Introduction
Lung cancer is the leading cause of cancer-related
mortality in humans, and is attributed to the death of more than 1
million individuals each year. New cases of lung cancer account for
13% (~1.6 million) of the total new cancer cases diagnosed each
year (1). In China, many of these
new cases of lung cancer often exhibit malignant characteristics
such as multiple tumor sites and high propensity to metastasize
(2). Despite recent advances in the
development of multidisciplinary treatment regimens for lung
cancer, the prognosis of lung cancer patients remains poor and the
5-year survival rate is only ~15% (3).
Approximately 85 to 90% of lung cancer cases are
non-small cell lung cancer (NSCLC), which includes three major
subtypes: squamous cell (epidermoid) carcinoma (25–30%),
adenocarcinoma (40%) and large cell (undifferentiated) carcinoma
(10–15%). While there exist discernible differences in size, shape
and chemical make-up between these three subtypes, the clinical
approaches to treatment and prognosis are often very similar. The
standard treatments for patients presenting with advanced stages of
lung cancer are chemotherapy and radiotherapy. However, these
therapies often bring only marginal benefits in regards to patient
survival; the median survival time after receiving chemotherapy is
<1 year. As normal cells are also adversely affected by these
therapies, the treatment is often accompanied by undesirable
side-effects, which, along with the limited improvement in patient
survival, results in the poor quality of life for lung cancer
patients receiving standard care. Novel therapeutics with higher
specificity towards cancer cells and less toxicity are urgently
needed for treating lung cancer patients.
Small chemicals extracted from plants, recognized
for low toxicity and often better bioavailability than synthetic
drugs, have become a hotspot in the search for novel drugs. In
particular, herbs used in Traditional Chinese Medicine (TCM) are
gaining wider recognition in recent years as an underexploited
source for potential therapeutic compounds (4,5).
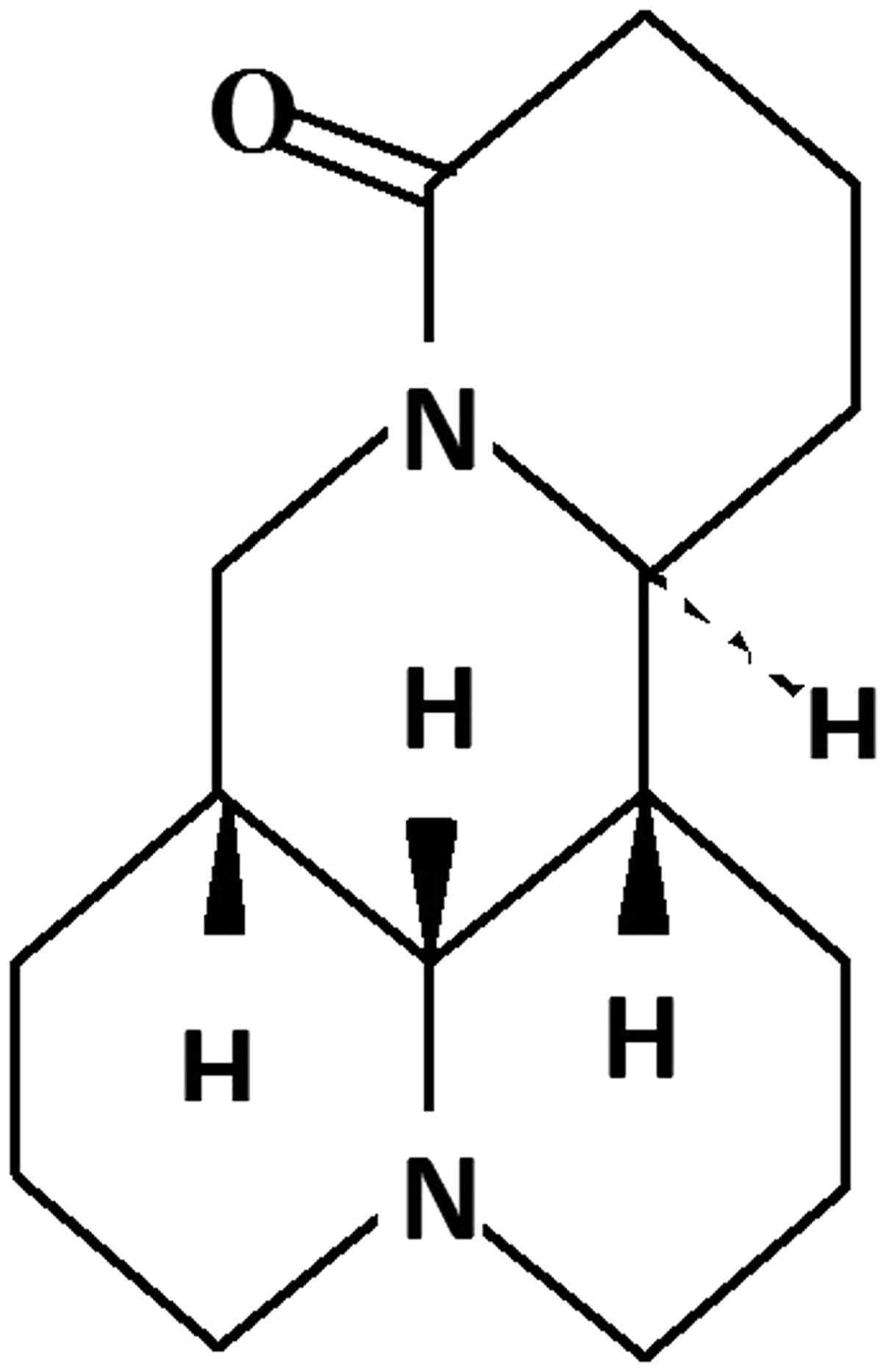
Matrine (Fig. 1) is an alkaloid
extracted as the major active ingredient of the traditional Chinese
herb Sophora flavescens Ait. (6). Presently, there are over 300
commercial concoctions containing matrine or its derivative
oxymatrine in the database of drug manufacturing licenses approved
by the China Food and Drug Administration (http://www.sda.gov.cn/). These drug preparations have
been prescribed for the treatment of cancer, arrhythmia (7–9), viral
hepatitis (10–12), hepatic fibrosis (13) and skin diseases such as atopic
dermatitis and eczema (14). In the
past few years, a growing body of evidence suggests that matrine
and oxymatrine may have some promising potential in cancer
treatment as demonstrated by their tumoricidal effects on many
cancer cell lines including those derived from gastric cancer,
cervical cancer, leukemia, hepatocellular carcinoma, breast cancer,
pancreatic cancer and lung cancer (3,15–25).
