Introduction
Angiogenesis, the development of novel blood
vessels, is a critical event in many important disease states
including ischemic cardiovascular disease, wound healing,
inflammation, psoriasis, primary tumor growth and formation of
metastases. It is a highly complex process involving endothelial
cell (EC) activation, disruption of vascular basement membranes,
and migration and proliferation of ECs (1). This process is orchestrated by a large
number of cytokines and associated receptors and proteinases, such
as vascular endothelial growth factor (VEGF), fibroblast growth
factor, interleukin 8 and matrix metalloproteinases (MMPs)
(2). Tumor-induced angiogenesis is
a process of irregular blood vessel formation, which results in
structurally and functionally abnormal blood vessels. This
abnormality impairs effective delivery of therapeutic agents to all
regions of tumors, it creates abnormal properties of cancer cells,
such as survival, resistance of radiation and chemotherapy, and
selects for more malignant cells (3). Several decades ago, Dr Folkman
proposed anti-angiogenic therapy as an effective method for
treatment of cancer (4,5). Nowadays, a number of inhibitors
targeting the tumor vasculature have been identified to improve the
sensitivity of cancer cells to cytotoxic chemotherapy and other
therapeutics (6–8).
Saururus chinensis (S. chinensis) is a
perennial herbaceous plant that grows wild in moist and shady
places throughout East Asia (9). It
has a long history of use in China as a folk medicine to remedy
various edema, gonorrhea, jaundice and inflammatory diseases
(9–11). Previous phytochemical and
pharmacological studies on this plant have revealed several types
of secondary metabolites, such as lignans, neolignans,
aristolactams and flavonoids, as the biologically active compounds
(12,13). Numerous studies reported anticancer
activities of lignans isolated from S. chinensis. Seo et
al (14) reported significant
effects of Saucernetin-7, a dineolignan from this herb, on
proliferation, cell cycle regulation and differentiation of HL-60
cells. Other groups described the anticancer-related effects of
these lignans, such as anti-invasive effects and anti-adhesive
properties. Manassantin A and B, dineolignan compounds isolated
from S. chinensis roots, inhibited PMA-induced ICAM-1
expression in HL-60 cells in a dose-dependent manner (15). Overexpression of MMP-9 induced by
lipopolysaccharide (LPS) was suppressed by Saucerneol G, a new
lignan from S. chinensis, in RAW264.7 cells (16). These findings suggested that
bioactive lignans from S. chinensis exhibit anti-adhesion,
anti-migration and anti-invasion activities in several cell types
and may contain properties to inhibit the malignant progression of
tumors.
Based on these findings, we investigated whether
bioactive lignans from S. chinensis impair EC activation and
angiogenesis in the progression of solid tumors. We focused on the
anti-angiogenic effects of lignans isolated from S.
chinensis. By invasion-inhibition-guide fractionation, we found
that MB, one of the dineolignans isolated from S. chinensis,
exhibited strong inhibitory effects on EC invasion in vitro.
In the present study, we demonstrated that MB exhibited
anti-angiogenic effects in ECs, and these effects may be mediated
by inhibition of MMPs via downregulation of the transcriptional
activity of RUNX2.
Materials and methods
Materials
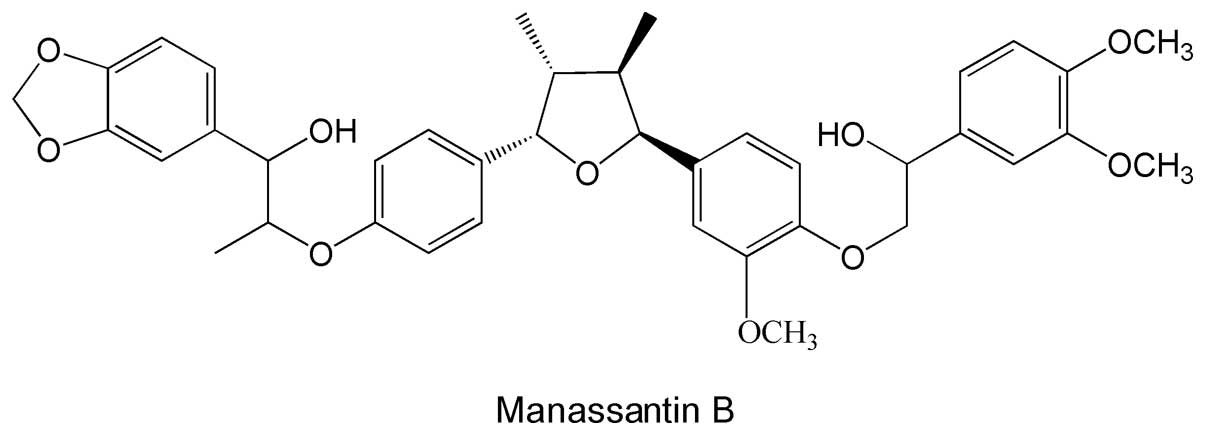
HPLC grade MB (Fig.
1) was purchased from AppliChem GmbH (Cat. No. A9007; St.
Louis, MO, USA). A stock solution (1 mM) was prepared by dissolving
MB in dimethyl sulfoxide (DMSO). Recombinant human VEGF165 (Cat.
No. 293-VE) is a product of R&D Systems (Minneapolis, MN, USA).
L-sulforaphane (SFP; Cat. No. S6317; Sigma-Aldrich, Beijing,
China), a potent inducer of inhibition of angiogenesis, and
batimastat (Bat; Cat. No. SC-203833; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA), a broad spectrum MMP inhibitor, were
used in the present study and served as positive controls.
Cell culture and animals
Human umbilical vein endothelial cells (HUVECs) were
isolated as previously described (17) from umbilical cords obtained from a
parturient at the Department of Maternity at the First Affiliated
Hospital of Nanchang University, who provided written informed
consent. The study protocol was approved by the Institutional
Review Board (IRB) of Nanchang University. HUVECs were routinely
grown in M199 supplemented with 10% fetal bovine serum (FBS) (both
from Gibco, Grand Island, NY, USA) and EC growth supplement (BD
Biosciences, Bedford, MA, USA) at 37°C and 5% CO2.
HUVECs between P3 and P4 were used for all experiments. The human
umbilical vein cell line, EA.hy 926, was purchased from the cell
bank of Shanghai Institute for Biological Sciences, CAS. EA.hy 926
cells were maintained in Dulbecco’s modified Eagle’s medium
supplemented with 10% FBS, 100 IU/ml penicillin and 100 μg/ml
streptomycin (Invitrogen, Beijing, China) in a humidified incubator
containing 5% CO2 at 37°C. Prior to performing each
assay, the ECs were serum starved for 4 h.
Five to six-week-old male Sprague Dawley (SD) rats
were purchased from the Vital River Laboratories (Beijing, China)
to obtain thoracic aorta in the rat aortic ring angiogenesis assay.
