Introduction
Gastric cancer is one of the major causes of
cancer-related death worldwide; however, recent advances in
systemic chemotherapy regimens have shown encouraging tumor
response rates and increased survival in patients with unresectable
or metastatic gastric cancer (1).
However, treatment outcomes for patients with peritoneal
metastasis, which is the most frequent metastatic pattern of
recurrence, have not improved sufficiently (2).
Paclitaxel is an anticancer agent with a wide
spectrum of antitumor activity in cancers that include ovarian,
breast, gastric and lung (3–5). This
drug stabilizes polymerized microtubules and enhances microtubule
assembly, and thus arrests cells in the cell cycle in the G0/G1 and
G2/M phases leading to cell death (6).
In unresectable or recurrent gastric cancer,
including cases with malignant ascites, treatment with paclitaxel
has achieved relatively good response rates (7–9).
Intraperitoneal (i.p.) administration of paclitaxel is now
attracting attention as an effective treatment for peritoneal
metastasis, since a high concentration of the drug is maintained in
the peritoneal cavity over a long period of time due to its high
molecular weight and bulky structure (10,11).
The i.p. paclitaxel regimen has been shown to prolong survival in a
phase III study involving ovarian cancer with peritoneal
metastasis, and has been approved as a recommended regimen by the
National Cancer Institute in the US (12). In gastric cancer, a recent phase II
study of intravenous (i.v.) and i.p. paclitaxel combined with S-1
(an oral fluoropyrimidine derivative, combining tegafur with two
modulators) showed a 1-year overall survival rate of 78% with a
median survival time (MST) of 22.5 months for patients with
peritoneal metastasis from gastric cancer (13). In addition, it has been reported
that other clinical trials involving i.p. chemotherapy with taxane
agents have shown favorable prognoses with an MST of 16.2–24.6
months (14–16). Therefore, a multicenter randomized
clinical trial is now ongoing to generate evidence regarding i.p.
chemotherapy for gastric cancer patients with peritoneal
metastasis.
The conventional formulation of paclitaxel, which
has poor solubility in water, requires the solubilization of the
drug in a 1:1 solution of cremophor-EL (Cr-EL) and dehydrated
ethanol to arrive at solvent-based PTX (Sb-PTX: Taxol®;
Bristol-Myers Squibb, New York, NY, USA). Because of the large
amount of Cr-EL used and the non-specific biodistribution of the
drug in both tumors and normal tissues, Sb-PTX has been associated
with serious side-effects, including severe hypersensitivity
reactions, myelosuppression and neurotoxicity (17–19).
In particular, Cr-EL has a negative impact on the efficacy of
paclitaxel by forming micelles that entrap the drug in the plasma
compartment (20).
Nanoparticle albumin-bound PTX (Nab-PTX:
Abraxane®; American BioScience, Inc., Santa Monica, CA,
USA) is an albumin-bound, 130-nm particle formulation of
paclitaxel, which is devoid of any solvents or ethanol.
Nab-PTX was developed to take advantage of the antitumor
activity of paclitaxel while decreasing or eliminating the
toxicities typically associated with Cr-EL (19,21).
Thus, since Nab-PTX delivery is not complicated by solvents,
a higher dose can be administered relative to Sb-PTX. In a pivotal
phase III trial of Nab-PTX and Sb-PTX as first-line therapy
in patients with metastatic breast cancer at the label-indicated
doses, the dose of paclitaxel delivered was 49% higher for patients
receiving Nab-PTX than Sb-PTX; this suggested that a higher
dose intensity is feasible with Nab-PTX. In addition,
albumin has the natural ability to promote drug delivery to tumors
by initiating albumin receptor (gp60)-mediated transcytosis across
endothelial cells (22,23), and facilitates the accumulation of
drugs in tumors via binding to secreted protein acidic and rich in
cysteine (SPARC) (24,25).
Nab-PTX and Sb-PTX have been extensively
investigated in comparative clinical and experimental studies, and
have exhibited unequivocal antitumor activity and minor
side-effects in the treatment of breast cancer and non-small cell
lung carcinoma (21,26).
In gastric cancer, second line chemotherapy with
Nab-PTX has shown a response rate of 27.8% and a disease
control rate of 59.3% in a phase II study involving patients with
unresectable and metastatic gastric cancer (27). Nonetheless, little is known
concerning the efficacy of Nab-PTX with regard to peritoneal
metastasis, which is biologically more malignant and has a severe
prognosis.
The aim of the present study was to investigate for
the first time the antitumor effects of Nab-PTX as compared
with i.p. Sb-PTX using a preclinical model of peritoneal
metastasis. In addition, we evaluated the difference in antitumor
activity between i.p. and i.v. administration of Nab-PTX.
This was because i.p. chemotherapy, in spite of its survival
advantages, is limited by the complicated procedure involved in
positioning the access port and several other complications,
including infection due to prolonged use of the in-dwelling
catheter and local toxicity (e.g. abdominal pain and other
gastrointestinal toxicities). For these purposes, we used four
treatment groups in the present study: control group; i.p.
Sb-PTX-treated group; i.p. Nab-PTX-treated group; and i.v.
Nab-PTX-treated group. Antitumor activity was compared among
these four groups at equitoxic and equal doses in nude mice bearing
OCUM-2MD3 subcutaneous and peritoneal xenografts.
Materials and methods
Study drugs
Nab-PTX (ABI-007, Abraxane®;
American BioScience) and Sb-PTX (Taxol®; Bristol-Myers
Squibb) was purchased from a hospital pharmacy. Both drugs were
reconstituted in normal saline, prepared fresh daily as required,
and administered within 1 h of preparation.
Cell lines and cell culture
OCUM-2MD3, a high peritoneal-seeding cell line from
human scirrhous gastric cancer, was kindly provided by the
Department of Surgical Oncology of Osaka City University of
Medicine (28). These cells were
cultured in 10 ml of medium at 37°C in a humidified atmosphere of
5% CO2 in air. OCUM-2MD3 cells were grown in Dulbecco’s
modified Eagle’s medium (DMEM) (Life Technologies, Tokyo, Japan)
supplemented with 10% heat-inactivated fetal bovine serum, 100
IU/ml penicillin, 100 mg/ml streptomycin, 2 mM glutamine and 0.5 mM
sodium pyruvate. Cells were grown to confluency and harvested by
trypsinization with 0.25 mg/ml trypsin/EDTA (Life Technologies) and
suspended in culture medium before use.