The major mechanisms that have been postulated to underlie the
antitumor effects of matrine center on the regulation of apoptosis-
and proliferation-related genes and proteins, such as Bcl-2 family
members, caspases, E2F-1, Akt and Fas/FasL (23,26–29).
Although available data suggest that the cytotoxic
effect of matrine may be wide-spectrum against multiple types of
cancer cells, it has not been thoroughly documented whether and how
matrine may affect the growth of different types of lung cancer
cells. In this study, we investigated the effect of matrine on two
highly invasive lung cancer cell lines, A549 and 95D. Our results
indicated that matrine is mildly effective in killing both of these
types of lung cancer cells, suggesting that matrine may be more
useful as an adjuvant therapy in combination with other treatments
of lung cancer. Furthermore, our results suggest that matrine
exerts its cancer-killing effect via promoting apoptosis in lung
cancer cells, possibly through the downregulation of cIAPs and the
Akt signaling pathway.
Materials and methods
Therapeutic compounds and reagents
Lung cancer cell lines A549 and 95D were purchased
from the Shanghai Institute of Biochemistry and Cell Biology,
Chinese Academy of Sciences. Matrine was purchased from the
National Institute for the Control of Pharmaceutical and Biological
Products (Beijing, China). DMSO and MTT were purchased from Sigma
(St. Louis, MO, USA). Annexin V-FITC apoptosis detection kit was
from KeyGene (Nanjing, China). RPMI tissue culture medium and fetal
bovine serum (FBS) were purchased from Gibco (USA). Anti-survivin,
anti-cIAP1, anti-cIAP2, anti-Smac, anti-Akt, anti-p-Akt, anti-GAPDH
and HRP-conjugated secondary antibodies were purchased from Abcam
Biotechnology (USA). Chemiluminescence (ECL) reagent kit was
purchased from Pierce Biotechnology (Rockford, IL, USA).
Cell culture
A549 and 95D cells were cultured in RPMI-1640 medium
containing 10% FBS, 100 IU/ml penicillin and 100 μg/ml
streptomycin. The cells were grown in a humidified incubator at
37°C in an atmosphere of 5% CO2 in air. Cells were grown
on sterile tissue culture Petri dishes and passaged once every 2 to
3 days.
MTT cell viability assay
Cells were seeded in a 96-well plate at a density of
1×105/ml and cultured in medium overnight. Cell
viability was determined using the conversion of MTT to formazan
via mitochondrial oxidation. Various treatments of cells included
the addition of matrine (0, 0.25, 0.5, 1.0, 1.5 and 2.0 g/l) for
12, 24 and 48 h. MTT solution was then added to each well at a
final concentration of 1 mg/ml/well, and the plates were incubated
at 37°C for another 4 h. After incubation, 150 μl DMSO was added to
each well to dissolve the formazan formed, and the absorbance was
read at 490 nm using a spectrophotometer.
Flow cytometric assay of apoptosis
Cellular apoptosis was determined using the Annexin
V-FITC and propidium iodide (PI) double staining kit according to
the manufacturer’s protocol with minor modification. Briefly, A549
cells were seeded in 6-well plates and allowed to attach overnight;
matrine (0, 0.25, 0.5, 1.0, 1.5 and 2.0 g/l) was then added to the
medium, and cells were incubated for 48 h. Cells were harvested,
washed twice with cold PBS, resuspended in 250 μl of binding buffer
and stained with a staining solution containing Annexin V/FITC and
PI. After incubation in the dark for 30 min, cells were analyzed by
FACSCalibur flow cytometry (BD Biosciences, USA).
Fluorescence microscopy
A549 cells were treated with a 1.0 g/l concentration
of matrine for 48 h. Cells were washed twice with PBS and fixed
with cold methanol and acetic acid before being stained with
Hoechst 33342 for 30 min in the dark. Stained cells were observed
with a fluorescence microscope (Nikon, Japan).
Western blotting
Western blotting was performed using standard
techniques as previously described (30,31).
Generally, cells were washed twice with PBS buffer and lysed in
RIPA lysis buffer (50 mM Tris-Cl pH 7.4, 150 mM NaCl, 0.5% sodium
deoxycholate, 1% NP-40, 0.1% SDS, 1 mM EDTA, 100 mM NaF, 1 mM
Na3VO4, 1 mM PMSF and 2 μg/ml aprotinin) on
ice. Total proteins (50 μg) were subjected to sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred to polyvinylidene difluoride (PVDF) membranes. The PVDF
membranes were blocked with 5% nonfat milk in TBST (10 mM Tris, pH
7.4, 150 mM NaCl and 0.1% Tween-20) at room temperature for 2 h and
incubated with the indicated primary antibodies at 4°C overnight
with gentle rocking. After washing with TBST, the membranes were
reacted with appropriate horseradish peroxidase (HRP)-conjugated
secondary antibodies for 1 h at room temperature. After extensive
washing with TBST, the presence of proteins was visualized by the
enhanced chemiluminescence (ECL) detection kit in accordance with
the manufacturer’s recommendations.
Statistical analysis
Each experiment involving tissue culture was
performed in triplicates. All analyses were performed using the
SPSS 13.0 software. Results are expressed as mean ± SD. The one-way
analysis of variance (ANOVA) was used to compare the difference
between treatment groups. Differences were considered significant
at a P-value <0.05.
Results
Growth inhibitory effect of matrine on
lung cancer cells
Matrine has been previously shown to have an
inhibitory effect on the growth of several cancer cell lines in
vitro (3,15–18,20,21,23–25).
