoblotted with the specific
antibody.
Subcellular localization
293T cells were cultured in Millicell® EZ
Slide 4-well (Millipore). The full-length cDNAs of RPL22 and CK2α
were cloned into the expression vector pcDNA3.1(+). These
constructs were transfected into the 293T cells using
FuGENE® HD transfection reagent (Roche) according to the
manufacturer’s instructions. RPL22 proteins were immunostained with
polyclonal anti-RPL22 antibody (Abcam) and Alexa Fluor®
488 goat anti-rabbit IgG (H+L) (Invitrogen); CK2α proteins were
immunostained with monoclonal anti-CK2α antibody (Sigma) and
tetramethyl rhodamine goat anti-mouse IgG (H+L) (Invitrogen). The
nucleus was stained by DAPI (Sigma). Fluorescence was visualized on
an LSM 710 confocal microscope (Zeiss).
CK2α kinase assay
To evaluate the interaction between RPL22 and CK2α,
we analyzed CK2α kinase activity. According to the manufacturer’s
instructions (Promega), samples (10 μg) were adjusted to a 5-μl
final volume in CK2α kinase buffer [100 mM Tris (pH 8.0), 100 mM
NaCl, 50 mM KCl, 20 mM MgCl2 and 100 μM
Na3VO4]. Following addition of 15 μl
incubation buffer, including CK2α kinase buffer, 50 μCi
[γ-32P]GTP, and 200 ng sample of each reaction group
(blank group using CK2α kinase buffer), reactions were incubated at
30°C for 30 min. RPL22-GST fusion proteins were prepared as