Animals and development of the gastric
cancer model
Female athymic NCr-nu nude mice (4–6 weeks of age)
were purchased from Charles River Laboratories (Yokohama, Japan).
All animal experiments were performed in accordance with the
guidelines of the Institutional Animal Care and Use Committee of
Kanazawa University. They were housed in specific pathogen-free
conditions and fed standard chow pellets and water ad
libitum. At the start of the treatment, body weights ranged
from 21 to 25 g and ages ranged from 6 to 8 weeks. To evaluate
systemic and intraperitoneal antitumor activity, the mice were
inoculated simultaneously with 1×107 OCUM-2MD3 cells
suspended in 1 ml PBS intraperitoneally and 2×106
OCUM-2MD3 cells suspended in 200 μl PBS subcutaneously in the
flank.
Mice for examination of the survival rate were
inoculated with 1×107 OCUM-2MD3 cells intraperitoneally
as a peritoneal metastatic model.
Evaluation of antitumor activity
Treatment schedule
After tumor inoculation (day 0) the mice were
randomly divided into four groups, each consisting of five animals
that received different treatments: control group; i.p.
Sb-PTX-treated group; i.p. Nab-PTX-treated group; and i.v.
Nab-PTX-treated group.
In the control and i.p. treatment groups,
phosphate-buffered saline or the PTX formulations were given i.p.,
and the injection volume was 1 ml/mouse to optimize spreading of
the drugs throughout the entire peritoneal cavity. In the i.v.
treatment group, Nab-PTX was injected into the tail vein,
and the injection volume was 100 μl/mouse. These four groups were
used in each evaluation as described below.
Drug treatment was initiated on day 7, and each drug
was administered once daily for 7 consecutive days. Sb-PTX and
Nab-PTX were administered at equitoxic doses (MTDs: 13.4 and
30 mg PTX/kg/day, respectively) that were previously reported in a
mouse study (29) and at an equal
dose (10 mg PTX/kg/day).
Assessment of tumor response to Nab-PTX
and Sb-PTX
Therapeutic efficacy of Sb-PTX and Nab-PTX
was evaluated as inhibition of tumor growth using a peritoneal
metastatic model with subcutaneous xenografts at equitoxic and
equal doses. Each group consisted of five mice with respect to each
treatment.
For subcutaneous tumors, the size was measured with
a digital caliper twice weekly. Tumor growth was calculated using
the formula (L × W2)/2, where L is the longest and W is
the shortest tumor diameter.
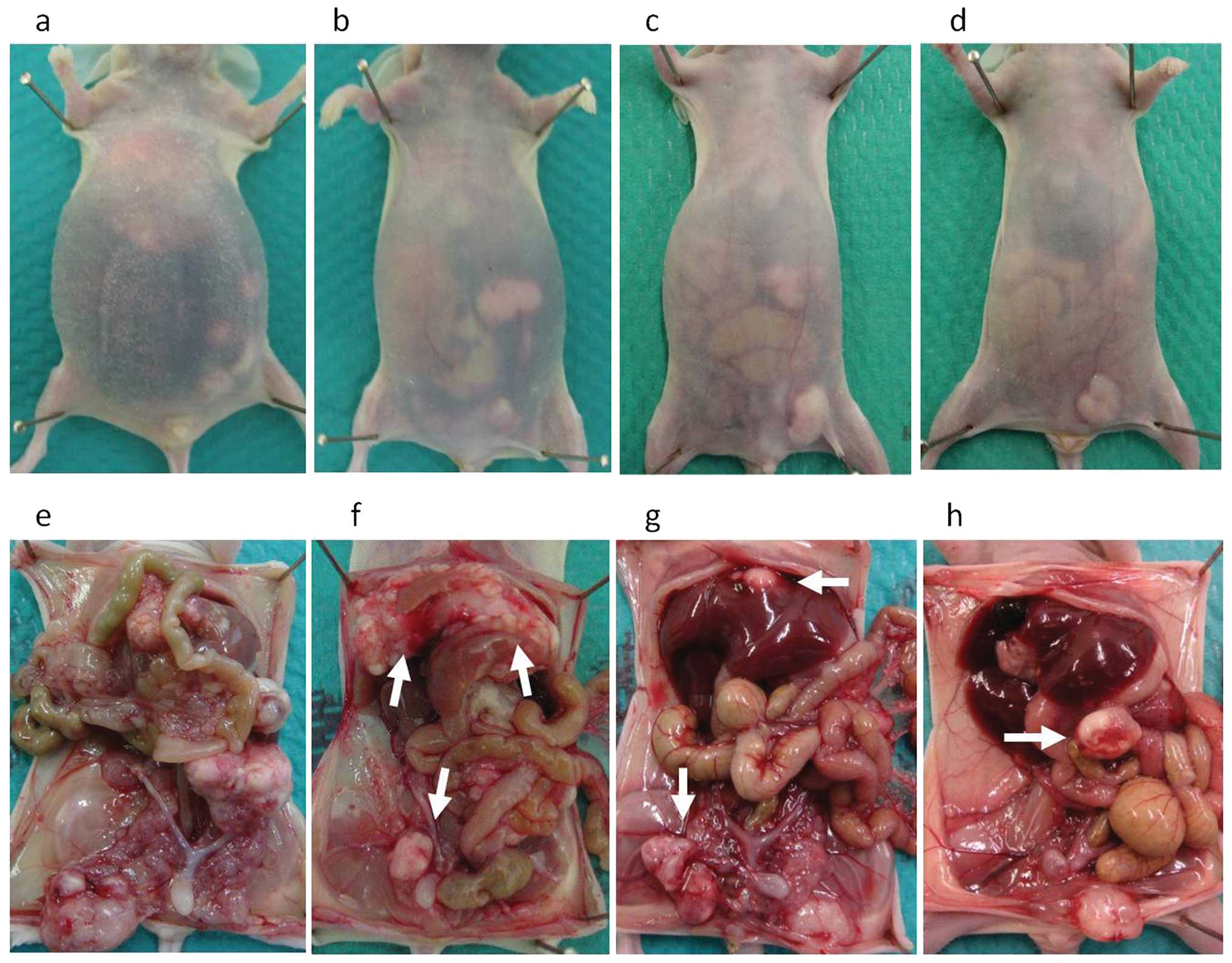
For assessment of peritoneal metastasis, the mice
were sacrificed using isoflurane inhalation and necropsied on day
25, and samples of ascites and peritoneal nodules were collected.