However, it has not been thoroughly explored whether the antitumor
property of matrine is also effective on different lung
cancer-derived cells. We first set out to examine the effects of
matrine on the proliferation of lung cancer cell lines A549 and
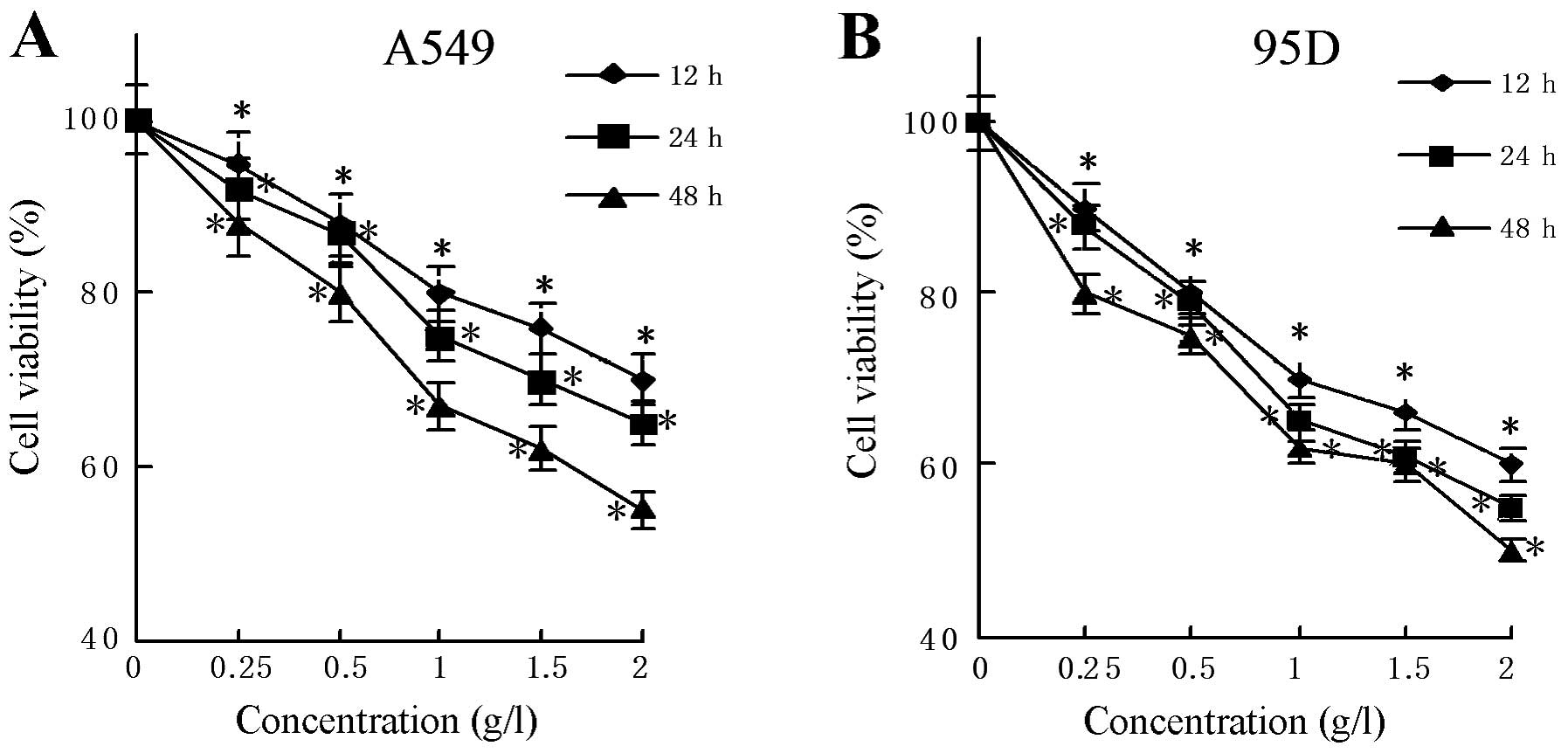
95D. As shown in Fig. 2A and B,
there was a significant reduction in the number of viable cells in
correspondence to the increasing concentration of matrine used in
the experiments. This holds true for the 12, 24 and 48 h
observations; the reduction in cell viability was even more
pronounced in the 48-h treatment group. After a 24-h treatment with
matrine at a concentration of 1.5 or 2.0 g/l, both A549 and 95D
cells showed a ~30% reduction in cell viability. The 48-h treatment
with matrine further reduced the cell viability, especially at the
higher concentrations, e.g. by an additional 20% at 2.0 g/l.
Therefore, matrine treatment exerted a modest inhibitory effect on
the growth of these two lung cancer cell lines in both a dose- and
time-dependent manner.
Matrine induces the apoptosis of A549
cells
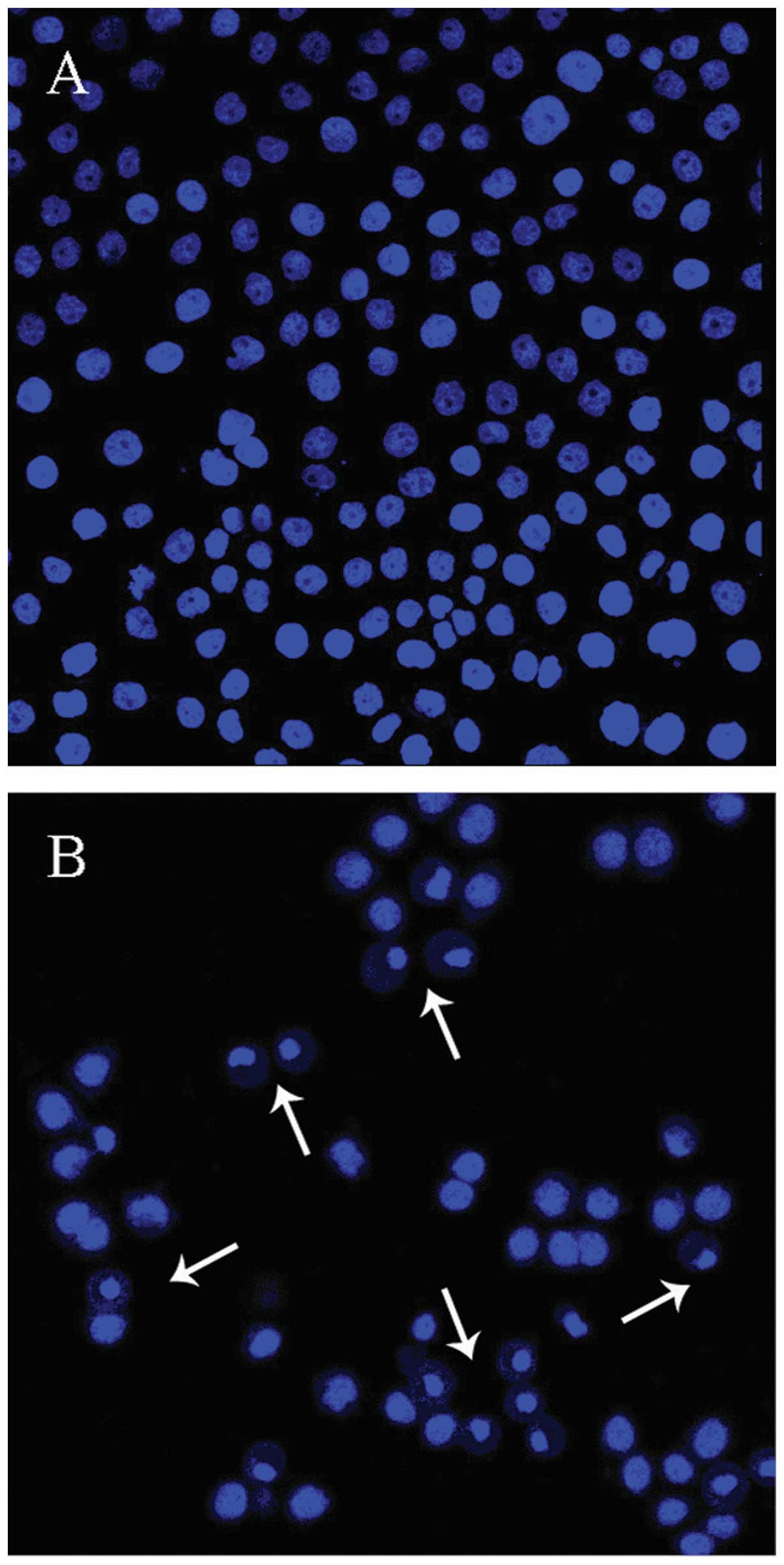
To ascertain that the reduction in viable cells post
matrine treatment is the result of apoptosis, A549 cells were
incubated with 1.0 g/l matrine for 48 h and then examined by
fluorescence microscopy. As shown in Fig. 3B, there were clear signs of
chromatin condensation, nuclear fragmentation and the formation of
apoptotic bodies in the matrine-treated cells (arrows), whereas the
untreated A549 cells (Fig. 3A)
showed mostly healthy nuclei. In line with the cell viability data,
the percentage of matrine-treated cells showing apoptotic
characteristics increased with higher concentrations of matrine
used in the incubation (data not shown). Taken together, these
results indicate that the cancer cell-killing mechanism of matrine
involves apoptosis in A549 cells and it occurs in a dose-dependent
manner.