previously described. The CK2α-specific peptide substrate
RRRADDSDDDDD (1 mM; Promega) was added to the kinase reaction
according to the manufacturer’s instructions. The reactions
included a blank (CK2α kinase buffer), peptide substrate, GST +
peptide substrate, GST-RPL22 or GST-RPL22 + peptide substrate. The
reactions were stopped by adding 25 μl 100 mM ATP in 4 N HCl.
Samples were spotted onto a P81 Whatman filter (Whatman, Inc.,
Clifton, NJ, USA), washed 4 times in 150 mM
H3PO4, and incorporated radioactivity was
measured by scintillation counting.
Statistical methods
Experiments were performed in triplicate and each
experiment was performed 3 times. Data are expressed as mean ± SEM.
Statistical analyses were performed by unpaired non-parametric
Mann-Whitney test. Statistical significance was indicated at
P<0.05 (SPSS, Inc., Chicago, IL, USA).
Results
Tandem affinity purification of the
RPL22-protein complexes
To study RPL22 protein function in lung cancer, we
fused the RPL22 gene into pCeMM-NTAP(GS) with tandem affinity
peptides (GS) and overexpressed this ‘bait’ protein in LTEP-a-2
cells. GFP-positive cells were identified by flow cytometry
(Fig. 1). We purified RPL22-binding
proteins through 2 consecutive affinity steps from 4×108
GFP-positive cells, and lysates containing putative protein