The weight of ascitic fluid and the total peritoneal tumor burden
were measured for each individual mouse.
In addition, the survival time with respect to each
treatment was evaluated in the peritoneal metastatic models. The
mice were euthanized when they became moribund (the day of death
being considered the limit of survival).
Statistical analyses
Statistical analyses were carried out using the
computer software package SPSS 10.0. Statistical differences for
two groups were evaluated using the Student’s t-test, and one-way
ANOVA for multiple groups. Survival rates were expressed using
Kaplan-Meier curves and their comparison was analyzed using the
log-rank test. A value of P<0.05 was considered to indicate a
statistically significant result.
Results
Antitumor activity of Sb-PTX and Nab-PTX
at equitoxic doses
The antitumor effects of i.p. Sb-PTX, i.p.
Nab-PTX, and i.v. Nab-PTX were evaluated at equitoxic
doses in the subcutaneous and intraperitoneal OCUM-2MD3 xenograft
models.
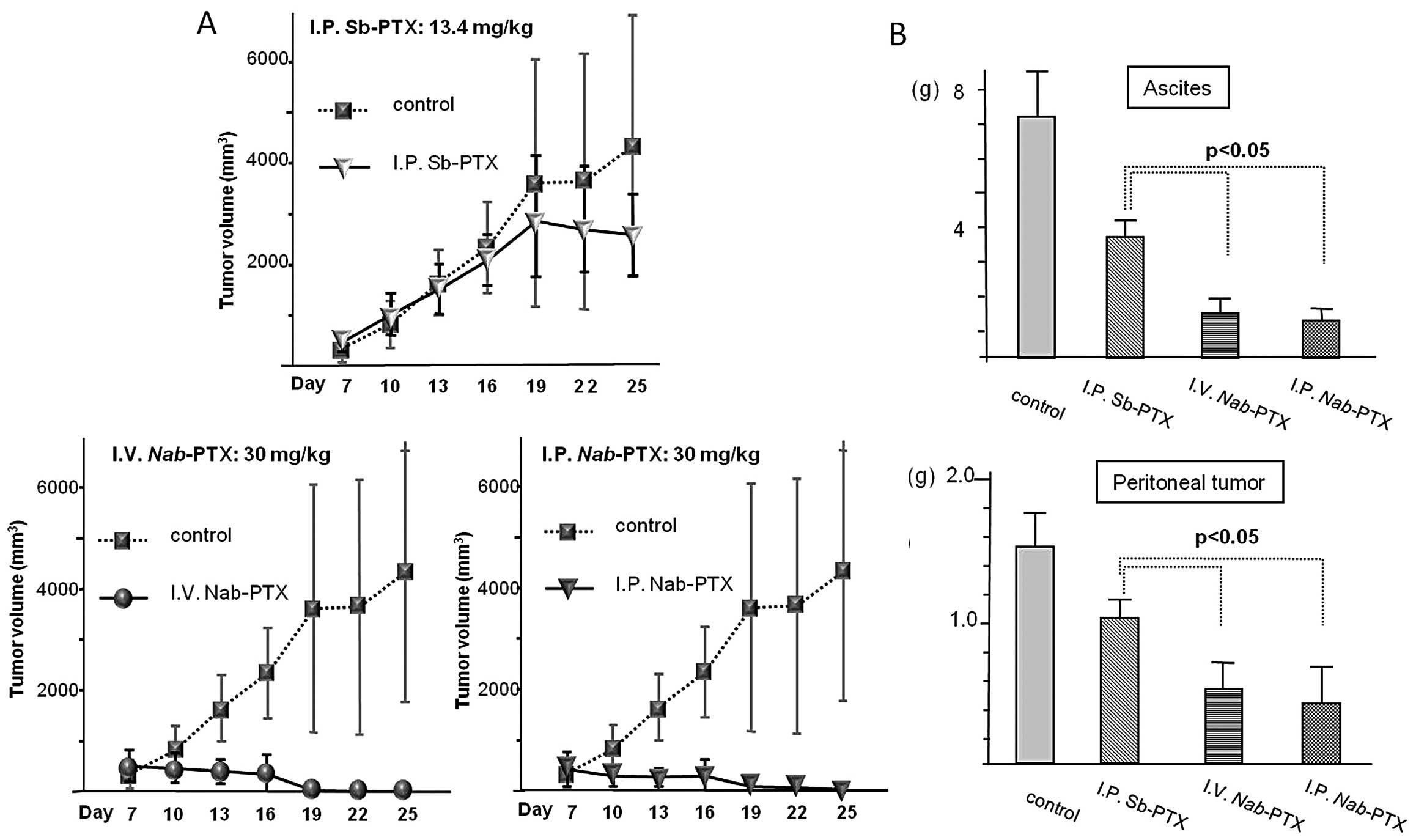
Regarding the subcutaneous tumors, the
Nab-PTX-treated group showed greater antitumor activity than
that of the i.p. Sb-PTX-treated group (P<0.01). Complete
regression was observed in both the i.p. and i.v.
Nab-PTX-treated groups. There was no significant difference
in the measurement of the subcutaneous tumor volume between the
routes of administration (i.v./i.p.) following treatment with
Nab-PTX (Fig. 1A).
Following assessment of the peritoneal metastasis,
complete regression was not observed in either the Nab-PTX
or Sb-PTX-treated group (Fig. 1B).