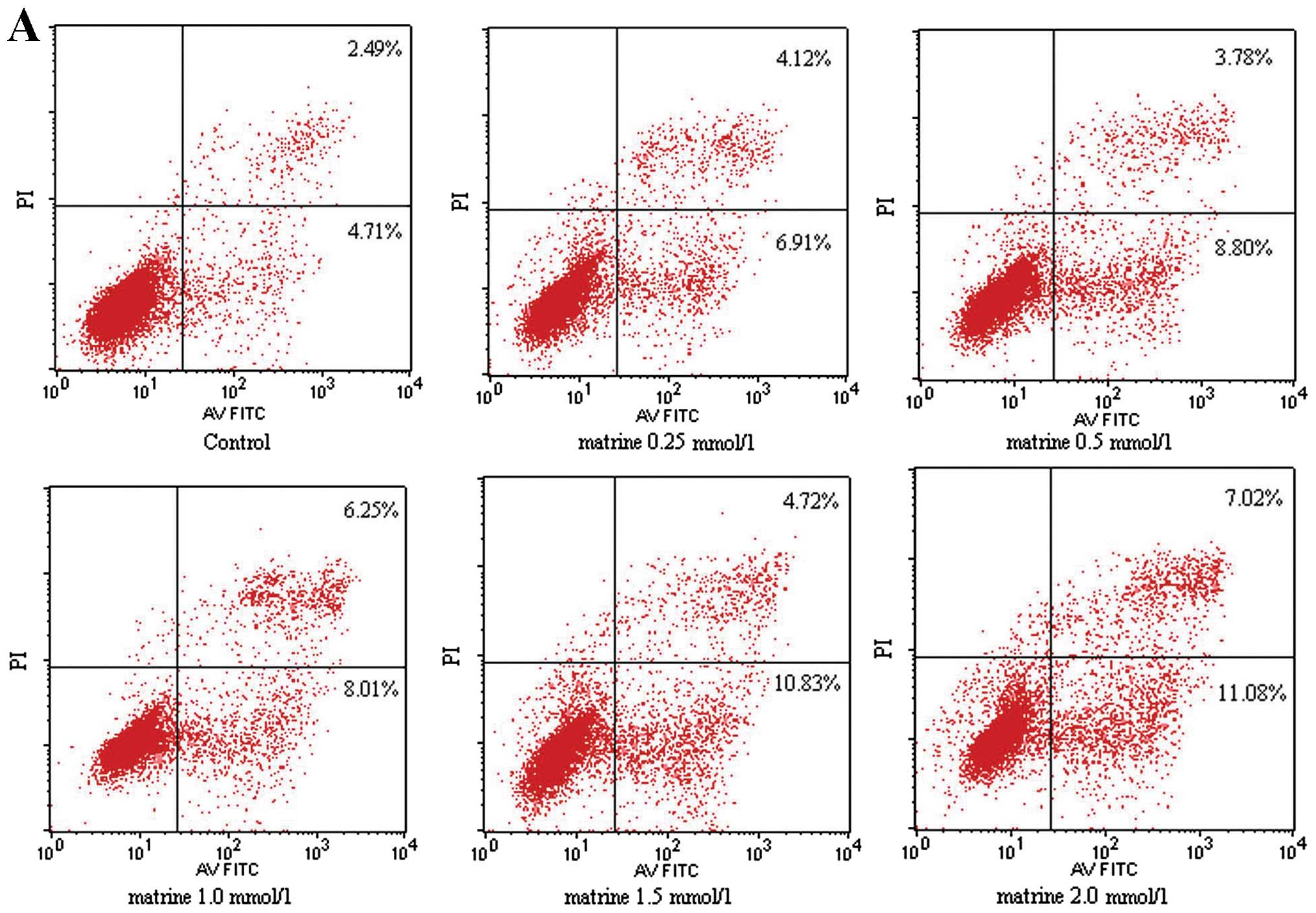
In order to gain further insight into the dynamic
progression from apoptosis to eventual cell death caused by matrine
treatment, FACS analysis was employed to monitor the cell
population undergoing apoptosis during matrine treatment. A549
cells were treated with different concentrations of matrine (0,
0.25, 0.5, 1.0, 1.5 and 2.0 g/l, respectively) for 48 h and were
analyzed by flow cytometry. As shown in Fig. 4A, the numbers of both the early
(bottom right quadrant) and late (top right quadrant) apoptotic
cells increased significantly with regard to increasing
concentrations of matrine used in the study (P<0.05). In
contrast, the ratio between early and late (dead) apoptotic cells
did not seem to change significantly in relation to the
concentration of matrine, suggesting that the major effect of
matrine occurs through the promotion of cancer cells to enter the
apoptotic pathway, rather than at some stages during the apoptotic
cascades. The pro-apoptotic effect of matrine was observed in both
A549 and 95D cells in both a dose- and time-dependent manner
(Fig. 4B–E).