complexes were purified over successive affinity columns as
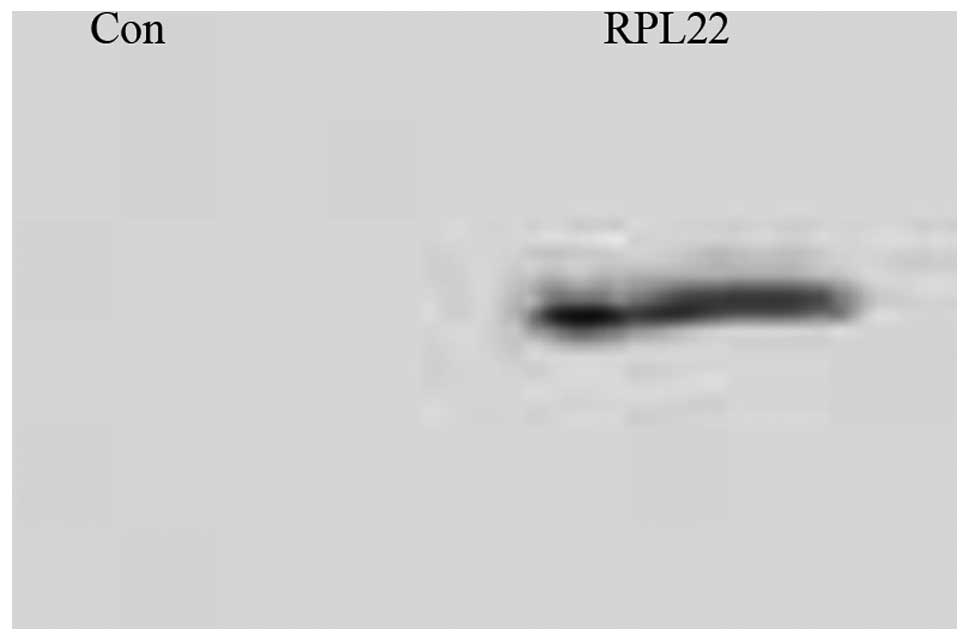
previously described (17).
Proteins detached from TAP-streptavidin agarose beads were western
blotted for RPL22 (Fig. 2).
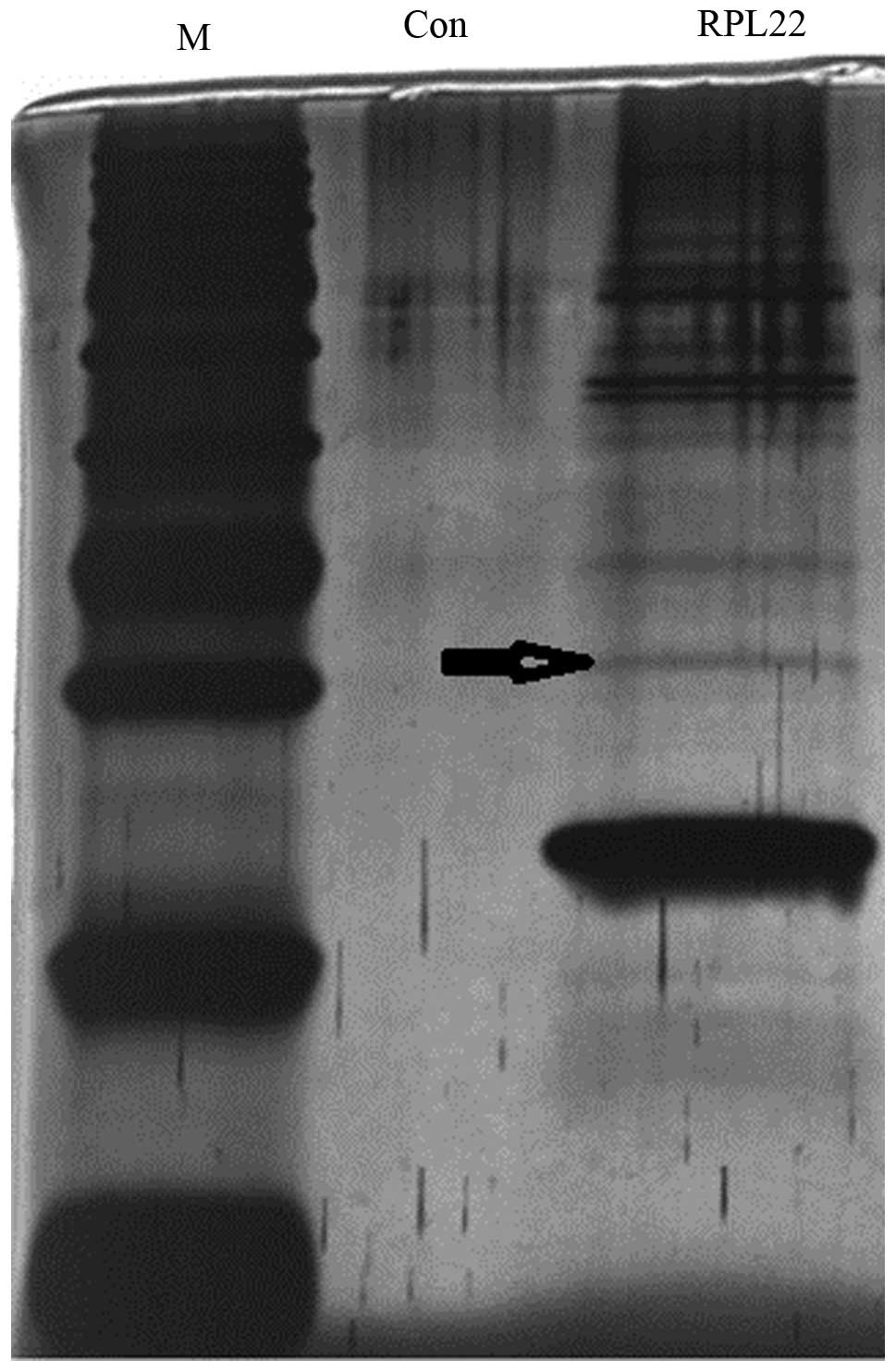
Proteins from the final elution were separated by SDS-PAGE and
visualized by silver staining (Fig.
3). Several differentially expressed proteins were isolated and
digested in situ with trypsin prior to identification by
LC-MS/MS, which revealed that one of the protein complexes included
casein kinase 2α (CK2α) (arrow in Fig.
3).
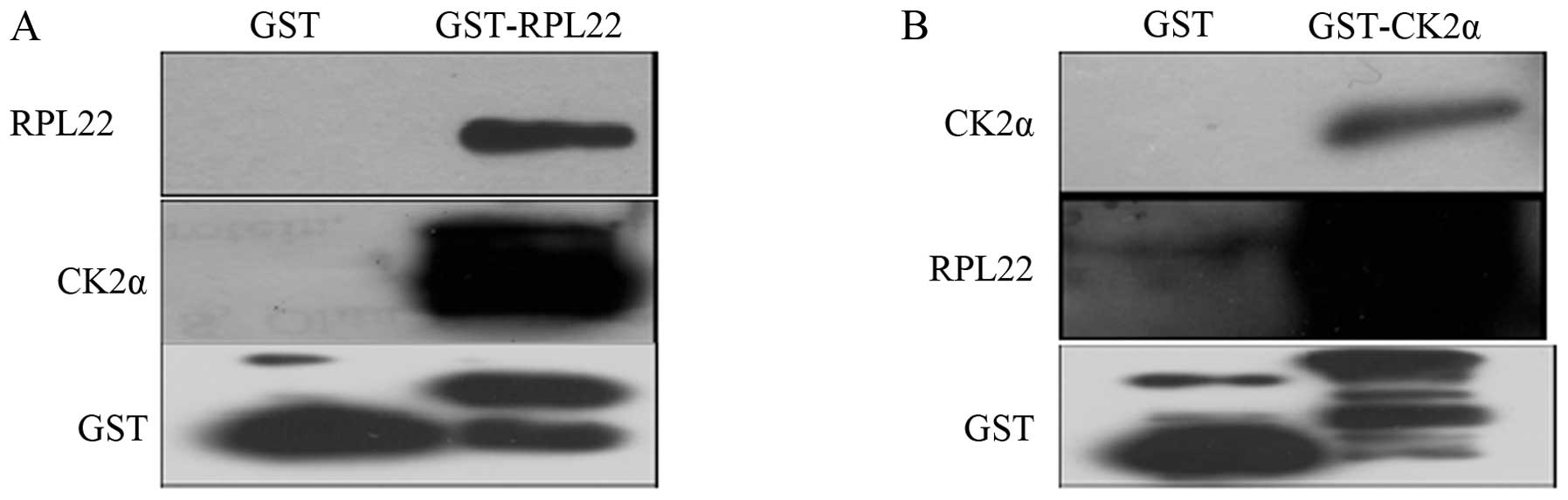
GST pull-down assay
GST pull-down experiments were used to verify the
interaction between the GST-RPL22 fusion protein (bait) and the
CK2α target, and between the GST-CK2α fusion protein (bait) and the
RPL22 target. GST-RPL22/GST-CK2α glutathione agarose beads were
incubated with LTEP-a-2 cell lysates and then washed. Eluted
proteins bound to GST-RPL22/GST-CK2α were identified by western
blotting using the antibody against RPL22/CK2α. As shown in
Fig. 4, CK2α was detected in the
eluent containing the GST-RPL22 fusion protein as bait, and RPL22
was detected in the eluent containing the GST-CK2α fusion protein
as bait, thus confirming the interaction between RPL22 and CK2α in
lung cancer cells in vitro.