As compared with the control group, all treatment groups displayed
significantly slower growth of peritoneal tumors and relief from
the accumulation of ascites. Representative images in the four
groups are shown in Fig. 2. Among
the treatment groups, both the i.p. and i.v. Nab-PTX-treated
groups displayed significant tumor reduction relative to the i.p.
Sb-PTX-treated group (P<0.05). With regard to the accumulation
of ascites, both Nab-PTX-treated groups also showed
significantly greater antitumor activity than the i.p.
Sb-PTX-treated group (P<0.05). In the Nab-PTX-treated
groups, there was no significant difference in the weights of
ascites and the peritoneal tumors between the i.p. and i.v.
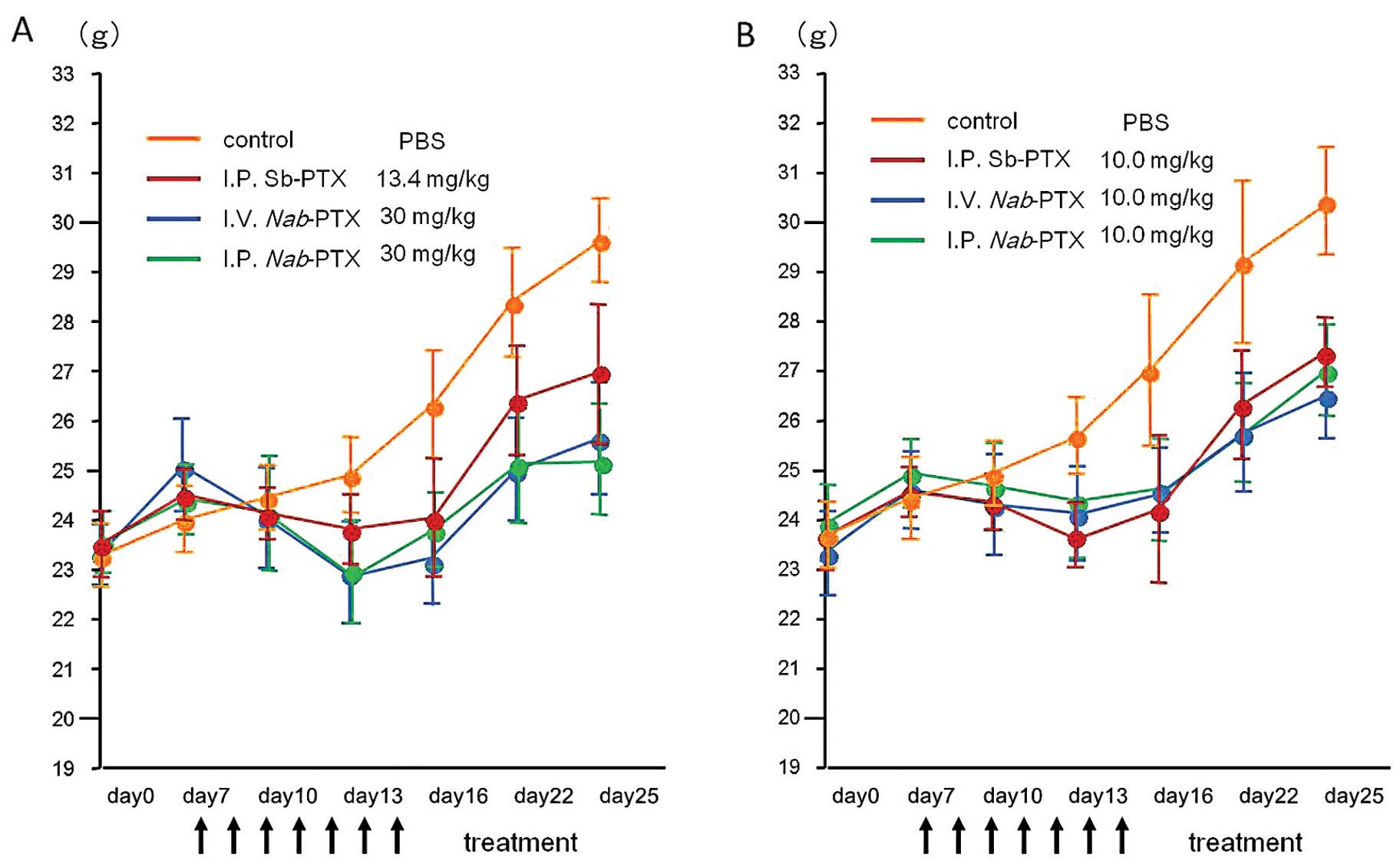
administration routes. All treatment sequences were well tolerated,
and mean body weights were not significantly different among the
three treatment groups (Fig. 3A).
The body weight in the control group was significantly higher than
the body weight in all of the treatment groups (P<0.05). This
was mainly due to the accumulation of ascites and peritoneal
tumors.