All animal experiments were conducted in strict accordance with
full ethical approval of the Animal Ethics Committees of Jiangxi
University of Traditional Chinese Medicines. All surgery was
performed under sodium pentobarbital anesthesia, and all efforts
were made to minimize suffering.
Tube formation assay
The tube formation assay was used to investigate the
effects of MB on angiogenesis in vitro. Briefly, 80 μl of
liquid growth factor-reduced Matrigel (BD Biosciences, San Jose,
CA, USA) were added to each well of a 96-well plate. After 45 min
incubation at 37°C, 3×104 ECs/well in 100 μl complete
culture medium containing vehicle, MB or SFP was seeded in each
well. Then, 100 μl serum-free medium containing VEGF (final
concentration 2 ng/ml) was added. After 24 h incubation at 37°C and
5% CO2, the images of each well were recorded by an
inverted microscope (Leica DMI3000B, Germany).
Cell proliferation assay
Cell proliferation was assayed by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. Briefly, log-phase cells were seeded in 96-well plates 24 h
before initiation of treatment with MB in the presence of 2 ng/ml
VEGF. The vehicle-treated cells served as controls. Three duplicate
wells were set up in each sample. After incubation with a range of
MB or vehicle for 24 h, cells were incubated with MTT (final
concentration 0.5 mg/ml) for 4 h at 37°C (Sigma-Aldrich). The media
was carefully removed from each well and 150 μl of DMSO was added.
The plates were gently agitated and optical absorbance at OD 570 nm
and OD 450 nm was determined using a microplate reader (ELx800;
BioTek, Winooski, VT, USA). Vehicle only-treated cells served as
the indicator of 100% cell viability. Each experiment was repeated
three times.
Rat aortic ring angiogenesis assay
In the present study, an ex vivo tube
formation system was used as previously described (18) to evaluate the anti-angiogenic effect
of MB. Male SD rats (5–6 weeks old) were euthanized and thoracic
aortas were retrieved. After rinsing with 1% antibiotic/antimycotic
cocktail in 1X PBS (100 U/ml penicillin, 100 μg/ml streptomycin and
0.25 μg/ml amphotericin B; Invitrogen), the surrounding
fibroadipose tissue of thoracic aorta was completely removed with
fine microdissection scissors, and the thoracic aorta was cut into
1 mm rings with a scalpel blade. Then, all the rings were implanted
on a Matrigel-coated 96-well microtiter plate. Matrigel was added
again to embed and fix rings. After 30 min incubation in 5%
CO2 at 37°C, the aorta rings were incubated in human
endothelial serum-free medium (Gibco, Carlsbad, CA, USA)
supplemented with 2% FBS, 50 U/ml penicillin and 50 μg/ml
streptomycin (Invitrogen) for 24 h and then treated with different
doses of MB for 13 days. Finally, 8 μg/ml Calcein AM (BD
Biosciences) was added to stain microvessels. Images of the
microvessels were obtained using an inverted fluorescence
microscope (Leica DMI3000B).
In vitro assay of EC invasion
Effects of MB on invasion of ECs were measured by a
48-well microchemotaxis system (AP48; Neuro Probe, Gaithersburg,
MD, USA). Briefly, 5 μg of fibronectin in a volume of 50 μl was
applied on the rough (lower) surface of the polycarbonate membrane,
and 5 μg/filter Matrigel was plated to the smooth (upper) surface.
The lower chambers of the plates were then filled with 30 μl medium
containing 0.1% BSA. Log-phase cells were harvested and
re-suspended in culture medium with 0.1% BSA. Cell suspensions (100
μl containing 1×105 cells) and 2 ng/ml VEGF were added
to the upper compartment and incubated for 16 h at 37°C in a 5%
CO2 atmosphere. After incubation, the filters were fixed
with methanol and stained with 0.5% crystal violet for 60 min. The
cells on the upper surface of the filters were removed by wiping
with cotton swabs. The cells invading to the lower surface of the
filter through Matrigel and filter were quantified with the
Image-Pro Plus software 5.0 (Media Cybernetics Inc., Silver Spring,
MD, USA) and the most representative results are illustrated in the
figures. Each assay was performed in triplicate.
Zymography analysis
MMP activity was analyzed with gelatin zymograms.
Following 24 h treatment with MB, Bat or vehicle in the presence of
2 ng/ml VEGF, ECs were harvested and lysed. Cell lysates were
diluted in 50 mM Tris-HCl, pH 7.4, and separated by electrophoresis
in 7.5% sodium dodecyl sulfate polyacrylamide gels containing 2
mg/ml gelatin. After electrophoresis, the gels were washed in 2.5%
Triton X-100 for 30 min and then incubated for 16 h at 37°C in 50
mM Tris-HCl, pH 7.4, 200 mM NaCl, 10 mM CaCl2. Gels were
then stained with Coomassie brilliant blue R-250 and destained with
40% methanol-10% acetic acid until clear bands appeared. Images
were captured with the UVP EC3 gel imaging system.
Fluorescence resonance energy
transfer-based MMP activity assay
MMP activities were also measured by the
SensoLyte® 570 Generic MMP Assay kit (AnaSpec, Fremont,
CA, USA). This kit uses a fluorescence resonance energy transfer
(FRET)-based method to detect the activities of a variety of MMPs
including MMP-1, -2, -3, -7, -8, -9, -10, -11, -12, -13 and -14. It
uses 5-FAM (fluorophore) and QXL520™ (quencher) labeled FRET
peptide substrates for continuous measurement of MMP activities. In
an intact FRET peptide, the fluorescence of 5-FAM is quenched by
SensoLyte®. Upon cleavage of FRET peptide by MMPs, the
fluorescence of 5-FAM is recovered. Analyses were performed
according to the manufacturer’s instructions. Briefly, supernatants
of ECs were collected after incubation with or without MB for 24 h.
MMPs in supernatants were activated by incubation with
4-aminophenylmercuric acetate for 1 h at 37°C. Fifty microliter
MMP-containing samples and 50 μl MMP substrate solution were added
into a 96-well plate. The reagents were mixed by shaking the plate
gently for 30 sec. After a 50-min incubation period at 37°C, the
reaction was stopped by adding stop solution, and fluorescence
intensity was measured by a multilabel counter (Victor3™;
Perkin-Elmer, Waltham, MA, USA) at Ex/Em = 540/575 nm.
Western blot analysis
Cells were rinsed twice with PBS, and total proteins
were extracted in 500 μl lysis buffer. Aliquots of whole cell
lysates were separated by 10% SDS-PAGE and then transferred to
Hybond nitro blotting membranes. The membranes were blocked with 3%
BSA in Tris-buffered saline containing 0.5 ml/l Tween-20 and then
incubated with primary antibodies against MMP-9 (SC-6840), RUNX2
(SC-10758) (both from Santa Cruz), and phospho-RUNX2 (AP3559a;
Abgent), followed by incubation with horseradish peroxidase
(HRP)-conjugated secondary antibodies. Immunoreactive proteins were
detected using an enhanced chemiluminescence kit (Millipore).
