Introduction
Human papillomavirus (HPV) contributes to the
development of cancer of the uterine cervix. It has been reported
that the E6 oncoproteins of high-risk HPV types inhibit the
activity of p53 tumor suppressor protein, while the E7 oncoproteins
of high-risk HPV types inhibit pRB tumor suppressor protein
(1,2). Cervical cancer is the second most
frequent cancer among women worldwide (3) and concurrent chemoradiotherapy with
cisplatin has become standard treatment for locally advanced
cervical cancer (4–7). It has been confirmed that
cisplatin-based concurrent chemoradiotherapy (CCRT) significantly
decreases the risk of mortality due to cervical cancer by 30–50%
(8–11), and also improves both disease-free
survival and overall survival. However, virtually all chemotherapy
agents enhance radiation damage to normal tissues, leading to
severe adverse effects of concurrent chemoradiotherapy (12,13).
There has been a lack of animal studies on the
timing of administration of anticancer agents. Although in
vitro studies using cell lines can evaluate efficacy (14–16),
adverse effects cannot be properly evaluated. The aim of the
present study was to determine the most appropriate timing for the
administration of cisplatin with radiation through comparison of
the neoadjuvant, concurrent chemoradiotherapy (CCRT) and adjuvant
strategies by evaluating efficacy and adverse effects in αT3
transgenic mice (αT3 mice) with undifferentiated lens epithelial
tumors induced by the T antigen of SV40, which is a DNA virus
resembling HPV types 16 and 18 (HPV16/18) that cause cervical
cancer (17,18).
Materials and methods
Animals
We produced αT3 mice that developed crystalline lens
epithelial tumors (17,18). The mechanism of transformation by
SV40 T antigen (TAg) of is similar to that by the E6/E7
oncoproteins of HPV16/18 since both TAg and these oncoproteins
inhibit the activity of the p53 and pRB tumor suppressor proteins
(19). These mice developed lens
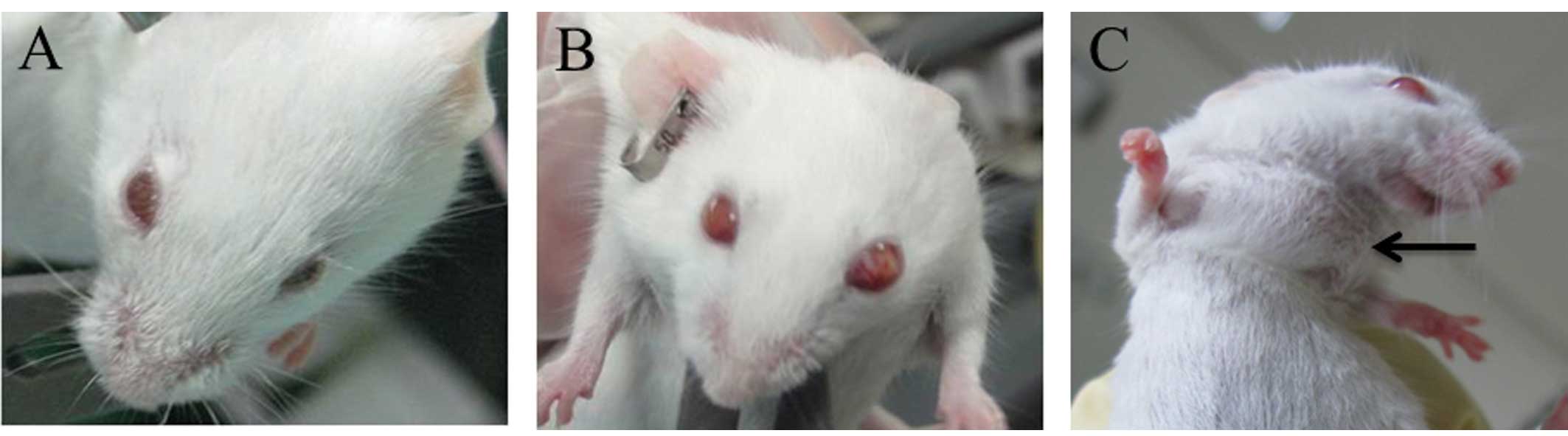
dysplasia at the embryonic stage and carcinoma in situ was
observed at 8 weeks after birth. The tumors subsequently showed
intraocular invasion (at 16 weeks of age), extraocular invasion (at
32 weeks of age), and metastasis to lymph nodes and other organs
(after 52 weeks of age) (Fig.
1).
All procedures were performed in accordance with the
Guide for the Care and Use of Laboratory Animals and were approved
by the Committee on Animal Experimentation of Kawasaki Medical
School. All mice had access to standard rodent chow (NMF; Oriental
Yeast Co., Ltd., Japan) and water ad libitum, and were
housed under pathogen-free conditions in a temperature-controlled
animal room with a 12-h light/dark cycle.
Harvesting of specimens
The mice (N=88; body weight, 28.5±6.5 g) were
sacrificed at 32–36 weeks of age as extraocular invasion occurred
after 32 weeks. Animals were anesthetized by intraperitoneal (i.p.)
injection of sodium pentobarbital (40 mg/kg), blood was collected
from the internal jugular vein and the mice were euthanized. Then
the eyeballs were carefully resected, fixed in 4% formalin,
embedded in paraffin and cut into 4 μm sections. These sections
were deparaffinized and stained with hematoxylin and eosin
(H&E) staining or were processed for terminal deoxynucleotidyl
transferase dUTP nick end-labeling (TUNEL) staining.
Chemotherapy
Cisplatin (Nippon Kayaku, Tokyo, Japan) was
reconstituted with sterile 0.9% saline in a laminar air-flow hood
under sterile conditions. Our preliminary experiment showed that
the 50% lethal dose (LD50) of cisplatin was 16 mg/kg,
therefore animals received i.p. chemotherapy with cisplatin at a
dose of 2 mg/kg (1/8 of the LD50). This dose was
approximately equivalent to the clinical dose used for treatment of
cervical cancer in humans (40 mg/m2) (20) based on the ratio of mass and body
surface area between mice and adult human females (21).
Irradiation
Whole-body irradiation was performed using an
MBR-1520R3 X-Ray generator (Hitachi Medical Co., Tokyo, Japan) and
the mice received daily fractions of 2.0 Gy from day 0 to 4 (total,
10.0 Gy). The radiation dose and schedule were selected to be
similar to those used to treat cervical cancer in humans. (In our
preliminary experiment, mice received 5.0–10.0 Gy of whole-body
irradiation as a single dose and animals administered 10.0 Gy died
within two weeks.)