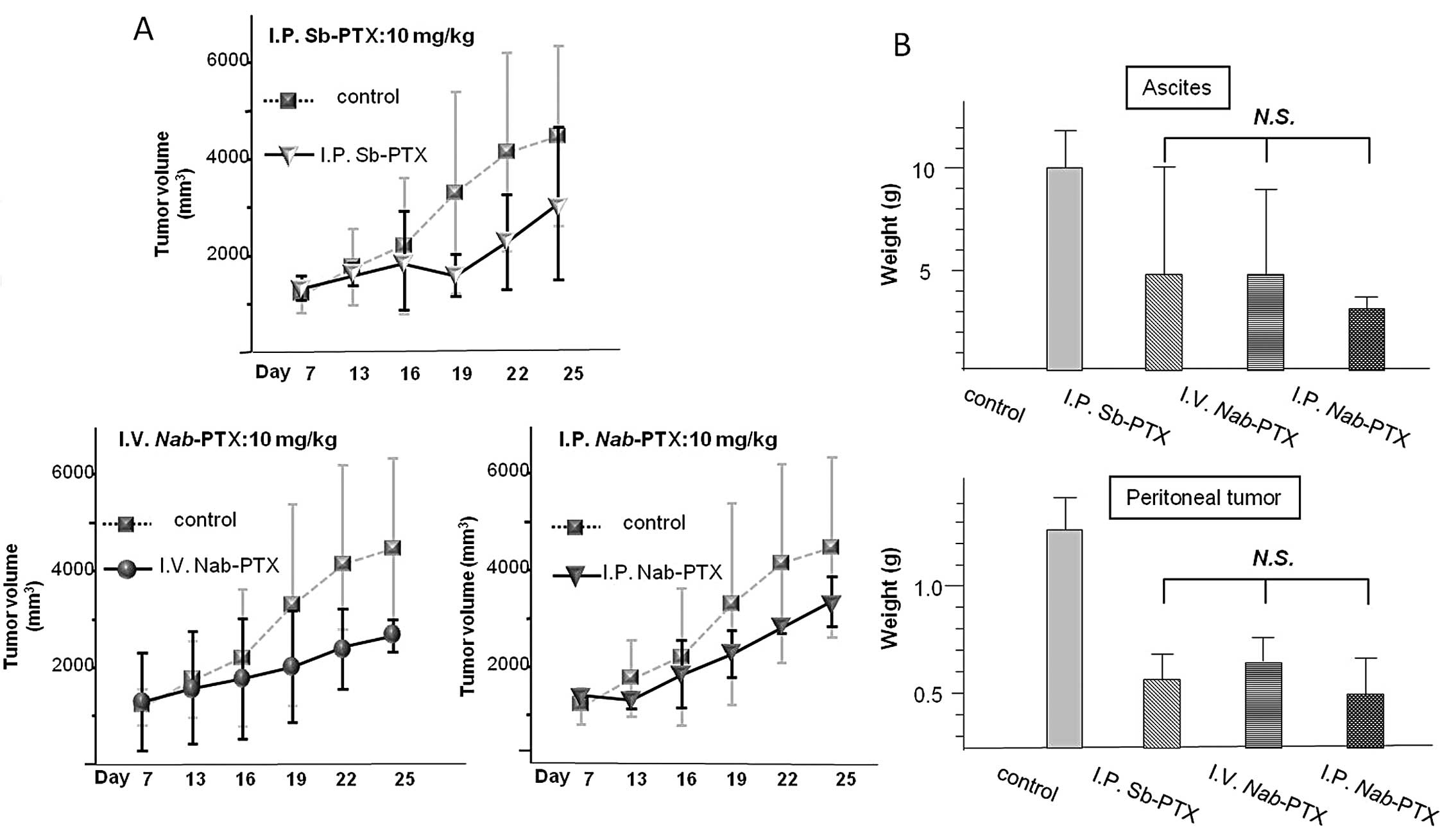
Antitumor activity of PTX and Nab-PTX in
the subcutaneous tumors administered at an equal dose
The antitumor effects of the two PTX formulations
were investigated at an equal dose (10.0 mg/kg/day for Sb-PTX and
Nab-PTX, concurrently) in the same manner as the equitoxic
study. As shown in Fig. 4A, the
volume of the subcutaneous tumors was significantly reduced in all
of the treatment groups relative to the control group (P<0.01),
whereas no significant reduction in tumor volume was observed when
comparing the three treatment groups.
In the assessment of peritoneal metastasis, the
weights of ascites and the peritoneal tumors were significantly
reduced in all treatment groups relative to the control group
(P<0.01). However, there was no significant difference among the
three treatment groups (Fig.
4B).
All treatment sequences were well tolerated, and
mean body weights were not significantly different among the three
treatment groups as well as in the equitoxic dose study (Fig. 3B).
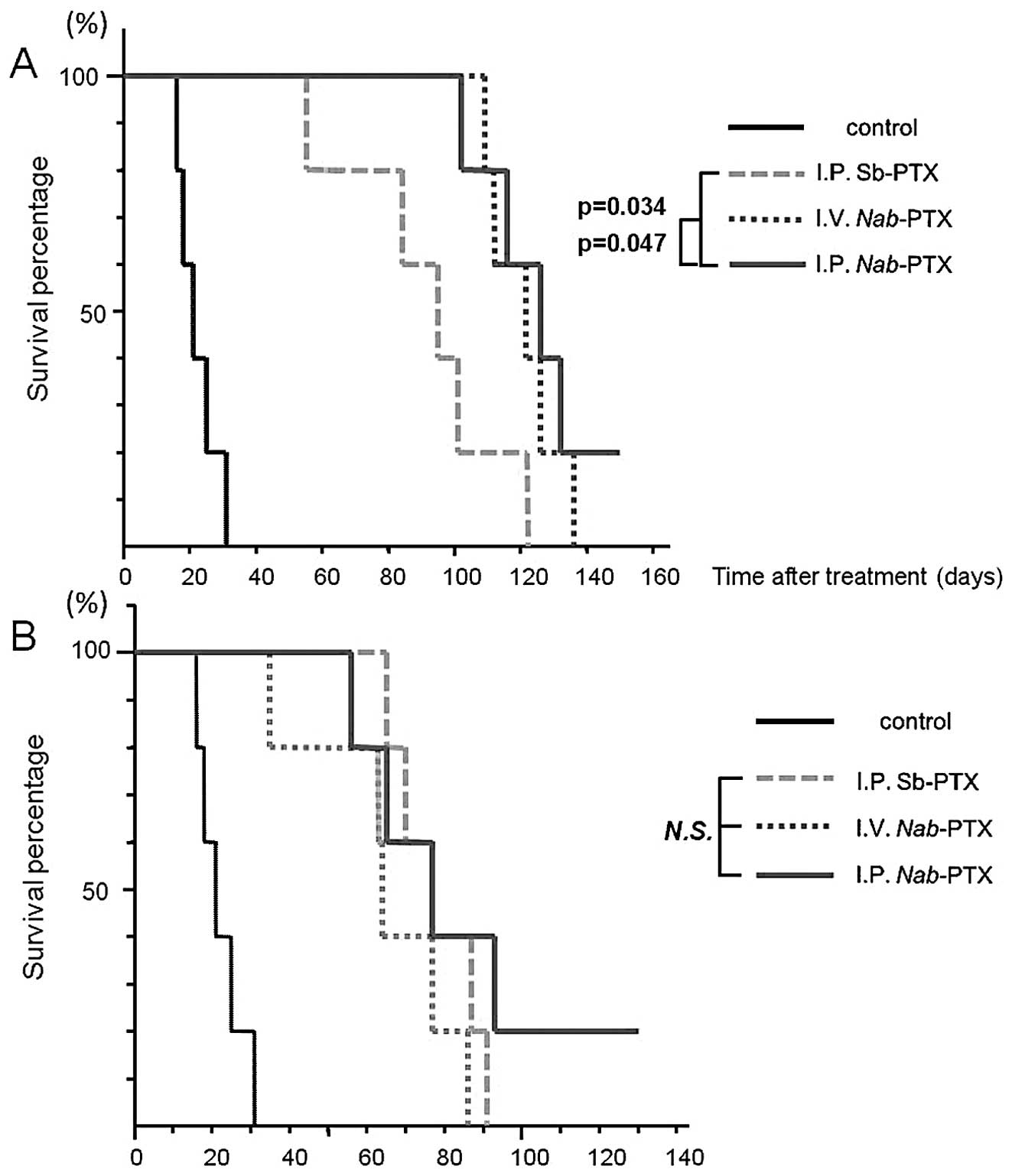
Survival rate
The survival rate in the peritoneal metastatic model
was also evaluated using Kaplan-Meyer survival curves at equitoxic
and equal doses (Fig. 5). All five
mice in the control group developed ascites and died within 19–32
days after tumor cell inoculation; the median survival time was 25
days. At equitoxic doses, the median survival time was 96 days for
the i.p. Sb-PTX-treated group, 122 days for the i.v.