β-actin (SC-130301; Santa Cruz) served as an internal control.
RUNX2 transcription factor assay
RUNX2 activity in EC nuclear extracts was detected
using the TransAM™ AML-3/RUNX2 kit (Active Motif North America,
Carlsbad, CA, USA) following the manufacturer’s instructions. Cell
extracts were prepared using the Nuclear Extract kit (Active Motif)
with ECs that were treated with either MB or DMSO for 24 h. Then,
20 μl of extracts diluted in complete lysis buffer and containing
15 μg nuclear extract were added into a 96-well plate. This plate
immobilizes oligonucleotides containing RUNX2 consensus binding
sites. Saos-2 nuclear extract served as a positive control for
RUNX2 activation, and 20 μl complete lysis buffer served as the
blank. The wild-type consensus oligonucleotide was provided as a
competitor for RUNX2 binding to monitor the specificity of the
assay. After 1 h incubation at room temperature, the plate was
washed three times with washing buffer. Diluted primary antibody
(100 μl) was added into wells and incubated for 1 h at room
temperature without agitation. After three washes, HRP-labeled
secondary antibody was added and incubated for 1 h at room
temperature. Then, 100 μl developing solution was added to initiate
the color reaction. After 100 μl stop solution was added, the
absorbance was measured within 5 min at 450 nm with a reference
wavelength of 655 nm using an ELx800 microplate reader
(BioTek).
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation assay (ChIP) was
performed using the agarose ChIP kit as described in the
manufacturer’s instructions (Pierce™ Agarose ChIP kit; Thermo
Scientific). Briefly, crosslinking was performed for 10 min using
formaldehyde (final concentration 1%). Crosslinked cells then were
lysed with lysis buffer containing protease inhibitors. To obtain
DNA fragments with an average size of 0.3 kb, micrococcal nuclease
was added and samples were incubated for 5 min. Protein-DNA
complexes were immunoprecipitated using anti-RUNX2 antibody
(SC-10758; Santa Cruz). Normal rabbit IgG served as a negative
control and anti-RNA polymerase II antibody served as positive
control. After DNA recovery, purified DNA was subjected to PCR
amplification on the Mastercycler gradient (Eppendorf) using the
Phusion® High-Fidelity PCR kit. The primers designed to
amplify one of the RUNX2 binding regions (−220 bp; TGGGGTC) in the
human mmp-9 promoter (GeneBank no. NM_004994) were: forward, 5′-ACA
GTT CCC ACA AGC TCT GC-3′ and reverse, 5′-CAG CAT GAG AAA GGG CTT
ACA-3′. The PCR profile was: 15 min at 95°C, followed by 30 three
step cycles of 15 sec at 95°C, 30 sec at 58°C and 30 sec at 72°C.
PCR reactions were subjected to a final extension at 72°C for 10
min. PCR analysis was performed using the Mastercycler gradient.
Aliquots of total DNA before immunoprecipitation were saved as
input, and these input lysates were also processed as above. All
ChIP assays were performed at least three times, and the most
representative results are illustrated in the figures.
Statistical analysis
The data are presented as means ± SD and were
analyzed with SPSS for Windows (13.0) software program (Chicago,
IL, USA). Comparison among different groups was carried out by
one-way analysis of variance (the one-way ANOVA). Differences
between means were considered statistically significant at
P<0.05.
Results
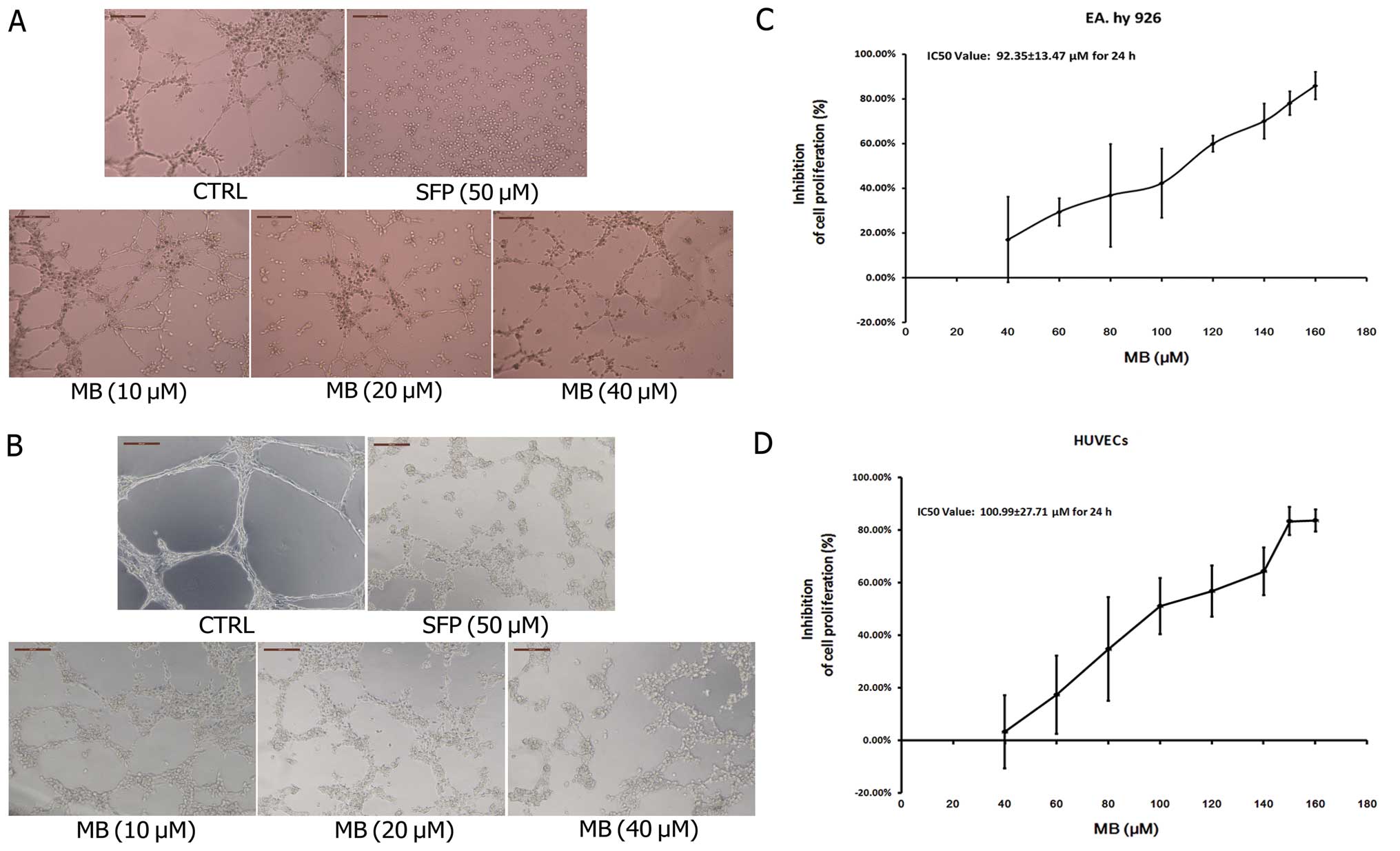
Effects of MB on tube formation of ECs in
vitro and ex vivo
We first used an in vitro tube formation
assay to assess the effects of MB on tumor-induced angiogenesis. As
shown in Fig. 2A, when EA.hy 926
cells were seeded on Matrigel, capillary-like structures with a
lumen were formed. After exposure to MB solution at various
concentrations for 24 h, these structures were destroyed in a
dose-dependent manner. MB treatment of HUVECs, a primary EC line
derived from the endothelium of umbilical vein, showed that normal
capillary-like structures were also significantly impaired by MB
(Fig. 2B). Fig. 2C and D shows the growth inhibition
effects of MB on EA.hy 926 and HUVECs; the IC50 values
of MB were 92.35±13.47 and 100.99±27.71 μM and the inhibitory rates
of 40 μM MB were 17.06 and 3.24%, respectively. These data indicate
that the observed ability of MB to suppress tube formation was not
due to growth inhibition.