Treatment plan
To determine the optimum timing for administration
of cisplatin and irradiation, we divided the mice into an
irradiation-first group (adjuvant group), a concurrent
chemoradiotherapy group (CCRT group) and a cisplatin-first group
(neoadjuvant group). Three control groups were also studied, and
they received no treatment, cisplatin alone and irradiation alone.
Specimens were obtained at three weeks after administration of
cisplatin or after starting irradiation (three weeks after starting
the second treatment in the neoadjuvant and adjuvant groups).
The mice were divided into the following six groups.
Group 1 (N=11) was the untreated control group, Group 2 (N=17) was
the cisplatin control group that received i.p. cisplatin on day 0,
and Group 3 (N=18) was the irradiation control group that received
2 Gy/day from day 0 to 4. Group 4 (N=14) was the CCRT group, which
received i.p. cisplatin on day 0 and radiation at 2 Gy/day on days
0–4. Group 5 (N=13) was designated as the irradiation-first group,
and received radiation at 2 Gy/day on days 0–4 and was administered
i.p. cisplatin on day 7. Group 6 (N=15) was designated as the
cisplatin-first group, and received i.p. cisplatin on day 0 and
radiation at 2 Gy/day on days 7 to 11. In all groups, specimens
were harvested on day 20. Before treatment (on day 0) and after
treatment (on the day of harvesting), the body weight and eyeball
size in all mice were measured.
To investigate the antitumor activity of each
treatment, we determined the reduction rate of eyeball diameter and
assessed apoptosis of tumor cells by TUNEL staining. To investigate
adverse effects, we assessed the mortality rate, the changes of
body weight, and the hemoglobin and leukocyte count. The hemoglobin
was measured in venous blood obtained at the time of sacrifice
using an ABL800 (Radiometer Medical, Tokyo, Japan), while
leukocytes were counted by S.K., N.U. and H.I. using an
erythrocytometer and the average of their results was
calculated.
Detection of apoptosis
Apoptosis of tumor cells was detected by the TUNEL
method using an ApopTag Plus Peroxidase In situ Apoptosis
Detection kit (Chemicon International, Temecula, CA, USA). Briefly,
after deparaffinization and rehydration, samples were pretreated by
incubation with proteinase K (2 μg/ml; Merck, Darmstadt, Germany)
for 15 min at 37°C. After endogenous peroxidase was inactivated by
incubation with 3% H2O2 in phosphate-buffered
saline (PBS) for 5 min, sections were rinsed with PBS and then
incubated with terminal deoxynucleotidyl transferase (TdT) buffer
containing 1 mM of cobalt-HCl, 0.5 U/l terminal transferase and 0.4
μM of digoxigenin-11-deoxyuridine triphosphate (dUTP) in a
humidified chamber for 60 min at 37°C. The reaction was stopped by
adding TdT stop buffer, anti-digoxigenin peroxidase conjugate was
added, and incubation was carried out for 30 min. As a negative
control, slides were incubated without TdT. After visualization of
the reaction products with diaminobenzidine (Sigma Chemical Co.,
St. Louis, MO, USA), nuclei were counterstained with methyl green.
Since many tumors showed central necrosis (Fig. 5A-a), even in the control group, we
counted the number of apoptotic cells (TUNEL-positive cells)
outside the central necrotic area.
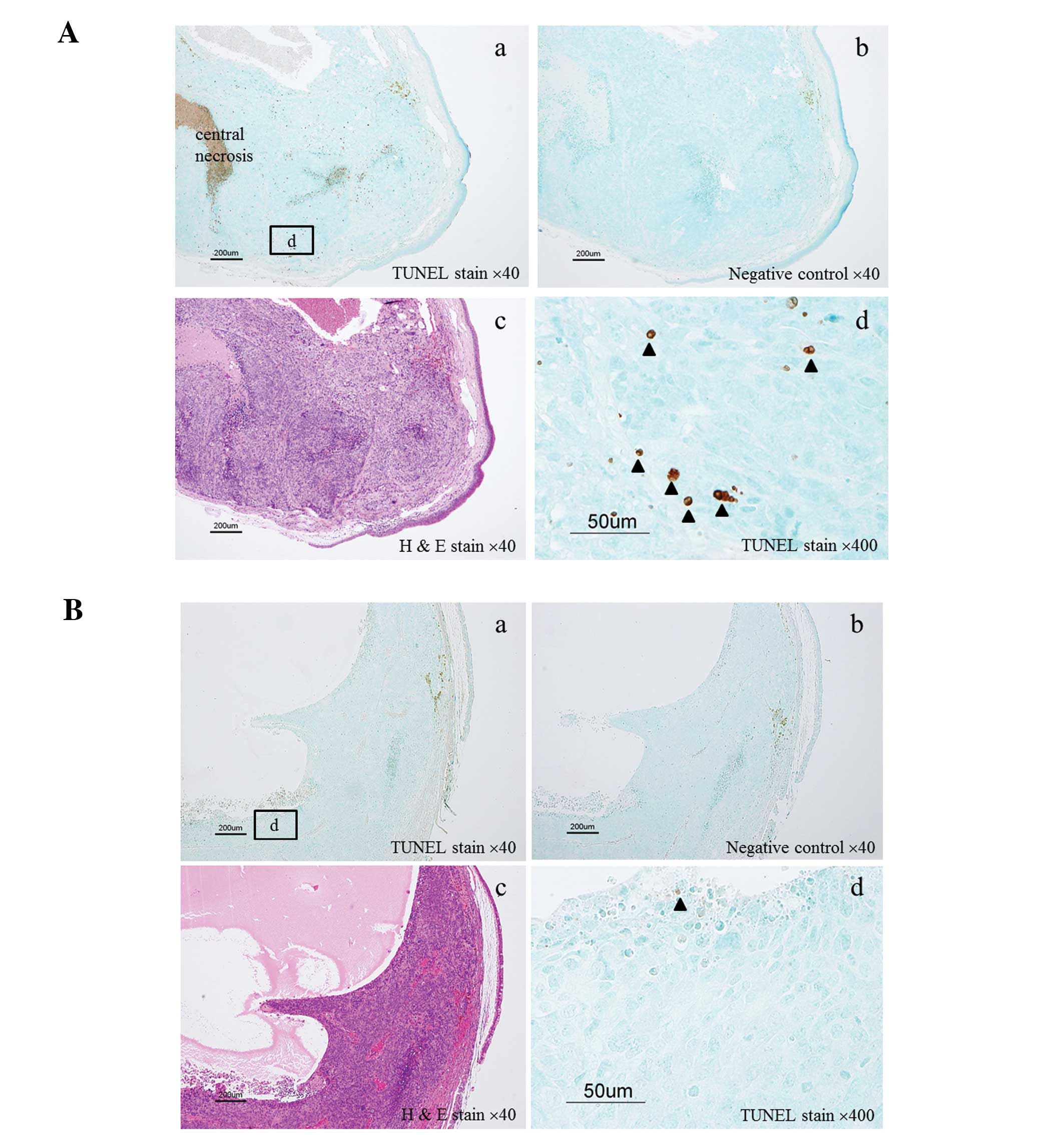 | Figure 5Detection of TUNEL-positive cells in
lens tissue. (A) Eyeball of a mouse from Group 4 with numerous
TUNEL-positive cells in the lens tissue. (B) Eyeball of a mouse
from Group 2 with few TUNEL-positive cells in the lens tissue. a,
TUNEL staining, magnification, ×40; b, negative control,
magnification, ×40; c, H&E staining, magnification, ×40 and d,
TUNEL staining, magnification, ×400. |
Statistical analysis
Data were analyzed by the Chi-square test and the
Mann-Whitney U test using StatFlex version 6.0 software (Artech
Co., Ltd., Osaka, Japan). P<0.05 was considered to indicate
statistically significant differences.