Subcellular localization
We used confocal microscopy to characterize the
subcellular localization of RPL22 and CK2α. Recombinant plasmids
were transfected into 293T cells and proteins were detected with
fluorescent antibodies (Fig. 5).
RPL22 expression is showed in green, CK2α in red and DAPI-stained
nuclei in blue. The results showed that RPL22- and CK2α-specific
antibodies produced overlapping signals in the cytoplasm,
demonstrating co-localization of the two proteins in cells.
CK2α kinase assay
Protein kinases are central components of signal
transduction cascades. CK2α is an important protein kinase in cell
proliferation. To explore the potential role of RPL22 and CK2α
interaction in lung cancer, we developed a CK2α kinase assay. We
measured CK2α activity using blank (CK2α kinase buffer), peptide,
GST + peptide, and GST-RPL22 or GST-RPL22 + peptide as kinase
reaction substrates (Fig. 6A).
Compared to the peptide substrate group, significant downregulation
of CK2α activity was observed in the GST-RPL22 + peptide substrate
group. Thus, GST-RPL22 inhibited CK2α activity in vitro
(P<0.05). Notably, GST-RPL22 was not phosphorylated by CK2α in
comparison to the blank (P<0.05; Fig. 6A). GST-RPL22 caused a dose-dependent
decrease in CK2α activity with the CK2α-specific peptide substrate
RRRADDSDDDDD (Fig. 6B).
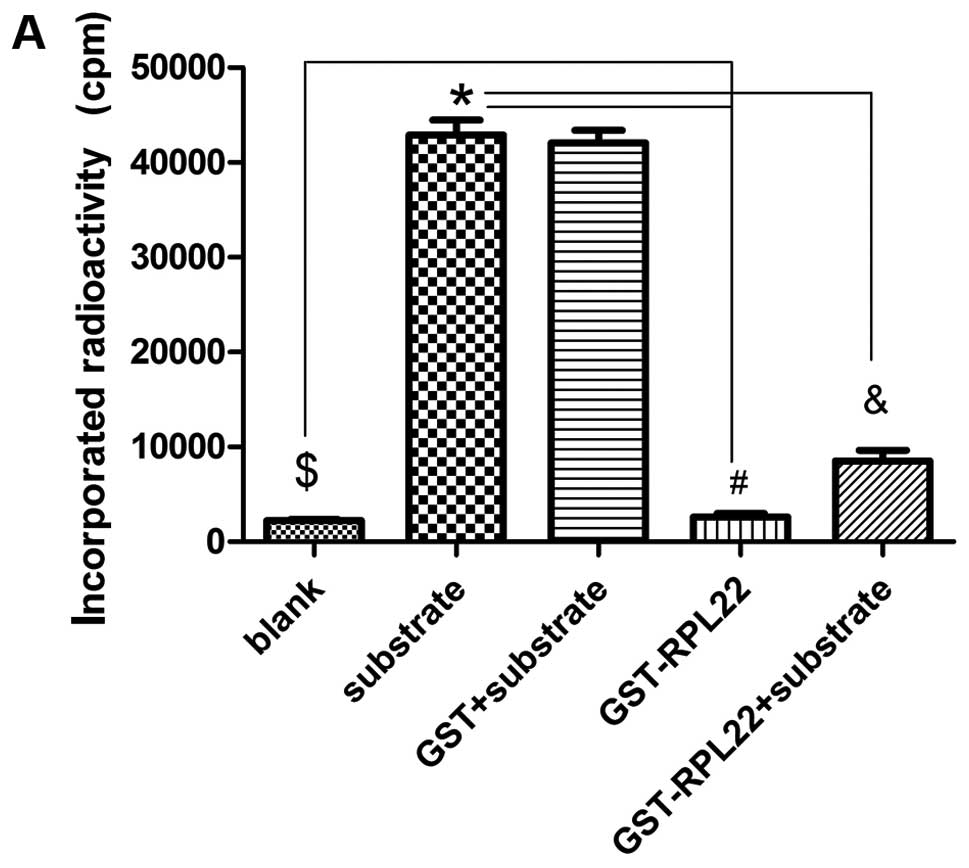 | Figure 6CK2α kinase assay. RPL22 inhibits
CK2α activity in vitro. (A) CK2α kinase assay used either
200 ng sample of each reaction group (blank group using CK2α kinase
buffer) as substrate and 50 μCi [γ-32P] GTP as phosphate
donor, and reactions were incubated at 30°C for 30 min. Where
indicated, 1 mM of the CK2α-specific peptide substrate RRRADDSDDDDD
was added to the kinase reaction. The reactions included a blank
(CK2α kinase buffer), peptide substrate, GST + peptide substrate,
GST-RPL22 or GST-RPL22 + peptide substrate. GST-RPL22 inhibited