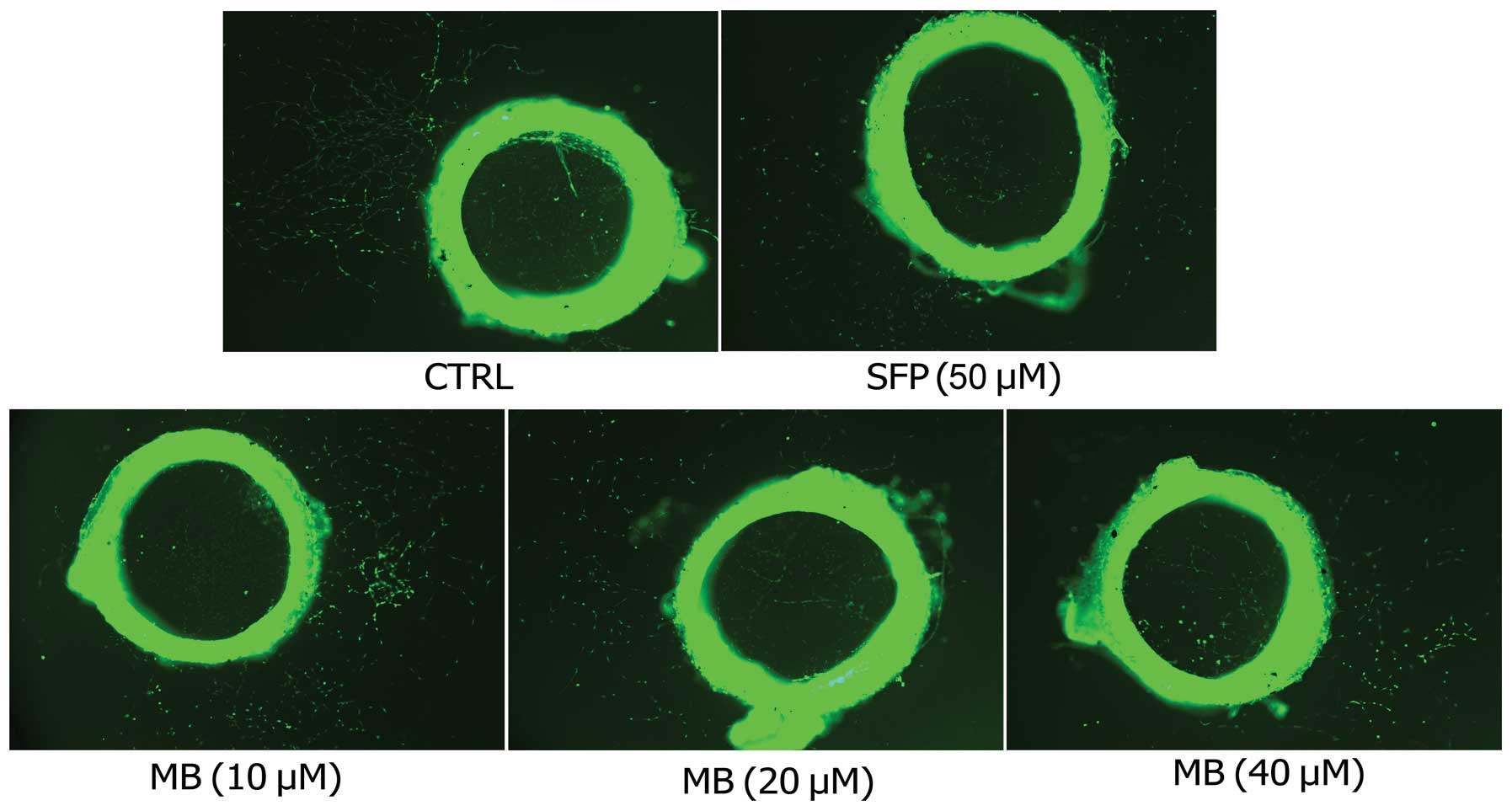
Next, the rat aortic ring angiogenesis assay was
employed to verify the anti-angiogenic effects of MB. New blood
vessels, triggered by the injury of the dissection procedure and
mediated by growth factors produced from the aortic ring, were
demolished by MB in a dose-dependent manner (Fig. 3).