Effect of matrine on early apoptotic
processes
The fate of cells is determined dynamically by the
competing pro- and anti-apoptotic processes involving members of
the IAP (inhibitor of apoptosis) family. In order to better
understand the molecular basis of matrine-induced apoptosis, we
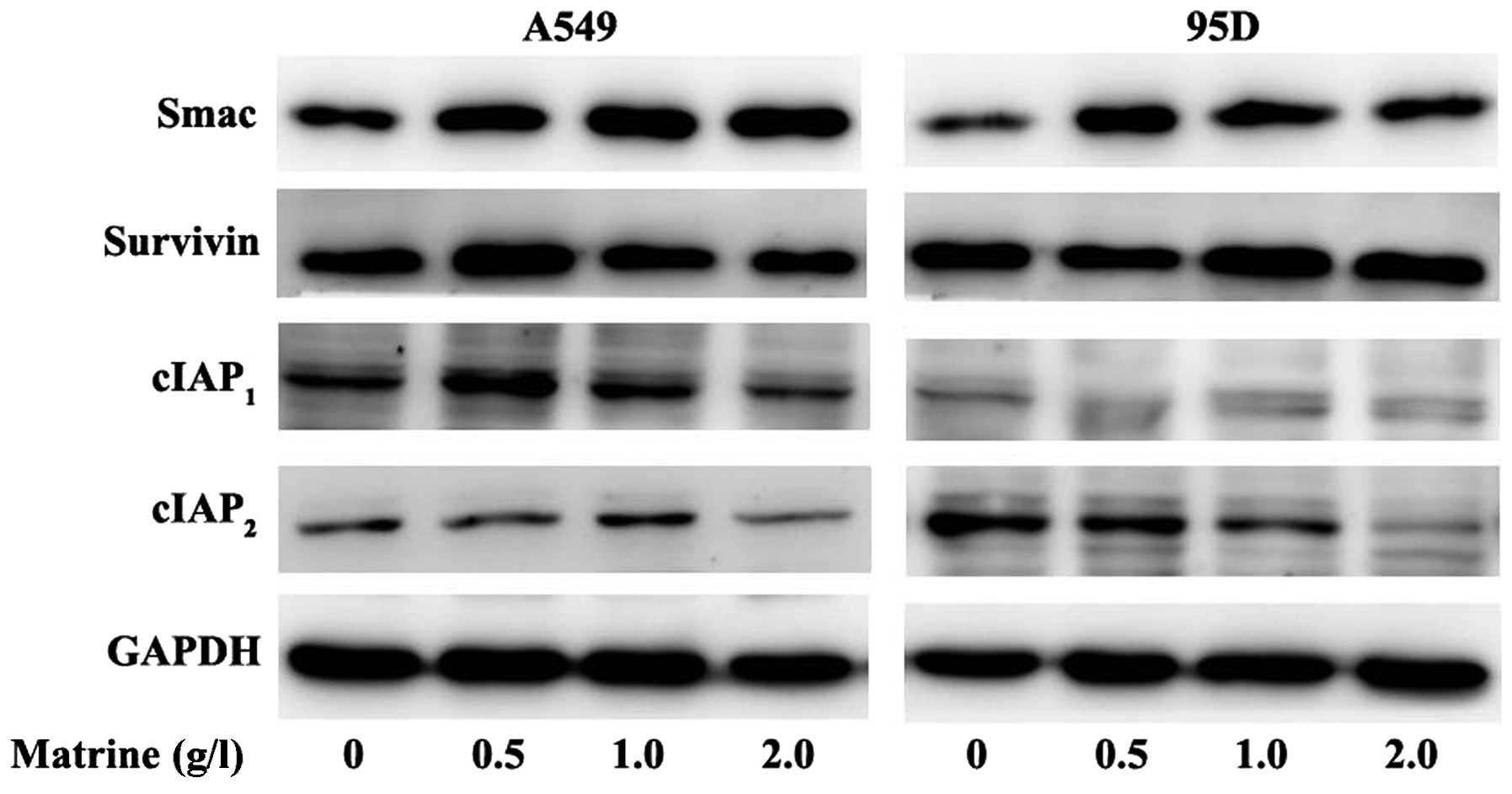
investigated the expression levels of members of the IAP family.
A549 cells were treated with different concentrations of matrine
(0, 0.5, 1.0 and 2.0 g/l) for 48 h, and the total cellular proteins
were extracted and analyzed on SDS-PAGE by western blotting.
Interestingly, the level of Smac increased in concert with
increasing concentrations of matrine in both A549 and 95D cells,
hinting that matrine may actively promote pro-apoptotic processes
(Fig. 5). Concomitantly, levels of
cIAPs decreased in response to increasing concentrations of
matrine, suggesting that matrine acted on the inhibition of
anti-apoptotic processes (Fig. 5).
The expression levels of survivin did not seem to change
significantly in response to matrine treatment, however, its
anti-apoptotic activity may have been blocked as a result of the
inhibition of the Akt pathway (discussed below).
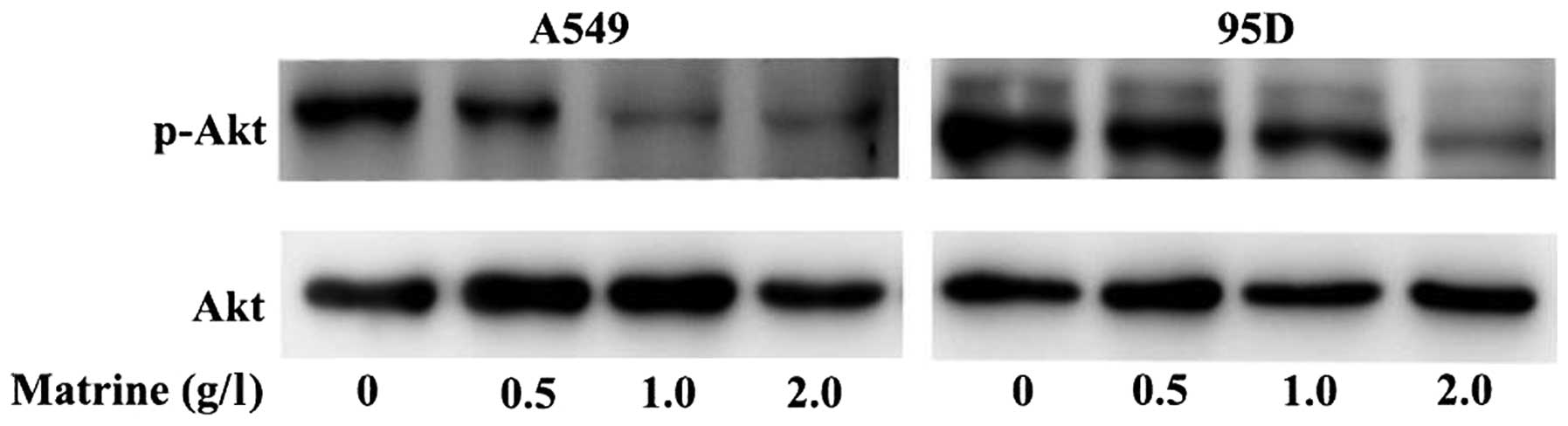
Matrine induces apoptosis in lung cancer
cells through Akt inactivation
Activation of the Akt signaling pathway is one of
the major mechanisms through which cancer cells promote cell
survival. Our previous results indicated that the Akt pathway is
involved not only in pathogenesis but also in drug-resistance of
lung cancer (31). We were
therefore interested in determining whether matrine treatment has
any effect on the Akt pathway. As shown in Fig. 6, the expression levels of
phosphorylated Akt (p-Akt) and total Akt (Akt) after treatment with
various concentrations of matrine (0, 0.5, 1.0, 2.0 g/l) for 48 h
decreased in a dose-dependent manner in response to increasing
concentrations of matrine. Combined with our results concerning the
matrine-induced reduction in cIAP expression, this result indicated
that matrine may lower the anti-apoptotic activity of cIAPs via
inhibiting the Akt pathway.