CK2α activity in vitro (&P<0.05 vs.
*substrate) and GST-RPL22 was not phosphorylated by CK2α
(#P<0.05 vs. $blank). (B) Concentration
curve of RPL22 inhibition using CK2α-specific peptide substrate.
Where indicated, 0, 5, 10, 20, 40, 80 and 160 ng GST-RPL22 and 1 mM
of the CK2α-specific peptide substrate RRRADDSDDDDD were added to
the kinase reaction. The results showed that GST-RPL22 inhibits
CK2α activity in vitro in a dose-dependent fashion. |
Discussion
In the present study, we confirmed RPL22 and CK2α
interactions in lung cancer cells in vitro by tandem
affinity purification, GST pull-down experiments and confocal
microscopy. RPL22 inhibited CK2α substrate phosphorylation through
this direct interaction.
RPL22, a small protein consisting of 128 amino
acids, is a component of the 60S large ribosomal subunit and
co-localizes with ribosomal RNA in the nucleolus and cytoplasm
(23,24). Although it is incorporated into the
ribosome subunit, RPL22 is not required for protein synthesis
(23,25). RPL22 inactivation enhances the
transformation potential of lymphoblastic leukemias (13). RPL22 associates with viral RNAs and
proteins; it was identified as an EBER-associated protein that
protects against tumorigenic transformation of EBV-infected cells
(26). Another report showed that
RPL22 may act as a repressor of transcription (27). In Drosophila, RPL22 interacts
with histone H1 and co-localizes on condensed chromatin;
upregulation of RPL22 expression inhibits transcription, while
depletion of RPL22 reverses this effect (28). RPL22 is also associated with human
telomerase (29) and regulates the
progress of cell apoptosis in part by selectively regulating p53
expression (25). Our previous
study confirmed that RPL22 expression is downregulated in NSCLC
cells (15). These data provide
insight into the mechanistic basis by which RPL22 may regulate
tumorigenesis, particularly in lung cancer.