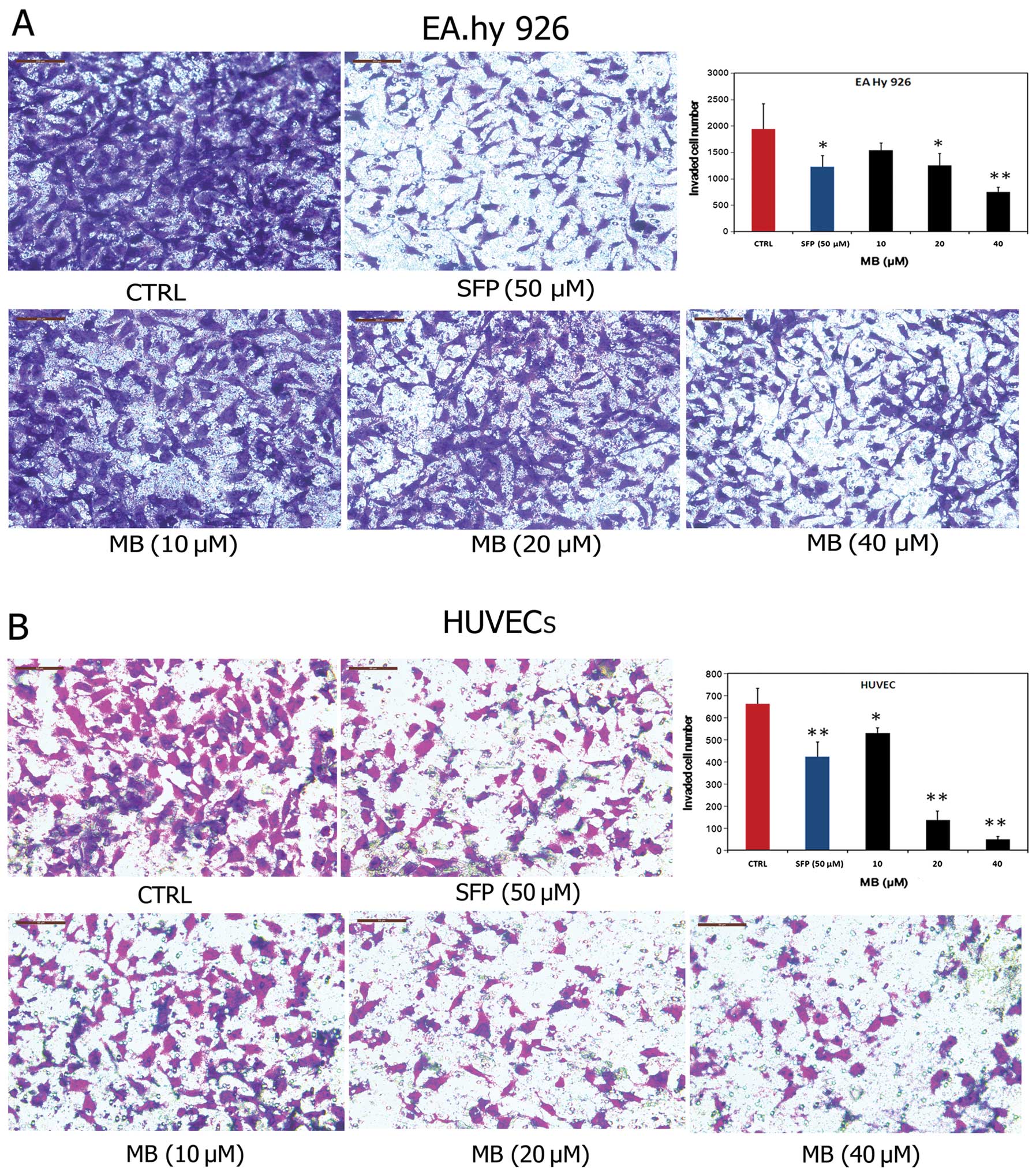
Effects of MB on tumor-induced invasion
of ECs in vitro
Invasion of ECs through the basement membrane to
form sprouting vessels is essential to angiogenesis. Thus, we next
examined the anti-invasive effects of MB on ECs. As shown in
Fig. 4A, MB significantly blocked
the transmembrane invasion of EA.hy 926 cells. The invasion rate
across the reconstituted basement membrane was 79.43%, 64.60% and
38.49% when the cells were incubated with 10, 20 and 40 μM MB for
14 h, respectively, compared with controls. Similar results were
obtained with HUVECs (Fig. 4B).
Collectively, these results demonstrated that MB significantly
inhibited the invasion through the basement membrane of ECs, and
this effect may contribute to the anti-angiogenic properties of
MB.
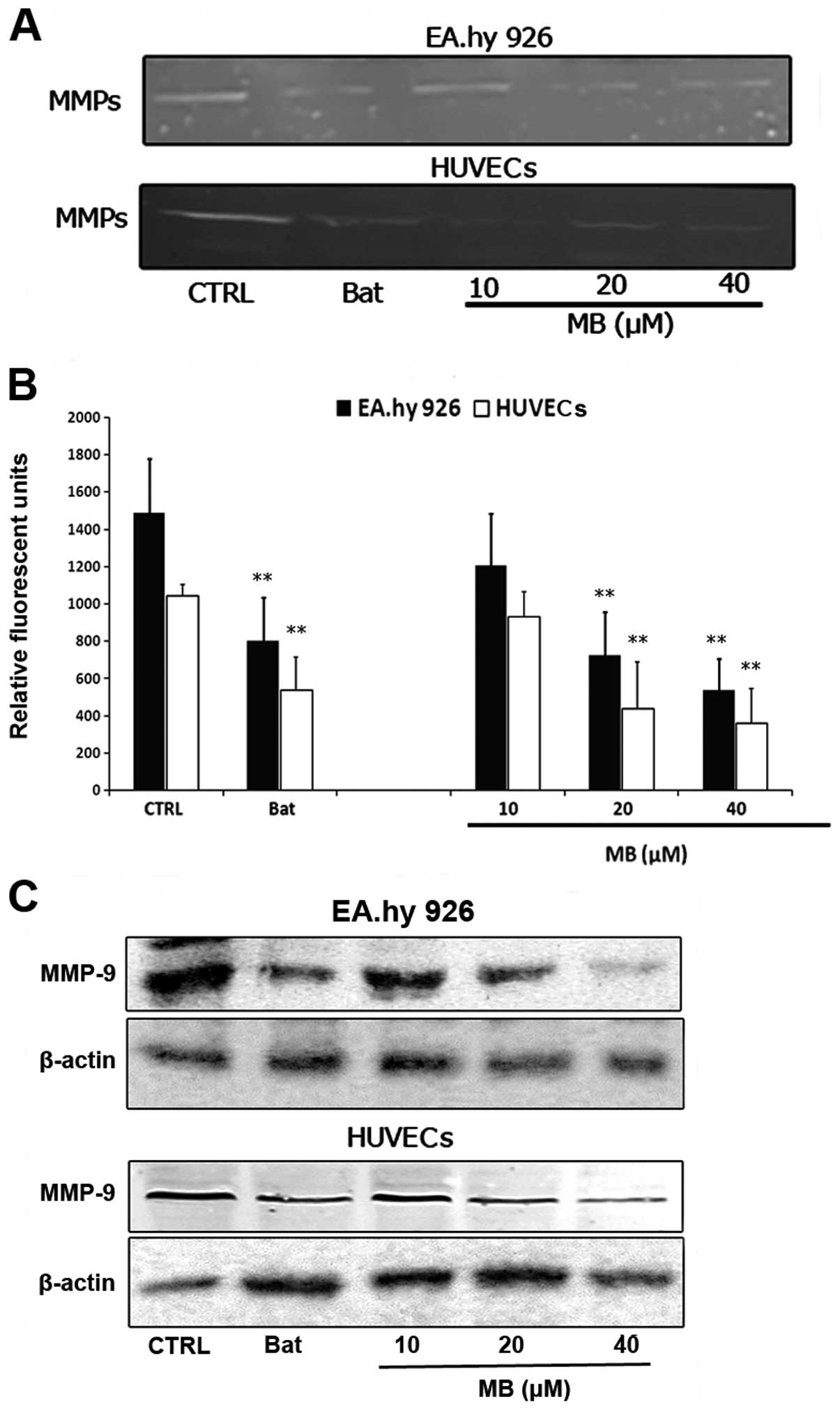
Effects of MB on MMP-9 activity and
expression in ECs
Breakdown of the extracellular matrix by MMPs in
surrounding tissues is a fundamental step of EC invasion in
tumor-induced angiogenesis. Therefore, we examined the effects of
MB on MMPs in ECs. Fig. 5 shows the
effects of MB on MMPs in EA.hy 926 and HUVECs. Data from the
zymography analysis showed that MB significantly inhibited
hydrolyzation of gelatin in both EC lines (Fig. 5A). Using a FRET-based analysis, we
found that MB exhibited significant suppression of FRET substrate
cleavage of MMPs in a dose-dependent manner (Fig. 5B). To further determine whether MB
inhibited the functional activity or expression of MMPs in ECs, we
analyzed the expression of MMP-9, one of the important MMPs, in
EA.hy 926 and HUVECs treated with MB. As shown in Fig. 5C, MB significantly decreased MMP-9
expression in both EC lines in a dose-dependent manner. These data
suggested that the anti-angiogenic properties of MB may be due to
downregulation of expression of MMP-9 in ECs.