Results
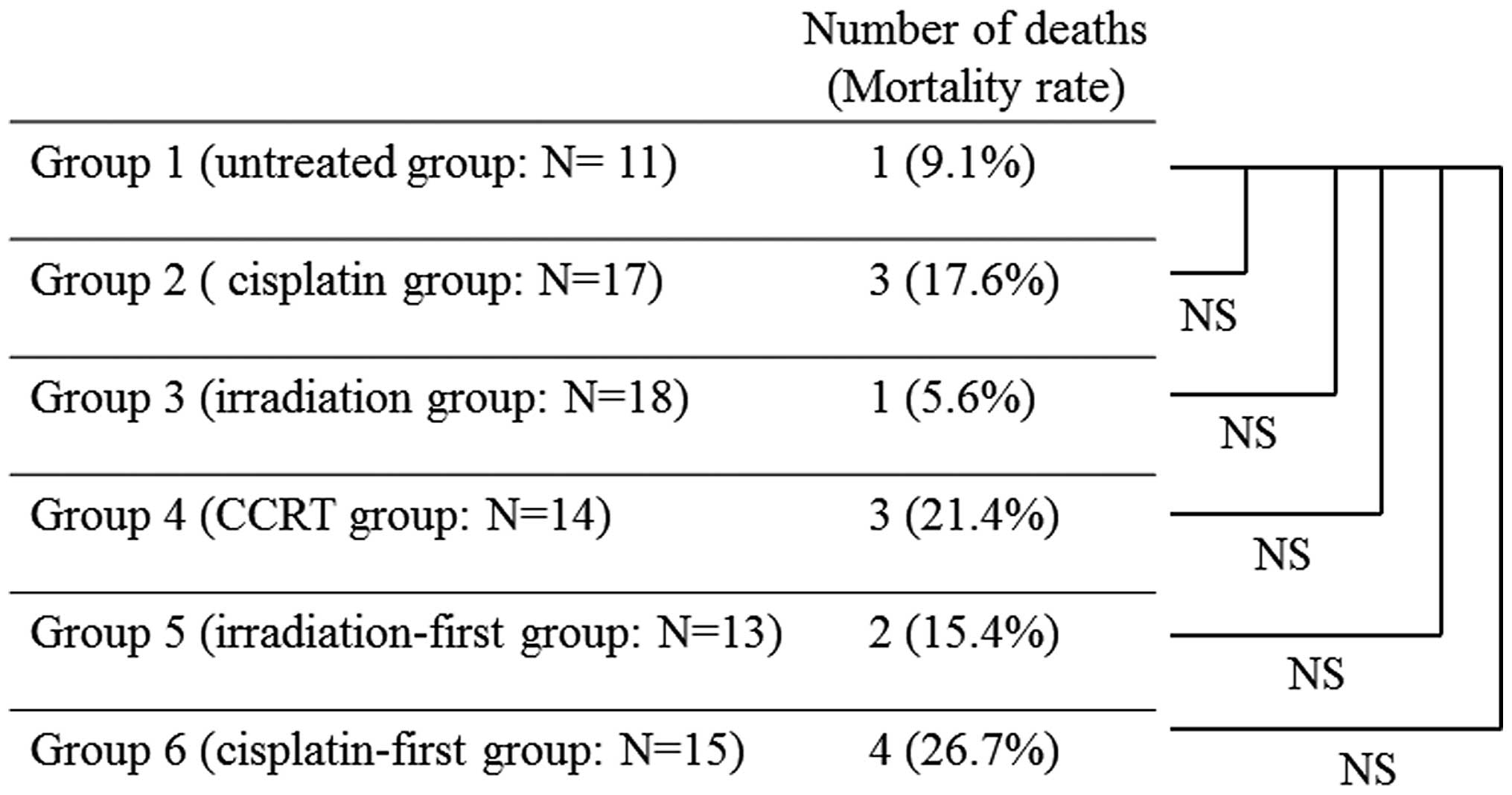
The number of mice that died before the scheduled
day for harvesting specimens was 1/11 (9.1%) in Group 1, 3/17
(17.6%) in Group 2, 1/18 (5.6%) in Group 3, 3/14 (21.4%) in Group
4, 2/13 (15.4%) in Group 5 and 4/15 (26.7%) in Group 6. The
mortality rate was the highest in Group 6, but there was no
significant difference from the rate in Group 1 (P=0.261; Fig. 2). Mice that died early were excluded
from the analysis of the antitumor activity and adverse effects,
except mortality. Thus, the number of animals analyzed in each
group was 10 in Group 1, 14 in Group 2, 17 in Group 3, 11 in Group
4, 11 in Group 5 and 11 in Group 6.