Nab-PTX-treated group and 126 days for the i.p.
Nab-PTX-treated group. The survival benefit was greater in
the i.p. and i.v. Nab-PTX-treated groups than that in the
i.p. Sb-PTX-treated group (P=0.034 and P=0.047, respectively).
Regarding the route of drug delivery, i.p. administration resulted
in no significant improvement in survival when compared with i.v.
administration in the Nab-PTX treated groups. In addition,
there was no significant difference in relation to survival time
among the treated groups at an equal dose.
Discussion
The primary aims of the present study were to
clarify whether or not Nab-PTX had any advantages over i.p.
Sb-PTX in the treatment of peritoneal metastasis from gastric
cancer, and to evaluate potential differences in the antitumor
activity of Nab-PTX between i.v. and i.p. administration
routes. Nab-PTX demonstrated significantly greater antitumor
efficacy in both peritoneal and subcutaneous tumors as compared
with i.p. Sb-PTX at equitoxic doses (Nab-PTX, 30 mg/kg/day;
Sb-PTX, 13.4 mg/kg/day) using a mouse model. In survival studies, a
significant improvement in survival time was observed in the
Nab-PTX-treated groups relative to the i.p. Sb-PTX-treated
group under the same conditions.
Additional studies involving other tumor models and
in vivo mechanism-related studies have confirmed the high
accumulation characteristics of Nab-PTX (24,25,29,32).
It is generally considered that the free or unbound form of the
drug is the active fraction, since drug bound to proteins or other
macromolecules may be unable to cross cell membranes (30). In clinical studies, Sb-PTX has been
shown to be highly bound to protein in plasma, with Cre-EL further
decreasing the free/unbound fraction of the drug (30,31).
The advantage of Nab-PTX is its water solubility, achieved
without the use of Cre-EL and ethanol. Indeed, Gardner et al
(32) reported that the formulation
of Nab-PTX allowed a much higher fraction of unbound
paclitaxel than that of Sb-PTX, and that the maximal concentration
of unbound paclitaxel was ~10-fold higher for Nab-PTX in
their pharmacokinetic study.
An additional advantage of Nab-PTX is its
albumin-bound particle formulation. Albumin is assumed to be a
ubiquitous carrier of biomolecules in human blood, which accumulate
in tumors by means of a receptor-mediated transport mechanism; this
involves an albumin-specific receptor such as glycoprotein 60, or
the permeation and retention effect (22,23).
Another molecular mechanism proposed to play a
potential role in the accumulation of Nab-PTX in tumors
involves albumin-binding proteins such as SPARC in proximity to
tumors (24). Clinical data suggest
a correlation between tumoral SPARC expression and the positive
clinical outcome of patients treated with Nab-PTX (24,25).
Indeed, SPARC expression has been confirmed in both gastric cancer
tissues and the microenvironment of peritoneal metastasis (33–35).
Accordingly, it has been hypothesized that Nab-PTX may take
advantage of each of these mechanisms to reach the microenvironment
of the tumor.
For these reasons, we initially hypothesized that
Nab-PTX would show greater antitumor effects than Sb-PTX,
even at an equal dose (10 mg/kg/day). However, in the present
study, the equal dose comparison did not indicate a significant
difference between Nab-PTX and Sb-PTX in terms of the
shrinkage of subcutaneous and peritoneal tumors. In addition,
Nab-PTX did not increase the median survival time as
compared with Sb-PTX at an equal dose. This finding indicates that
the greater antitumor activity of Nab-PTX was mainly
attributable to a higher-dose administration relative to Sb-PTX. We
could not clarify the reason why the higher tumor accumulation of
Nab-PTX relative to Sb-PTX did not reflect antitumor
efficacy in our study. However, we consider the comparison of
equitoxic doses to be more clinically applicable rather than
comparison at an equal dose, since chemotherapy is generally
administered at the highest tolerated dose. Indeed, these findings
are supported by a recent phase III clinical study, which showed
significantly higher therapeutic efficacy using Nab-PTX as
compared with Sb-PTX at equitoxic doses (21).
One of the limitations of the present study was the
inadequacy to evaluate the toxicity of both drugs. Although we
measured the body weight of mice during the course of treatment,
blood examination for neutropenia, liver or renal dysfunctions was
not performed. Because body weight is critically affected by tumor
burden, ascites and cachexia, further investigation is required to
validate the side-effects of Nab-PTX.