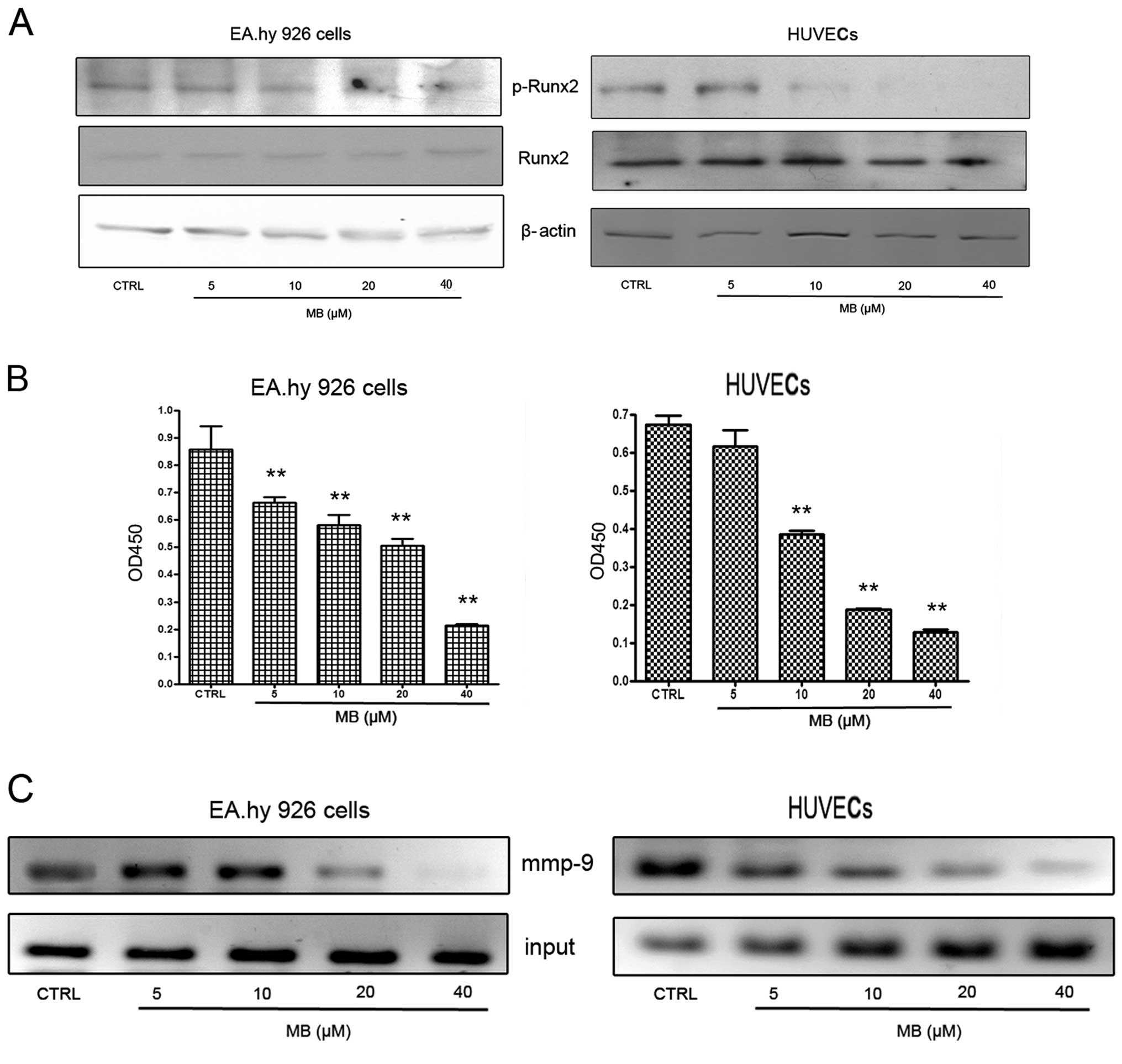
Effects of MB on RUNX2 activation in
ECs
From current observations, we understood that Runx2
is a ‘master’ transcriptional factor of metastatic growth of breast
cancer cells. Several genes required for the formation of
metastatic foci, including mmp-9, bsp, opn and
vegf, are targets of this transcriptional factor. Therefore,
next we examined RUNX2 activities in ECs when cells were
co-incubated with VEGF at 2 ng/ml. As shown in Fig. 6A, MB had no effect on total RUNX2
expression, but caused a significant decrease of phospho-RUNX2
expression. These results indicate that MB-induced downregulation
of MMP-9 expression may be associated with the suppression of RUNX2
activation.
To confirm the western blot results, Runx2
transcription factor assay and ChIP assay were designed. First, an
enzyme-linked immunoabsorbent assay (ELISA)-based kit for the Runx2
transcription factor was used to analyze the effects of MB on
Runx2. EC nuclear extracts incubated with MB or vehicle were
prepared and the binding activity between RUNX2 with its target
sequence was determined. The results showed that MB decreased the
binding activity of RUNX2 to its target sequences in a
dose-dependent manner (Fig. 6B).
Using ChIP assay, we found that binding of RUNX2 to one of the
RUNX2 binding domains (−220 bp; TGGGGTC) in the mmp-9
promoter region was inhibited by MB (Fig. 6C). Collectively, these findings
suggested that the inhibitory effect of MB on MMP-9 was caused by
MB inhibition of RUNX2 binding to the mmp-9 promoter, which
may be associated with suppression of RUNX2 phosphorylation.