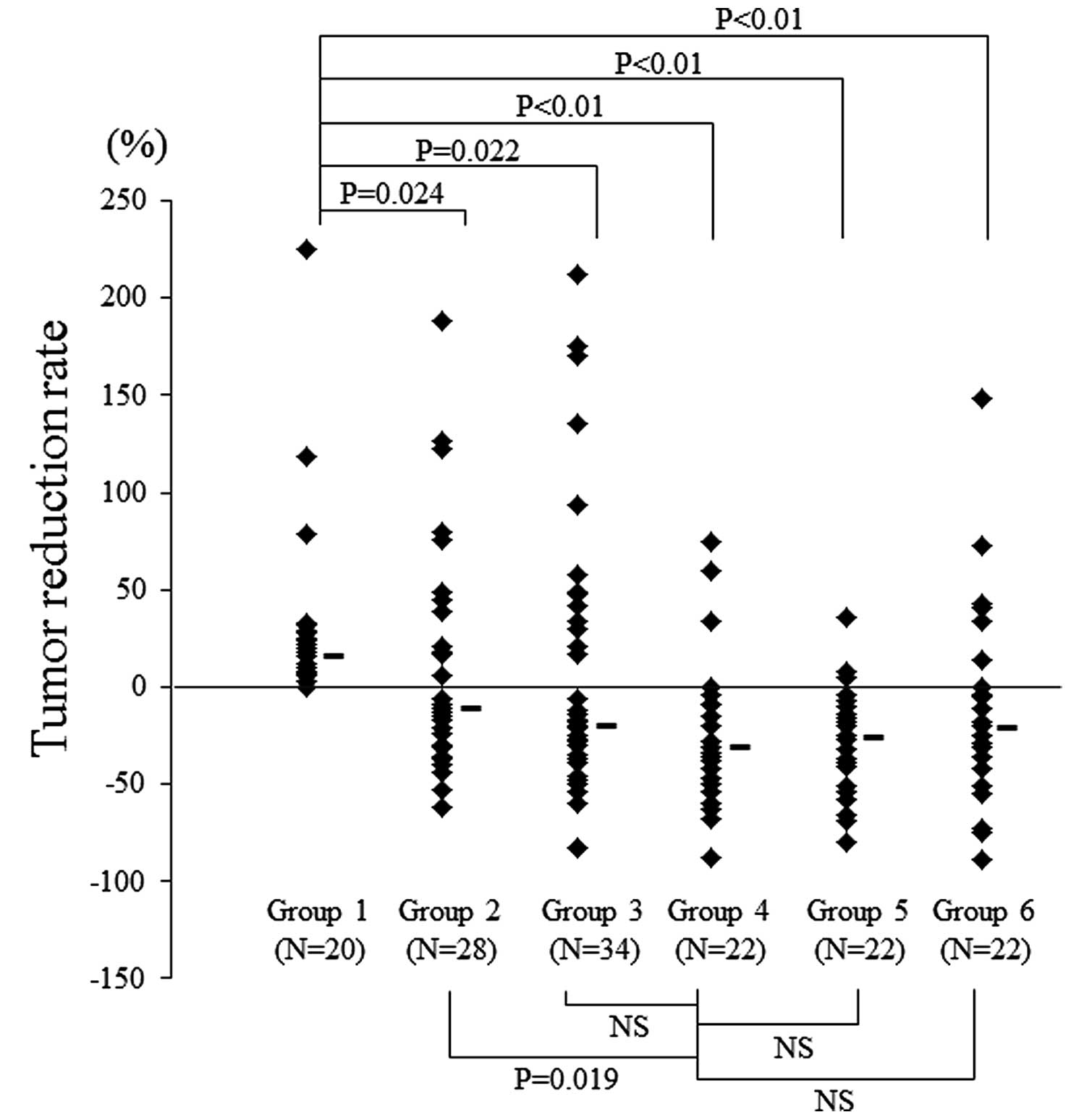
In each group, antitumor activity was assessed by
comparison of the tumor diameter reduction rate between before and
after treatment (Fig. 3). In Group
4, the tumors showed the most marked decrease in size and there was
a significant difference between Group 4 and 2 (P=0.019), although
there was no significant difference between Group 4 and Groups 3, 5
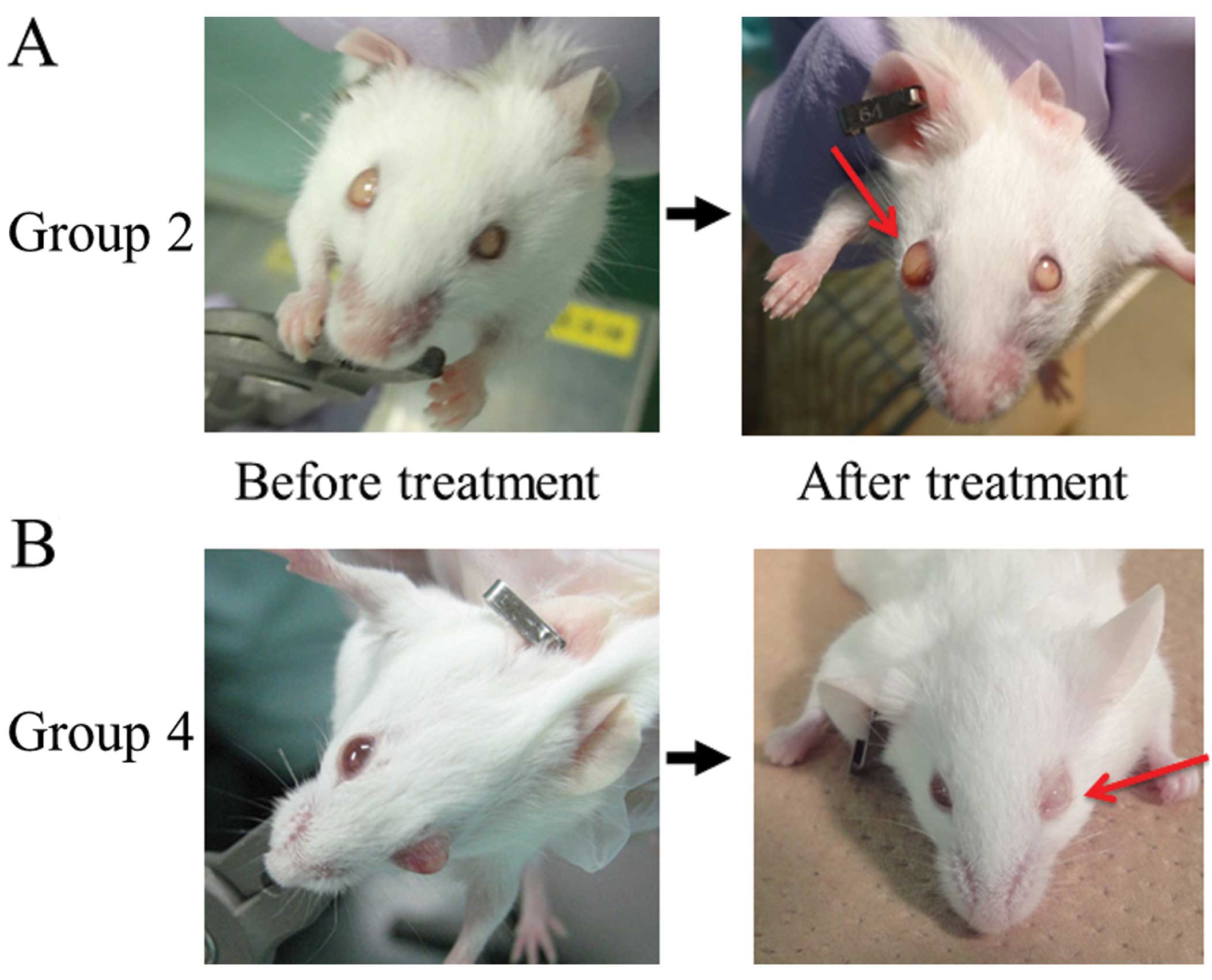
or 6. Representative images obtained from Groups 2 and 4 before and
after therapy are shown in Fig. 4.