Virtually, all cellular functions require
protein-protein interactions (22,30,31).
RPs modulate the trans-activation function of important regulatory
proteins (32); they specifically
bind to the central regions of the MDM2 oncoprotein and inhibit
MDM2 E3 ligase activity towards p53 to regulate cell cycle
progression (32,33). The involvement of RPs in tumors
represents a new oncogenic pathway associated with their
extraribosomal functions through protein-protein interactions with
important signal molecules (34).
In the present study, we found that RPL22 binds CK2α
in lung cancer cells. Protein kinase CK2 is a highly conserved
tetrameric serine/threonine protein kinase (35). It regulates several important
signaling pathways including the PI3K/Akt and WNT signaling
cascades, NF-κB transcription and the DNA damage response (35,36).
CK2α is one of the catalytic subunits of the protein kinase CK2
tetrameric complex. Accumulating evidence confirms that CK2α has a
vast range of physiological targets and appears to be highly
pleiotropic (37). It is involved
in many key biological progresses, including growth and cell cycle
control, signal transduction, circadian rhythms and gene expression
(36). Upregulation of protein
kinase CK2 expression is associated with increased cell growth and
proliferation in normal and cancer cells (38). Dysregulated CK2 may impact cell
proliferation and apoptosis, key features of cancer cell biology
(39). Our results showed that
RPL22 protein binds CK2α in lung cancer cells, inhibiting the CK2α
phosphorylation reaction in a dose-dependent fashion, and RPL22
expression is downregulated in NSCLC. It remains unclear whether
RPL22 could contribute to relatively upregulate the expression and
the function of CK2 in lung cancer. The RPL22-CK2α pathway might be
associated with lung tumorigenesis.
GST-RPL22 was not phosphorylated by CK2α in the
present study, although RPL22 phosphorylation by CK2 has been
reported in Drosophila (40). It is, therefore, possible that
different modifications regulate the RPL22 function in different
species. The regulatory motif of the Drosophila RPL22
ortholog has a large unique N-terminal extension not present in
vertebrates (23,26). The C-terminal acidic region of human
RPL22 also differs from Drosophila RPL22 and may be used to
traffic the protein from the nucleoplasm to the nucleolus (24). The nuclear matrix is a key locus for
CK2 signaling in cell proliferation and cell death. RPL22 may be a
phosphoregulated substrate of CK2. Our data suggest that RPL22
directly binds and regulates CK2. The impact of CK2 on diverse
molecular pathways may control cell proliferation and cell death in
cancer (38). Downregulation of CK2
results in induction of apoptosis in cultured cells and xenograft
cancer models, suggesting its potential as a therapeutic target
(41). Protein-protein interactions
generate specificity in signal transduction (42). RPL22 could be a useful anticancer
agent that functions by binding and inhibiting CK2 function in
human lung cancer.
In summary, RPL22 and CK2α interact in lung cancer
cells. RPL22 inhibits CK2α kinase activity. Given the function of
these proteins, we expect the present study will shed light on
their regulatory role in lung cancer. We are continuing to
characterize the interaction between RPL22 and CK2 and their
related signaling pathways in lung cancer.
Acknowledgements
We thank the Austrian Academy of Sciences Center for
Molecular Medicine, Professor Giulio Superti-Furga, and the
creators of the plasmid for kindly providing the pCeMM-NTAP (GS)
plasmid. The present study was supported by the National Natural
Science Foundation of China (M.Y.) under contract no. 30901708.
References
|
1
|
Herbst RS, Heymach JV and Lippman SM: Lung
cancer. N Engl J Med. 359:1367–1380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hinchliffe SR, Rutherford MJ, Crowther MJ,
et al: Should relative survival be used with lung cancer data? Br J
Cancer. 106:1854–1859. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yoshida T, Ishii G, Goto K, et al: Solid
predominant histology predicts EGFR tyrosine kinase inhibitor
response in patients with EGFR mutation-positive lung
adenocarcinoma. J Cancer Res Clin Oncol. 139:1691–1700. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brambilla E, Travis WD, Colby TV, et al:
The new World Health Organization classification of lung tumours.