Discussion
Currently, the prognosis for inoperable or recurrent
lung cancer patients remains dismal in spite of the development of
novel chemotherapeutic strategies. While patients with early stage
lung cancer can be potentially cured, most lung cancer patients are
initially diagnosed at advanced stages. In addition, most advanced
lung cancer patients are habitual smokers, whose health condition
often leads to comorbidity in both cardiovascular and pulmonary
systems, rendering aggressive surgery and multimodality therapy
unfeasible. The severely unpleasant side-effects resulting from the
standard care for advanced lung cancer can further reduce the
quality of life of patients; at times it may lead to therapy
noncompliance. Urgently, the high prevalence and death rate of lung
cancer cases in China calls for alternative therapeutics with
improved effectiveness against the advanced stages and with lower
toxicity.
The antitumor effect of matrine, an alkaloid
extracted from Traditional Chinese herbal plants, has been studied
in several cancer cell lines (3,15–18,20,21,23–25).
Matrine was found to inhibit the growth of cell lines derived from
hepatoma, acute myeloid leukemia, melanoma, gastric carcinoma and
lung cancer. Furthermore, matrine can inhibit the migration of lung
cancer cells as well as that of human umbilical vein epithelial
cells cultured in lung cancer cell conditioned media (32). The ability of matrine to inhibit the
proliferation of cancer cells of many different types and origins
suggests that it may act on a common pathway involved in the
survival and growth of these cancer cells. Our finding that matrine
inhibited the growth of both A549 and 95D lung cancer cells
strongly supports this hypothesis.
Apoptosis, induced by chemotherapy, radiation and
cytokines, is a major mechanism underlying the killing of tumor
cells during cancer therapy. In this study, we found that apoptosis
was also the point of action by which matrine prevents lung cancer
cell growth. The proportion of both early and late apoptotic cells
increased significantly after treatment of matrine at a
concentration of 1.0 g/l or higher. A few reports have implicated
matrine in the induction of the pro-apoptotic pathways via lowering
the ratio of Bcl-2/Bax (27) or by
inhibiting human telomerase reverse transcriptase (hTERT), the
catalytic subunit of telomerase (33). Activation of the
phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin
(PI3K-Akt-mTOR) signaling pathway has been observed in many types
of tumors including lung cancer, which is considered to be
important for the survival, proliferation, angiogenesis of cancer
cells as well as their resistance to chemotherapy (34). Our previous results indicated that
upregulation of the PI3K-Akt-mTOR pathway plays an important role
in the growth and migration of lung cancer cells (30,31).
In particular, this pathway seems to be involved in the adapted
tolerance of cancer cells to chemotherapeutic compounds such as
docetaxel. The present study suggests that matrine can contribute
to the effectiveness of chemotherapy by inhibiting the
PI3K-Akt-mTOR pathway in lung cancer cells.
Second mitochondrial-derived activator of caspases
(Smac) and inhibitor of apoptosis (IAP) family members, such as
XIAP, c-IAP1, c-IAP2 and survivin, are key
regulators of apoptosis. In the cell, these factors promote
competing pathways that ultimately determine the fate of the cell;
Smac is pro-apoptotic and promotes cell death, IAPs are
anti-apoptotic and promote cell survival. IAPs usually have three
baculoviral IAP repeats (BIR) and a significant new gene (RING)
zinc finger domain (35). BIR1,
BIR2 and BIR3 domains are each capable of binding and inhibiting
caspases independently and each also interacts with Smac; both
interactions serve to halt the apoptotic cascade and promote cell
survival. C-IAP1 and c-IAP2 are E3 ubiquitin ligases for Smac; they
stimulate Smac degradation both in vivo and in vitro
(36). Survivin, typically absent
in most normal tissues, is often found to be highly expressed in
fetal tissues, perpetuated cell lines and cancer cells (37–41).
Clinically, overexpression of survivin in cancer is associated with
the high propensity to metastasize and to develop resistance to
chemotherapy; hence it is often considered as a biomarker for
malignant characteristics of cancer (42–47).
Consistent with the aforementioned hypothesis, matrine was found to
upregulate the expression of Smac while downregulating the
expression of IAPs. The expression of survivin did not seem to be
significantly affected by the treatment of matrine; however, its
anti-apoptotic activity was likely blocked due to the inhibition of
the Akt pathway and the downregulation of cIAPs, which are known to
stimulate the anti-apoptotic activity of survivin (48). Whether matrine acts directly or
indirectly on these protein regulators of apoptosis remains an open
question. Taken together, it hints at the notion that matrine may
affect the expression of Akt, Bcl-2/Bax and Fas/FasL in various
cancer cells, perhaps through a common mechanism. Identifying the
direct target of matrine would help to delineate such a mechanism
that links the PI3K-Akt-mTOR pathway with the apoptosis
cascades.
Our results reported here substantiated the
therapeutic potential of matrine in lung cancer treatment. Given
the dosage of matrine used in TCM concoctions, concentrations of
matrine used in the present study should be well tolerated by the
human body. In a mouse model for squamous cell carcinoma, matrine
was administered at a dosage of 250 g/kg with no apparent adverse
impact on health (49).
Furthermore, it has been reported that matrine could ease the pain
of cancer patients receiving chemotherapy, enhance the body’s
immunity and protect major organs such as the heart, liver and
kidney from being damaged by chemicals/drugs during chemotherapy
(50–52), all of which could help improve the
quality of life of lung cancer patients receiving chemotherapy.