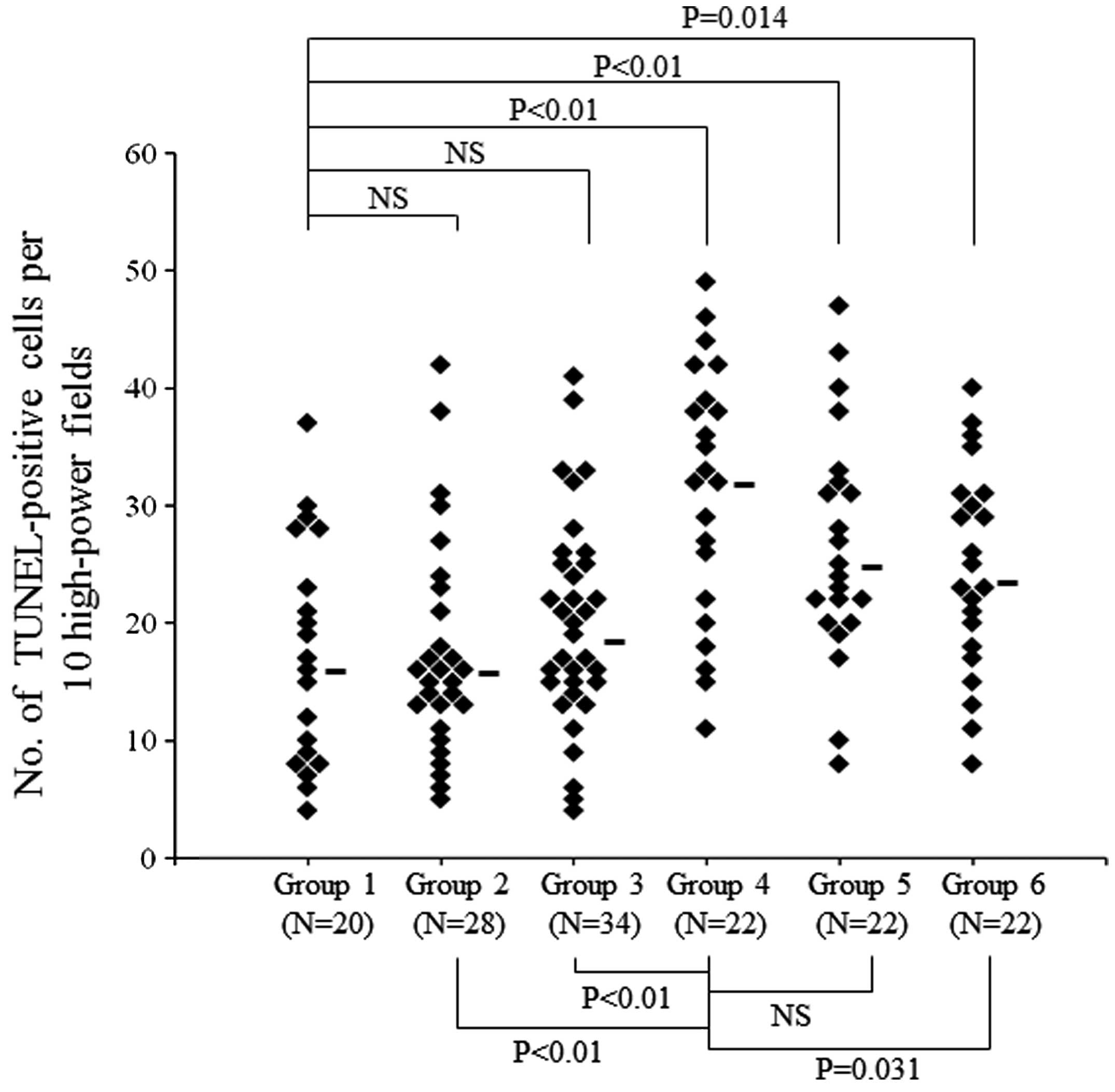
We also evaluated apoptosis in each group to examine the effect of
treatment. Apoptotic cells were defined as TUNEL-positive cells
with obvious nuclear immunoreactivity (Fig. 5A and B). Immunohistochemical
analysis revealed that the median number of TUNEL-positive cells in
lens tissue per 10 high-power fields was 15.5 (range, 4–37) in
Group 1, 15.5 (range, 5–42) in Group 2, 18 (range, 4–41) in Group
3, 32 (range, 11–49) in Group 4, 24.5 (range, 8–47) in Group 5 and
23 (range, 8–40) in Group 6. The number of TUNEL-positive cells was
the highest in Group 4, and there was a significant difference
between the number in Group 4 and that in Groups 2, 3 or 6
(P<0.01, P<0.01 and P=0.031, respectively) (Fig. 6).