Eur Respir J. 18:1059–1068. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giaccone G: Oncogenes and antioncogenes in
lung tumorigenesis. Chest. 109:130S–134S. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zajac-Kaye M: Myc oncogene: a key
component in cell cycle regulation and its implication for lung
cancer. Lung Cancer. 34(Suppl 2): S43–S46. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Warner JR and McIntosh KB: How common are
extraribosomal functions of ribosomal proteins? Mol Cell. 34:3–11.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jang CY, Lee JY and Kim J: RpS3, a DNA
repair endonuclease and ribosomal protein, is involved in
apoptosis. FEBS Lett. 560:81–85. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Q, Yang C, Zhou J, et al: Cloning and
characterization of full-length human ribosomal protein L15 cDNA
which was overexpressed in esophageal cancer. Gene. 263:205–209.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Henry JL, Coggin DL and King CR:
High-level expression of the ribosomal protein L19 in human breast
tumors that overexpress erbB-2. Cancer Res. 53:1403–1048. 1993.
|
|
11
|
Zheng SE, Yao Y, Dong Y, et al:
Down-regulation of ribosomal protein L7A in human osteosarcoma. J
Cancer Res Clin Oncol. 135:1025–2031. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang S, Huang J, He J, et al: RPL41, a
small ribosomal peptide deregulated in tumors, is essential for
mitosis and centrosome integrity. Neoplasia. 12:284–293.
2010.PubMed/NCBI
|
|
13
|
Rao S, Lee SY, Gutierrez A, et al:
Inactivation of ribosomal protein L22 promotes transformation by
induction of the stemness factor, Lin28B. Blood. 120:3764–3773.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Novetsky AP, Zighelboim I, Thompson DM Jr,
et al: Frequent mutations in the RPL22 gene and its clinical
and functional implications. Gynecol Oncol. 128:470–474. 2013.
|
|
15
|
Yang M, Sun H, Wang H, et al:
Down-regulation of ribosomal protein L22 in non-small cell lung
cancer. Med Oncol. 30:6462013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rigaut G, Shevchenko A, Rutz B, et al: A
generic protein purification method for protein complex
characterization and proteome exploration. Nat Biotechnol.
17:1030–1032. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Burckstummer T, Bennett KL, Preradovic A,
et al: An efficient tandem affinity purification procedure for
interaction proteomics in mammalian cells. Nat Methods.
3:1013–1019. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen D, Zhang Z, Li M, et al: Ribosomal
protein S7 as a novel modulator of p53-MDM2 interaction: binding to
MDM2, stabilization of p53 protein, and activation of p53 function.
Oncogene. 26:5029–5037. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun XX, Wang YG, Xirodimas DP, et al:
Perturbation of 60 S ribosomal biogenesis results in ribosomal
protein L5- and L11-dependent p53 activation. J Biol Chem.
285:25812–25821. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lohrum MA, Ludwig RL, Kubbutat MH, et al:
Regulation of HDM2 activity by the ribosomal protein L11. Cancer
Cell. 3:577–587. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vazquez A, Bond EE, Levine AJ, et al: The
genetics of the p53 pathway, apoptosis and cancer therapy. Nat Rev
Drug Discov. 7:979–987. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Westermarck J, Ivaska J and Corthals GL:
Identification of protein interactions involved in cellular
signaling. Mol Cell Proteomics. 2:1752–1763. 2013. View Article : Google Scholar
|
|
23
|
Lavergne JP, Conquet F, Reboud JP, et al:
Role of acidic phosphoproteins in the partial reconstitution of the
active 60 S ribosomal subunit. FEBS Lett. 216:83–88. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shu-Nu C, Lin CH and Lin A: An acidic
amino acid cluster regulates the nucleolar localization and
ribosome assembly of human ribosomal protein L22. FEBS Lett.