Regarding the route of administration, there was no
significant difference between i.p. and i.v. administration of
Nab-PTX in any of the parameters evaluated, such as volume
change in the subcutaneous tumors, the weight of ascites and
peritoneal tumors and survival times of the mice. Although the
effects of i.v. Nab-PTX have been reported in several
studies, the effects of this nanoparticulate paclitaxel following
i.p. administration remain unclear.
Intraperitoneal Sb-PTX was expected to demonstrate
high efficacy for peritoneal metastasis, since Sb-PTX is retained
for long periods in the peritoneal cavity after i.p. administration
due to its large molecular weight and fat solubility (36). However, two shortcomings are that
the drug infiltrates only the surface of the peritoneal tumor and
that the drug is not fully absorbed into the systemic
circulation.
On the other hand, our results suggest that
Nab-PTX following i.v. administration may infiltrate into
the peritoneal tumor to the same degree as i.p. injection.
Conversely, Nab-PTX administered intraperitoneally might be
absorbed into the systemic circulation more easily than Sb-PTX.
We speculate that one of the reasons for these
findings is that Nab-PTX is unaffected by the ability of
Cre-EL to inhibit transport into the bloodstream and binding to
endothelial cells around the tumor. In addition, the enhanced
permeability and retention (EPR) effect, which is known as
selective accumulation of nanoparticle drugs by passive targeting
is thought to be another reason (37). Through the EPR effect, nanoparticle
drugs are retained for a long period in the systemic circulation,
are easily extravasated from tumor vessels into the interstitium of
tumor tissue, and accumulate there for longer periods than
conventional small-molecule agents (38–40).
On the basis of these findings, i.v. administration is considered
to be a more feasible and simplified treatment than i.p.
administration for Nab-PTX. This is because i.p.
chemotherapy requires surgical intervention to position the access
port and has several complications and local toxicities. In the
next stage of evaluation, we will investigate the paclitaxel
concentrations in plasma and ascites after i.v. administration of
Nab-PTX in patients with ascites due to peritoneal
metastasis resulting from gastric cancer.
In conclusion, we demonstrated, using a peritoneal
metastatic model of gastric cancer, that Nab-PTX showed
greater efficacy than i.p. Sb-PTX at equitoxic doses involving a
subcutaneous xenograft model. The antitumor efficacy of
Nab-PTX regarding peritoneal metastasis after i.v.
administration was equivalent to i.p. administration. Although
further studies are necessary for a more detailed evaluation, i.v.
Nab-PTX treatment might be another encouraging option for
targeting not only peritoneal metastasis, but also primary sites or
other metastatic sites. The present preclinical study suggests the
need for a clinical study to evaluate the antitumor effects of
Nab-PTX in gastric cancer patients with peritoneal
metastasis.
References
|
1
|
Siewert JR, Bottcher K, Roder JD, Busch R,
Hermanek P and Meyer HJ: Prognostic relevance of systematic lymph
node dissection in gastric carcinoma. German Gastric Carcinoma
Study Group. Br J Surg. 80:1015–1018. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Allum WH, Powell DJ, McConkey CC and
Fielding JW: Gastric cancer: a 25-year review. Br J Surg.
76:535–540. 1989.PubMed/NCBI
|
|
3
|
Rowinsky EK, Cazenave LA and Donehower RC:
Taxol: a novel investigational antimicrotubule agent. J Natl Cancer
Inst. 82:1247–1259. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carney DN: Chemotherapy in the management
of patients with inoperable non-small cell lung cancer. Semin
Oncol. 23:71–75. 1996.PubMed/NCBI
|
|
5
|
Crown J and O’Leary M: The taxanes: an
update. Lancet. 355:1176–1178. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gelmon K: The taxoids: paclitaxel and
docetaxel. Lancet. 344:1267–1272. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ohtsu A, Boku N, Tamura F, et al: An early
phase II study of a 3-hour infusion of paclitaxel for advanced
gastric cancer. Am J Clin Oncol. 21:416–419. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamada Y, Shirao K, Ohtsu A, et al: Phase
II trial of paclitaxel by three-hour infusion for advanced gastric
cancer with short premedication for prophylaxis against
paclitaxel-associated hypersensitivity reactions. Ann Oncol.
12:1133–1137. 2001. View Article : Google Scholar
|
|
9
|
Hironaka S, Zenda S, Boku N, et al: Weekly
paclitaxel as second-line chemotherapy for advanced or recurrent
gastric cancer. Gastric Cancer. 9:14–18. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Francis P, Rowinsky E, Schneider J, Hakes
T, Hoskins W and Markman M: Phase I feasibility and pharmacologic
study of weekly intraperitoneal paclitaxel: a Gynecologic Oncology
Group pilot study. J Clin Oncol. 13:2961–2967. 1995.PubMed/NCBI
|
|
11
|
Markman M, Brady MF, Spirtos NM, Hanjani P
and Rubin SC: Phase II trial of intraperitoneal paclitaxel in
carcinoma of the ovary, tube, and peritoneum: a Gynecologic
Oncology Group Study. J Clin Oncol. 16:2620–2624. 1998.PubMed/NCBI
|
|
12
|
Armstrong DK, Bundy B, Wenzel L, Huang HQ,
Baergen R, Lele S, Copeland LJ, Walker JL and Burger RA;
Gynecologic Oncology Group. Intraperitoneal cisplatin and
paclitaxel in ovarian cancer. N Engl J Med. 354:34–43. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ishigami H, Kitayama J, Kaisaki S, et al:
Phase II study of weekly intravenous and intraperitoneal paclitaxel
combined with S-1 for advanced gastric cancer with peritoneal
metastasis. Ann Oncol. 21:67–70. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fushida S, Kinoshita J, Kaji M, Hirono Y,
Goda F, Yagi Y, Oyama K, Sudo Y, Watanabe Y and Fujimura T; Society
for the Study of Peritoneal Carcinomatosis in Gastric Cancer. Phase
I/II study of intraperitoneal docetaxel plus S-1 for the gastric
cancer patients with peritoneal carcinomatosis. Cancer Chemother
Pharmacol. 71:1265–1272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fujiwara Y, Takiguchi S, Nakajima K, et
al: Intraperitoneal docetaxel combined with S-1 for advanced
gastric cancer with peritoneal dissemination. J Surg Oncol.