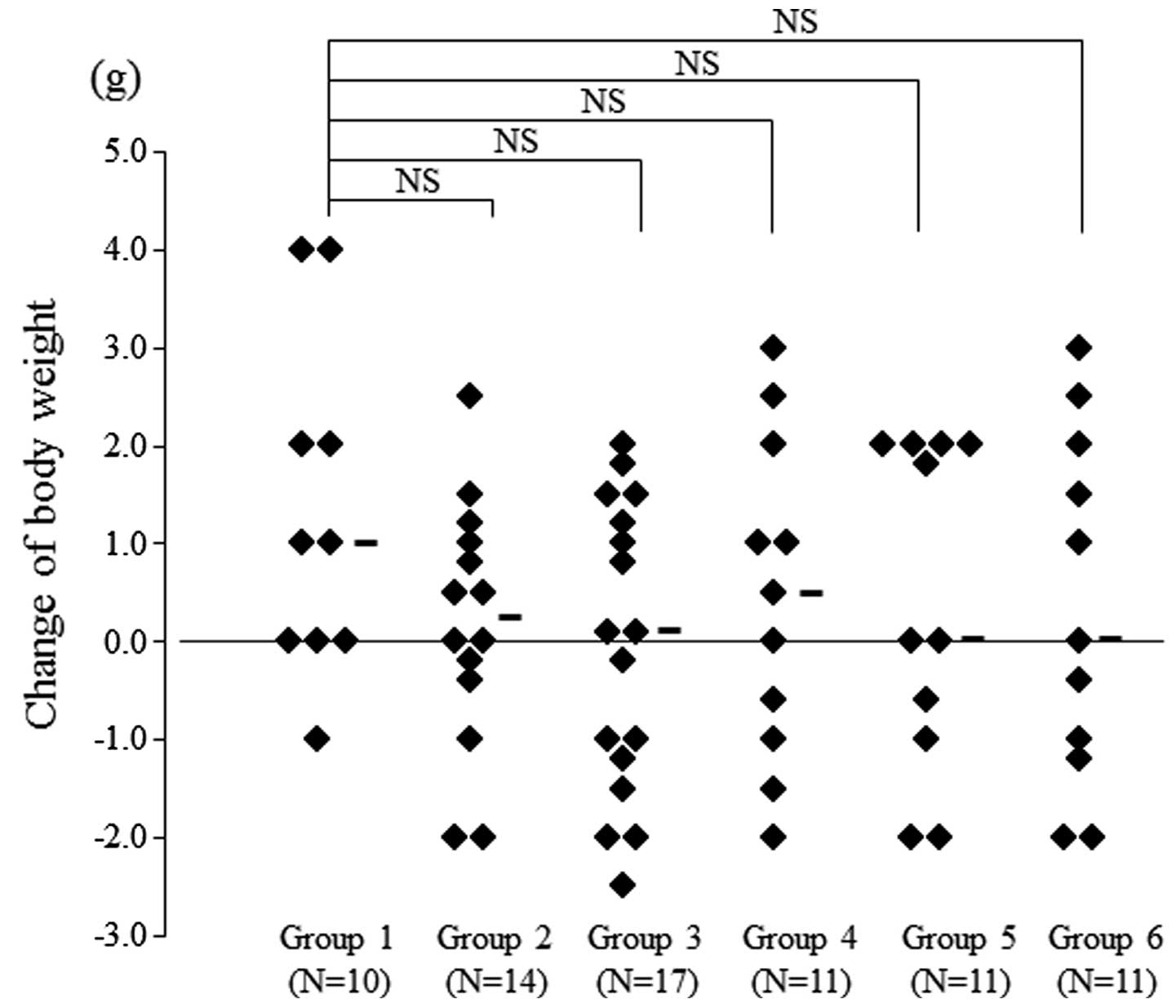
Finally, we compared the adverse effects of each
regimen. Investigation of the changes of body weight showed that
there were no significant differences between Group 1 and any other
group (Fig. 7). Therefore, the
anorectic effect of treatment did not show marked differences among
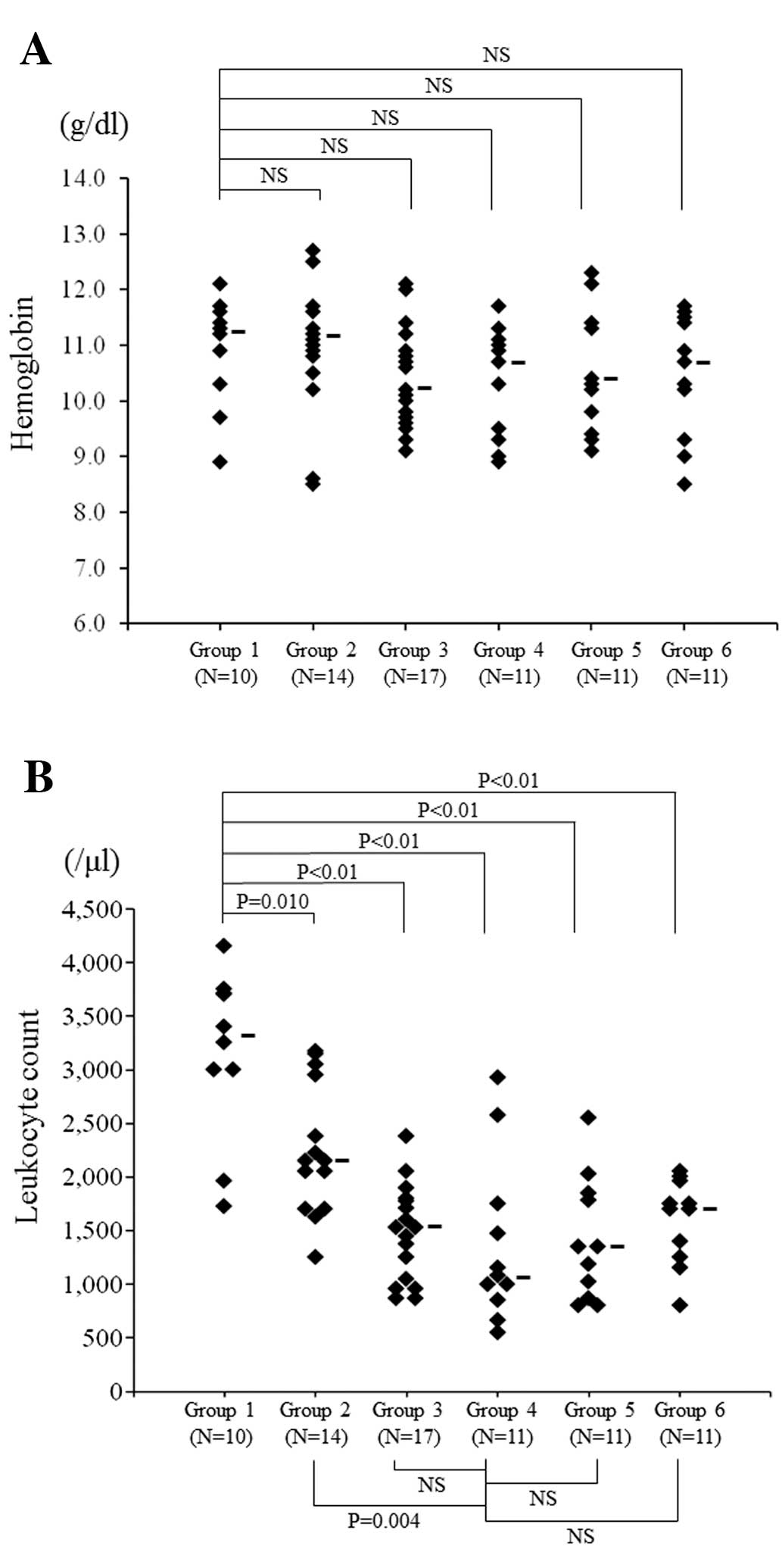
the groups. In addition, we examined the severity of
myelosuppression by measuring the hemoglobin and leukocyte count
after treatment. We found that the hemoglobin did not show a
significant difference between Group 1 and any of the other groups
(Fig. 8A). The leukocyte count was
the lowest in Group 4 and there was a significant difference
compared with Group 2 (P=0.004), but there was no significant
difference between Group 4 and Groups 3, 5 or 6 (Fig. 8B).