Discussion
MB, a neolignan isolated from the roots of S.
chinensis, has been demonstrated to exhibit a range of
activities, including anti-inflammatory (10,19–21),
antiseptic (22) and antitumor
activities (11,23). Furthermore, MB was shown to inhibit
PMA-induced ICAM-1 expression in HL-60 cells (15) and to prevent monocyte adhesion to
HUVECs through the inhibition of ICAM-1, VCAM-1 and E-selectin
expression stimulated by TNF-α (24). Due to the important roles of these
adhesion molecules in tumor-induced angiogenesis, we sought to
determine whether MB exerts its effects directly on ECs in
angiogenesis and to identify the underlying mechanisms. In the
present study, we used tube formation assay of ECs and rat aortic
ring angiogenesis assay to evaluate the anti-angiogenic effects of
MB. To mimic tumor angiogenesis, VEGF (2 ng/ml) was added into the
culture system. Results showed that mesh-like structure formation
on Matrigel was significantly impaired by MB both in tube formation
assay and in rat aortic ring angiogenesis assay.
Angiogenesis is the physiological process through
which new capillary blood vessels grow from pre-existing vessels
(1–3). However, it is also a fundamental step
in the transition of tumors from a benign state to a malignant one.
Tumor angiogenesis starts with cancerous tumor cells releasing
signal molecules, such as VEGF or bFGF, to surrounding normal host
tissues. These pro-angiogenic molecules activate ECs in the host
tissues that in turn stimulate a series of steps toward the
creation of new blood vessels (1–3).
Breakdown of matrix by MMPs in surrounding tissues is one of the
critical steps that facilitate the migration of ECs. As they
migrate into the surrounding tissues, activated ECs begin to divide
and organize into hollow tubes that gradually evolve into a mature
network of blood vessels (25,26).
Based on these findings, we tested whether MB exhibits effects on
the invasive and migratory behaviors of ECs. Our data showed that,
in the presence of 2 ng/ml VEGF, MB significantly inhibited
invasion of HUVECs and EA.hy 926 cells through the reconstituted
basement membrane.
MMPs are another major contributor to angiogenesis.
These proteolytic enzymes break down matrix and allow the ECs to
escape into the interstitial matrix, as seen in sprouting
angiogenesis (25,26). Over the past 30 years, the role of
MMPs in human cancer has been widely investigated. These enzymes
participate in the proteolysis of the extracellular matrix,
modulation of cell adhesion, migration (27), the epithelial to mesenchymal
transition (EMT) (28), processing
of growth factors, and tumor-induced angiogenesis. Based on these
findings, we evaluated the effects of MB on MMPs. MB treatment
showed overt inhibition on MMP activities and MMP-9 expression.
Collectively, these results indicate that anti-angiogenic effects
of MB may be due to the suppression of expression of matrix-related
proteases in ECs, resulting in inhibition of EC invasion and
migration.
RUNX2, also termed PEBP2αA/AML3/Cbfa1, is a critical
transcription factor for osteoblastic differentiation and skeletal
morphogenesis (29–31). RUNX2 belongs to the Runx family that
encodes proteins homologous to Drosophila runt and has a
conserved runt DNA-binding domain. RUNX2 was originally found to
act as a master regulatory factor in skeletal development (32). To date, extensive evidence shows a
close association between RUNX2 and cancer, and this
transcriptional factor is becoming a potential target of novel
anticancer agents and diagnostic approaches to cancer control
(33,34). RUNX2 is involved in the regulation
of tumor-induced angiogenesis (35,36).
Sun et al (35) first
reported that RUNX2 effects on EC migration and invasion may occur
through the activation of protease expression, which regulates
angiogenesis and tumor growth. Furthermore, Qiao et al found
that RUNX2 is phosphorylated by cdc2, which may facilitate cell
cycle progression and promote EC proliferation (36).
RUNX2 is also involved in the regulation of MMP in
metastatic breast cancer cells. Pratap et al investigated
the role of RUNX2 in the regulation of the mmp-9 promoter in
MDA-MB-231 and MCF-7 cells. MMP-9 was found to be a direct target
of RUNX2 in metastatic breast cancer cells, and the modulation of
RUNX2 activity by either forced expression or RNA interference
directly affected MMP-9 expression and the invasive properties of
metastatic cancer cells (37).
Jiménez et al found that MMP-13, also known as
collagenase-3, was highly expressed in MDA-MB-231 cells, and
identified it as another target gene of RUNX2 (38). These results were also confirmed by
Selvamurugan et al (39,40).
Thus, RUNX2 acts a ‘master’ transcription factor of MMP expression.
Therefore, we speculated whether the inhibitory effects of MB on
MMPs in ECs were associated with the downregulation of RUNX2
activity. Our western blot results demonstrated that MB
significantly inhibited the levels of phospho-RUNX2, indicating
that the transcriptional ability of RUNX2 on the mmp-9
promoter is impaired by MB. To confirm these findings, we used
ELISA-based RUNX2 transcription factor and ChIP assays, and the
results revealed that the interaction between RUNX2 and sequences
in the mmp-9 promoter was significantly inhibited by MB.
In summary, here we report that MB, a neolignan from
S. chinensis, inhibits the angiogenic potential induced by
tumors, and the inhibitory effects are due to its ability to reduce
the expression of MMP-9, a target of the RUNX2 transcription
factor. These effects may be associated with inhibition of the
transcriptional activity of RUNX2.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 81160530 and
81260656), the Key Research Project from the Ministry of Education
of China (grant no. 211091), and the Natural Science Foundation of
Jiangxi Province (grant no. 2010GQY0147).
References
|
1
|
Folkman J: Fundamental concepts of the
angiogenic process. Curr Mol Med. 3:643–651. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Adams RH and Alitalo K: Molecular
regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell
Biol. 8:464–478. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carmeliet P: Angiogenesis in life, disease
and medicine. Nature. 438:932–936. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Folkman J: Anti-angiogenesis: new concept
for therapy of solid tumors. Ann Surg. 175:409–416. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Holleb AI and Folkman J: Tumor
angiogenesis. CA Cancer J Clin. 22:226–229. 1972. View Article : Google Scholar
|
|
6
|
Ferrara N and Kerbel RS: Angiogenesis as a
therapeutic target. Nature. 438:967–974. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weis SM and Cheresh DA: Tumor
angiogenesis: molecular pathways and therapeutic targets. Nat Med.
17:1359–1370. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bridges EM and Harris AL: The angiogenic
process as a therapeutic target in cancer. Biochem Pharmacol.
81:1183–1191. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Editorial Committee of the Administration
Bureau of Traditional Chinese Medicine. Saururus Chinensis. Chinese
Materia Medica (Zhonghua Bencao). Shanghai Science and Technology
Press; Shanghai: pp. 2016–2017. 1998
|
|
10
|
Hwang BY, Lee JH, Nam JB, et al: Lignans
from Saururus chinensis inhibiting the transcription factor
NF-κB. Phytochemistry. 64:765–771. 2003.PubMed/NCBI
|
|
11
|
Lee YK, Seo CS, Lee CS, et al: Inhibition
of DNA topoisomerases I and II and cytotoxicity by lignans from
Saururus chinensis. Arch Pharm Res. 32:1409–1415. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sung SH, Kwon SH, Cho NJ and Kim YC:
Hepatoprotective flavonol glycosides of Saururus chinensis
herbs. Phytother Res. 11:500–503. 1997. View Article : Google Scholar
|
|
13
|
Wang EC, Shih MH, Liu MC, et al: Studies
on constituents of Saururus chinensis. Heterocycles.