105:38–42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Imano M, Yasuda A, Itoh T, et al: Phase II
study of single intraperitoneal chemotherapy followed by systemic
chemotherapy for gastric cancer with peritoneal metastasis. J
Gastrointest Surg. 16:2190–2196. 2012. View Article : Google Scholar
|
|
17
|
Weiss RB, Donehower RC, Wiernik PH, et al:
Hypersensitivity reactions from Taxol. J Clin Oncol. 8:1263–1268.
1990.PubMed/NCBI
|
|
18
|
Kloover JS, den Bakker MA, Gelderblom H
and van Meerbeeck JP: Fatal outcome of a hypersensitivity reaction
to paclitaxel: a critical review of premedication regimens. Br J
Cancer. 90:304–305. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
ten Tije AJ, Verweij J, Loos WJ and
Sparreboom A: Pharmacological effects of formulation vehicles:
implications for cancer chemotherapy. Clin Pharmacokinet.
42:665–685. 2003.PubMed/NCBI
|
|
20
|
Sparreboom A, van Zuylen L, Brouwer E, et
al: Cremophor EL- mediated alteration of paclitaxel distribution in
human blood: clinical pharmacokinetic implications. Cancer Res.
59:1454–1457. 1999.
|
|
21
|
Gradishar WJ, Tjulandin S, Davidson N, et
al: Phase III trial of nanoparticle albumin-bound paclitaxel
compared with polyethylated castor oil-based paclitaxel in women
with breast cancer. J Clin Oncol. 23:7794–7803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Simionescu M, Gafencu A and Antohe F:
Transcytosis of plasma macromolecules in endothelial cells: a cell
biological survey. Microsc Res Tech. 57:269–288. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
John TA, Vogel SM, Tiruppathi C, Malik AB
and Minshall RD: Quantitative analysis of albumin uptake and
transport in the rat microvessel endothelial monolayer. Am J
Physiol Lung Cell Mol Physiol. 284:L187–L196. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Desai N, Trieu V, Damascelli B and
Soon-Shiong P: SPARC expression correlates with tumor response to
albumin-bound paclitaxel in head and neck cancer patients. Transl
Oncol. 2:59–64. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Von Hoff DD, Ramanathan RK, Borad MJ, et
al: Gemcitabine plus nab-paclitaxel is an active regimen in
patients with advanced pancreatic cancer: a phase I/II trial. J
Clin Oncol. 29:4548–4554. 2011.
|
|
26
|
Socinski MA, Bondarenko I, Karaseva NA, et
al: Weekly nab-paclitaxel in combination with carboplatin versus
solvent-based paclitaxel plus carboplatin as first-line therapy in
patients with advanced non-small-cell lung cancer: final results of
a phase III trial. J Clin Oncol. 30:2055–2062. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takiuchi H, Sasaki Y, Nishina T, et al:
ABI-007 in the treatment of unresectable or recurrent gastric
cancer refractory to fluoropyrimidine-containing regimen: Updated
data from the multicenter phase II study. J Clin Oncol (Meeting
Abstracts). 30(Suppl 4): 902012.
|
|
28
|
Yashiro M, Chung YS, Nishimura S, Inoue T
and Sowa M: Peritoneal metastatic model for human scirrhous gastric
carcinoma in nude mice. Clin Exp Metastasis. 14:43–54. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Desai N, Trieu V, Yao Z, et al: Increased
antitumor activity, intratumor paclitaxel concentrations, and
endothelial cell transport of cremophor-free, albumin-bound
paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin
Cancer Res. 12:1317–1324. 2006. View Article : Google Scholar
|
|
30
|
Brouwer E, Verweij J, De Bruijn P, et al:
Measurement of fraction unbound paclitaxel in human plasma. Drug
Metab Dispos. 28:1141–1145. 2000.PubMed/NCBI
|
|
31
|
Kumar GN, Walle UK, Bhalla KN and Walle T:
Binding of taxol to human plasma, albumin and alpha 1-acid
glycoprotein. Res Commun Chem Pathol Pharmacol. 80:337–344.
1993.PubMed/NCBI
|
|
32
|
Gardner ER, Dahut WL, Scripture CD, et al:
Randomized crossover pharmacokinetic study of solvent-based
paclitaxel and nab-paclitaxel. Clin Cancer Res.
14:4200–4205. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang CS, Lin KH, Chen SL, Chan YF and
Hsueh S: Overexpression of SPARC gene in human gastric carcinoma
and its clinic-pathologic significance. Br J Cancer. 91:1924–1930.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao ZS, Wang YY, Chu YQ, Ye ZY and Tao
HQ: SPARC is associated with gastric cancer progression and poor
survival of patients. Clin Cancer Res. 16:260–268. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Said N and Motamed K: Absence of
host-secreted protein acidic and rich in cysteine (SPARC) augments
peritoneal ovarian carcinomatosis. Am J Pathol. 167:1739–1752.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kamijo Y, Ito C, Sai Y and Miyamoto K:
Surfactants influence the distribution of taxanes in peritoneal
dissemination tumor-bearing rats. Cancer Lett. 287:182–186. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Matsumura Y and Maeda H: A new concept for
macromolecular therapeutics in cancer chemotherapy: mechanism of
tumoritropic accumulation of proteins and the antitumor agent
smancs. Cancer Res. 46:6387–6392. 1986.
|
|
38
|
Vicent MJ and Duncan R: Polymer
conjugates: nanosized medicines for treating cancer (Review).
Trends Biotechnol. 24:39–47. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Duncan R: Polymer conjugates as anticancer
nanomedicines (Review). Nat Rev Cancer. 6:688–701. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Emoto S, Yamaguchi H, Kishikawa J,
Yamashita H, Ishigami H and Kitayama J: Antitumor effect and
pharmacokinetics of intraperitoneal NK105, a nanomicellar
paclitaxel formulation for peritoneal dissemination. Cancer Sci.
103:1304–1310. 2012. View Article : Google Scholar : PubMed/NCBI
|