484:22–28. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Anderson SJ, Lauritsen JP, Hartman MG, et
al: Ablation of ribosomal protein L22 selectively impairs αβ T cell
development by activation of a p53-dependent checkpoint. Immunity.
26:759–772. 2007.PubMed/NCBI
|
|
26
|
Elia A, Vyas J, Laing KG, et al: Ribosomal
protein L22 inhibits regulation of cellular activities by the
Epstein-Barr virus small RNA EBER-1. Eur J Biochem. 271:1895–1905.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fok V, Mitton-Fry RM, Grech A, et al:
Multiple domains of EBER 1, an Epstein-Barr virus noncoding RNA,
recruit human ribosomal protein L22. RNA. 12:872–882. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ni JQ, Liu LP, Hess D, et al:
Drosophila ribosomal proteins are associated with linker
histone H1 and suppress gene transcription. Genes Dev.
20:1959–1973. 2006. View Article : Google Scholar
|
|
29
|
Le S, Sternglanz R and Greider CW:
Identification of two RNA-binding proteins associated with human
telomerase RNA. Mol Biol Cell. 11:999–1010. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stelzl U, Worm U, Lalowski M, et al: A
human protein-protein interaction network: a resource for
annotating the proteome. Cell. 122:957–968. 2005.PubMed/NCBI
|
|
31
|
Gallegos Ruiz MI, Floor K, Rijmen F, et
al: EGFR and K-ras mutation analysis in non-small cell lung cancer:
comparison of paraffin embedded versus frozen specimens. Cell
Oncol. 29:257–264. 2007.PubMed/NCBI
|
|
32
|
Lee NY, Choi HK, Shim JH, et al: The
prolyl isomerase Pin1 interacts with a ribosomal protein S6 kinase
to enhance insulin-induced AP-1 activity and cellular
transformation. Carcinogenesis. 30:671–681. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Deisenroth C and Zhang Y: Ribosome
biogenesis surveillance: probing the ribosomal protein-Mdm2-p53
pathway. Oncogene. 29:4253–4260. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Komili S, Farny NG, Roth FP, et al:
Functional specificity among ribosomal proteins regulates gene
expression. Cell. 131:557–571. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Poole A, Poore T, Bandhakavi S, et al: A
global view of CK2 function and regulation. Mol Cell Biochem.
274:163–170. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Trembley JH, Wang G, Unger G, et al:
Protein kinase CK2 in health and disease: CK2: a key player in
cancer biology. Cell Mol Life Sci. 66:1858–1867. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Meggio F and Pinna LA:
One-thousand-and-one substrates of protein kinase CK2? FASEB J.
17:349–368. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lin KY, Tai C, Hsu JC, et al:
Overexpression of nuclear protein kinase CK2 α catalytic subunit
(CK2α) as a poor prognosticator in human colorectal cancer. PLoS
One. 6:e171932011.
|
|
39
|
Siddiqui-Jain A, Drygin D, Streiner N, et
al: CX-4945, an orally bioavailable selective inhibitor of protein
kinase CK2, inhibits prosurvival and angiogenic signaling and
exhibits antitumor efficacy. Cancer Res. 70:10288–10298. 2010.
View Article : Google Scholar
|
|
40
|
Zhao W, Bidwai AP and Glover CV:
Interaction of casein kinase II with ribosomal protein L22 of
Drosophila melanogaster. Biochem Biophys Res Commun.
298:60–66. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Prudent R and Cochet C: New protein kinase
CK2 inhibitors: jumping out of the catalytic box. Chem Biol.
16:112–120. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pawson T and Nash P: Protein-protein
interactions define specificity in signal transduction. Genes Dev.
14:1027–1047. 2000.PubMed/NCBI
|