Discussion
To improve the outcome of treatment for locally
advanced cervical cancer, radiotherapy has become mainstream and,
to increase the effect of radiation chemotherapy, agents are
administered before radiotherapy, concurrently with radiotherapy or
after radiotherapy. However, only concurrent chemoradiation has
been proven to improve disease-free survival and overall survival
in patients with cervical cancer (8–11),
while there have been a number of reports that performing
chemotherapy before radiotherapy does not improve survival
(22–26). The anticancer drug that has proved
to be most effective with radiation is cisplatin either alone or in
combination with other agents such as 5-fluorouracil (4–7).
Therefore, CCRT with cisplatin has become the standard treatment
for locally advanced cervical cancer.
The effect of irradiation is presumably enhanced by
performing concurrent chemotherapy due to a radiosensitizing effect
of anticancer drugs that enhances initial radiation damage to DNA.
For example, cisplatin interacts with nucleophilic sites on DNA or
RNA to form intra-and inter-strand crosslinks (12). Second, chemotherapy agents inhibit
cellular repair processes and exacerbate radiation damage. Grégoire
et al reported that the effect of fludarabine on
radiocurability in mice was greater when it was combined with
fractionated radiation than when it was combined with a single dose
of radiation (27). Third,
chemotherapy can cause the accumulation of cells in the
radiosensitive phases of the cell cycle (the G2 and M phases) or
eliminate cells in the radioresistant phase (S-phase) (28–30).
Some in vitro studies using human cervical squamous cell
carcinoma cell lines have already investigated the timing of
anticancer drug administration (15,16).
Tanaka et al reported that sensitivity to nedaplatin was
enhanced by irradiation and this effect was significantly greater
when cells were treated 8 h before or 8 h after irradiation than
when they were treated concurrently with irradiation (16). They also reported that 5 of the 6
etoposide-resistant subclones established from ME180 cells showed
significant radioresistance, indicating that etoposide should be
administered to patients with advanced cervical squamous cancer
after the completion of radiotherapy (15).
Although it is inevitable that CCRT will be
associated with enhanced acute toxicity (12,13),
there has been a lack of animal studies on the relation between
adverse effects and the timing of administration of anticancer
drugs. Therefore, we performed the present investigation using αT3
transgenic mice bearing SV40-induced undifferentiated lens
epithelial tumors (17,18). Comparison of the three combined
treatment groups showed that the antitumor activity of CCRT was
superior with respect to the tumor reduction rate and the apoptotic
effect, although leukopenia was also most severe. In contrast, when
cisplatin was administered before radiotherapy the antitumor
activity (both tumor reduction rate and the apoptotic effect) was
lower than with CCRT or with administration of cisplatin after
radiotherapy, and there was a significant difference in the extent
of apoptosis between the CCRT group and the cisplatin-first group
(P=0.031). Although leukopenia was less severe in the
cisplatin-first group, there was no significant difference from the
other groups. These results suggest that it may be unwise to
administer cisplatin before radiotherapy. It was recently reported
that neoadjuvant chemotherapy with weekly paclitaxel and
carboplatin before CCRT is beneficial for locally advanced cervical
carcinoma (31,32). Therefore, further studies are needed
to examine the effectiveness of such agents with radiotherapy in
our animal model.
In conclusion, the present study performed on mice
did not show the superiority of CCRT over administration of
chemotherapy after radiotherapy with respect to efficacy and
adverse effects, therefore we could not demonstrate that CCRT is
the optimum treatment. However, our findings in this animal model
demonstrated that chemotherapy with cisplatin should probably not
be performed before irradiation for the treatment of cancer.
Acknowledgements
We thank Miss Yoshimi Harada for handling of the
mice and Mr. Nobuhisa Iwachidou for the technical assistance with
immunostaining. This study was supported by multiple Research
Project Grants (nos. 20-111N, 21-122, 22-A68, 24Base-27 and
25Base-99) from Kawasaki Medical School.
References
|
1
|
Kessis TD, Slebos RJ, Nelson WG, et al:
Human papillomavirus 16 E6 expression disrupts the p53-mediated
cellular response to DNA damage. Proc Natl Acad Sci USA.
90:3988–3992. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nees M, Geoghegan JM, Munson P, et al:
Human papillomavirus type 16 E6 and E7 proteins inhibit
differentiation-dependent expression of transforming growth
factor-β2 in cervical keratinocytes. Cancer Res. 60:4289–4298.
2000.PubMed/NCBI
|
|
3
|
Parkin DM, Pisani P and Ferlay J:
Estimates of the worldwide incidence of eighteen major cancers in
1985. Int J Cancer. 54:594–606. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Runowicz CD, Wadler S, Rodriguez-Rodriguez
L, et al: Concomitant cisplatin and radiotherapy in locally
advanced cervical carcinoma. Gynecol Oncol. 34:395–401. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alberts DS, Garcia D and Mason-Liddil N:
Cisplatin in advanced cancer of the cervix: an update. Semin Oncol.
18:11–24. 1991.PubMed/NCBI
|
|
6
|
Malfetano J, Keys H, Kredentser D,
Cunningham M, Kotlove D and Weiss L: Weekly cisplatin and radical
radiation therapy for advanced, recurrent, and poor prognosis
cervical carcinoma. Cancer. 71:3703–3706. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Whitney CW, Sause W, Bundy BN, et al:
Randomized comparison of fluorouracil plus cisplatin versus
hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA
carcinoma of the cervix with negative para-aortic lymph nodes: a
Gynecologic Oncology Group and Southwest Oncology Group study. J
Clin Oncol. 17:1339–1348. 1999.
|
|
8
|
Sorbe B, Bohr L, Karlsson L and Bermark B:
Combined external and intracavitary irradiation in treatment of
advanced cervical carcinomas: Predictive factors for local tumor
control and early recurrences. Int J Oncol. 36:371–378. 2010.
|
|
9
|
Morris M, Eifel PJ, Lu J, et al: Pelvic
radiation with concurrent chemotherapy compared with pelvic and
para-aortic radiation for high-risk cervical cancer. N Engl J Med.