43:969–975. 1996. View Article : Google Scholar
|
|
14
|
Seo BR, Lee KW, Ha J, et al: Saucernetin-7
isolated from Saururus chinensis inhibits proliferation of
human promyelocytic HL-60 leukemia cells via
G0/G1 phase arrest and induction of
differentiation. Carcinogenesis. 25:1387–1394. 2004.
|
|
15
|
Rho MC, Kwon OE, Kim K, et al: Inhibitory
effects of manassantin A and B isolated from the roots of
Saururus chinensis on PMA-induced ICAM-1 expression. Planta
Med. 69:1147–1149. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu Y, Hong TG, Jin M, et al: Saucerneol G,
a new lignan, from Saururus chinensis inhibits matrix
metalloproteinase-9 induction via a nuclear factor κB and
mitogen activated protein kinases in lipopolysaccharide-stimulated
RAW264.7 cells. Biol Pharm Bull. 33:1944–1948. 2010.PubMed/NCBI
|
|
17
|
Watanabe K and Jaffe EA: Hypoglycemia
stimulates thrombin-induced PGI2 production by cultured human
umbilical vein endothelial cells. Prostaglandins Leukot Essent
Fatty Acids. 52:251–254. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bellacen K and Lewis ECJ: Aortic ring
assay. J Vis Exp. 33:15642009.
|
|
19
|
Lu Y, Hwang SL, Son JK and Chang HW:
Manassantin B isolated from Saururus chinensis inhibits
cyclooxygenase-2-dependent prostaglandin D2 generation
by blocking Fyn-mediated nuclear factor-kappaB and mitogen
activated protein kinase pathways in bone marrow derived-mast
cells. Biol Pharm Bull. 36:1370–1374. 2013.PubMed/NCBI
|
|
20
|
Park HC, Bae HB, Jeong CW, et al: Effect
of manassantin B, a lignan isolated from Saururus chinensis,
on lipopolysaccharide-induced interleukin-1β in RAW 264.7 cells.
Korean J Anesthesiol. 62:161–165. 2012.PubMed/NCBI
|
|
21
|
Son KN, Song IS, Shin YH, et al:
Inhibition of NF-IL6 activity by manassantin B, a dilignan isolated
from Saururus chinensis, in phorbol myristate
acetate-stimulated U937 promonocytic cells. Mol Cells. 20:105–111.
2005.PubMed/NCBI
|
|
22
|
Seo CS, Lee YK, Kim YJ, et al: Protective
effect of lignans against sepsis from the roots of Saururus
chinensis. Biol Pharm Bull. 31:523–526. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hodges TW, Hossain CF, Kim YP, et al:
Molecular-targeted antitumor agents: the Saururus cernuus
dineolignans manassantin B and 4-O-demethylmanassantin B are
potent inhibitors of hypoxia-activated HIF-1. J Nat Prod.
67:767–771. 2004.PubMed/NCBI
|
|
24
|
Kwon OE, Lee HS, Lee SW, et al:
Manassantin A and B isolated from Saururus chinensis inhibit
TNF-α-induced cell adhesion molecule expression of human umbilical
vein endothelial cells. Arch Pharm Res. 28:55–60. 2005.PubMed/NCBI
|
|
25
|
Stetler-Stevenson WG: Matrix
metalloproteinases in angiogenesis: a moving target for therapeutic
intervention. J Clin Invest. 103:1237–1241. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rundhaug JE: Matrix metalloproteinases and
angiogenesis. J Cell Mol Med. 9:267–285. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Duffy MJ, Maguire TM, Hill A, et al:
Metalloproteinases: role in breast carcinogenesis, invasion and
metastasis. Breast Cancer Res. 2:252–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Radisky ES and Radisky DC: Matrix
metalloproteinase-induced epithelial-mesenchymal transition in
breast cancer. J Mammary Gland Biol Neoplasia. 15:201–212. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lian JB, Stein JL, Stein GS, et al:
Runx2/Cbfa1 functions: diverse regulation of gene transcription by
chromatin remodeling and co-regulatory protein interactions.
Connect Tissue Res. 44(Suppl 1): S141–S148. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Karsenty G: Role of Cbfa1 in osteoblast
differentiation and function. Semin Cell Dev Biol. 11:343–346.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Komori T: Runx2, a multifunctional
transcription factor in skeletal development. J Cell Biochem.
87:1–8. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stein GS, Lian JB, van Wijnen AJ, et al:
Runx2 control of organization, assembly and activity of the
regulatory machinery for skeletal gene expression. Oncogene.
23:4315–4329. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Barnes GL, Javed A, Waller SM, et al:
Osteoblast-related transcription factors Runx2 (Cbfa1/AML3) and
MSX2 mediate the expression of bone sialoprotein in human
metastatic breast cancer cells. Cancer Res. 63:2631–2637.
2003.PubMed/NCBI
|
|
34
|
Shore P: A role for Runx2 in normal
mammary gland and breast cancer bone metastasis. J Cell Biochem.
96:484–489. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun L, Vitolo M and Passaniti A:
Runt-related gene 2 in endothelial cells: inducible expression and
specific regulation of cell migration and invasion. Cancer Res.
61:4994–5001. 2001.PubMed/NCBI
|
|
36
|
Qiao M, Shapiro P, Fosbrink M, et al: Cell
cycle-dependent phosphorylation of the RUNX2 transcription factor
by cdc2 regulates endothelial cell proliferation. J Biol Chem.
281:7118–7128. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pratap J, Javed A, Languino LR, et al: The
Runx2 osteogenic transcription factor regulates matrix
metalloproteinase 9 in bone metastatic cancer cells and controls
cell invasion. Mol Cell Biol. 25:8581–8591. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jiménez MJ, Balbín M, López JM, et al:
Collagenase 3 is a target of Cbfa1, a transcription factor of the
runt gene family involved in bone formation. Mol Cell Biol.
19:4431–4442. 1999.PubMed/NCBI
|
|
39
|
Selvamurugan N and Partridge NC:
Constitutive expression and regulation of collagenase-3 in human
breast cancer cells. Mol Cell Biol Res Commun. 3:218–223. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Selvamurugan N, Kwok S and Partridge NC:
Smad3 interacts with JunB and Cbfa1/Runx2 for transforming growth
factor-beta1-stimulated collagenase-3 expression in human breast
cancer cells. J Biol Chem. 279:27764–27773. 2004. View Article : Google Scholar : PubMed/NCBI
|