Clinically, lung cancer manifests in different
forms, and the underlying biology dictates the progression and
malignancy of the cancer such as the propensity to metastasize and
to be resistant to conventional therapies. While matrine has shown
some promise in several types of lung cancer in a tissue culture
system, it does not seem to kill cancer cells completely. This may
be due to the low concentration of matrine used in our experiments.
On the other hand, these data suggest that probably the most
effective use of matrine in lung cancer treatment is as an
adjuvant. Investigation of whether there exists any additive and/or
synergistic therapeutic benefits when applying a combination of
matrine and conventional chemotherapies to treat cancer cell
cultures or in animal models is warranted.
Our findings reported here corroborate previous
results that reveal matrine-induced anticancer activity in tissue
culture systems. The cancer-killing effect is likely through
inhibition of the PI3K-Akt-mTOR pathway and promotion of apoptosis
via downregulation of cIAPs. Given the prevalence of the
deregulated PI3K-Akt-mTOR pathway and overexpression of cIAPs in
many types of cancer, perhaps it is not surprising to find that
matrine is effective in killing a spectrum of cancer cells of
varying origins. A better understanding of the molecular basis of
matrine-induced apoptosis in lung cancer cells may not only shed
light on the pathogenesis of NSCLC but may also contribute to the
development of more effective combination therapies for the
treatment of advanced stage lung cancer.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (no. 81201832), the
Outstanding Scientific Fund of Shengjing Hospital and Specialized
Research Fund for the Doctoral Program of Higher Education (no.
20122104110011).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Y, Zhang H, Yu P, et al: Effects of
matrine against the growth of human lung cancer and hepatoma cells
as well as lung cancer cell migration. Cytotechnology. 59:191–200.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Efferth T, Konkimalla VB, Wang YF, et al:
Prediction of broad spectrum resistance of tumors towards
anticancer drugs. Clin Cancer Res. 14:2405–2412. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dong J, Su SY, Wang MY and Zhan Z: Shenqi
fuzheng, an injection concocted from Chinese medicinal herbs,
combined with platinum-based chemotherapy for advanced non-small
cell lung cancer: a systematic review. J Exp Clin Cancer Res.
29:1372010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu XS, Jiang J, Jiao XY, Wu YE and Lin
JH: Matrine-induced apoptosis in leukemia U937 cells: involvement
of caspases activation and MAPK-independent pathways. Planta Med.
72:501–506. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang HM and Li HQ: Anti-arrhythmic
effects of sophoridine and oxysophoridine. Zhongguo Yao Li Xue Bao.
20:517–520. 1999.
|
|
8
|
Ai J, Gao HH, He SZ, Wang L, Luo DL and
Yang BF: Effects of matrine, artemisinin, tetrandrine on cytosolic
[Ca2+]i in guinea pig ventricular myocytes. Acta
Pharmacol Sin. 22:512–515. 2001.PubMed/NCBI
|
|
9
|
Xu CQ, Dong DL, Du ZM, Chen QW, Gong DM
and Yang BF: Comparison of the anti-arrhythmic effects of matrine
and berbamine with amiodarone and RP58866. Yao Xue Xue Bao.
39:691–694. 2004.(In Chinese).
|
|
10
|
Hu ZL, Zhang JP, Yu XB, Lin W, Qian DH and
Wan MB: Effect of matrine on
lipopolysaccharides/D-galactosamine-induced hepatitis and tumor
necrosis factor release from macrophages in vitro. Zhongguo Yao Li
Xue Bao. 17:351–353. 1996.(In Chinese).
|
|
11
|
Liu J, Zhu M, Shi R and Yang M: Radix
Sophorae flavescentis for chronic hepatitis B: a systematic
review of randomized trials. Am J Chin Med. 31:337–354. 2003.
View Article : Google Scholar
|
|
12
|
Long Y, Lin XT, Zeng KL and Zhang L:
Efficacy of intramuscular matrine in the treatment of chronic
hepatitis B. Hepatobiliary Pancreat Dis Int. 3:69–72.
2004.PubMed/NCBI
|
|
13
|
Gao HY, Li GY, Lou MM, Li XY, Wei XY and
Wang JH: Hepato-protective effect of matrine salvianolic acid B
salt on carbon tetrachloride-induced hepatic fibrosis. J Inflamm.
9:162012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu JY, Hu JH, Zhu QG, Li FQ, Wang J and
Sun HJ: Effect of matrine on the expression of substance P receptor
and inflammatory cytokines production in human skin keratinocytes
and fibroblasts. Int Immunopharmacol. 7:816–823. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li H, Tan G, Jiang X, et al: Therapeutic
effects of matrine on primary and metastatic breast cancer. Am J
Chin Med. 38:1115–1130. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li LQ, Li XL, Wang L, et al: Matrine
inhibits breast cancer growth via miR-21/PTEN/Akt pathway in MCF-7
cells. Cell Physiol Biochem. 30:631–641. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu T, Song Y, Chen H, Pan S and Sun X:
Matrine inhibits proliferation and induces apoptosis of pancreatic
cancer cells in vitro and in vivo. Biol Pharm Bull. 33:1740–1745.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luo C, Zhong HJ, Zhu LM, et al: Inhibition
of matrine against gastric cancer cell line MNK45 growth and its
anti-tumor mechanism. Mol Biol Rep. 39:5459–5464. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang CL, Liu SS, Ma YG, Liu YY, Xue YX and
Huang B: The influence of intraoperative pleural perfusion with
matrine-cisplatin or cisplatin on stromal cell-derived factor-1 in
non-small cell lung cancer patients with subclinical pleural
metastasis. Med Oncol. 29:574–581. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang J, Li Y, Chen X, et al: Autophagy is
involved in anticancer effects of matrine on SGC-7901 human gastric
cancer cells. Oncol Rep. 26:115–124. 2011.PubMed/NCBI
|
|
21
|
Chui CH, Lau FY, Tang JC, et al:
Activities of fresh juice of Scutellaria barbata and warmed
water extract of Radix Sophorae Tonkinensis on
anti-proliferation and apoptosis of human cancer cell lines. Int J
Mol Med. 16:337–341. 2005.PubMed/NCBI
|
|
22
|
Hu MJ, Zeng H, Wu YL, et al: Synergistic
effects of matrine and 5-fluorouracil on tumor growth of the
implanted gastric cancer in nude mice. Chin J Dig Dis. 6:68–71.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Luo C, Zhu Y, Jiang T, et al: Matrine
induced gastric cancer MKN45 cells apoptosis via increasing
pro-apoptotic molecules of Bcl-2 family. Toxicology. 229:245–252.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma L, Wen S, Zhan Y, He Y, Liu X and Jiang
J: Anticancer effects of the Chinese medicine matrine on murine
hepatocellular carcinoma cells. Planta Med. 74:245–251. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu P, Liu Q, Liu K, Yagasaki K, Wu E and
Zhang G: Matrine suppresses breast cancer cell proliferation and
invasion via VEGF-Akt-NF-kappaB signaling. Cytotechnology.