340:1137–1143. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rose PG, Bundy BN, Watkins EB, et al:
Concurrent cisplatin-based radiotherapy and chemotherapy for
locally advanced cervical cancer. N Engl J Med. 340:1144–1153.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eifel PJ, Winter K, Morris M, et al:
Pelvic irradiation with concurrent chemotherapy versus pelvic and
para-aortic irradiation for high-risk cervical cancer: an update of
radiation therapy oncology group trial (RTOG) 90-01. J Clin Oncol.
22:872–880. 2004. View Article : Google Scholar
|
|
12
|
Vokes EE and Weichselbaum RR: Concomitant
chemoradiotherapy: rationale and clinical experience in patients
with solid tumors. J Clin Oncol. 8:911–934. 1990.PubMed/NCBI
|
|
13
|
Tannock IF: Treatment of cancer with
radiation and drugs. J Clin Oncol. 14:3156–3174. 1996.PubMed/NCBI
|
|
14
|
Randall LM, Monk BJ, Moon J, et al:
Prospective evaluation of an in vitro radiation resistance assay in
locally advanced cancer of the uterine cervix: a Southwest Oncology
Group Study. Gynecol Oncol. 119:417–421. 2010. View Article : Google Scholar
|
|
15
|
Tanaka T, Bai T, Yukawa K and Umesaki N:
Optimal combination chemotherapy and chemoradiotherapy with
etoposide for advanced cervical squamous cancer cells in
vitro. Oncol Rep. 15:939–947. 2006.PubMed/NCBI
|
|
16
|
Tanaka T, Yukawa K and Umesaki N:
Radiation reduces carboplatin sensitivity and enhances nedaplatin
sensitivity in cervical squamous cell carcinoma in vitro. Eur J
Gynaecol Oncol. 28:352–355. 2007.
|
|
17
|
Egwuagu CE, Li W, Yu CR, et al:
Interferon-γ induces regression of epithelial cell carcinoma:
critical roles of IRF-1 and ICSBP transcription factors. Oncogene.
25:3670–3679. 2006.
|
|
18
|
Zheng HC, Nakamura T, Zheng Y, et al: SV40
T antigen disrupted the cell metabolism and the balance between
proliferation and apoptosis in lens tumors of transgenic mice. J
Cancer Res Clin Oncol. 135:1521–1532. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fujii M, Ide A, Nakabayashi K, Joguchi A,
Ogino H and Ayusawa D: The introduction of dominant-negative p53
mutants suppresses temperature shift-induced senescence in immortal
human fibroblasts expressing a thermolabile SV40 large T antigen. J
Biochem. 125:531–536. 1999. View Article : Google Scholar
|
|
20
|
Dueñas-González A, Cetina-Perez L,
Lopez-Graniel C, et al: Pathologic response and toxicity assessment
of chemoradiotherapy with cisplatin versus cisplatin plus
gemcitabine in cervical cancer: a randomized Phase II study. Int J
Radiat Oncol Biol Phys. 61:817–823. 2005.PubMed/NCBI
|
|
21
|
Verbraecken J, Van de Heyning P, De Backer
W and Van Gaal L: Body surface area in normal-weight, overweight,
and obese adults. A comparison study. Metabolism. 55:515–524. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kumar L, Kaushal R, Nandy M, et al:
Chemotherapy followed by radiotherapy versus radiotherapy alone in
locally advanced cervical cancer: a randomized study. Gynecol
Oncol. 54:307–315. 1994. View Article : Google Scholar
|
|
23
|
Tattersall MH, Lorvidhaya V, Vootiprux V,
et al: Randomized trial of epirubicin and cisplatin chemotherapy
followed by pelvic radiation in locally advanced cervical cancer.
Cervical Cancer Study Group of the Asian Oceanian Clinical Oncology
Association. J Clin Oncol. 13:444–451. 1995.
|
|
24
|
Sundfør K, Tropé CG, Högberg T, et al:
Radiotherapy and neoadjuvant chemotherapy for cervical carcinoma. A
randomized multicenter study of sequential cisplatin and
5-fluorouracil and radiotherapy in advanced cervical carcinoma
stage 3B and 4A. Cancer. 77:2371–2378. 1996.
|
|
25
|
Shueng PW, Hsu WL, Jen YM, Wu CJ and Liu
HS: Neoadjuvant chemotherapy followed by radiotherapy should not be
a standard approach for locally advanced cervical cancer. Int J
Radiat Oncol Biol Phys. 40:889–896. 1998. View Article : Google Scholar
|
|
26
|
Neoadjuvant Chemotherapy for Locally
Advanced Cervical Cancer Meta-analysis Collaboration. Neoadjuvant
chemotherapy for locally advanced cervical cancer: a systematic
review and meta-analysis of individual patient data from 21
randomised trials. Eur J Cancer. 39:2470–2486. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Grégoire V, Hunter NR, Brock WA, Hittelman
WN, Plunkett W and Milas L: Improvement in the therapeutic ratio of
radiotherapy for a murine sarcoma by indomethacin plus fludarabine.
Radiat Res. 146:548–553. 1996.
|
|
28
|
Milas L, Hunter N, Mason KA, Milross C and
Peters LJ: Tumor reoxygenation as a mechanism of taxol-induced
enhancement of tumor radioresponse. Acta Oncol. 34:409–412. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Milross CG, Mason KA, Hunter NR, et al:
Enhanced radioresponse of paclitaxel-sensitive and-resistant
tumours in vivo. Eur J Cancer. 33:1299–1308. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Suzuki M, Nakamatsu K, Kanamori S,
Masunaga S and Nishimura Y: Additive effects of radiation and
docetaxel on murine SCCVII tumors in vivo: special reference to
changes in the cell cycle. Radiat Res. 159:799–804. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Singh RB, Chander S, Mohanti BK, et al:
Neoadjuvant chemotherapy with weekly paclitaxel and carboplatin
followed by chemoradiation in locally advanced cervical carcinoma:
a pilot study. Gynecol Oncol. 129:124–128. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
McCormack M, Kadalayil L, Hackshaw A, et
al: A phase II study of weekly neoadjuvant chemotherapy followed by
radical chemoradiation for locally advanced cervical cancer. Br J
Cancer. 108:2464–2469. 2013. View Article : Google Scholar
|