59:219–229. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dai ZJ, Gao J, Ji ZZ, et al: Matrine
induces apoptosis in gastric carcinoma cells via alteration of
Fas/FasL and activation of caspase-3. J Ethnopharmacol. 123:91–96.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang S, Zhang Y, Zhuang Y, et al: Matrine
induces apoptosis in human acute myeloid leukemia cells via the
mitochondrial pathway and Akt inactivation. PLoS One. 7:e468532012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liang CZ, Zhang JK, Shi Z, Liu B, Shen CQ
and Tao HM: Matrine induces caspase-dependent apoptosis in human
osteosarcoma cells in vitro and in vivo through the upregulation of
Bax and Fas/FasL and downregulation of Bcl-2. Cancer Chemother
Pharmacol. 69:317–331. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang H, Hou C, Zhang S, et al: Matrine
upregulates the cell cycle protein E2F-1 and triggers apoptosis via
the mitochondrial pathway in K562 cells. Eur J Pharmacol.
559:98–108. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Niu H, Li H, Xu C and He P: Expression
profile of RhoGDI2 in lung cancers and role of RhoGDI2 in lung
cancer metastasis. Oncol Rep. 24:465–471. 2010.PubMed/NCBI
|
|
31
|
Niu H, Wang J, Li H and He P: Rapamycin
potentiates cytotoxicity by docetaxel possibly through
downregulation of survivin in lung cancer cells. J Exp Clin Cancer
Res. 30:282011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lu J, Luo Q, Cheng P, Liu X, Bai M and Tu
M: The role of matrine and mitogen-ativated protein
kinase/extracellular signal-regulated kinase signal transduction in
the inhibition of the proliferation and migration of human
umbilical veins endothelial cells induced by lung cancer cells.
Zhongguo Fei Ai Za Zhi. 12:747–752. 2009.(In Chinese).
|
|
33
|
Chen Q, Liu L and Cao H: Effects of
matrine on the growth inhibition, c-myc and hTERT protein
expression in human adenocarcinoma lung cancer cell line A549.
Zhongguo Fei Ai Za Zhi. 11:559–562. 2008.(In Chinese).
|
|
34
|
Nicholson KM and Anderson NG: The protein
kinase B/Akt signalling pathway in human malignancy. Cell Signal.
14:381–395. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Deveraux QL and Reed JC: IAP family
proteins - suppressors of apoptosis. Genes Dev. 13:239–252. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hu S and Yang X: Cellular inhibitor of
apoptosis 1 and 2 are ubiquitin ligases for the apoptosis inducer
Smac/DIABLO. J Biol Chem. 278:10055–10060. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Altieri DC: The molecular basis and
potential role of survivin in cancer diagnosis and therapy. Trends
Mol Med. 7:542–547. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Marioni G, Bertolin A, Giacomelli L, et
al: Expression of the apoptosis inhibitor protein survivin in
primary laryngeal carcinoma and cervical lymph node metastasis.
Anticancer Res. 26:3813–3817. 2006.PubMed/NCBI
|
|
39
|
Osaka E, Suzuki T, Osaka S, et al:
Survivin as a prognostic factor for osteosarcoma patients. Acta
Histochem Cytochem. 39:95–100. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tran J, Rak J, Sheehan C, et al: Marked
induction of the IAP family antiapoptotic proteins survivin and
XIAP by VEGF in vascular endothelial cells. Biochem Biophys Res
Commun. 264:781–788. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Harfouche R, Hassessian HM, Guo Y, et al:
Mechanisms which mediate the antiapoptotic effects of
angiopoietin-1 on endothelial cells. Microvasc Res. 64:135–147.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Als AB, Dyrskjot L, von der Maase H, et
al: Emmprin and survivin predict response and survival following
cisplatin-containing chemotherapy in patients with advanced bladder
cancer. Clin Cancer Res. 13:4407–4414. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hinnis AR, Luckett JC and Walker RA:
Survivin is an independent predictor of short-term survival in poor
prognostic breast cancer patients. Br J Cancer. 96:639–645. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nakagawa Y, Abe S, Kurata M, et al: IAP
family protein expression correlates with poor outcome of multiple
myeloma patients in association with chemotherapy-induced
overexpression of multidrug resistance genes. Am J Hematol.
81:824–831. 2006. View Article : Google Scholar
|
|
45
|
Watanuki-Miyauchi R, Kojima Y, Tsurumi H,
et al: Expression of survivin and of antigen detected by a novel
monoclonal antibody, T332, is associated with outcome of diffuse
large B-cell lymphoma and its subtypes. Pathol Int. 55:324–330.
2005. View Article : Google Scholar
|
|
46
|
Schlette EJ, Medeiros LJ, Goy A, Lai R and
Rassidakis GZ: Survivin expression predicts poorer prognosis in
anaplastic large-cell lymphoma. J Clin Oncol. 22:1682–1688. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Adida C, Haioun C, Gaulard P, et al:
Prognostic significance of survivin expression in diffuse large
B-cell lymphomas. Blood. 96:1921–1925. 2000.PubMed/NCBI
|
|
48
|
Altieri DC: Survivin, cancer networks and
pathway-directed drug discovery. Nat Rev Cancer. 8:61–70. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang Y, Zhang Z, Garbow JR, et al:
Chemoprevention of lung squamous cell carcinoma in mice by a
mixture of Chinese herbs. Cancer Prev Res. 2:634–640. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu Y, Xu Y, Ji W, et al: Anti-tumor
activities of matrine and oxymatrine: literature review. Tumour
Biol. Feb 14–2014.(Epub ahead of print).
|
|
51
|
Linglu D, Yuxiang L, Yaqiong X, et al:
Antinociceptive effect of matrine on vincristine-induced
neuropathic pain model in mice. Neurol Sci. Dec 14–2013.(Epub ahead
of print).
|
|
52
|
Haiyan W, Yuxiang L, Linglu D, et al:
Antinociceptive effects of matrine on neuropathic pain induced by
chronic constriction injury. Pharm Biol. 51:844–850. 2013.
View Article : Google Scholar : PubMed/NCBI
|